Abstract
Cardiac tissue engineering will remain only a prospect unless large numbers of therapeutic cells can be provided, either from small samples of cardiac cells or from stem cell sources. In contrast to most adult cells, cardiomyocytes are terminally differentiated and cannot be expanded in culture. We explored the feasibility of enabling the in vitro expansion of primary neonatal rat cardiomyocytes by lentivector-mediated cell immortalization, and then reverting the phenotype of the expanded cells back to the cardiomyocyte state. Primary rat cardiomyocytes were transduced with simian virus 40 large T antigen (TAg), or with Bmi-1 followed by the human telomerase reverse transcriptase (hTERT) gene; the cells were expanded; and the transduced genes were removed by adenoviral vector expressing Cre recombinase. The TAg gene was more efficient in cell transduction than the Bmi-1/hTERT gene, based on the rate of cell proliferation. Immortalized cells exhibited the morphological features of dedifferentiation (increased vimentin expression, and reduced expression of troponin I and Nkx2.5) along with the continued expression of cardiac markers (α-actin, connexin-43, and calcium transients). After the immortalization was reversed, cells returned to their differentiated state. This strategy for controlled expansion of primary cardiomyocytes by gene transfer has potential for providing large amounts of a patient's own cardiomyocytes for cell therapy, and the cardiomyocytes derived by this method could be a useful cellular model by which to study cardiogenesis.
Introduction
Myocardial infarction affects more than 500,000 patients in the United States each year (Kirkpatrick et al., 2007), largely because of minimal endogenous cardiac regeneration that cannot compensate for the massive loss of cardiomyocytes after myocardial infarction. Tissue engineering of a functional cardiac patch is being studied as a potential way to replace damaged myocardium. However, engineering a clinically sized cardiac patch (centimeters in size, millimeters thick) requires large numbers of cells, because of the high cell density (∼108 cells/cm3) that needs to be established for functional cell coupling and signaling (Gerecht-Nir et al., 2006).
A small fraction of adult heart cells has stem cell-like properties (Moretti et al., 2006); however, it is difficult to isolate and expand these cells in vitro, because of their low frequency and limited access. Transplantation of exogenous adult and embryonic stem cells showed improved myocardial function by increasing vascularization and reducing ventricular remodeling (Orlic et al., 2001; Kehat et al., 2004). However, these cells may generate immunogenic or tumorigenic problems. Breakthroughs resulting in the derivation of induced pluripotent stem cells (iPS) by the introduction of four transcription factors (Oct3/4, Sox2, Klf4, and c-Myc) into adult cells represents a potential way to address this issue (Takahashi and Yamanaka, 2006; Takahashi et al., 2007), and may offer a source of autologous functional cardiomyocytes (Mauritz et al., 2008; Narazaki et al., 2008). It remains to be determined whether these cells have all the properties of embryonic stem cells, and how much phenotypic stabilization needs to be done in vitro to avoid the risk of teratoma formation.
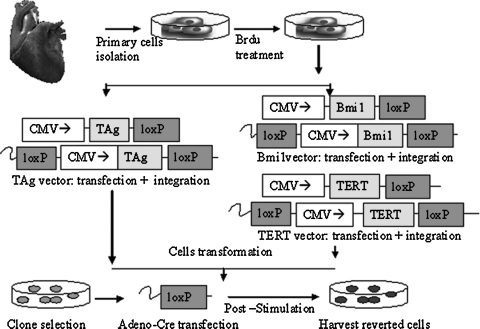
A potential alternative approach is that of expanding nonproliferating primary cardiomyocytes, by step-wise immortalization that requires a combination of immortalization genes (Hahn et al., 1999), followed by reversal into the terminally differentiated phenotype after the cells are expanded in culture. The process of reversible immortalization involves transfer and expression of specific immortalization genes in primary cells, and the subsequently removal of these genes by Cre–loxP site recombination. So far, reversible immortalization had been successfully applied to primary fibroblasts (Westerman and Leboulch 1996), human muscle satellite cells (Cudré-Mauroux et al., 2003), human insulin secreting beta cells (Narushima et al., 2005), and other types of cells (Kobayashi et al., 2001; Noguchi et al., 2002; Kowolik et al., 2004; Hashimoto et al., 2006). Here, we explore whether this method of gene transfer may be used to expand primary cardiac cells to numbers that are sufficient for basic research and regenerative medicine applications. We selected neonatal rat cardiomyocytes as model cells because they represent a widely used “standard” for cardiac tissue engineering studies. It has been established that neonatal rat cardiomyocytes undergo terminal differentiation stage during early neonatal myocardial growth, after which cellular proliferation no longer occurs (Simpson and Savion, 1982; Long et al., 1990). For example, the 8-day culture of cell populations harvested from 1-day-old neonatal rat hearts resulted in a 6-fold increase in numbers of vascular cells and fibroblasts, and no increase in the number of cardiomyocytes (Long et al., 1990). This is consistent with our experience in studies of cardiac tissue engineering using neonatal rat heart cells (Radisic et al. 2008). We studied reversible immortalization of primary neonatal rat cardiomyocytes that were (1) immortalized by two different combinations of genes (simian virus 40 large T antigen [TAg], or Bmi-1/human telomerase reverse transcriptase [hTERT]), (2) expanded in culture, and (3) reversed back to cardiac phenotype (Fig. 1).
FIG. 1.
Experimental design for reversible immortalization of cardiac myocytes. Freshly isolated neonatal rat cardiomyocytes were preplated for 1 hr and cultured in monolayer, using DMEM supplemented with bromodeoxyuridine (BrdU, 100 μM) for 3–4 days to eliminate fibroblast contamination. The cells were then transduced either with lentiviral vector expressing simian virus 40 large T antigen (TAg) under the control of the cytomegalovirus (CMV) promoter, or with Bmi-1 vector and then human telomerase reverse transcriptase (hTERT) vector. The transformed cells were subjected to single cell cloning to yield TAg clone 8 or Bmi-1 clone 4. The cells were then infected with a recombinant adenovirus expressing Cre recombinase (Ad-CMV-Cre), to remove the TAg, Bmi-1, and hTERT genes that were flanked by loxP sites. Biophysical stimulation (100 μM phenylephrine [PE], 10 μM norepinephrine [NE], vascular endothelial growth factor [VEGF, 10 ng/ml], and dickkopf homolog-1 [DKK1, 150 ng/ml]) was applied to the reverted cells.
Materials and Methods
Lentiviral vector production
loxP-containing human immunodeficiency virus (HIV)-derived lentiviral vectors expressing enhanced fluorescent green protein (GFP), TAg, Bmi-1, and hTERT from an internal human cytomegalovirus (CMV) immediate promoter (Salmon et al., 2000) were obtained from Addgene (plasmids 12243, 12246, 12240, and 12245; Addgene, Cambridge, MA). The recombinant retroviruses were produced by established methods (Naghavi et al., 2005). In brief, the lentiviral vector expressing the target gene, the lentiviral packaging construct (pCMVR8.91) encoding the HIV-1 Gag and Pol precursors, and the vesicular stomatitis virus (VSV) G envelope construct (pMD.G) (kindly provided by S. Goff, Department of Microbiology, Columbia University, NY) were cotransfected into 293T cells in the presence of FuGENE 6 (Roche, Basel, Switzerland). The culture fluid was harvested 48–72 hr posttransfection and stored at −80°C. The titer of recombinant viruses was >5 × 104 transduction units/ml, as determined by flow cytometric analysis of 293T cells or diluted colonies, counting after selection in hypoxanthine–aminopterin–thymidine (HAT) medium (Sigma-Aldrich, St. Louis, MO).
Isolation, culture, and transduction of primary neonatal cardiomyocytes
Primary cultures of neonatal rat ventricular cardiomyocytes were derived according to previously published procedures (Radisic et al., 2008). In brief, cardiomyocytes were obtained from 1- to 2-day-old neonatal Sprague-Dawley rats by means of a protocol approved by the committee on animal care at Columbia University (New York, NY). The ventricles were quartered, dissociated overnight at 4°C in a 0.06% (w/v) solution of trypsin in Hanks' balanced salt solution (HBSS; GIBCO, Grand Island, NY), washed in culture medium (Dulbecco's modified Eagle's medium [DMEM] supplemented with 10–20% fetal bovine serum [FBS], 10 mM HEPES, 2 mM l-glutamine, and penicillin–streptomycin [100 units/ml]; GIBCO), and then subjected to a series of digestions (8 min at 37°C; 75 rpm) in a 0.1% (w/v) solution of collagenase type II in HBSS. The harvested cells were then pooled and resuspended in culture medium. Cells were preplated for one 60-min period to enrich for cardiomyocytes. The cells were seeded at a density of 20 × 103 cells/cm2 in 6-well plates. Bromodeoxyuridine (BrdU, 100 μM; Sigma-Aldrich) was added to the growth medium for 3–4 days to eliminate proliferating fibroblast cells and progenitor cells (Lokuta et al., 1994; Miragoli et al., 2006).
For transduction, cardiomyocytes were exposed to lentiviral vectors at a multiplicity of infection (MOI) of 0.5–2 in the presence of Polybrene (10 μg/ml; Sigma-Aldrich) at 37°C for 6 hr (protocol adapted from Noguchi et al., 2002; Naghavi et al., 2005). For Bmi-1 and hTERT transduction, the infection was repeated for 3–4 days. For single cell cloning, 1000 transduced cells were plated on a 100-mm culture dish coated with methylcellulose-based medium (R&D Systems, Minneapolis, MN). Colonies were isolated and expanded for experimentation.
Western blot
Cells were lysed in cell extraction buffer (Invitrogen, Carlsbad, CA). Total protein from lysate (15 μg per sample) was loaded for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and the proteins were transferred to nitrocellulose membranes. Membranes were blocked in 10% milk and incubated with primary antibodies: mouse monoclonal antibodies against TAg and Bmi-1 (Santa Cruz Biotechnology, Santa Cruz, CA), and against TERT and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) (Abcam, Cambridge, MA) at dilutions of 1:200–1:500, followed by secondary antibodies against mouse IgG conjugated with horseradish peroxidase (GE Healthcare, Buckinghamshire, UK). Signal was detected with enhanced chemiluminescence (ECL) reagent (GE Healthcare).
Measurement of cell growth kinetics
Primary heart cells isolated from neonatal rat were transduced with lentiviral vectors expressing TAg or Bmi-1/hTERT genes, and their growth kinetics were monitored by measuring the cell number in each of the first 6–8 passages of culture in the laboratory. The number of population doublings at each passage was defined as log N/log 2, where N is the number of cells harvested at confluence divided by the number of cells initially seeded. Alternatively, DNA was measured with a PicoGreen dsDNA kit (Invitrogen) according to the manufacturer's protocol. The cytotoxicity of adenovirus was tested in a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich).
Reversal to differentiated cell phenotype
Purified adenoviral vector expressing Cre recombinase under the control of the CMV promoter (Ad-CMV-Cre) (Vector Biolabs, Philadelphia, PA) was stored at −80°C at a titer of 2 × 107 transduction units/ml. TAg-transduced or Bmi-1/hTERT-transduced cells were infected with Ad-CMV-Cre at an MOI of 100–500 for 6–12 hr. The medium was then replaced with growth medium and the Cre-transduced cells were continuously cultured for another 4 days and passaged, to measure the proliferation capability of cells after Ad-CMV-Cre transduction. For further phenotype characterization experiments on the transduced cells after reversal, the cells were transduced by Ad-CMV-Cre at the required concentration (TAg clone 8, MOI: 300; Bmi-1/hTERT clone 4, MOI: 400) and were maintained in differentiation medium: DMEM–F12 containing penicillin–streptomycin (100 units/ml), 2 mM l-glutamine, 5% heat-inactivated FBS, insulin (10 μg/ml), transferrin (5.5 μg/ml), and sodium selenite (6.7 ng/ml) (Sigma-Aldrich) and further supplemented with 100 μM phenylephrine (PE) and 10 μM norepinephrine (NE) (Sigma-Aldrich) or with vascular endothelial growth factor (VEGF, 10 ng/ml)/dickkopf homolog-1 (DKK1, 150 ng/ml; R&D Systems).
Immunofluorescence studies of marker expression
Cells were fixed in 4% paraformaldehyde for 20 min at room temperature, blocked with 10% horse serum (Vector Biolabs) for 40 min at room temperature, and then incubated for 1 hr at 37°C with primary antibodies: mouse anti-cardiac troponin I (diluted 1:150; Biodesign, Saco, ME), mouse anti-sarcomeric α-actin (diluted 1:150; Sigma-Aldrich), rabbit anti-connexin-43 (Cx-43, diluted 1:150; Chemicon, Temecula, CA), rabbit anti-vimentin (diluted 1:150; Chemicon), and rabbit anti-Nkx2.5 (diluted 1:50; Santa Cruz Biotechnology) in phosphate-buffered saline (PBS) containing 0.5% Tween 20 and 1.5% horse serum. Subsequently, the cells were incubated with secondary antibodies: Texas red-conjugated horse anti-IgG (diluted 1:200; Chemicon) and fluorescein-conjugated horse anti-mouse IgG (diluted 1:200; Chemicon). The nuclei were costained with 4′,6-diamidino-2-phenylindole (DAPI, diluted 1:1000; Vector Biolabs). The images were captured by fluorescence microscopy (IX81; Olympus, Tokyo, Japan) and the MetaMorph program (Molecular Devices, Downingtown, PA). The Matlab program CONNEXINMEASUREMENT, developed by our laboratory (Tandon et al., 2009), was adapted to quantify the presence of Cx-43 protein. The number of instances of Cx-43 was measured in n > 40 sections per experimental group, and normalized by the cell number (measured by counting DAPI-stained nuclei in the same sections).
Measurements of calcium transients
The excitation–contraction coupling between cells was evaluated by measuring calcium transients. Briefly, cells were grown in 35-mm tissue culture dishes for 2–3 days until 70–80% confluent and loaded with 2 μM Fluo-4 (Molecular Probes/Invitrogen, Carlsbad, CA) in PBS for 30 min at 37°C, washed with PBS, and incubated in growth medium for another 30 min to allow complete deesterification of intracellular acetoxymethyl ester (AM). Last, the cells were loaded with pulsing buffer containing 255 mM sucrose, 1 mM CaCl2, 1 mM MgCl2, and 5 mM HEPES (conductivity, 500 μS/cm). A pair of carbon electrodes (1/8-in. diameter, spaced 3.2 in. apart; Ladd Research Industries, Williston, VT) was connected to an electrical stimulator (Grass Technologies, West Warwick, RI) via a platinum wire (Ladd Research Industries) and placed in the tissue culture chamber. For excitation, field stimulation was applied at a frequency of 0.3–1 Hz, current pulses of 2 to 100 msec in duration, and a signal amplitude of 1–34.2 V/cm. Calcium transient images were acquired with a fluorescence microscope (IX81; Olympus), stored, and analyzed digitally with the ImageJ program (National Institutes of Health, Bethesda, MD). For each image, fluorescence intensity was integrated over the entire cell culture space and corrected by subtracting the background fluorescence measured for an area of the same size but without cultured cells.
Results
Lentivector-mediated conditional immortalization of primary cardiomyocytes
Cardiomyocytes isolated from neonatal rat left ventricles were treated with BrdU (100 μM), a mitotic inhibitor, which binds to the newly synthesized DNA during the S phase of the cell cycle, substituting for thymidine during DNA replication. A high concentration of BrdU in vitro effectively blocked the proliferation of dividing cells (see Supplementary Fig. 2 at www.liebertonline.com/hum), including fibroblasts and progenitor cells, presumably because of activation of classical apoptosis pathways in BrdU-incorporating cells (Xia et al., 1995; Caldwell et al., 2005). After BrdU incorporation, the cardiac fibroblasts could not be passaged (Lokuta et al., 1994; Miragoli et al., 2006) or transduced by lentivirus-mediated TAg or Bmi-1/hTERT transfection. Likewise, we were not able to produce any clones from the BrdU-treated fibroblasts.
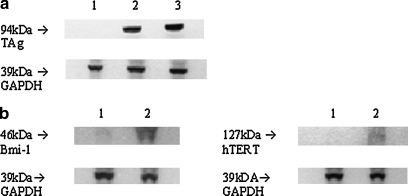
Approximately 10–20% of the purified cardiomyocytes exposed to lentiviral vector expressing GFP from the CMV promoter at an MOI of 2 were transduced (see Supplementary Fig. 1C at www.liebertonline.com/hum). By the same cell preparation protocol, DNAs encoding TAg, Bmi-1, hTERT were introduced by lentivector-mediated transduction into cardiomyocytes, either alone or in combinations. Insertion of the gene was confirmed by Western blot analysis of the expressed protein (Fig. 2).
FIG. 2.
Western blot analysis of lentiviral vectors. Expression of TAg and Bmi-1 followed by hTERT in infected cells was determined before and after Cre expression. (a) Lane 1, TAg clone 8 cells (passage 11) after Cre expression; lanes 2 and 3, TAg clone 8 cells before Cre expression (passage 11 and passage 3, respectively). (b) Lane 1, Bmi-1/hTERT clone 4 cells (passage 8) after Cre expression; lane 2, Bmi-1/hTERT clone 4 cells before Cre expression.
Cells quickly responded to TAg gene by increased cell proliferation, within 1–2 weeks of transduction. In contrast, cells transduced with Bmi-1/hTERT exhibited a significant delay before starting to form stabilized proliferative clones (Fig. 3). The delay was probably due to the low Bmi-1-induced immortalization frequency (Hashimoto et al., 2006) and slow cell proliferation rate in the single cell cloning process. Therefore, we repeatedly transduced cells with Bmi-1 vector (three or four times), followed by transduction of hTERT three or four times to increase the immortalization frequency (protocol adapted from Noguchi et al., 2002; Kowolik et al., 2004). Without hTERT transduction, Bmi-1-transformed cells undergo replicative senescence within five passages. With only hTERT, primary cardiomyocytes cannot be transduced, as evidenced by the lack of clone induction with up to five rounds of transfection in multiple experiments.
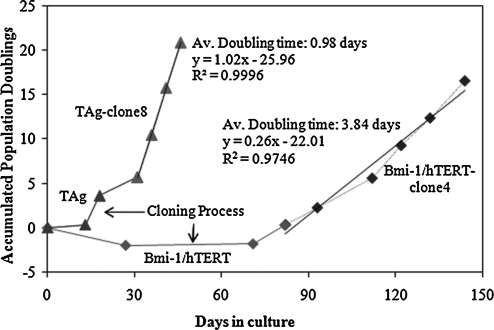
FIG. 3.
Proliferation of immortalized cardiomyocytes. The proliferation of cardiac myocytes transduced with TAg or with Bmi-1/hTERT in the first six to eight passages of culture in the laboratory. Cell growth kinetics were monitored by measuring accumulated population doublings. Cell culture medium was DMEM supplemented with 10% FBS, 10 mM HEPES, 2 mM l-glutamine, and penicillin–streptomycin (100 U/ml).
The average doubling time of TAg-containing cells (TAg clone 8) was 0.98 days, whereas the average doubling time of Bmi-1/hTERT-containing cells (Bmi-1/hTERT clone 4) was 3.84 days (Fig. 3). When cells were seeded at a higher density of 104 cells/cm2 and cultured in growth medium containing 20% FBS, the doubling time fell to 2.5 days for Bmi-1/hTERT-transduced cells (Bmi-1/hTERT clone 4). TAg-transduced cells had more compact cell morphology, a lower tendency to align or flatten (Fig. 4), and tended to form nodules, whereas Bmi-1/hTERT-transduced cells maintained contact inhibition and did not grow on top of each other.
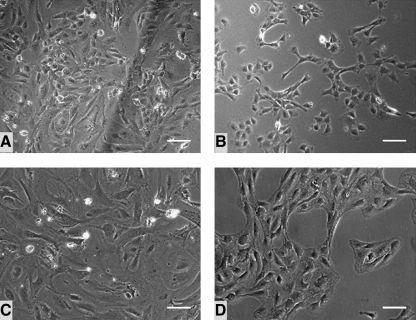
FIG. 4.
Cell morphology. (A) TAg-transduced cells on day 7. (B) TAg clone 8 cells. (C) Bmi-1/hTERT-transduced cells on day 13. (D) Bmi-1/hTERT clone 4 cells. Cells immortalized with Bmi-1 and hTERT appeared large and elongated, whereas TAg-containing cells were smaller in size and more rounded in shape. Scale bars: 100 μm.
Remarkably, some of the Bmi-1/hTERT-transduced cells maintained contractile activity until passage 3 or 4. When the derived cells were seeded at low density onto methylcellulose-coated plates, they proliferated and formed well-defined colonies. In total, 28 clones of TAg-transduced cells and 7 clones of Bmi-1/hTERT-transduced cells were isolated. None of the colonies showed contractile activity beyond passage 3 or 4. We scanned all the clones on the basis of immunostaining (Cx-43, α-actin, and vimentin) and morphology, and then selected the best clones, TAg clone 8 and Bmi-1/hTERT clone 4, for in-depth analysis.
Reversal of cell immortalization after Cre recombinase expression
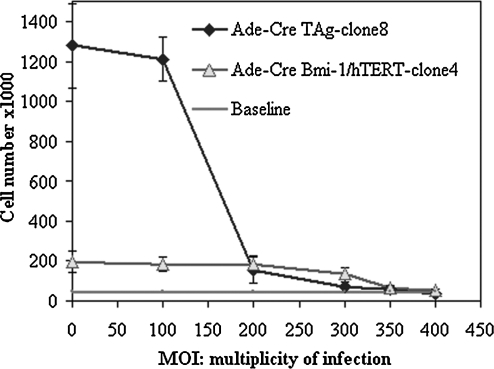
Both TAg- and Bmi-1/hTERT-transduced cells proliferated without apparent senescence for more than 6 months of culture. To totally reverse the immortalization by Cre–loxP site recombination, we tested the infection of the transduced cells with adenovirus expressing Cre recombinase (Ad-CMV-Cre) at various concentrations (MOI, 100–500). Only at a high concentration (TAg clone 8, MOI ≥ 300; Bmi-1/hTERT clone 4, MOI: 400) of Ad-CMV-Cre transduction did cell proliferation completely cease for the duration of the study (Fig. 5). The high MOI adenovirus transduction was probably due to the limited expression of certain interaction receptors on the transduced cell surface, such as coxsackievirus and adenovirus receptor (CAR) (Li et al., 1999) and heparan sulfate proteoglycans (HSPGs) (Fender et al., 2008). The removal of immortalization genes from the cellular genome was confirmed by Western blot (Fig. 2). After 6 hr of incubation with adenoviral vector Ad-CMV-Cre, the bioactivity of control cells (nontransduced cells) was reduced by 12.30% (MOI, 300) or 35.71% (MOI, 400) by MTT measurement.
FIG. 5.
Effect of multiplicity of infection (MOI) on cell proliferation. Cells were harvested, counted, and passaged 4 days after Cre transduction. Baseline: initial seeding cell number. After high-dose Cre transduction (MOI, 300 for TAg clone 8; MOI, 400 for Bmi-1/hTERT clone 4), the transfected cells stopped growing as confirmed by lack of proliferation in the next passage. Data are shown as averages ± SD (n = 4).
Phenotype characterization of transduced cells before and after reversal
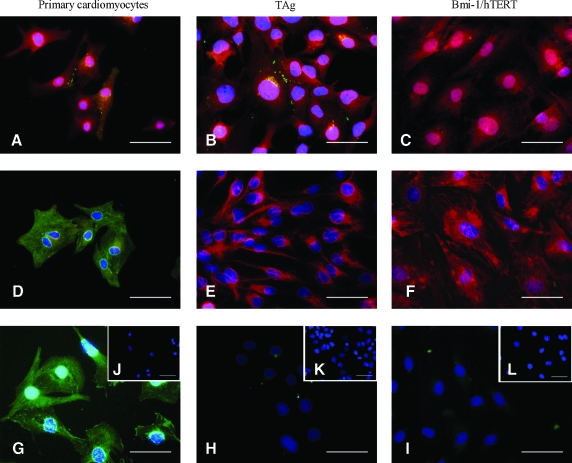
Immunofluorescence analysis of transduced cells showed expression of α-actin, a prototypical Z-line protein in cardiomyocytes, and Cx-43, a connexin gap junction protein in cardiac muscles (Fig. 6). TAg clone 8 cells exhibited normal patterns of Cx-43 expression in cell junctions, whereas Bmi-1/hTERT clone 4 cells did not exhibit these cardiac-specific patterns. Both clones had low expression of troponin I, a regulatory protein for cardiac muscle contraction; low expression of Nkx2.5, a transcription factor related to cardiac differentiation; and high expression of vimentin, which was consistent with the dedifferentiation of cells during transformation (LaFramboise et al., 2007; Nakamura et al., 2008). Both clones maintained Cx-43 and α-actin expression; however, only TAg clone 8 exhibited localized Cx-43 expression in cell–cell junctions.
FIG. 6.
Phenotypic characterization of transduced cardiomyocytes. (A–C) Cx-43 (FITC) and α-actin (Texas red) staining of (A) primary cardiomyocytes, (B) TAg clone 8 cells, and (C) Bmi-1/hTERT clone 4 cells. (D–F) Troponin (FITC) and vimentin (Texas red) staining of (D) primary cardiomyocytes, (E) TAg clone 8 cells and (F) Bmi-1/hTERT clone 4 cells. (G–I) Nkx2.5 (FITC) staining of (G) primary cardiomyocytes, (H) TAg clone 8 cells, and (I) Bmi-1/hTERT clone 4 cells. (J–K) Isotype controls for (J) primary cardiomyocytes, (K) TAg clone 8 cells, and (L) Bmi-1/hTERT clone 4 cells. Scale bars: 50 μm. Color images available online at www.liebertonline.com/hum.
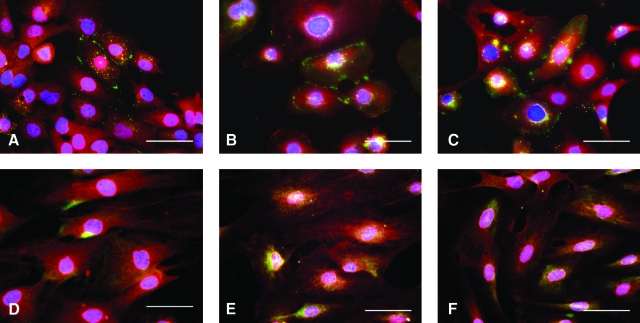
We tested a group of biochemical simulation factors including 100 μM PE and 10 μM NE, and growth factors including VEGF (10 ng/ml) and DKK1 (150 ng/ml), on postexpansion differentiation. Cx-43 quantification, using a Matlab program (Tandon et al., 2009), showed that the frequency of Cx-43 in primary cardiomyocytes (2.87 per cell) decreased after TAg-induced transformation (to 1.52 per cell) and then increased again after Cre recombinase reversion (to 1.62 per cell) as well as after stimulation with PE (to 1.72 per cell) and growth factors DKK1 and VEGF (to 2 per cell). Growth factor stimulation and NE also led to increases in α-actin and Cx-43 in Bmi-1/hTERT-transduced cells after reversion (Fig. 7); however, these stimulations still could not generate well-developed cardiac-specific patterned Cx-43.
FIG. 7.
Phenotypic characterization of reverted clones after Cre transduction. (A–C) Cx-43 (FITC) and α-actin (Texas red) staining of TAg clone 8 cells after Cre transduction (A), TAg clone 8 cells after Cre transduction and PE stimulation (B), and TAg clone 8 cells after Cre transduction and GF stimulation (C). (D–F) Cx-43 (FITC) and α-actin (Texas red) staining of Bmi-1/hTERT clone 4 cells after Cre transduction (D), Bmi-1/hTERT clone 4 cells after Cre transduction and NE stimulation (E), and Bmi-1/hTERT clone 4 cells after Cre transduction and GF stimulation (F). PE, phenylephrine (100 μM); NE, norepinephrine (10 μM); GF, VEGF (10 ng/ml) and DKK1 (150 ng/ml). Scale bars: 50 μm. Color images available online at www.liebertonline.com/hum.
Spontaneous and induced calcium transients in immortalized cells
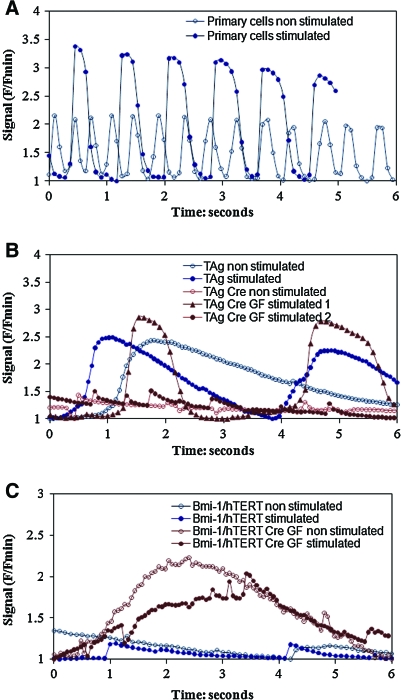
Primary cardiomyocytes exhibited spontaneous contractions associated with calcium transients, with the fastest transient rate observed at a frequency of 2 Hz, which is in the physiologic range for rats (Fig. 8A). When contractile function was induced by electrical stimulation, primary cardiomyocytes showed excitable calcium transients in a relatively lower electrical field (20 V/cm) and short pulse duration (2 msec), with capture rates of up to 1 Hz. TAg clone 8 cells also exhibit spontaneous calcium transients at much lower frequency (<0.2 Hz), and they could only be excited at lower frequency (0.3 Hz) with a higher electrical field (34.2 V/cm) and longer pulse duration (100 msec) (Fig. 8B). Bmi-1/hTERT clone 4 cells were quiescent and did not undergo any apparent changes in calcium content, when given the same stimulation as TAg clone 8 cells (Fig. 8C).
FIG. 8.
Calcium transients in primary cardiomyocytes and transduced cells. (A) Spontaneous and paced calcium transient in primary cells (stimulation: 20 V/cm, 1 Hz, 2-msec duration). (B) Spontaneous and paced calcium transient in TAg clone 8 cells before and after Cre expression with growth factor treatment. (Before Cre expression, stimulation: 34.2 V/cm, 0.3 Hz, 100-msec duration; After Cre expression and growth factor treatment, stimulation 1: 34.2 V/cm, 0.3 Hz, 100-msec duration; stimulation 2: 34.2 V/cm, 1 Hz, 2-msec duration). (C) Spontaneous and paced calcium transient in Bmi-1/hTERT clone 4 cells before and after Cre expression with growth factor treatment (stimulation: 34.2 V/cm, 0.3 Hz, 100-msec duration). Color images available online at www.liebertonline.com/hum.
After Cre transduction, the reverted TAg-transduced cells showed increased spontaneous calcium transients at a frequency of 1 Hz (VEGF and DKK1 treatment; Fig. 8B) or ∼0.25 Hz (NE, PE, or Cre only; see Supplementary Fig. 3E, G, and C at www.liebertonline.com/hum). With electrical stimulation at 34.2 V/cm and a pulse duration of 100 msec, all reverted TAg-transduced cells could be excited except for the nontreatment group (Supplementary Fig. 3C, E, G, and I). After VEGF and DKK1 treatment, reverted TAg-transduced cells exhibited an increased excitable calcium response frequency up to 1 Hz, suggesting enhanced cell excitability after removing the TAg gene and growth factor stimulation (Supplementary Fig. 3I). In contrast, after removing the Bmi-1/hTERT gene, these effects were not seen—the cells exhibited enhanced spontaneous transients (<0.2 Hz) and increased amplitude of calcium changes, but could not respond to electrical stimulation. After VEGF and DKK1, PE, or NE treatment, reverted Bmi-1/hTERT-transduced cells exhibited enhanced spontaneous transients (<0.2 Hz), and an increased amplitude of calcium changes; however, the cells did not show paced transients in response to electrical stimulation (Supplementary Fig. 3D, F, H, and J).
Discussion
Our experiments show that reversible immortalization has the potential to generate large numbers of patient-specific cells for cardiac tissue engineering. First, we proved lentiviral vectors were efficient in introducing genes into primary cardiomyocytes; approximately 10–20% of the purified primary cardiomyocytes could be infected with lentiviral vector at an MOI of 2. The high transduction efficiency of lentiviral vector was the result of their ability to mediate stable integration and long-term expression of transgenes in a wide variety of targets irrespective of their proliferative status (Salmon et al., 2000). However, potential insertional mutagenesis may occur through the random integration of inserted lentiviral vectors; site-specific insertion by adeno-associated virus (AAV)-mediated transduction may be a promising option for future work.
Second, we compared the application of two frequently used immortalizing genes (Cudré-Mauroux et al., 2003): TAg, which acts through the binding of retinoblastoma protein (Rb) and p53 (Ali and DeCaprio, 2001), and Bmi-1, which downregulates the p16 and p19Arf tumor suppressor genes encoded by the ink4a locus (Jacobs et al., 1999), and is often combined with human telomerase (hTERT) (Hashimoto et al., 2006). Lentivector-mediated transduction of TAg alone, or of Bmi-1/hTERT, resulted in the immortalization of primary neonatal rat cardiomyocytes such that they acquired the ability to proliferate over prolonged periods of time (up to 6 months of continuous culture during our studies). In contrast, Bmi-1 alone was not able to sustain continuous cell growth. Consistently, the cell phenotypes associated with the transduction of these two sets of immortalization genes were distinctly different. The TAg gene was expressed at high levels, and induced fast cell proliferation without a lag phase, which led to cells arranged in colonies and round in shape, aggregating and growing without contact inhibition in most clones. In contrast, Bmi-1/hTERT transduction delayed cell proliferation, which occurred at a much slower rate, and Bmi-1/hTERT-transduced cells exhibited more extended cell morphology, and maintained the contact inhibition growth pattern in all clones, which was possibly due to the low levels of gene expression. The method established in this study enabled us to start with a small number of primary cells (<600,000), transform the cells, and derive stable TAg-transduced clones within approximately 3 weeks. The method could potentially be used to derive large numbers of repair cells from a small biopsy sample, for use in cardiac therapy.
Interestingly, certain phenotype characteristics of primary cardiomyocytes (expression of α-actin, patterned Cx-43, and spontaneous and induced calcium transients) were conserved through multiple generations of TAg-transduced cells. In particular, the excitation–contraction coupling measured as calcium transients showed that the sarcoplasmic reticulum of TAg clone 8 cells was still able to release calcium in response to electrical stimulation. The lack of response to electrical stimulation at higher frequencies may be attributed to delayed restoration of excitability, because intracellular calcium in these cells may return to the basal level more slowly than in primary cardiomyocytes. The presence of cardiac-specific features in Bmi-1/hTERT-transduced cells was less evident than in TAg-transduced cells (the expression of α-actin was conserved, but Cx-43 patterning and calcium responses were not).
After adenovirus-mediated Cre recombinase expression, which spliced the inserted genes, we noticed a complete arrest of cell proliferation and increased expression of Cx-43 in TAg-transduced cells. To redifferentiate cardiac phenotypes in those reverted cells, we tested a group of biochemical stimulatory molecules including PE, NE, VEGF, and DKK1. PE and NE are pharmacologic agents, used in clinics to elicit varied effects on vascular resistance, myocardial contractility, and heart rate (Coons and Seidl, 2007). Cellular studies showed that PE or NE could further induce sarcomere formation in some transformed cardiac cells (Rybkin et al., 2003). In our studies, the supplementation of culture medium with PE or NE enhanced the expression of α-actin as well as Cx-43 in reverted cells. VEGF and DKK1 are growth factors used in the cardiovascular differentiation of embryonic stem cells, through stage-specific inhibition of Wnt signaling (Yang et al., 2008). VEGF and DKK1 enhanced the expression of α-actin and Cx-43 in both TAg-transduced and Bmi-1/hTERT-transduced cells after reversion. Stimulation with VEGF and DKK1 also increased the calcium transient frequency in reverted TAg-transduced cells, suggesting improved capacity of the cells for electrical communication.
Our findings are consistent with the previous findings that Wnt signaling and VEGF are important modulators of Cx-43 expression in cardiomyocytes (Ai et al., 2000; Yamada et al., 2005). Because inappropriate expression of Wnt signaling had been found in a variety of cell transformations (Smalley and Dale, 1999), we hypothesized that Wnt signaling was triggered during cell immortalization, causing the loss of some properties of the cardiac phenotype, such as the decrease in Cx-43. Therefore, modulation of Wnt signaling with growth factors may present a method to redifferentiate the cardiac phenotype after reversion. Taken together, these data suggest that the immortalization of terminally differentiated cardiac myocytes could have practical value. Further phenotypic analysis of the cells in vivo should be included for future study, in order to better characterize the functional value of the reversible immortalization of cardiomyocytes.
This study shares some similarities with studies of reprogrammed (iPS) cells (Mauritz et al., 2008; Narazaki et al., 2008). Both strategies use lentivirus-based gene transduction to induce adult primary cells into dedifferentiated proliferating status, and apply growth factors to redifferentiate the expanded cells into specific lineages. Notably, when reversible immortalization is used, the expanded cells are depleted of the inserted genes via loxP–Cre recombination. As a result of complete gene splicing, cell proliferation was completely arrested, enabling the elimination of potential tumorigenic problems in transplantation practice.
The iPS research suggested various strategies of gene transfer toward deriving fully functional, mature cell phenotypes. The TAg gene interacts with cell cycling-related factors, such as p53 (Schultz et al., 2000), which induces cell proliferation, but is also involved in the control of DNA repair and recombination. In iPS studies, differentiated cell phenotypes are derived through a series of steps starting with early cell differentiation. In studies of reversible immortalization, the process is being shortened by picking the most “promising” clones that express specific key phenotypes and by regaining the lost cell phenotypes by proper stimulation after gene splicing. This “shortcut” strategy worked well with many cell types, such as human insulin-secreting beta cells (Narushima et al., 2005). If, however, the full phenotypic characteristics of cardiomyocytes are not restored (in particular, the contractile function), we may need to implement some of the strategies used to derive iPS cells.
In summary, the strategy for controlled expansion of primary cardiomyocytes by gene transfer has the potential to provide large amounts of a patient's own cardiomyocytes for cell therapy. Further studies are needed to determine whether the phenotypic properties of reversed cells are sufficient for cell therapy of the heart. In turn, because of the partial expression of the cardiac phenotype, these cells could serve as an interesting model for studies of cardiac differentiation.
Supplementary Material
Acknowledgments
The authors are thankful to Dr. Stephen Goff, Dr. Susana T. Valente, and Dr. Ettaly K. Franke for their kind help with the DNA extraction and virus production. The authors also gratefully acknowledge the funding of this work by the National Institutes of Health (grants R01 HL076485 and R21 HL089913).
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- Ai Z. Fischer A. Spray D.C. Brown A.M. Fishman G.I. Wnt-1 regulation of connexin43 in cardiac myocytes. J. Clin. Invest. 2000;105:161–171. doi: 10.1172/JCI7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S.H. DeCaprio J.A. Cellular transformation by SV40 large T antigen: Interaction with host proteins. Semin. Cancer Biol. 2001;11:15–23. doi: 10.1006/scbi.2000.0342. [DOI] [PubMed] [Google Scholar]
- Caldwell M.A. He X. Svendsen C.N. 5-Bromo-2′-deoxyuridine is selectively toxic to neuronal precursors in vitro. Eur. J. Neurosci. 2005;22:2965–2970. doi: 10.1111/j.1460-9568.2005.04504.x. [DOI] [PubMed] [Google Scholar]
- Coons J.C. Seidl E. Cardiovascular pharmacotherapy update for the intensive care unit. Crit. Care Nurs. Q. 2007;30:44–57. doi: 10.1097/00002727-200701000-00006. [DOI] [PubMed] [Google Scholar]
- Cudré-Mauroux C. Occhiodoro T. Knig S. Salmon P. Bernheim L. Trono D. Lentivector-mediated transfer of Bmi-1 and telomerase in muscle satellite cells yields a Duchenne myoblast cell line with long-term genotypic and phenotypic stability. Hum. Gene Ther. 2003;14:1525–1533. doi: 10.1089/104303403322495034. [DOI] [PubMed] [Google Scholar]
- Fender P. Schoehn G. Perron-Sierra F. Tucker G.C. Lortat-Jacob H. Adenovirus dodecahedron cell attachment and entry are mediated by heparan sulfate and integrins and vary along the cell cycle. Virology. 2008;371:155–164. doi: 10.1016/j.virol.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Gerecht-Nir S. Radisic M. Park H. Cannizzaro C. Boublik J. Langer R. Vunjak-Novakovic G. Biophysical regulation during cardiac development and application to tissue engineering. Int. J. Dev. Biol. 2006;50:233–243. doi: 10.1387/ijdb.052041sg. [DOI] [PubMed] [Google Scholar]
- Hahn W.C. Counter C.M. Lundberg A.S. Beijersbergen R.L. Brooks M.W. Weinberg R.A. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- Hashimoto N. Kiyono T. Wada M.R. Shimizu S. Yasumoto S. Inagawa M. Immortalization of human myogenic progenitor cell clone retaining multipotentiality. Biochem. Biophys. Res. Commun. 2006;348:1383–1388. doi: 10.1016/j.bbrc.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Jacobs J.J. Kieboom K. Marino S. DePinho R.A. van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- Kehat I. Khimovich L. Caspi O. Gepstein A. Shofti R. Arbel G. Huber I. Satin J. Itskovitz-Eldor J. Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat. Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick J.N. Vannan M.A. Narula J. Lang R.M. Echocardiography in heart failure: Applications, utility, and new horizons. J. Am. Coll. Cardiol. 2007;50:381–396. doi: 10.1016/j.jacc.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Kobayashi N. Noguchi H. Westerman K.A. Watanabe T. Matsumura T. Totsugawa T. Fujiwara T. Leboulch P. Tanaka N. Cre/loxP-based reversible immortalization of human hepatocytes. Cell Transplant. 2001;10:383–386. [PubMed] [Google Scholar]
- Kowolik C.M. Liang S. Yu Y. Yee J.K. Cre-mediated reversible immortalization of human renal proximal tubular epithelial cells. Oncogene. 2004;23:5950–5957. doi: 10.1038/sj.onc.1207801. [DOI] [PubMed] [Google Scholar]
- LaFramboise W.A. Scalise D. Stoodley P. Graner S.R. Guthrie R.D. Magovern J.A. Becich M.J. Cardiac fibroblasts influence cardiomyocyte phenotype in vitro. Am. J. Physiol. Cell Physiol. 2007;292:C1799–C1808. doi: 10.1152/ajpcell.00166.2006. [DOI] [PubMed] [Google Scholar]
- Li Y. Pong R.C. Bergelson J.M. Hall M.C. Sagalowsky A.I. Tseng C.P. Wang Z. Hsieh J.T. Loss of adenoviral receptor expression in human bladder cancer cells: A potential impact on the efficacy of gene therapy. Cancer Res. 1999;59:325–330. [PubMed] [Google Scholar]
- Lokuta A. Kirby M.S. Gaa S.T. Lederer W.J. Rogers T.B. On establishing primary cultures of neonatal rat ventricular myocytes for analysis over long periods. J. Cardiovasc. Electrophysiol. 1994;5:50–62. doi: 10.1111/j.1540-8167.1994.tb01114.x. [DOI] [PubMed] [Google Scholar]
- Long C.S. Kariya K. Karns L. Simpson P.C. Trophic factors for cardiac myocytes. J. Hypertens. Suppl. 1990;8:S219–S224. [PubMed] [Google Scholar]
- Mauritz C. Schwanke K. Reppel M. Neef S. Katsirntaki K. Maier L.S. Nguemo F. Menke S. Haustein M. Hescheler J. Hasenfuss G. Martin U. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- Miragoli M. Gaudesius G. Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ. Res. 2006;98:801–810. doi: 10.1161/01.RES.0000214537.44195.a3. [DOI] [PubMed] [Google Scholar]
- Moretti A. Caron L. Nakano A. Lam J.T. Bernshausen A. Chen Y. Qyang Y. Bu L. Sasaki M. Martin-Puig S. Sun Y. Evans S.M. Laugwitz K.L. Chien K.R. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Naghavi M.H. Hatziioannou T. Gao G. Goff S.P. Overexpression of fasciculation and elongation protein ζ-1 (FEZ1) induces a post-entry block to retroviruses in cultured cells. Genes Dev. 2005;19:1105–1115. doi: 10.1101/gad.1290005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T. Feng Z. Honda T. Nomura Y. Kitajima T. Umezu M. Comparison of mRNA expression of transcriptional factors and intercalated disk constituent proteins between in vivo and cultured cardiomyocytes. J. Artif. Organs. 2008;11:134–140. doi: 10.1007/s10047-008-0414-7. [DOI] [PubMed] [Google Scholar]
- Narazaki G. Uosaki H. Teranishi M. Okita K. Kim B. Matsuoka S. Yamanaka S. Yamashita J.K. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118:498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- Narushima M. Kobayashi N. Okitsu T. Tanaka Y. Li S.A. Chen Y. Miki A. Tanaka K. Nakaji S. Takei K. Gutierrez A.S. Rivas-Carrillo J.D. Navarro-Alvarez N. Jun H.S. Westerman K.A. Noguchi H. Lakey J.R. Leboulch P. Tanaka N. Yoon J.W. A human beta-cell line for transplantation therapy to control type 1 diabetes. Nat. Biotechnol. 2005;23:1274–1282. doi: 10.1038/nbt1145. [DOI] [PubMed] [Google Scholar]
- Noguchi H. Kobayashi N. Westerman K.A. Sakaguchi M. Okitsu T. Totsugawa T. Watanabe T. Matsumura T. Fujiwara T. Ueda T. Miyazaki M. Tanaka N. Leboulch P. Controlled expansion of human endothelial cell populations by Cre–loxP-based reversible immortalization. Hum. Gene Ther. 2002;13:321–334. doi: 10.1089/10430340252769833. [DOI] [PubMed] [Google Scholar]
- Orlic D. Kajstura J. Chimenti S. Jakoniuk I. Anderson S.M. Li B. Pickel J. McKay R. Nadal-Ginard B. Bodine D.M. Leri A. Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Radisic M. Marsano A. Maidhof R. Wang Y. Vunjak-Novakovic G. Cardiac tissue engineering using perfusion bioreactor systems. Nat. Protoc. 2008;3:719–738. doi: 10.1038/nprot.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybkin I.I. Markham D.W. Yan Z. Bassel-Duby R. Williams R.S. Olson E.N. Conditional expression of SV40 T-antigen in mouse cardiomyocytes facilitates an inducible switch from proliferation to differentiation. J. Biol. Chem. 2003;278:15927–15934. doi: 10.1074/jbc.M213102200. [DOI] [PubMed] [Google Scholar]
- Salmon P. Oberholzer J. Occhiodoro T. Morel P. Lou J. Trono D. Reversible immortalization of human primary cells by lentivector-mediated transfer of specific genes. Mol. Ther. 2000;2:404–414. doi: 10.1006/mthe.2000.0141. [DOI] [PubMed] [Google Scholar]
- Schultz L.B. Chehab N.H. Malikzay A. Halazonetis T.D. p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J. Cell Biol. 2000;151:1381–1390. doi: 10.1083/jcb.151.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P. Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells: Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ. Res. 1982;50:101–116. doi: 10.1161/01.res.50.1.101. [DOI] [PubMed] [Google Scholar]
- Smalley M.J. Dale T.C. Wnt signalling in mammalian development and cancer. Cancer Metastasis Rev. 1999;18:215–230. doi: 10.1023/a:1006369223282. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tandon N. Cannizzaro C. Chao P.H. Maidhof R. Marsano A. Au H.T. Radisic M. Vunjak-Novakovic G. Electrical stimulation systems for cardiac tissue engineering. Nat. Protoc. 2009;4:155–173. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman K.A. Leboulch P. Reversible immortalization of mammalian cells mediated by retroviral transfer and site-specific recombination. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8971–8976. doi: 10.1073/pnas.93.17.8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z. Dickens M. Raingeaud J. Davis R.J. Greenberg M.E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yamada K. Green K.G. Samarel A.M. Saffitz J.E. Distinct pathways regulate expression of cardiac electrical and mechanical junction proteins in response to stretch. Circ. Res. 2005;97:346–353. doi: 10.1161/01.RES.0000178788.76568.8a. [DOI] [PubMed] [Google Scholar]
- Yang L. Soonpaa M.H. Adler E.D. Roepke T.K. Kattman S.J. Kennedy M. Henckaerts E. Bonham K. Abbott G.W. Linden R.M. Field L.J. Keller G.M. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.