Abstract
Background
Pediatric gliomas are rare and heterogeneous tumors. The Surveillance, Epidemiology, and End Results (SEER) database allows a large-scale analysis of the clinical characteristics and prognostic features of these tumors.
Methods
We analyzed available SEER data on 6212 patients less than 20 years old at diagnosis of glioma (1973–2005), according to 4 age categories: age < 1 year, 1–3 years, 3–5 years, and 5–20 years.
Results
The overall 5- and 10-year survival estimates were 71%±0.62% (SE) and 68%±0.67%, respectively. More than two fifths (41%) of gliomas were cerebral; the frequency of cerebellar tumors (22%–32% of gliomas) increased sharply after the first year of life. Of the tumors for which grade was available, 77% were low-grade (grade I or II). Tumor grade emerged as the most significant independent prognostic factor in all age groups except the youngest age group, in which extent of resection was most significant. Surgery other than gross total resection was an adverse prognostic factor (hazard ratio, 2.18; 95% CI, 1.78–2.67). Age <3 years predicted a greater likelihood of survival in patients with high-grade gliomas and brainstem tumors. On the other hand, Age < 3 years predicted a lower likelihood of survival in patients with low-grade gliomas. Children <1 year old received less radiotherapy than older patients (P< 0.0001) and were less likely to undergo gross total resection (p < 0.0001)
Conclusions
The survival of children with gliomas is influenced by histologic subtype, age, and extent of resection. Despite its limitations, the SEER database provides a useful tool for studies of rare tumors such as pediatric gliomas.
Keywords: Gliomas, SEER, low-grade, high-grade, pediatric
INTRODUCTION
Gliomas comprise a broad, heterogeneous spectrum of central nervous system (CNS) tumors that account for 56% to 70% of all pediatric CNS tumors, depending on the registry and the histologic criteria used.1, 2 In pediatric patients, these tumors are classified as low-grade gliomas (LGGs) and high-grade gliomas (HGGs). The former group includes tumors such as pilocytic astrocytoma (PA, WHO grade I),3 and fibrillary astrocytoma (FA, WHO grade II), while the latter group includes mainly anaplastic astrocytoma (AA, WHO grade III) and glioblastoma multiforme (GBM, WHO grade IV).
Historically, clinical trials and retrospective studies have been small. Therefore, LGG4–8 studies have historically included patients with different tumor histologies, as have studies of HGGs.9, 10 Whereas some studies have found the specific tumor grade (i.e., grade I vs. II in LGG and grade III vs. IV in HGG) to be a predictor of survival,4, 9, 10 others have not.5, 8, 11 Age was shown to influence survival in both HGG and LGG.12–15 Hence it is imperative to determine prognostic factors in a large cohort of pediatric glioma patients.
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database is the gold standard for cancer registries 16 and provides an opportunity for large-scale analysis of rare tumors such as pediatric glioma. We used the SEER database to analyze possible prognostic factors in pediatric patients with glioma.
PATIENTS AND METHODS
Data Source and Study Population
Data on pediatric patients (<20 years old) who had a reported diagnosis of glioma during the period January 1973 through December 2005 were obtained from the SEER 17 database (http://seer.cancer.gov/data/) which was accessed in November 2008 for the purpose of this study. We used a case listing session of the SEER*Stat 6.4.4 program to generate a matrix of cases that fit our study criteria. The selection query, based on the International Classification of Childhood Cancer, third edition (ICCC-3), 17 was as follows: astrocytomas, oligodendrogliomas, mixed and unspecified gliomas, and neuroepithelial glial tumors of uncertain origin. Patients who were registered only on the basis of autopsy or death certificate were excluded. The number of all pediatric patients with CNS tumors was retrieved for comparison.
Data Analysis
Primary tumor sites were grouped in the following categories: cerebrum, cerebellum, brainstem, spine, ventricles, and other, including not otherwise specified (NOS). (The term NOS is entered in the SEER database when tumor site, surgery, or pathology data are not available). Tumors were assigned to grades according to the current (2007) WHO classification.3 When grade assignment was not possible, e.g. for glioma NOS, the grade provided in the SEER database was used if available. Patients were grouped as follows by age at the time of diagnosis: age <1 year, 1–3 years, 3–5 years, and 5–20 years; the age groups were arbitrarily defined.
We used MedCalc for Windows version 9.4.2.0 (MedCalc Software, Mariakerke, Belgium) for statistical calculations and survival estimates. Death from any cause was used to calculate survival, as death from causes other than the malignancy is rare in the studied population. Survival estimates were calculated by the Kaplan-Meier method and compared by using the log-rank test. The chi-square test was used to compare demographic and clinical data. When appropriate, the log-rank test for trend and the chi-square test for trend were used to compare variables across the age groups. In these models, successive age groups were labeled with numbers (1 to 4) and the presence of each variable was tested in a 2×4 table (present/absent in the 4 age groups). Cox proportional-hazards regression was used to conduct multivariate analysis.
RESULTS
Patient Characteristics
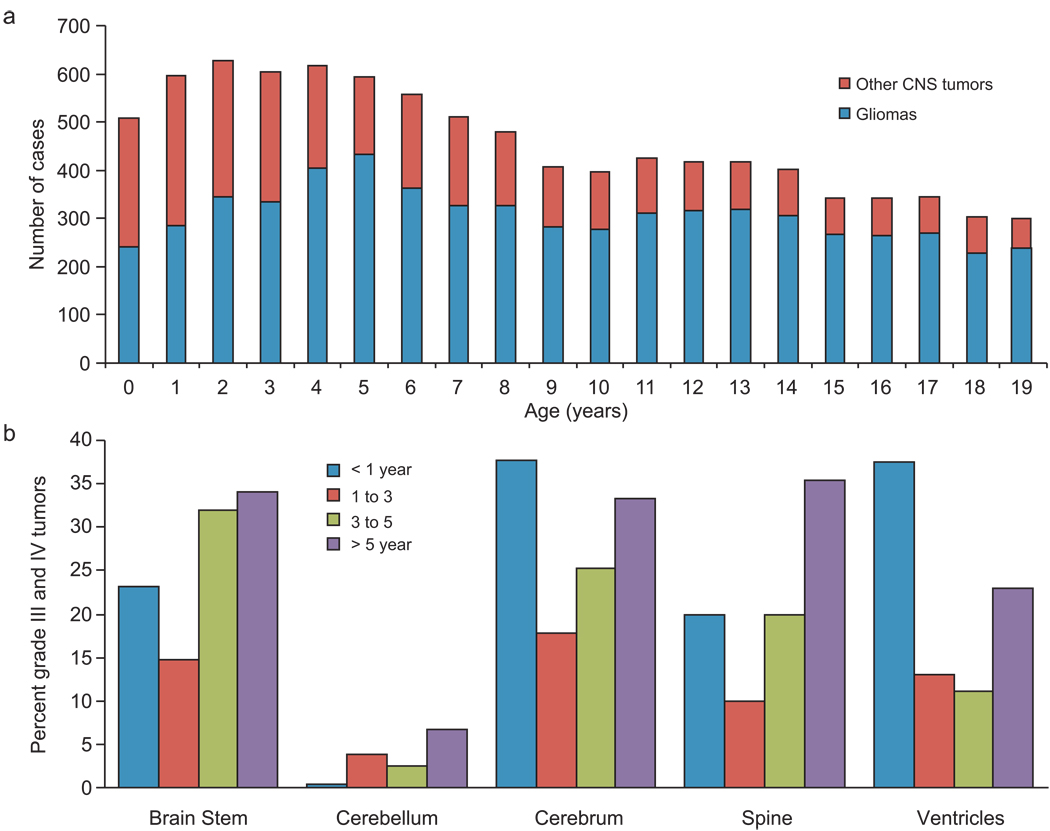
The SEER database included 9951 pediatric patients with CNS tumors, 6250 of whom met our search criteria. Thirty-eight patients characterized on the basis of autopsy/death certificate were excluded, leaving 6212 patients for this analysis. The median age at diagnosis was 9 years; male sex (53%) and white race (83%) were predominant. Approximately half of all pediatric CNS tumors diagnosed during the first 4 years of life were gliomas; the proportion of gliomas then increased with age as the frequency of other CNS tumors declined (Fig. 1A).
Figure 1.
(A) Bar histogram showing the relative frequency of gliomas versus other CNS tumors in 9192 pediatric patients with CNS tumors diagnosed between 1973 and 2005. (B) Distribution of the proportion of gliomas that were high-grade (grade III and IV) according to primary site and age group.
While the International Classification of Diseases for Oncology, version 3 (ICD-O-3) codes were available for all 6212 tumors, histologic grade was available for only 41% (n=2547). We used the WHO 2007 classification for CNS tumors 3 to grade 3247 (52%) of the 6212 tumors according to their histologic subtype (i.e., pilocytic astrocytoma = grade I). We were unable to grade 2965 (48%) of the tumors on this basis; we used the original SEER-assigned grade for 1209 of these tumors, leaving 1756 (28.3%) ungraded (Table 1). When tumors were categorized as LGG (grades I and II) and HGG (grades III and IV), we found only 4% discordance between the grades we assigned and the original SEER-assigned grades. Twenty-three percent of the tumors that could be assigned to a grade were HGG (n=1037; 17%; Table 1), although this proportion varied according to age and primary site (Fig. 1B).
Table 1.
Histologic subtypes (preceded by ICD-O-3 codes) of the studied tumors
| ICCC | Astrocytomas (n=4605) |
Oligodendrogliomas (n=315) |
Mixed and unspecified gliomas (n=1222) |
Neuroepithelial glial tumors of uncertain origin (n=70) |
Total (6212) |
|---|---|---|---|---|---|
| Tumor grade | |||||
| I | 9384/3: subependymal giant cell | 9380/3: glioma, | 2074 | ||
| astrocytoma, malignant (n=1) | malignant | ||||
| 9421/3: pilocytic astrocytoma, malignant | (n=37)* | ||||
| (n=1819) | 9382/3: mixed | ||||
| 9380/3: glioma, malignant (n=4)* | glioma (n=11)* | ||||
| 9400/3: ASTROCYTOMA, NOS (n=202)* | |||||
| II | 9410/3: protoplasmic astrocytoma (n=22) | 9450/3: | 9380/3: glioma, | 9430/3: | 1345 |
| 9411/3: gemistocytic astrocytoma (n=17) | oligodendroglioma, NOS | malignant | astroblastoma | ||
| 9420/3: fibrillary astrocytoma (n=183) | (n=288) | (n=78)* | (n=62) | ||
| 9424/3: pleomorphic xanthoastrocytoma | 9382/3: mixed | ||||
| (n=84) | glioma (n=49)* | ||||
| 9380/3: glioma, malignant (n=4)* | |||||
| 9400/3: ASTROCYTOMA, NOS (n=558)* | |||||
| III | 9401/3: astrocytoma, anaplastic (n=311) | 9451/3: | 9380/3: glioma, | 485 | |
| 9400/3: ASTROCYTOMA, NOS (n=124)* | oligodendroglioma, | malignant | |||
| anaplastic (n=25) | (n=12)* | ||||
| 9382/3: mixed | |||||
| glioma (n=13)* | |||||
| IV | 9440/3: glioblastoma, NOS (n=404) | 9460/3: | 9380/3: glioma, | 9381/3: | 552 |
| 9441/3: giant cell glioblastoma (n=24) | oligodendroblastoma | malignant | gliomatosis | ||
| 9442/3: gliosarcoma (n=7) | (n=1)* | (n=34)* | cerebri (n=1)* | ||
| 9380/3: glioma, malignant (n=1)* | 9382/3: mixed | ||||
| 9400/3: ASTROCYTOMA, NOS (n=56)* | glioma (n=24)* | ||||
| Not graded | 9380/3: glioma, malignant (n=179) | 9460/3: | 9380/3: glioma, | 9381/3: | 1756 |
| 9400/3: ASTROCYTOMA, NOS (n=601) | oligodendroblastoma | malignant | gliomatosis | ||
| 9423/3: polar spongioblastoma (n=4) ** | (n=1) ** | (n=890) | cerebri (n=7) | ||
| 9382/3: mixed | |||||
| glioma (n=74) |
ICCC, International Classification of Childhood Cancer, Third edition; NOS, not otherwise specified
SEER grade was used (available for 1209 tumors in the grade related row) for histologic subtypes not associated with specific grades
subtypes no longer recognized in the current WHO classification.
Most tumors were histologically confirmed (n=5177, 84%), while a minority (n=1012, 16%) were diagnosed on the basis of radiologic or clinical information. More than half of the latter group of tumors (57%) were located in the brainstem, 28% were located in NOS sites, and 10% were in the cerebrum.
The SEER database offered limited information about treatment. Radiation was used to treat 37% of the tumors overall; among graded tumors, more than half of those irradiated (52%) were LGG. Most irradiated tumors were located in the brainstem (34%) or cerebrum (32%).
Information about primary site–directed surgery was available for 4168 patients. One third of these (n=1431) underwent gross total resection (GTR). GTR was performed for 44% of cerebellar tumors, 25% of cerebral tumors, 22% of ventricular tumors, 21% of spinal tumors, and 6% of brainstem tumors.
Tumor Characteristics and Treatment According to Age Group
In the 4 age categories, there were 242 patients (3.9%) less than 1 year old, 640 patients (10%) 1–3 years old, 745 patients (12%) 3–5 years old, and 4585 patients (74%) 5–20 years old. Table 2 lists patient characteristics according to age group.
TABLE 2.
Patient Characteristics and Treatment Modalities
| Variable | All | <1 year | 1 to 3 | 3 to 5 | 5 to 20 | Pa | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | ||
| Total | 6212 | 242 | 640 | 745 | 4585 | ||||||
| Sex | |||||||||||
| Female | 2931 | (47.6) | 125 | (51.7) | 301 | (47.0) | 362 | (48.6) | 2143 | (46.7) | 0.41 |
| Male | 3281 | (53.2) | 117 | (48.3) | 339 | (53.0) | 383 | (51.4) | 2442 | (53.3) | |
| Race | |||||||||||
| White | 5089 | (82.6) | 202 | (83.5) | 537 | (83.9) | 591 | (79.3) | 3759 | (82.0) | 0.28 |
| Black | 659 | (10.7) | 19 | (7.9) | 61 | (9.5) | 92 | (12.3) | 487 | (10.6) | |
| Others | 464 | (7.5) | 21 | (8.7) | 42 | (6.6) | 62 | (8.3) | 339 | (7.4) | |
| Histology | |||||||||||
| Astrocytoma | 4605 | (74.7) | 188 | (77.7) | 512 | (80.0) | 544 | (73.0) | 3361 | (73.3) | 0.0001 |
| Oligodendroglioma | 315 | (5.1) | 4 | (1.7) | 21 | (3.3) | 26 | (3.5) | 264 | (5.8) | |
| Mixed and unspecified gliomas | 1222 | (19.8) | 48 | (19.8) | 97 | (15.2) | 170 | (22.8) | 907 | (19.8) | |
| Neuroepithelial glial tumors of uncertain origin |
70 | (1.1) | 2 | (0.8) | 10 | (1.6) | 5 | (0.7) | 53 | (1.2) | |
| Grade | |||||||||||
| I | 2074 | (33.7) | 55 | (22.7) | 262 | (40.9) | 290 | (38.9) | 1467 | (32.0) | <0.0001 |
| II | 1345 | (21.8) | 48 | (19.8) | 131 | (20.5) | 129 | (17.3) | 1037 | (22.6) | |
| III | 485 | (7.9) | 22 | (9.1) | 28 | (4.4) | 41 | (5.5) | 394 | (8.6) | |
| IV | 552 | (9.0) | 29 | (12.0) | 20 | (3.1) | 36 | (4.8) | 467 | (10.2) | |
| Unknown | 1756 | (28.5) | 88 | (36.4) | 199 | (31.1) | 249 | (33.4) | 1220 | (26.6) | |
| Site | |||||||||||
| Cerebrum | 1979 | (32.1) | 85 | (35.1) | 138 | (21.6) | 145 | (19.5) | 1611 | (35.1) | <0.0001 |
| Cerebellum | 1118 | (18.1) | 7 | (2.9) | 128 | (20.0) | 180 | (24.2) | 803 | (17.5) | |
| Brain stem | 1217 | (19.8) | 26 | (10.7) | 110 | (17.2) | 197 | (26.4) | 884 | (19.3) | |
| Ventricles | 246 | (4.0) | 12 | (5.0) | 26 | (4.1) | 24 | (3.2) | 184 | (4.0) | |
| Spine | 254 | (4.1) | 8 | (3.3) | 44 | (6.9) | 18 | (2.4) | 184 | (4.0) | |
| NOS/Others | 1398 | (22.7) | 104 | (43.0) | 194 | (30.3) | 181 | (24.3) | 919 | (20.0) | |
| Histologic confirmation | |||||||||||
| Done | 5177 | (84.0) | 206 | (85.1) | 534 | (83.4) | 577 | (77.4) | 3860 | (84.2) | 0.27 |
| Not done | 1012 | (16.4) | 34 | (14.0) | 104 | (16.3) | 164 | (22.0) | 710 | (15.5) | |
| Unknown | 23 | (0.4) | 2 | (0.8) | 2 | (0.3) | 4 | (0.5) | 15 | (0.3) | |
| Radiation | |||||||||||
| EBRT | 2297 | (37.3) | 30 | (12.4) | 113 | (17.7) | 245 | (32.9) | 1909 | (41.6) | <0.0001 |
| None | 3727 | (60.5) | 204 | (84.3) | 509 | (79.5) | 474 | (63.6) | 2540 | (55.4) | |
| Others | 188 | (3.1) | 8 | (3.3) | 18 | (2.8) | 26 | (3.5) | 136 | (3.0) | |
| Surgery | |||||||||||
| None | 1196 | (19.4) | 52 | (21.5) | 135 | (21.1) | 191 | (25.6) | 818 | (17.8) | <0.0001 |
| Biopsy | 213 | (3.5) | 11 | (4.5) | 11 | (1.7) | 35 | (4.7) | 156 | (3.4) | |
| Partial Resection | 1328 | (21.6) | 59 | (24.4) | 173 | (27.0) | 139 | (18.7) | 957 | (20.9) | |
| NOS/unknown/others | 2044 | (33.2) | 90 | (37.2) | 179 | (28.0) | 201 | (27.0) | 1574 | (34.3) | |
| GTR | 1431 | (23.2) | 30 | (12.4) | 142 | (22.2) | 179 | (24.0) | 1080 | (23.6) | |
Chi-square test was used to compare variables in different age groups.
NOS, not otherwise specified; GTR, gross total resection; EBRT, external-beam radiotherapy.
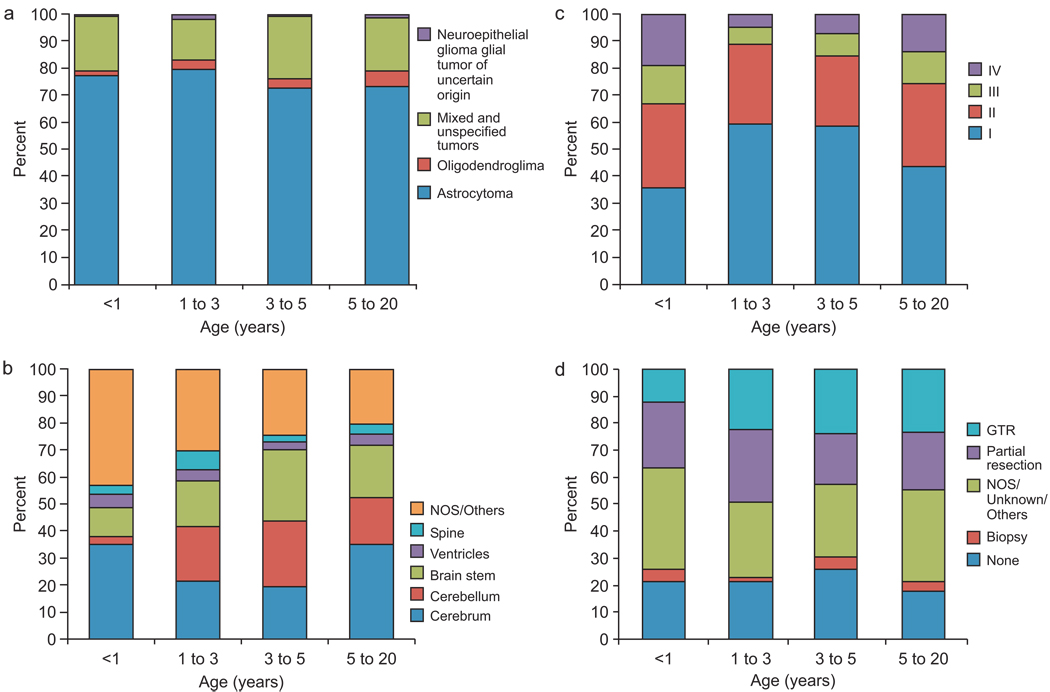
Astrocytoma was the most common histologic subtype (75%) (Fig. 2A). Only 5.1% of the tumors overall were oligodendrogliomas. The proportion of oligodendroglioma varied across the age groups and was less than 2% in the youngest group. Among tumors diagnosed during the first year of life, approximately one third (36%) were grade I, one third (31%) were grade II, and the remainder were grade III (14%) or IV (19%) (Fig. 2B). More than half of tumors were grade I in the 1–3 and 3–5–year age groups, but the proportion was slightly lower in patients older than 5 years. The tumor sites differed substantially across the age groups. During the first year of life, cerebral and brainstem sites were most common (Fig. 2C). After the first year of life the cerebellum became a more prominent site (22% to 32% of known tumor sites).
Figure 2.
Relative frequency of glioma (A) grade, (B) site, (C) histology, and (D) extent of surgery, according to age group.
Only 12% of tumors (n=30) diagnosed during the first year of life were irradiated. Most of these (n=19) were not graded. The chi-square test for trend showed significantly more use of radiotherapy in patients older than 1 year than in younger patients (P<0.0001); in children older than 5 years, 42% of tumors were irradiated.
Twelve percent (n=30) of patients diagnosed during the first year of life had GTR (Fig. 2D), and 19% of cerebral tumors in this group were grossly resected. Among patients older than 1 year, 22%–24% had GTR (chi-square test for trend comparing GTR vs. other surgeries in this age group, P=0.0001) and 26% of cerebral tumors were grossly resected. Only 14% of cerebellar tumors in children <1 year old received GTR, vs. 45% of cerebellar tumors in older children. A similar percentage of tumors (19%–27%) were partially resected in all age groups.
Outcome
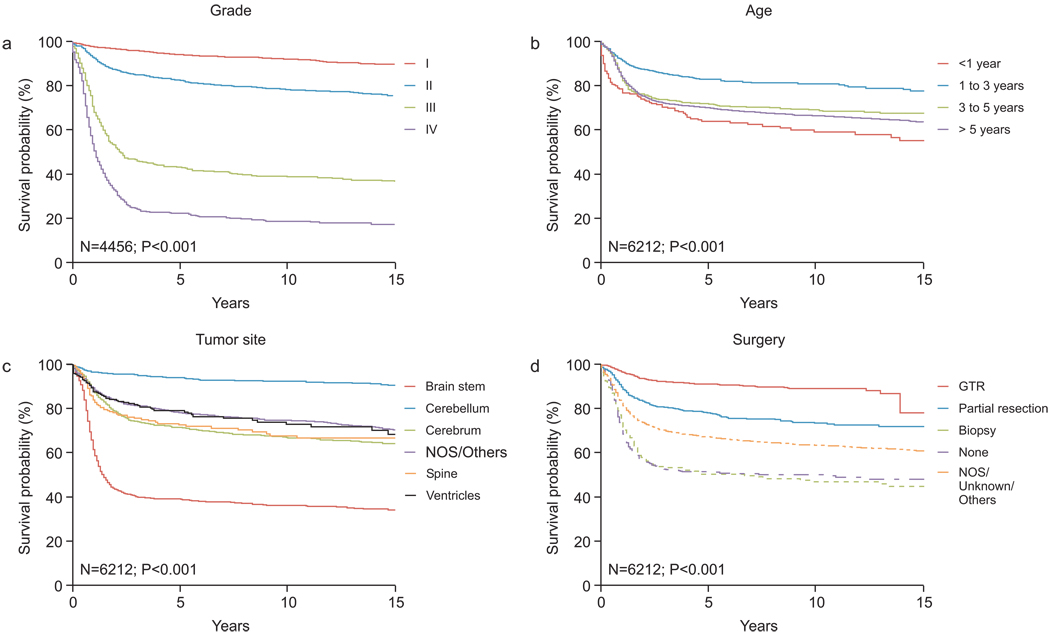
The 5-, 10-, and 15-year survival estimates (±SE) for all studied patients were 71% ± 0.62%, 68% ± 0.67% and 65% ± 0.73%. In a univariate analysis using the log-rank test, the following factors were significant (P<0.05) adverse prognostic factors: higher tumor grade (Fig. 3A), age < 1 year at diagnosis (Fig. 3B), brainstem primary tumor site (Fig. 3C), surgical biopsy only or no surgery ( Fig. 3D), and mixed or unspecified histologic subtype. This later group of tumors as defined by the ICCC-3 encompasses both mixed gliomas (ICD-O-3, 9382/3; n=171) and unspecified gliomas (ICD-O-3, 9380/3; n=1051). The outcome of patients with the former histology is significantly better than unspecified gliomas within this group (5-year survival, 73% ± 3.6% and 40% ±1.6%, respectively; P<0.001).
Figure 3.
Survival of pediatric patients with gliomas according to (A) tumor grade (n=4456), (B) age (n=6212), (C) tumor site (n=6212), and (D) extent of surgery (GTR, gross total resection) (n=6212). The log-rank test was used to compare survival curves.
Non-white race, absence of histologic confirmation, and use of radiation therapy were also significant adverse prognostic factors in univariate analysis (data not shown).
Of all prognostic factors identified in univariate analysis, tumor grade was the best predictor of survival; when viewed as an “internal control,” this finding showed that tumor grading as applied in this study worked well to stratify the studied tumors, minimizing the impact of lack of central pathology review. Further, the specific tumor grades within the LGG and HGG categories were predictive of survival (grade I vs. grade II; P<0.0001 and grade III vs. grade IV; P<0.0001). Similar results were found when analysis was applied on original grading as provided by the SEER (data not shown).
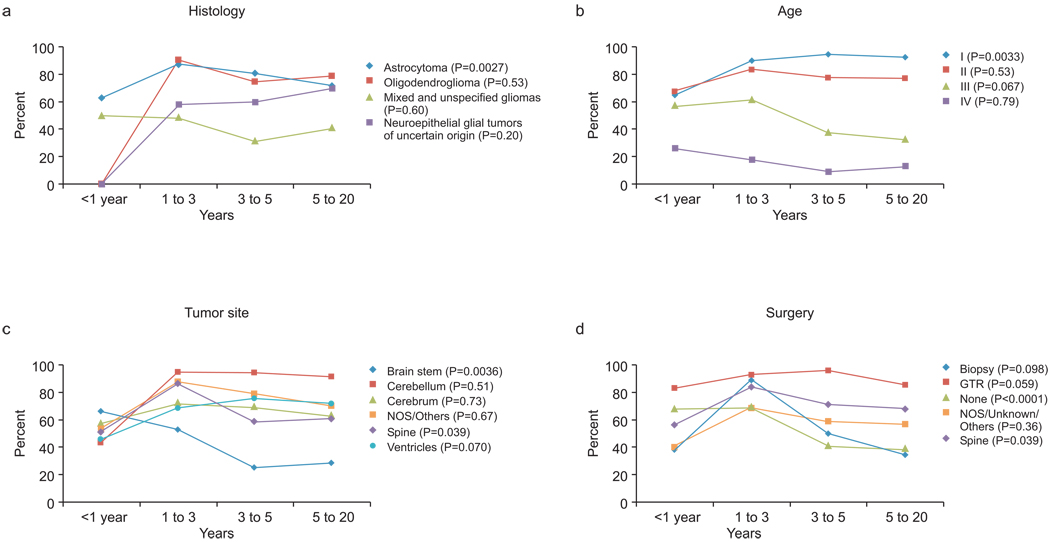
The 10-year survival estimates were lower for patients < 1 year old (59% ± 3.6%) than for older patients: 81% ±1.8% for those 1–3 years old, 69% ±1.9% for those 3 –5 years old, and 66% ±0.8% for those > 5 years old. Figure 4A–D shows the 10-year survival estimates for the age groups according to histologic subtype, tumor grade, tumor site, and extent of surgery.
Figure 4.
Line charts comparing the 10-year Kaplan-Meier survival estimates of pediatric patients with gliomas in the 4 age groups according to (A) histology, (B) tumor grade, (C) primary tumor site, and (D) extent of surgery. The log-rank test for trend was used to test the association of age with survival in each subset of patients.
In a multivariate analysis using the Cox proportional-hazards regression model, the following factors were independent adverse prognostic factors (P<0.05): age <1 year at diagnosis (hazard ratio [HR], 1.96; 95% CI, 1.49–2.59), surgery other than GTR (HR, 2.18; 95% CI, 1.78–2.67), histology other than astrocytoma (HR, 1.42; 95% CI, 1.19–1.67), radiotherapy (HR, 1.92; 95% CI, 1.67– 2.20), brainstem tumor (HR, 1.86; 95% CI, 1.62–2.19), and high tumor grade (HR, 6.13; 95% CI, 5.39–6.98).
The same model was tested separately using original SEER grading or histology-based grading for tumors not graded through by the SEER. High grade tumor remained significant in both models but with variable hazard ratios (2.61 for SEER grades and 4.83 for histology-based grading); all other factors remained significant.
The findings within each age group were similar to the overall findings; tumor grade was the most significant prognostic factor in all patients older than 1 year. Among patients < 1 year old at diagnosis, surgery other than GTR was the most significant adverse prognostic factor (HR, 5.05; 95% CI, 1.56–16.37) and high tumor grade (HR, 3.58; 95% CI, 2.08–6.18) was the second most significant adverse prognostic factor.
Improved outcome over time
We analyzed the outcomes of patients who were diagnosed in 3 arbitrary eras; 1) from 1973 to 1985 (n=1359, 22%), 2) from 1986 to 1995 (n=1617, 26%) and 3) from 1996 to 2005 (n=3236, 52%). The 5-year survival estimates for patients diagnosed in these 3 eras were 63% ±1.3%, 72% ±1.1% and 75% ± 0.9%, respectively (P<0.001). Interestingly, the use of radiotherapy decreased dramatically from 64% in the first era to 25% in the last era (P<0.001). When tested in the multivariate model mentioned above, diagnosis before 1986 was not a significant factor in the model (P=0.15).
Changing pathology over time
We looked at the histologies of gliomas diagnosed in the 3 eras. Significant changes occurred in the distribution of reported CNS gliomas; most noticeable, was the decrease of NOS astrocytoma (from 47% of reported gliomas in era 1 to 13% in era 3). This generic diagnosis was largely replaced with more specific pilocytic astrocytoma and anaplastic astrocytoma which together accounted for 10% of gliomas in era 1 and 45 % of gliomas in the last era. Astroblastoma accounted for 3.6% of tumors reported in the first era but only of 0.2% of tumors reported in the 3rd era.
DISCUSSION
The 6212 cases of pediatric glioma in the SEER 17 database (70% of all pediatric CNS tumors diagnosed during the years 1973 through 2005) allowed us to perform a population-based analysis of clinical features and outcome in the arbitrarily defined age groups (<1 year, 1–3 years, 3–5 years, and 5–20 years). We observed significant differences across these groups in tumor histology, grade, and primary site, although the distribution of tumor grade and the predominance of cerebral tumors were similar in patients < 1 year old and those 5 to 20 years old (Fig. 2B, C).
Among patients with low-grade gliomas (n=3419, 55.5%), grade I predicted better survival than grade II (Fig. 3 A) except among infants (Fig 4B). Reports of the prognostic value of grade I vs. grade II LGG are inconsistent. Some studies have found no difference in outcome between grades I and II,6–8 possibly because of the small number of subjects. A larger study (n= 278) showed that tumor grade (after pathology review) significantly influenced survival estimates, which were 92% for pilocytic astrocytoma, 86% for glioma not otherwise specified, and 48% for diffuse astrocytoma (P< 0.0001).4 Interestingly, our study found that age less than one year at diagnosis of grade I and II glioma had a negative impact on survival (Fig. 4B). Further, survival of patients with grade I gliomas was significantly better in the 1–3 and 3–5–-year age groups than in patients <1 year old (log-rank test for trend, P=0.0033; Fig. 4B). Few previous studies have addressed the negative impact of age less than 5 years on progression-free survival, but not on overall survival in pediatric LGG. 5,14
High-grade gliomas represented 17% of the studied tumors; however, their frequency differed according to primary site and age (Fig. 1B). Most high-grade tumors occurred in the cerebrum and brainstem, and fewer occurred in children 1 to 5 years old than in other age groups. Younger patients with high-grade tumors, particularly grade III, had higher survival estimates than did older patients (log-rank test for trend, P=0.067; Fig. 4B). Only 3 studies of children younger than three years with HGG have been reported, and they included only 16 to 39 subjects.15, 18, 19 Ours reports on the largest group to date of patients less than 3 years old with HGG (99 patients) (Table 2) to show improved survival in this age group.
Among patients with HGG, the extent of surgery was a major factor in survival. Patients with HGG who underwent GTR (23%) had the best survival in all age groups. Both GTR and grade were shown to influence the survival of patients with HGG in the largest studies reported to date, the Children’s Cancer Group (CCG)-943, and CCG-945 studies. 9,10 CCG-943 included 58 patients, 18 with AA and 40 with GBM; the latter group had significantly worse survival (p = 0.012). Patients who underwent only biopsy (n=11) had significantly worse survival than those who had partial resection (n=39) or GTR (n=8) (p < 0.001). In CCG-945 (172 children aged 18 months to 21 years), histologic subtype (AA vs. GBM) and percent resection (> 90% vs. <90%) were significantly associated with better prognosis (p < 0.02 and p < 0.04, respectively). Our larger, population-based analysis of 485 patients with grade III glioma and 552 with grade IV glioma confirmed these findings.
As expected, we found that brainstem gliomas carried the poorest prognosis (Fig. 4C). Interestingly, however, survival was inversely associated with age. Patients less than one year old had the best outcome, followed by age 1–3 years (Fig. 4C; log-rank test for trend, P= 0.0036). There has been only one reported study to date of infantile brainstem glioma; this report describes 10 patients, only one of whom was less than one year old.12 In that study the 3-year survival was69% ± 19% which is higher than other groups. Another study on pontine gliomas by Wagner et al found that age younger than 4 years (n= 13) was also associated with better prognosis.22 The positive impact of age on brain stem gliomas has been an area of debate with cases of spontenous regression in the neonatal peroid.23 Our study reports on the largest group of infantile glioma so far; 136 less than 3-year old ( 26 less than one year old). We believe this provides a basis for further research on this unique group of patients.
This study, like other population-based studies, was limited by the lack of central pathology review, which is considered important in confirming both low-grade and high-grade pediatric gliomas.4, 20, 21 In the CCG-945 study, 70 (28%) of 250 tumors originally designated as high-grade gliomas were found to be low-grade gliomas on central pathology review, and these patients fared better than the rest of the HGG cohort.20 However, after these 70 tumors were excluded from analysis, the final conclusions remained the same.21 The much greater size of our study population suggests that the absence of central pathology review was unlikely to confound our analysis. In addition, the importance of pathology review of LGGs is controversial. Fisher et al. reported a 39% (97/246) rate of discordance in their study of LGGs, whereas Gajjar et al. had only 2% discordance in their cohort of 145 tumors.4, 5
Our study was also limited by the lack of complete data about radiotherapy and surgery. The use of radiotherapy increased gradually with age at diagnosis (from 12.4% in patients <1 year old to 42% in children > 5 years old, P<0.0001). The use of radiotherapy was independently associated with poorer survival, but this finding is likely to reflect other prognostic factors, including resectability, anatomic site, tumor grade, and relation to nearby critical structures, that influenced the decision to use radiotherapy. Further, the majority of tumors with available grade in our cohort were LGGs (77%), for which radiation therapy is usually deferred or used only for recurrent or high-risk tumors. Signs and symptoms at presentation and association with neurofibromatosis may also have been factors in considering radiotherapy but were not available for analysis.
Notwithstanding its limitations, the SEER database provides a useful tool for the study of rare pediatric CNS tumors. Inclusion of more data about treatment, associated conditions such as neurofibromatosis, tumor size, and extent of disease will allow a better understanding of these tumors as well as other rare childhood cancers.
Acknowledgments
This work was supported in part by grant CA21765 from the U.S. Publish Health Service; Musicians Against Childhood Cancer (MACC); The Noyes Brain Tumor Foundation; The Ryan McGhee Foundation; the American Lebanese Syrian Associated Charities (ALSAC); and the King Hussein Cancer Foundation (KHCF).
The authors thank Sharon Naron for the scientific editing of this manuscript.
References
- 1.Central Brain Tumor Registry of the United States. 2005–2006 Statistical Report. Available at http://www.cbtrus.org/2005-2006/2005-2006.html.
- 2.Gurney J, Smith M, Bunin G. CNS and miscellaneous intracranial and intraspinal neoplasms. [Monograph online] National Cancer Institute. Classification of Childhood Cancer. 1999 Available at http://seer.cancer.gov/publications/childhood/cns.pdf.
- 3.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher PG, Tihan T, Goldthwaite PT, et al. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008;51(2):245–250. doi: 10.1002/pbc.21563. [DOI] [PubMed] [Google Scholar]
- 5.Gajjar A, Sanford RA, Heideman R, et al. Low-grade astrocytoma: a decade of experience at St. Jude Children's Research Hospital. J Clin Onco. 1997;15(8):2792–2799. doi: 10.1200/JCO.1997.15.8.2792. [DOI] [PubMed] [Google Scholar]
- 6.Rivera-Luna R, Zapata-Tarres M, Medina-Sanson A, et al. Long-term survival in children under 3 years of age with low-grade astrocytoma. Childs Nerv Syst. 2007;23(5):543–547. doi: 10.1007/s00381-006-0287-0. [DOI] [PubMed] [Google Scholar]
- 7.Prados MD, Edwards MS, Rabbitt J, Lamborn K, Davis RL, Levin VA. Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol. 1997;32(3):235–241. doi: 10.1023/a:1005736104205. [DOI] [PubMed] [Google Scholar]
- 8.Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86(5):747–754. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- 9.Finlay JL, Boyett JM, Yates AJ, et al. Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen. Children's Cancer Group. J Clin Oncol. 1995;13(1):112–123. doi: 10.1200/JCO.1995.13.1.112. [DOI] [PubMed] [Google Scholar]
- 10.Sposto R, Ertel IJ, Jenkin RD, et al. The effectiveness of chemotherapy for treatment of high grade astrocytoma in children: results of a randomized trial. A report from the Children's Cancer Study Group. J Neurooncol. 1989;7(2):165–177. doi: 10.1007/BF00165101. [DOI] [PubMed] [Google Scholar]
- 11.Wolff JE, Gnekow AK, Kortmann RD, et al. Preradiation chemotherapy for pediatric patients with high-grade glioma. Cancer. 2002;94(1):264–271. doi: 10.1002/cncr.10114. [DOI] [PubMed] [Google Scholar]
- 12.Broniscer A, Laningham FH, Sanders RP, Kun LE, Ellison DW, Gajjar A. Young age may predict a better outcome for children with diffuse pontine glioma. Cancer. 2008;113(3):566–572. doi: 10.1002/cncr.23584. [DOI] [PubMed] [Google Scholar]
- 13.Laithier V, Grill J, Le Deley MC, et al. Progression-free survival in children with optic pathway tumors: dependence on age and the quality of the response to chemotherapy--results of the first French prospective study for the French Society of Pediatric Oncology. J Clin Oncol. 2003;21(24):4572–4578. doi: 10.1200/JCO.2003.03.043. [DOI] [PubMed] [Google Scholar]
- 14.Massimino M, Spreafico F, Cefalo G, et al. High response rate to isplatin/etoposide regimen in childhood low-grade glioma. J Clin Oncol. 2002;20(20):4209–4216. doi: 10.1200/JCO.2002.08.087. [DOI] [PubMed] [Google Scholar]
- 15.Sanders RP, Kocak M, Burger PC, Merchant TE, Gajjar A, Broniscer A. High-grade astrocytoma in very young children. Pediatr Blood Cancer. 2007;49(7):888–893. doi: 10.1002/pbc.21272. [DOI] [PubMed] [Google Scholar]
- 16.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 17 Regs Limited-Use + Hurricane Katrina Impacted Louisiana Cases, Nov 2007 Sub (1973–2005 varying) - Linked To County Attributes - Total U.S., 1969–2005 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2008, based on the November 2007 submission - – accessed on November 20th, 2008
- 17.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer. Cancer. (third edition) 2005;103(7):1457–1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 18.Duffner PK, Krischer JP, Burger PC, et al. Treatment of infants with malignant gliomas: the Pediatric Oncology Group experience. J Neurooncol. 1996;28(2–3):245–256. doi: 10.1007/BF00250203. [DOI] [PubMed] [Google Scholar]
- 19.Geyer JR, Finlay JL, Boyett JM, et al. Survival of infants with malignant astrocytomas. A Report from the Children's Cancer Group. Cancer. 1995;75(4):1045–1050. doi: 10.1002/1097-0142(19950215)75:4<1045::aid-cncr2820750422>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 20.Fouladi M, Hunt DL, Pollack IF, et al. Outcome of children with centrally reviewed low-grade gliomas treated with chemotherapy with or without radiotherapy on Children's Cancer Group high-grade glioma study CCG-945. Cancer. 2003;98(6):1243–1252. doi: 10.1002/cncr.11637. [DOI] [PubMed] [Google Scholar]
- 21.Pollack IF, Boyett JM, Yates AJ, et al. The influence of central review on outcome associations in childhood malignant gliomas: results from the CCG-945 experience. Neuro Oncol. 2003;5(3):197–207. doi: 10.1215/S1152-8517-03-00009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner S, Warmuth-Metz M, Emser A, et al. Treatment options in childhood pontine gliomas. J Neurooncol. 2006;79:281–287. doi: 10.1007/s11060-006-9133-1. [DOI] [PubMed] [Google Scholar]
- 23.Thompson WD, Kosnik EJ. Spontaneous regression of a diffuse brain stem lesion in the neonate. Report of two cases and review of the literature. J Neurosurg. 2005;102(1):65–71. doi: 10.3171/ped.2005.102.1.0065. [DOI] [PubMed] [Google Scholar]