Abstract
Accumulating evidence suggests that solution-phase conformations of small globular proteins and large molecular protein assemblies can be preserved for milliseconds after electrospray ionization. Thus, the study of proteins in the gas-phase on this time-scale is highly desirable. Here we demonstrate that a travelling wave ion guide (TWIG) of a Synapt mass spectrometer offers a highly suitable environment for rapid and efficient gas-phase hydrogen/deuterium exchange (HDX). Gaseous ND3 was introduced into either the source TWIG or the TWIG located just after the ion mobility cell, such that ions underwent HDX as they passed through the ND3 on the way to the time-of-flight analyzer. The extent of deuterium labeling could be controlled by varying the quantity of ND3 or the speed of the travelling wave. The gas-phase HDX of model peptides corresponded to labeling of primarily fast exchanging sites due to the short labeling times (ranging from 0.1 to 10 ms). In addition to peptides, gas-phase HDX of ubiquitin, cytochrome c, lysozyme and apomyoglobin were examined. We conclude that HDX of protein ions in a TWIG is highly sensitive to protein conformation, enables the detection of conformers present on sub-milliseconds timescales and can readily be combined with ion mobility spectrometry.
Introduction
Numerous studies have demonstrated that protein-ligand complexes and even large functional macromolecular protein assemblies can retain their non-covalent bonding in the gas-phase (1–4 and references therein). This has enabled the determination of stoichiometry and binding interactions by various gas-phase techniques such as limited collisional dissociation and ion mobility separations. In contrast, smaller globular proteins appear to adopt a multitude of gas-phase conformations depending on the condition of the electrospray process and the amount of time that elapses before detection. Although this conformational ensemble likely extends beyond that present in solution, the gas-phase conformations of globular proteins offer a window into the nonnative and solvent-free conformational landscape5 including intermediates along the folding pathway and trapped misfolded species. This information can be relevant for understanding important areas of biology such as protein folding, protein aggregation and amyloid formation. Furthermore, several recent experimental studies suggests that the solution-phase conformers of even small globular proteins can be largely preserved for 30–60 milliseconds following ESI 6–10. Thus, sensitive analytical tools are needed for the rapid characterization of conformations of both small globular proteins and large macromolecular complexes in the gas-phase.
Several techniques are available for interrogating the conformational properties of gaseous protein ions. These include: (1) ion mobility spectrometry (IMS) where ions are separated due to their drift-time in an inert bath gas at high pressure 11 and (2) the measurement of the kinetics of gas-phase chemistry such as proton transfer reactions 12–14 or hydrogen/deuterium exchange (HDX) 15, 16. While IMS has proved an invaluable tool and has recently been introduced in a commercially available instrument17, gas-phase HDX measurements provide an alternative dimension for conformational interrogation. In a pioneering study by McLafferty and coworkers, gas-phase HDX was used to provide some of the first experimental evidence for stable coexisting protein gas-phase conformations15. Other studies have shown that HDX can sometimes expose the presence of additional gas-phase protein conformers not resolved by IMS and vice versa 18–20. Measuring the HDX of proteins in solution 21, 22 with mass spectrometry is an established method with recent developments 23, 24 enabling the measurement of deuterium levels of individual amide hydrogens, similar to NMR spectroscopy 25–27. In contrast, mass spectrometric detection of gas-phase HDX has yet to see wide-spread use in biological research and the emerging field of native mass spectrometry 1, 28. Perhaps by combining the information obtained with solution HDX and those of gas-phase HDX experiments, it will be possible to determine more definitively which conformations, present in the gas-phase shortly after ionization, are the same as those existing in solution.
Isotopic labeling studies of gaseous proteins have typically been confined to custom-built ion traps/drift-tubes or FT-ICR instruments. We have modified the gas-inlets of the commercially available Waters Synapt HDMS mass spectrometer to enable gas-phase HDX to be conducted in the different travelling wave (T-wave) ion guides (TWIG) of the instrument as described recently 29. Performing gas-phase HDX in a TWIG offers a number of advantages: exchange occurs in an intermediate pressure environment ideal for efficient gas-phase HDX and there is precise control of the labeling times of ions across a time-range spanning two orders of magnitude (from 0.1 to approx. 10 milliseconds). In addition, ions can be labeled only a few milliseconds after ESI29, providing access to the study of gas-phase conformers that could closely resemble those present in solution 8. Finally, with the Synapt, gas-phase HDX experiments can be quickly and easily combined with other gas-phase techniques such as IMS by employing the ion mobility cell that is in tandem with the TWIG used for gas-phase HDX. Sequential ion mobility separation of protein ions followed by gas-phase deuterium labeling in the neighboring TWIG can independently measure both the shape and surface reactivity of gaseous protein states thus resolving the same ensemble of conformers in both a physical and a chemical dimension.
Experimental
Materials
99% deuterated ammonia gas was purchased from Cambridge Isotope Labs (Andover, MA) and contained in a lecture bottle fitted with a standard regulator (Model 3332, Matheson Tri-Gas, Montgomeryville, PA). All proteins and peptides were purchased from Sigma Aldrich (St. Louis, MO) and used without further purification.
Sample preparation
Lyophilized peptides were dissolved in water and diluted into 50% acetonitrile, 0.1% formic acid to 3 μM (Leucine Enkephalin), 0.5 μM (Glu-fibrinopeptide B) and 2.5 nM (Bradykinin). Equine cytochrome C was dissolved in water (290 μM) and diluted to 2 μM in 50% acetonitrile containing 0.2% acetic acid (pH 2.8). Lysozyme from chicken egg white was dissolved in water (300 μM) and either (a) diluted directly to 60 μM in 1mM ammonium acetate, pH 6.5 (disulfide-intact form) or (b) diluted to 60 μM in 20 mM TCEP, pH 2.5 and incubated at 90 °C for 5 min (disulfide-reduced form). Lysozyme samples were infused immediately into the mass spectrometer after preparation. Bovine ubiquitin was dissolved in water (39 μM) and diluted into 50% acetonitrile containing 0.1% formic acid (pH 2.3) to a concentration of 4.2 μM. Equine myoglobin was dissolved in water (200 μM) and diluted to 20 μM in 50% acetonitrile containing 0.1% formic acid (pH 2.3).
Prior to electrospray, protein solutions were occasionally mixed 1:1 with a solution of 3 μM Glu-fibrinopeptide B. Since the deuterium uptake as a function of ND3 pressure was determined for Glu-fibrinopeptide B (GFP) in a separate experiment, the peptide served as an internal reporter of gas-phase HDX when it was present in mixtures containing other peptides/proteins. A given deuterium uptake observed for GFP in mixtures could be correlated with a known ND3 pressure in the transfer TWIG when GFP was labeled by itself under conditions where the precise ND3 pressure was well characterized.
Mass spectrometry
Positive electrospray ionization (ESI) mass spectrometry was performed with a Waters Synapt HDMS mass spectrometer (Waters Corp., Milford, MA, USA). This instrument has been described in detail elsewhere 17. The ESI source was operated with a capillary voltage of 3.5 kV, a sampling cone voltage of 45 V, a source-block temperature of 100 °C and a desolvation temperature of 250 °C. In MS-mode, the quadrupole collision energy was set to 4V. Mass accuracy was ensured by external calibration in MS/MS mode with 100 fmol/mL GFP. Mass spectra were acquired over an m/z range of 100–2000. All protein and peptide samples were infused directly into the mass spectrometer at 5 μl/min via the auxiliary sample pump of the Synapt HDMS mass spectrometer.
Gas-phase HDX experiments
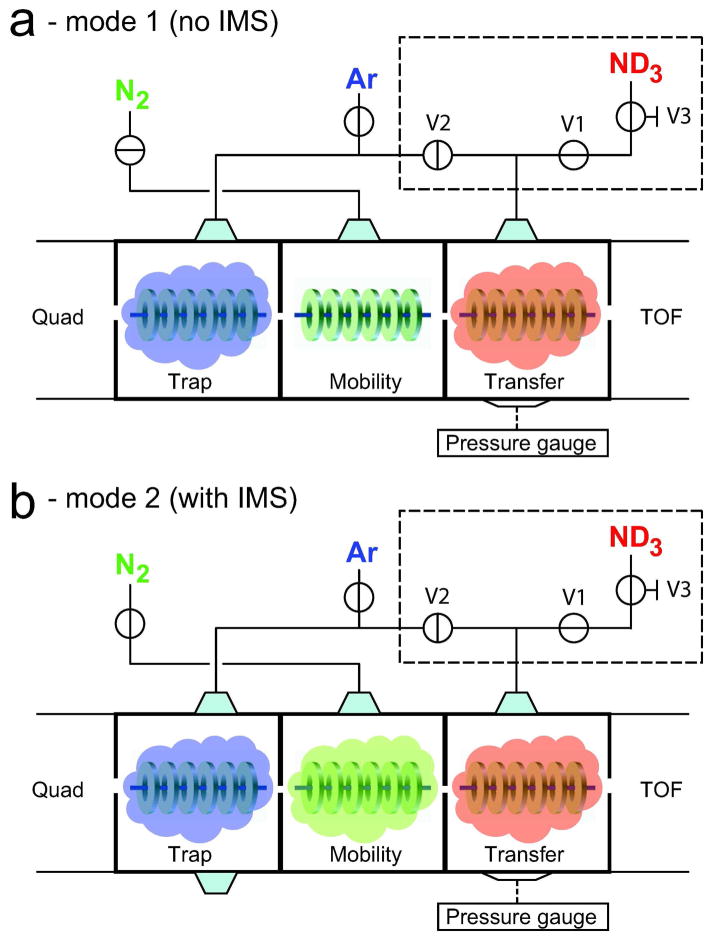
HDX was performed either in the source- or the transfer TWIG of the Synapt. The tubing connecting the gas inlet of the trap and the transfer TWIGs to the external Argon gas source was modified according to the schematic shown in Figure 1 (inside dashed box). All gas-tubing was stainless steel and connections were made using 1/8″ swagelok fittings (Swagelok, Billerica, MA). Valve 1 and valve 2 were two-way switching valves (Whitey SS-41S2, Swagelok, Billerica, MA). An additional needle valve (Valve 3, Meggitt Avionics, Hampshire, UK) was used for gradual infusion of ND3 gas into the mass spectrometer. Prior to fitting, all new valves and tubing were rinsed thoroughly in 50% isopropanol and flushed with N2 gas. The described modification of gas-inlets did not result in loss in sensitivity or changes in the pressure of the various vacuum stages of the instrument. By placing valve 1 in the “open” position and valve 2 in the “closed” position, valve 3 allowed the controlled infusion of ND3 gas (1–12 psi). Additionally, by opening valve 2, ND3 gas could be infused into both the trap- and transfer TWIGs if desired. An additional pressure gauge was fitted onto the Synapt tri-wave enclosure near the transfer TWIG. While not a requirement, the additional gauge enabled measurement of the pressure in the transfer TWIG. ND3 pressures were determined by subtracting the default background pressure in the transfer TWIG in the absence of ND3 gas from the pressure after infusion of ND3 gas. For experiments where gas-phase labeling was performed in the source TWIG (located in a partition between the ion source transfer optics and the quadrupole), valve 2 was opened and valve 1 was closed. The connector tubing between valve 1 and valve 3 was disconnected from valve 1 and connected instead to the source TWIG gas-inlet facilitating controlled infusion of ND3 gas into this front-end TWIG.
Figure 1.
Configuration of the gas-inlets of the Synapt HDMS mass spectrometer to enable gas-phase deuterium labeling both with or without anteceeding ion mobility separation. Selective infusion of Ar, N2 and ND3 into the three travelling wave ion guides (TWIG) was enabled by a simple modification of the existing gas-inlets (inside dashed box) involving two new switching valves (Valves 1 and 2) and a splitting tee. The ND3 gas was supplied from an external lecture bottle fitted with a needle valve (Valve 3) to provide better control of the infusion of deuterated gas. In mode 1 (a) Ar gas is led into the trap TWIG while ND3 gas is infused into the transfer TWIG for gas-phase HDX with no prior mobility separation. In mode 2 (b) Ar, N2 and ND3 gas are infused into the trap, mobility and transfer TWIGs, respectively. Ion mobility separation occurs in the mobility TWIG and subsequent gas-phase HDX of temporally separated ions occurs in the transfer TWIG.
Operation of the travelling wave ion guides during gas-phase reactions
Unless stated otherwise, all mode 1 HDX experiments (no ion mobility, see Figure 1) were performed in the default TOF-MS setting of the instrument but using a T-wave velocity of 300 m/s (source, trap, mobility & transfer TWIG) with T-wave heights of 3 V (source, trap and mobility TWIG) and 6 V (transfer TWIG). At these conditions, protein and peptide ions produced in the ion source, reach the transfer TWIG in approx. 1.2 ms (the lengths of the source, trap and mobility TWIGs are 10 cm, 10 cm and 18.7 cm, respectively). Argon gas flow to the trap TWIG was fixed at 1.5 mL/min with a variable ND3 gas flow to the transfer TWIG. The equilibration time between changing ND3 pressures in the TWIG was <5 seconds. Thus numerous gas-phase HDX experiments could be performed on the same continually infused sample enabling real-time measurement of deuterium uptake as a function of reagent gas pressure, or various other TWIG parameters
The limited number of mode 2 experiments (with ion mobility, see Figure 1) were performed using the default ion mobility settings of the instrument. Ions accumulated in the trap T-Wave were released over a period of 64 ms into the ion mobility T-Wave during each mobility separation cycle. The IMS gas (nitrogen) flow was set to 24 mL/min. The mobility T-wave parameters were varied for maximal mobility separation but using fixed T-wave parameters for the source-and trap TWIG (T-wave height, 3V and T-wave velocity of 300 m/s) and the transfer TWIG (T-wave height, 6V and T-wave velocity of 300 m/s).
Analyte ions were retained in the potential wells of the transfer T-wave and labeled at adjustable pressures of ND3 from 0.1 – 9 × 10−3 mbar with corresponding pressures in the time-of-flight (TOF) analyzer ranging from 3 × 10−7 to 1.4 × 10−6 mbar (the background pressure in the transfer TWIG was 0.1 × 10−3 mbar in the absence of ND3 gas). A further increase in the pressure of ND3 beyond 9 × 10−3 mbar caused a rapid decline in the performance of the TOF analyzer. The residence time of analyte ions in the transfer TWIG (i.e. labeling time) was controlled by changing the speed of the T-wave. By changing transfer T-wave speeds from 900 to 10 m/s, labeling times from 0.1 to 10 ms, respectively, could be achieved (the transfer TWIG has a length of 10 cm). Gas-phase HDX experiments in the source TWIG used similar T-wave settings as for the transfer TWIG. Source TWIG labeling experiments could be performed at significantly higher ND3 pressures (0.1 × 10−3- 1 × 10−1 mbar) due to the remote location of the source TWIG from the TOF analyzer.
Data analysis
Mass spectra were processed with MassLynx software (Waters Corporation, Milford, MA) and mass lists were exported to Excel (Microsoft, Redmond, WA). The gas-phase deuterium uptake of peptides and proteins was calculated from intensity-weighted average masses of deuterium labeled ions relative to the corresponding masses of non-labeled ions measured in the absence of ND3 gas. Replicate labeling experiments on ubiquitin at an ND3 pressure of 0.8 × 10−3 mbar indicated a standard deviation of 1 Da (n=3) in the measurement of the mass of deuterated species. Mobility data were processed in the Driftscope module of the MassLynx software package.
Results and Discussion
Gas-phase reactions in a travelling wave ion guide
A travelling wave ion guide (TWIG) enables well-defined ion propulsion through a background gas (10−3 to 10−1 mbar) by use of a travelling voltage wave 17, 30. Ions inside the TWIG are retained in a potential well within the RF ion guide by a stack of ring electrodes. Ions are propelled through the stack of electrodes by imposing a radially confining RF pulse to one set of electrodes and then moving this pulse to the next set of electrodes producing a moving electric field or a voltage wave that moves ions through the ion guide. In the presence of a significant background gas, ion mobility can be induced as ions roll over the sides of the potential well due to increased draft of ions in the bath gas. However, by increasing the heights of the potential well (i.e. the wave height) ions can be retained in the potential well and their residence time in the TWIG is determined by the speed of the T-wave (wave speed)30. As the TWIG operates at a much higher pressure relative to ion traps and ICR cells, and because the residence time of ions can be controlled, the TWIG is an ideal place to perform gas-phase HDX.
The ability to control the speed of the travelling wave allows very short labeling times to be performed (from 0.1 –10ms) which provide the means to probe the near-native compact folds of protein ions immediately after ESI and their evolution during the first few milli-seconds. Numerous gas-phase HDX studies have been performed on FT-ICR instruments where ions are labeled while stored in an external rf-only ion guide 31, 32 or contained in the ICR cell 15, 18 which allows defined ion-molecule reaction times from seconds to hours. Trapping of ions in multipole-type ion reservoirs as opposed to ICR cells enables the use of higher reagent gas-pressures and shorter gas-phase labeling times (>50ms) 31. The continuous accumulation of ions in an external ion reservoir during a gas-phase HDX reaction, however, can give rise to complex exchange kinetics as ions of the same origin are labeled for different amounts of time depending on their time of entry into the ion reservoir. Furthermore, filling of the ion reservoir beyond its space charge limit can result in vibrational excitation and dissociation33 which can further complicate interpretation of HDX kinetics. Notably, such issues have been addressed by the use of a custom designed gated-beam ESI source with an ion shutter 34 or by the use of a MALDI source which does not produce continuous ion beams35. In the present study, the transfer TWIG is not operated as an ion reservoir and ions are labeled “on-the-fly” while confined in the potential wells of a travelling wave that traverses the stacked ring ion guide. Thus, in contrast to external ion reservoirs, the unique properties of the TWIG can ensure that all ions are labeled for the same amount of time as a function of the speed of the travelling wave, without the need for a discontinuous ion-beam. The instrumental setup should therefore also be readily compatible with online liquid chromatography, enabling gas-phase HDX of individual peptide or protein components from complex mixtures.
HDX experiments were facilitated by modifying the gas-inlets of the transfer TWIG (Figure 1a–b) to allow the controlled infusion of ND3 gas via an external lecture bottle (1–12 psi). The presence of ND3 gas in the transfer TWIG created a “curtain” of deuterated gas through which ions must pass prior to analysis in the TOF analyzer. Because this curtain was placed in the transfer TWIG, which resides between the ion mobility cell and the TOF analyzer, gas-phase deuterium labeling of analyte ions could be performed within a few milliseconds after ionization either with no ion mobility separation (mode 1) or with IMS and HDX performed in tandem (mode 2).
Gas-phase HDX of model peptides
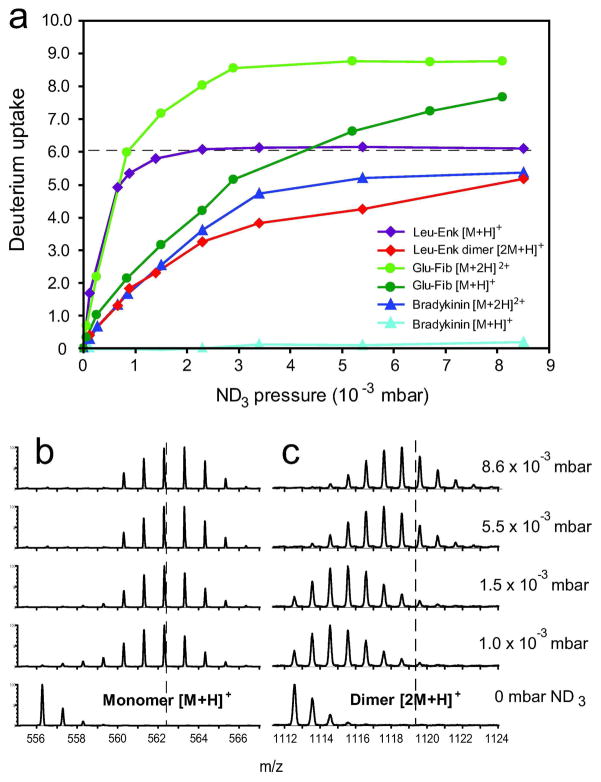
Protonated polypeptide ions were labeled in the transfer TWIG at various ND3 pressures (0.1 – 9 × 10−3 mbar). An increase in ND3 pressure resulted in an immediate increase in the deuterium uptake of peptide ions as observed upon infusion of Leucine enkephalin (Leu-Enk, seq.:YGGFL), Glu-fibrinopeptide B (GFP, seq.: EGVNDNEEGFFSAR) and Bradykinin (BK, seq.: RPPGFSPFR) (Fig. 2). At higher reagent gas pressure, more collisions occur between deuterated gas-molecules and analyte ions as they travel through the curtain gas. Increased ion-neutral collisions slow down the analyte ions and thus increase the likelihood for formation of stable exchange-competent ion-neutral complexes 36. By increasing ND3 pressure in the TWIG, the singly charged Leu-Enk peptide readily incorporated 5 deuteriums (to a maximum of 6 at the highest pressure), while singly charged BK did not exchange a single of its 18 theoretically labile hydrogens. These results are in good agreement with previous gas-phase HDX studies on these model peptides using ND3 gas 37, 38. Singly charged BK fails to exchange due to the sequestering of the single charge at the Arg-sidechain, thus making the proton unavailable to initiate exchange 37. Notably, the addition of one more proton to BK causes the doubly charged BK to exchange up to 5 hydrogens, in striking contrast to the singly charged counterpart. A similar difference in gas-phase HDX could also be observed for singly- versus doubly-charged GFP (Figure 2a).
Figure 2.
Gas-phase HDX of peptides with ND3 gas in the transfer TWIG. (a) The deuterium labeling of Leucine enkephalin, Glu-fibrinopeptide B and Bradykinin in the transfer TWIG as a function of ND3 pressure. (b–c) Mass spectra of the singly-charged monomer (b) and the homodimer (c) of Leucine enkephalin at different ND3 pressures with a constant T-wave velocity of 300 m/s and a wave height of 6V. The dashed line in (a) corresponds to a deuterium uptake of 6 Da (as calculated from the average masses of the labeled and unlabeled species) and is also indicated in (b–c) for reference. Notably, the Leucine enkephalin dimer undergoes significantly less gas-phase labeling (decreased D uptake) than the monomer.
In a prior study37 the gas-phase HDX of Leu-Enk was fully accounted for by 5 fast exchanging sites corresponding to hydrogens attached to the side-chains (i.e. the protonated N-terminal amino-group, the hydroxyl group of the Tyr sidechain, and the C-terminal carboxy-group) and 4 slower exchanging sites corresponding to the back-bone amide hydrogens. Based on this classification of exchangeable sites in Leu-Enk, it appears that primarily fast-exchanging sites on the side-chains are deuterium labeled in the travelling wave ion guide presumably due to the very short exchange times employed. Of note, at maximal ND3 pressure, exchange of a single amide hydrogen in Leu-Enk does occur. Due to proximity effects 39 this could preferentially be the N-terminal amide hydrogen in Leu-Enk as the charge on the N-terminal aminogroup would enhance exchange of this particular amide.
At the employed ESI conditions, the Leu-Enk peptide exists as both a monomer and a non-covalent homodimer in the gas-phase. We found that the singly charged non-covalent homodimer of leucine enkephalin exchanged significantly less at increasing pressures than the corresponding Leu-Enk monomer (Fig 2a,b,c). The dimer exchanged only 5 deuteriums even at maximal ND3 pressure indicating that several exchangeable sites on Leu-Enk were protected from exchange in the complex. This suggested that steric and conformational constraints significantly influence the deuterium labeling of gas-phase polypeptides in the transfer TWIG.
Gas-phase HDX of proteins in the TWIG
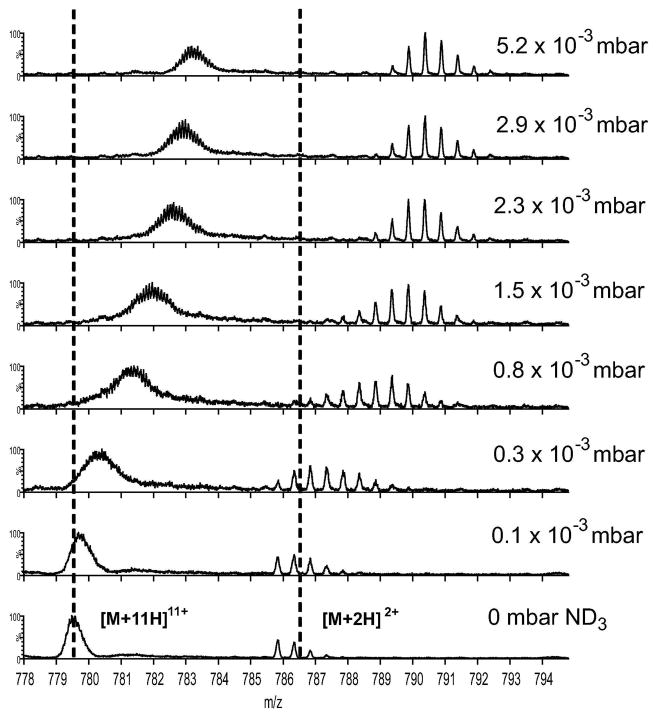
Gas-phase HDX experiments of small peptides with ND3 in the transfer TWIG were also extended to proteins. Mass spectra acquired at gradually increasing pressures of ND3 (mode 1, Fig. 1a) upon infusion of a mixture of ubiquitin and GFP in 50% acetonitrile and 0.1% formic acid are shown in Figure 3. Ubiquitin ions displayed considerable deuterium labeling in the transfer TWIG, with the [M+11H]11+ ion exchanging up to 40 deuteriums at maximal ND3 pressure using the default T-wave velocity (300 m/s). This corresponds to exchange of 50% of all labile side chain hydrogens or 25 % of all labile hydrogens in ubiquitin within the 0.33 ms the ions were exposed to ND3 in the TWIG (the length of the transfer TWIG is approx. 10 cm). The amplitude or height of the travelling wave can control whether protein ions in a TWIG are retained in the potential wells of the voltage wave or are able to roll over the top of the wave into the following potential wave. Ion roll-over causes mobility separation of ions according to ion shape and charge. Ion roll-over is not desired during HDX in the transfer TWIG as the time of labeling is then no longer equal to the transit of one wave through the TWIG but rather becomes a function of properties of each ion (e.g., shape and m/z of individual ions). To ensure that all protein ions were retained in the potential wells of the travelling wave and did not roll over, a sufficiently high wave height (a potential difference of 6V) was used. At a constant ND3 pressure of 3 × 10−3 mbar in the transfer TWIG, the deuterium uptake of ubiquitin ions remained constant at wave heights from 6V to 1V; however, a sudden increase in the observed deuterium uptake of ubiquitin ions was observed upon decreasing the wave height to 0.2 V (Fig. S1). A wave height of 0.2 V was no longer sufficient to carry the ubiquitin ions in the T-wave, thus causing a significantly slower transport through the TWIG and hence longer labeling times. Similar control experiments conducted on other proteins used in this study and at various gas pressures in the TWIG (data not shown) indicated that in all cases a wave height of 6 V in the transfer TWIG was sufficient to contain ions in the T-wave. In this way, we ensured that all peptide or protein ions from the same sample were exposed to ND3 for equal times, irrespective of differences in ion collisional cross section and m/z ratio.
Figure 3.
The effect of ND3 pressure on the gas-phase labeling of protein and peptide ions in the TWIG. Mass spectra of ubiquitin [M+11H]11+ and Glu-fibrinopeptide B [M+2H]2+ at different levels of ND3 gas infused into the transfer TWIG. Experiments were performed in mode 1 (see Figure 1a) with a transfer TWIG T-wave speed of 300 m/s and wave height of 6V.
The use of greatly elevated potentials in the TWIG (wave velocity and wave height) in the presence of high pressure background gas can cause substantial collisional activation and dissociation of analyte ions. In a recent systematic study, GFP was observed to undergo fragmentation in a TWIG at wave velocities >1000 m/s and a wave height of 8V in the presence of Argon gas at 5 × 10−3 mbar 30. We observed similar results using GFP and Argon gas on our instrumental setup (data not shown). However, the experimental conditions and pressures of ND3 at which the transfer TWIG was operated during gas-phase HDX experiment in the present study (i.e. wave velocities of 50–300 m/s, wave height of 3–6V, minimal TWIG collision- and injection voltages and a bath ND3 gas pressure below 8 × 10−3 mbar) were well below the observed threshold for fragmentation. This is supported by the fact that the deuterium uptake of ubiquitin ions were unaffected by increases in the wave height from 1V to 6V (Figure S1) and even up to 15V (data not shown). The multiple peak shapes occurring for individual charge states of apo-myo upon gas-phase HDX (as described below) where unaffected by an increase of the transfer TWIG from 4 to 15V (data not shown). Finally, ESI of myoglobin in deionized water at the conditions used for HDX experiments resulted in charge states only of the folded holo-form of the protein, indicating minimal activation of analyte ions at the conditions used in this study (data not shown). Taken together, these findings suggests that the present gas-phase HDX experiments were performed in a soft collisional regime where the internal energies of analyte ions were below the energy threshold required for structural unfolding/isomerization. At such gentle conditions, the exchange rate is limited by the frequency of formation of exchange competent ion-molecule complexes and not by unintentional activation of ions.
The residence time of analyte ions in the TWIG (i.e. labeling time) could be precisely controlled. Changing T-wave speeds from 900 to 10 m/s resulted in labeling times from 0.1 to 10 ms, respectively. The effect of wave velocity on deuterium uptake of ubiquitin and GFP at a fixed pressure of ND3 is shown in Figure S2. As the wave travels faster, there is less time for labeling and therefore less deuterium in both peptide and protein ions. In the present setup, a pressure gauge was added to a socket on the tri-wave enclosure to measure the pressure in the transfer TWIG. Figures 3 and S2 illustrate that co-infusion of a small peptide such as GFP can provide an internal labeling standard that gauges the efficiency of HDX in the TWIG. Such a simple internal standard can be used to correlate independent measurements on different protein samples as an alternative to measuring the pressure of ND3 gas via the pressure gauge presently fitted to the TWIG. In this way, one could also obtain identical conditions in different instruments independent of a pressure measurement or flow rate of ND3 by monitoring the amount of deuterium found in the GFP standard under identical instrumental parameters.
The predominant reaction pathway of ND3 with protonated polypeptides is exchange of labile hydrogens between sites of similar gas-phase basicity 36, 40. We also detected a minor degree of proton- transfer reactions (i.e. stripping of charge from multiply protonated protein ions) at elevated ND3 pressures of >5 × 10−3 mbar (data not shown). A similar effect was observed upon maximal exposure of protein ions to the deuterated gas (i.e. a minimal T-wave velocity of 10 m/s, data not shown). As the confluence of both gas-phase reactions could confound interpretation of exchange data, we assessed the extent of charge-stripping occurring prior to TOF detection during our HDX experiments. This was done by performing control experiments in which individual charge states of ubiquitin and apo-myoglobin were isolated in the quadrupole prior to gas-phase reactions in the TWIG. In this manner, the occurrence of proton-transfer reactions in a given experiment could be monitored by the emergence of charge-reduced peaks (e.g., z-1, z-2, z-3 etc) of the isolated protein ion in the resulting spectrum (data not shown). Measurements of proton-transfer reactions14 occurring in the TWIG with reagent bases such as ammonia could inherently provide an additional avenue for conformational detection using the present setup. However, while significant charge stripping by the ND3 gas could be induced at defined conditions discussed above, it did not occur to significant levels in HDX experiments reported in this study as the labeling times were very short and the pressure of reactant base (ND3) was too low.
Sequential ion mobility spectrometry and gas-phase HDX
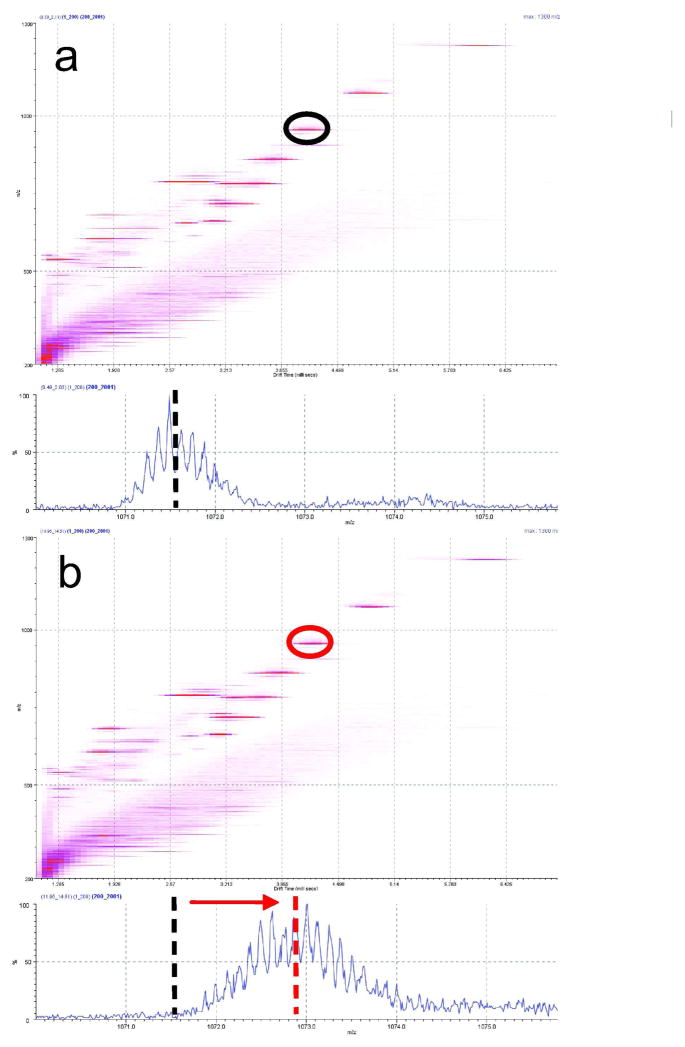
Previous pioneering work by Valentine and Clemmer 41 showed that infusion of small amounts of D2O gas into the driftube of a custom-made IMS instrument allowed deuterium labeling of protein ions simultaneously undergoing mobility separation in a He bath-gas within the driftube. The simultaneous ion mobility separation and labeling presented some complications with regard to data analysis as labeling times varied with the drift-times of different ions. Moreover the authors noted changes in mobility separation with the pressure of D2O gas in the drift tube. We have investigated the ability of the current setup to perform ion mobility separation in one TWIG and a chemical reaction (i.e. HDX) in the neighboring TWIG (Mode 2, see Fig. 1b). In such an experiment, ions are propelled by a travelling wave through the mobility TWIG containing a N2 background bath gas at high pressure (0.1 mbar). This separates ions according to collisional cross-section. Subsequently, these temporally separated ions are transported through the neighboring transfer TWIG which harbors a cloud of lower pressure ND3 gas that isotopically labels analyte ions in a sub-millisecond time-frame. Results from such an experiment using a mixture of ubiquitin and GFP are shown in Figure 4. Comparison of Figure 4a and 4b, shows that the drift times of ions in the mobility TWIG are unaffected by the presence of ND3 gas in the neighboring TWIG. Thus, both the collisional cross-section and the exchange reactivity of analyte ions can be measured in a single data acquisition. An extensive characterization of the ion mobility of conformers of ubiquitin was not the emphasis of the experiment as this has been covered in detail by prior studies 42, 43. The analysis of gas-phase HDX of ion-mobility separated conformers will be reported elsewhere. The results shown here serve to indicate the general versatility of the TWIG for gas-phase studies of proteins and how analytical approaches based on ion mobility or gas-phase reactivity can be compartmentalized in the same instrument by TWIGs placed in tandem.
Figure 4.
Tandem ion mobility separation and gas-phase HDX of mobility separated ubiquitin ions in a single data acquisition (mode 2, see Figure 1). Ion mobility drift-time chromatogram and corresponding mass spectra of the [M+8H]8+ ion of ubiquitin in the absence (a) and presence (b) of ND3 gas in the transfer TWIG at a pressure 1 × 10−3 mbar ND3.
In a limited number of related experiments, the gas-inlets were reconfigured to allow the infusion of ND3 gas into the source TWIG which is located in the interface between the transfer optics of the ion source assembly and the quadrupole of the Synapt mass spectrometer. The source TWIG provided similar control of reaction parameters (i.e. labeling times and labeling pressure). In contrast to the transfer TWIG, a higher pressure of ND3 gas (>9 × 10−3 mbar) in the source TWIG did not affect the performance of the TOF analyzer enabling HDX experiments at an expanded range of reagent gas pressures (0.1 × 10−3- 1 × 10−1 mbar). However, the efficiency of deuterium labeling of proteins and peptides in the source TWIG was much reduced relative to the transfer TWIG. Very high ND3 pressures in the source TWIG were required to achieve similar extents of exchange as corresponding experiments in the transfer TWIG (see Fig. S3 for the deuterium labeling of GFP in the source TWIG). A likely explanation is the interference to the exchange process of water vapor from the neighboring ion source region.
Interrogating the conformational properties of gaseous proteins
Having established the conditions for controlled gas-phase HDX of peptides and proteins in the TWIG, we explored the ability of our setup to study the conformational properties of proteins in the gas-phase. Upon exchange with ND3 gas, all peaks comprising the charge state distribution of ubiquitin gradually shifted to a higher m/z value due to deuterium uptake (see Fig. 3 and Fig. S2). Notably, this occurred with only a minor broadening of the isotopic envelope, indicating that ubiquitin ions populated a quite homogenous or very rapidly interconverting ensemble of structures in the milli-second time-frame after ESI. Gas-phase labeling experiments on cytochrome c, also a small globular protein, produced similar results to ubiquitin. Cytochrome c ions of all charge states incorporated deuterium upon exchange in the ND3 gas, resulting in gradual shifts of peaks in the mass spectra without significant peak broadening (data not shown). Prior studies have demonstrated that small globular proteins like cytochrome c and ubiquitin unfold from compact forms to several more unfolded forms approx. 30–60 ms after ESI depending on charge state 6, 7, 42. Our present results are in good agreement with these findings and indicate that gas-phase ubiquitin and cytochrome c populate a very narrow ensemble of structures at sub-millisecond timescales after ESI ionization (no significant change in peak widths or the emergence of bimodal peaks upon labeling).
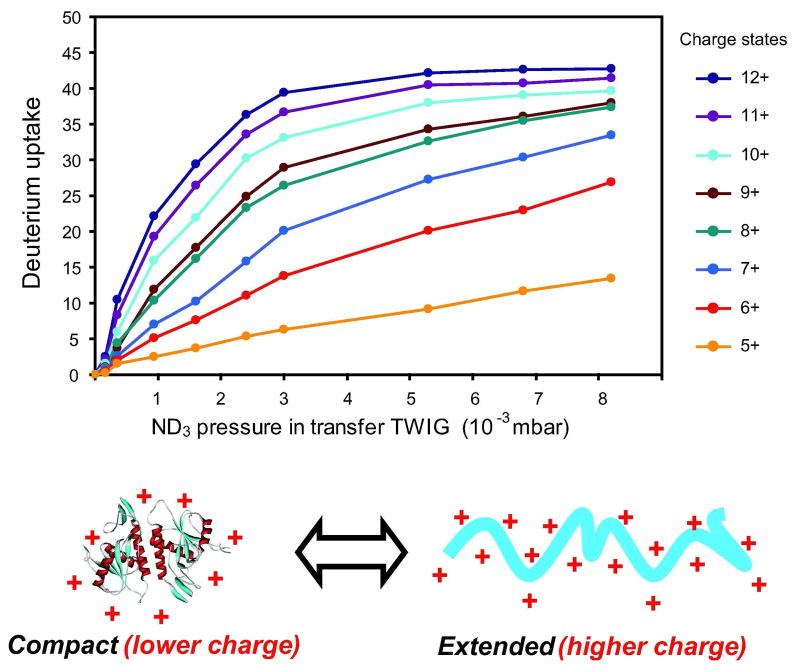
Deuterium uptake of ubiquitin increased smoothly as a function of charge state (Figure 5 and Fig. S1). Increased deuterium uptake was also observed for higher charge states of cytochrome c in a manner similar to ubiquitin (data not shown). This trend was furthermore reflected for all other proteins studied. The accumulation of charge in a gaseous protein ion has been shown to cause unfolding of the protein in order to reduce coulombic repulsion forces 44. Our current results appear to in be good agreement with charge-induced unfolding of ubiquitin, a hypothesis confirmed experimentally by ion mobility measurements of the collisional cross-sections of charged protein ions 43, 45. Gas-phase HDX studies of proteins on instrumental setups employing substantially longer labelling times (>1 s) and weaker reagent gasses (typically D2O) has shown no such clear correlation between the extent of deuterium uptake and protein ion charge state 15, 18, 20, 46. We speculate that the short residence time (0.1–10 ms) of ions in the gas-filled TWIG and the use of highly basic ND3 reagent gas, are the main reasons why a clear correlation between deuterium uptake and unfolding of protein ions is observed in our studies. From experiments on small peptides such as Leu-Enk (Fig. 2), short time-scales appear to primarily label facile sidechain hydrogens while slower exchanging sites do not have time to equilibrate in the deuterated gas before exiting the TWIG. In direct support of our results, prior gas-phase HDX experiments at a longer millisecond-timescale in a custom-built drift-tube filled with ND3 gas showed that the [M+10H]10+ charge state of cytochrome c exchanged fewer hydrogens than the [M+12H]12+ charge state 47. By the use of an entropic minimization algorithm 48 to extract grouped rate constants from experimentally derived exchange data, it was furthermore shown that the [M+12H]12+ state of cytochrome c had a unique population of fast-exchanging hydrogens not present for the lower charge state indicative of localized unfolding of the higher charged cytochrome c ions. On the same setup, these authors also studied the gas-phase HDX of ubiquitin with ND3 gas 49. In good agreement with our results, charge states +8 to +12 of ubiquitin exchanged as a single population with an increase in both extent and rate of HDX with increasing charge state. Note, the [M+13H]13+ charge state for which the authors did see evidence of two differently exchanging species could not be measured accurately in our study due to poor signal and overlap of an interfering ion in the mass spectrum.
Figure 5.
The deuterium uptake as a function of ND3 pressure of different charge states (see color guide) of ubiquitin ions. The schematic at the bottom illustrates the effect of charge (protons) on the conformation of gas-phase protein ions as measured by extensive ion mobility studies (references in text).
HDX of polypeptides with weaker reagent bases such as D2O and CH3OD occur via a so-called “relay” mechanism in which the weak reagent base needs to complex with both a charged site and a neighboring basic site on the polypeptide50. Accessibility of the weak reagent base to a charged site on the polypeptide therefore does not necessarily enable exchange. In contrast to weaker basic reagent gasses such as D2O and CH3OD, ND3 exchanges via an “onium” mechanism, in which complexation occurs exclusively with a charged site50. ND3 therefore poses no requirements for a nearby basic site in the polypeptide to facilitate HDX at an exposed site. It has been suggested that the difference in exchange mechanisms between D2O and ND3 might explain the previously (and presently) observed clear correlation between deuterium uptake of proteins in ND3 gas and their degree of folding 49. We hypothesize that the extent of HDX observed under our experimental conditions primarily depends on: (part a) the number and stability of long-lived exchange-competent ion-molecule complexes 36, 39, 51 formed per unit time and (part b) the accessibility of complex-bound ND3 molecules to exchangeable sites in the protein. While the ND3 pressure, T-wave velocity and the abundance of charge on the protein ion (i.e., protein charge state) influence part a, the gas-phase conformation of the protein influence part b.
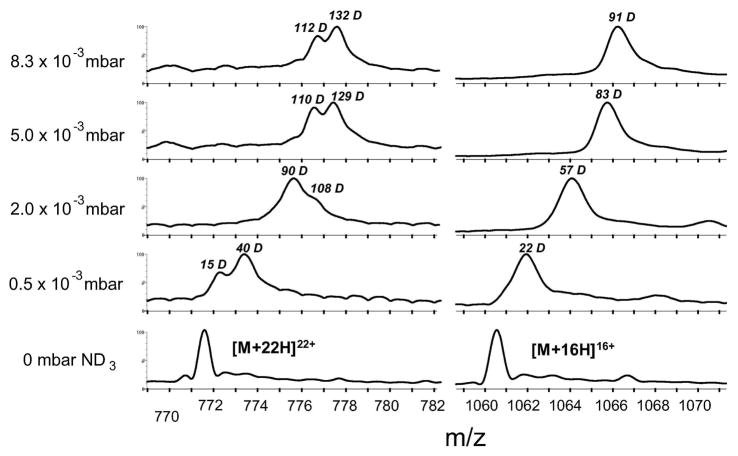
Myoglobin is a larger protein than either ubiquitin or cytochrome c and has been shown to display considerable conformational heterogeneity in the gas-phase52. To investigate if such heterogeneity could be detected shortly after ESI (approx. 1.5 ms), we performed gas-phase deuterium labeling of apo-myoglobin ions in mode 1 (Figure 1a). Mass spectra acquired at increasing ND3 pressure showed that while the lower charge states (+9 to +16) of apo-myoglobin were present in the mass spectrum as a single peak of gradually increasing m/z, higher charge states of apo-myoglobin (+16 to +23) displayed a distinct splitting of peaks into a bimodal pattern (Figure 6). A bimodal pattern indicates that these ions exist in at least two populations that exchange to different extents and do not interconvert during the labeling time in the TWIG (approx. 0.3 ms). The presence of such distinctly labeled populations of apo-myoglobin ions agree well with a prior ion mobility study performed at <10 ms timescales after ESI using a Synapt mass spectrometer 17. The fact that a bimodal exchange pattern was only observed for charge states >16+ suggests that the differences in HDX are due to local charge-induced unfolding of myoglobin to at least two different, partially folded or elongated states of the protein. Extensive experimental evidence 6, 9, 42, 52, 53 has suggested that even elongated gas-phase conformers of several proteins, including apo-myoglobin 52, can retain extensive secondary structure. Thus, the two differently-exchanging states of apo-myoglobin that are observed are likely the result of local unfolding of secondary structure elements, or changes to regional secondary structure.
Figure 6.
Detection of coexisting protein conformers of apo-myoglobin. Mass spectra obtained at increasing ND3 pressure reveals two conformations of the [M+22H]22+ charge state of apo-myoglobin (left). In contrast, the deuterium uptake of the [M+16H]16+ charge state indicates a single conformer (right).
Finally, we investigated the gas-phase HDX of protein states known to adopt different gas-phase conformers due to the presence or absence of well-defined covalent structural constraints such as intramolecular disulfide-bonds. Suckau et al. reported that the absence of native disulfide bonds in gas-phase RNase caused a significant increase in deuteration compared to the disulfide-intact form15. In related work, the presence of disulfide bonds in lysozyme has been shown to hinder the unfolding of highly charged ions of the disulfide-intact state whereas disulfide-reduced states of lysozyme were able to adopt significantly more elongated structures53. We have compared the gas-phase HDX of disulfide-reduced lysozyme and disulfide-intact lysozyme produced under denaturing and native conditions, respectively (Figure 7). Gas-phase deuterium uptake of both forms of lysozyme resulted in a gradual shift in m/z for all charge states with no significant peak broadening or splitting (data not shown). In contrast to proteins of similar size, such as ubiquitin and cytochrome c, native disulfide-intact lysozyme incorporated very few deuteriums at low ND3 pressures and required higher reagent-gas pressure or prolonged labeling times (i.e. slow T-wave velocity) to display only modest (up to 10–20 deuteriums) isotopic exchange. As shown for the [M+10H]10+ and [M+11H]11+ charge states, however, the deuterium uptake of disulfide-reduced lysozyme was significantly increased relative to the intact form (approximately a factor 3 e.g. 5 vs. 15 deuteriums at a pressure of ND3 of 8.3 × 10−3 mbar). The difference in HDX behavior between the two lysozyme forms is largely absent for the [M+12H]12+ charge state. We speculate that in this case, the increased number of charges on the protein causes sufficient columbic repulsion to unfold local regions of lysozyme structure that are indirectly stabilized by the presence of disulfide bonds. These experiments further indicate that the current method for gas-phase HDX is sensitive to the conformational properties of gaseous proteins.
Figure 7.
Gas-phase labeling of lysozyme with and without disulfide bonds. Deuterium uptake as a function of ND3 pressure of charge state +10 (circles), +11 (triangles) and +12 (boxes) of lysozyme in both reduced (open) and intact (solid) forms. The presence of intact disulfide bonds in lysozyme ions causes a significantly decreased deuterium uptake of the [M+10]10+ and [M+11H]11+ charge states.
The extent of HDX of the different proteins studied, at a given ND3 pressure and labeling time, cannot be compared directly as size and particularly protein charge greatly influences deuterium uptake. In order to partially compensate for this, we calculated the deuterium uptake per residue for the most abundant charge state of each protein under ESI at similar denaturing conditions. This value was defined as Dz(max). The Dz(max) values at a fixed labeling time (T-wave velocity of 300 m/s) and ND3 pressure of approx. 2 × 10−3 mbar for all four proteins was (in order of decreasing Dz(max)): apo-myoglobin (0.48), ubiquitin (0.39), cytochrome C (0.34), disulfide-reduced lysozyme (0.17) and disulfide-intact lysozyme (0.04). Although, the comparison is semi-quantitative and makes certain assumptions, it serves to illustrate the diversity in gas-phase HDX behavior of the different globular proteins in this study.
Conclusions
In this work we have shown that the intermediate pressure environment of a travelling wave ion is highly suited for very fast, localized deuterium labeling. Labeling gaseous protein ions with this methodology allows for controllable ion-molecule reaction times without interference from water vapor in air as has been observed for HDX experiments in the ion source 54. Furthermore, by performing HDX in the transfer TWIG of a Synapt HDMS mass spectrometer, it becomes possible to perform IMS and HDX analysis in tandem, thereby probing the same population of ions in two orthogonal dimensions of conformational detection. The utility of a TWIG for other types of gas-phase reactions could easily be envisioned for instance for harboring charge-stripping reagent gases or radical anions for ETD.
We selected ND3 for these labeling experiments because it is a strong reagent base. The weaker bases D2O and CH3OD might not label peptide and proteins to significant extents in the short time-scales employed. Evidence presented here and elsewhere38, 47, 49 suggests that the deuterium incorporation of proteins in ND3 gas is more directly correlated to surface accessibility and conformation due to the exchange mechanism employed by ND3 50. Our results indicate that by performing gas-phase HDX in a “curtain” of highly reactive deuterated gas, protein ions are primarily deuterium labeled at surface accessible facile sites. At sub-millisecond timescales and high pressures of ND3 gas, all exchangeable sites are probed continuously due to the high frequency of ion-molecule collisions; however, only facile sites have sufficient time to exchange. Slower exchanging sites such as backbone amide hydrogens do not appear to exchange significantly in this time-frame. The extent of HDX depends on the abundance of exchange-competent ND3-protein ion complexes formed per unit time (i.e. reaction parameters and protein charge state) and the accessibility of bound ND3 molecules to facile exchangeable sites in the protein (i.e. surface accessibility, intramolecular hydrogen bonding). Steric shielding of facile sites due to complex formation or protein conformation will give rise to changed deuterium uptake. Our present results on (1) the monomer and the homodimer of Leu-Enk, (2) the clear dependence of HDX reactivity on protein ion charge, (3) the detection of differentially labelled coexisting conformers of apo-myoglobin and (4) the increased HDX of disulfide-reduced lysozyme ions relative to the disulfide-intact form, all support this interpretation.
Curtain labeling in a TWIG samples protein ions within a few milliseconds after electrospray ionization and only probes them for 0.1 to 10 ms. By the use of highly reactive ND3 gas at elevated pressures, a high efficiency of gas-phase HDX of protein ions was achieved in the TWIG, corresponding to deuteration of 50–80% of side-chain positions or 25–50% of all labile hydrogens in less than a millisecond depending on the protein. This allowed us to extract information about gaseous ion structure from very short HDX reaction times. The conformations revealed in these short times should better reflect the conformational landscape of protein ions at the conditions of electrospray. At longer labeling timescales, such as in some other instrumental setups, gas-phase protein conformers have been shown to interconvert and labeling observed at such timescales would be affected by the presence of any exchange-competent states not present shortly after ionization 6–8, 42. We envision that the present setup for gas-phase HDX will be useful for probing biologically relevant states of single proteins and large protein-protein complexes occurring shortly after ESI at native state conditions 28. It will also facilitate better defining of what solution conformations are retained in the gas-phase.
Supplementary Material
Acknowledgments
We gratefully acknowledge financial support by The Danish Natural Science Research Council (K.D.R: grant 272-07-0276) and the National Institutes of Health (J.R.E: grants GM070590 and GM086507). We thank Robert H. Bateman and Bob Pfeifer for their valuable contributions to this work. This is contribution 946 from the Barnett Institute.
Footnotes
Supporting Information Available: This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Benesch JL, Robinson CV. Curr Opin Struct Biol. 2006;16:245–251. doi: 10.1016/j.sbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Loo AJ. J Mass Spectrom. 1995;30:180–183. [Google Scholar]
- 3.Loo JA. Mass Spectrom Rev. 1997;16:1–23. doi: 10.1002/(SICI)1098-2787(1997)16:1<1::AID-MAS1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Sobott F, McCammon MG, Hernandez H, Robinson CV. Phil Trans R Soc A. 2005;363:379–389. doi: 10.1098/rsta.2004.1498. [DOI] [PubMed] [Google Scholar]
- 5.Jarrold MF. Annu Rev Phys Chem. 2000;51:179–207. doi: 10.1146/annurev.physchem.51.1.179. [DOI] [PubMed] [Google Scholar]
- 6.Badman ER, Hoaglund-Hyzer CS, Clemmer DE. Anal Chem. 2001;73:6000–6007. doi: 10.1021/ac010744a. [DOI] [PubMed] [Google Scholar]
- 7.Badman ER, Myung S, Clemmer DE. J Am Soc Mass Spectrom. 2005;16:1493–1497. doi: 10.1016/j.jasms.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Breuker K, McLafferty FW. Proc Nat Acad Sci. 2008;105:18145–18152. doi: 10.1073/pnas.0807005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breuker K, Oh HB, Horn DM, Cerda BA, McLafferty FW. J Am Chem Soc. 2002;124:6407–6420. doi: 10.1021/ja012267j. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg MZ, Elber R, McLafferty FW, Gerber RB, Breuker K. Chembiochem. 2008;9:2417–2423. doi: 10.1002/cbic.200800167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohrer BC, Mererbloom SI, Koeniger SL, Hilderbrand AE, Clemmer DE. Annu Rev Anal Chem. 2008;1:293–327. doi: 10.1146/annurev.anchem.1.031207.113001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross DS, Schnier PD, Rodriguez-Cruz SE, Fagerquist CK, Williams ER. Proc Natl Acad Sci USA. 1996;93:3143–3148. doi: 10.1073/pnas.93.7.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loo RRO, Winger BE, Smith RD. J Am Soc Mass Spectrom. 1994;5:1064–1071. doi: 10.1016/1044-0305(94)85067-4. [DOI] [PubMed] [Google Scholar]
- 14.Williams ER. J Mass Spectrom. 1996;31:831–842. doi: 10.1002/(SICI)1096-9888(199608)31:8<831::AID-JMS392>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suckau D, Shi Y, Beu SC, Senko MW, Quinn JP, Wampler FM, 3rd, McLafferty FW. Proc Natl Acad Sci USA. 1993;90:790–793. doi: 10.1073/pnas.90.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winger BE, Light-Wahl KJ, Rockwood AL, Smith RD. J Am Chem Soc. 2002;114:5897. [Google Scholar]
- 17.Pringle SD, Giles K, Wildgoose JL, Williams JP, Slade SE, Thalassinos K, Bateman RH, Bowers MT, Scrivens JH. Int J Mass Spectrom. 2007;261:1–12. [Google Scholar]
- 18.Freitas MA, Hendrickson CL, Emmett MR, Marshall AG. Int J Mass Spectrom. 1999;187:565–575. [Google Scholar]
- 19.McLafferty FW, Guan Z, Haupts U, Wood TD, Kelleher NL. J Am Chem Soc. 1998;120:4732–4740. [Google Scholar]
- 20.Robinson EW, Williams ER. J Am Soc Mass Spectrom. 2005;16:1427–1437. doi: 10.1016/j.jasms.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wales TE, Engen JR. Mass Spectrom Rev. 2006;25:158–170. doi: 10.1002/mas.20064. [DOI] [PubMed] [Google Scholar]
- 22.Yan X, Maier CS. Mass Spectrometry of Proteins and Peptides. 2009:255–271. doi: 10.1007/978-1-59745-493-3_15. [DOI] [PubMed] [Google Scholar]
- 23.Rand KD, Adams CM, Zubarev RA, Jorgensen TJD. J Am Chem Soc. 2008;130:1341–1349. doi: 10.1021/ja076448i. [DOI] [PubMed] [Google Scholar]
- 24.Zehl M, Rand KD, Jensen ON, Jørgensen TJD. J Am Chem Soc. 2008;130:17453–17459. doi: 10.1021/ja805573h. [DOI] [PubMed] [Google Scholar]
- 25.Pan J, Han J, Borchers CH, Konermann L. J Am Chem Soc. 2008;130:11574–11575. doi: 10.1021/ja802871c. [DOI] [PubMed] [Google Scholar]
- 26.Pan J, Han J, Borchers CH, Konermann L. J Am Chem Soc. 2009 doi: 10.1021/ja904379w. [DOI] [PubMed] [Google Scholar]
- 27.Rand KD, Zehl M, Jensen ON, Jørgensen TJD. Anal Chem. 2009;81:5577–5584. doi: 10.1021/ac9008447. [DOI] [PubMed] [Google Scholar]
- 28.Heck AJR. Nat Meth. 2008;5:927–933. doi: 10.1038/nmeth.1265. [DOI] [PubMed] [Google Scholar]
- 29.Rand KD, Pringle SD, Murphy JP, III, Fadgen KE, Brown J, Engen JR. Proceedings of the 57th Conference on Mass Spectrometry and Allied Topics; May 31- June 4 2009; WPR 421. [Google Scholar]
- 30.Giles K, Pringle SD, Worthington KR, Little D, Wildgoose JL, Bateman RH. Rapid Commun Mass Spectrom. 2004;18:2401–2414. doi: 10.1002/rcm.1641. [DOI] [PubMed] [Google Scholar]
- 31.Hofstadler SA, Kristin ASL, Richard HG. J Mass Spectrom. 2000;35:62–70. doi: 10.1002/(SICI)1096-9888(200001)35:1<62::AID-JMS913>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Mo J, Hakansson K. Anal Chem. 2007;79:7893–7898. doi: 10.1021/ac0713095. [DOI] [PubMed] [Google Scholar]
- 33.Sannes-Lowery K, Griffey RH, Kruppa GH, Speir JP, Hofstadler SA. Rapid Commun Mass Spectrom. 1998;12:1957–1961. doi: 10.1002/(SICI)1097-0231(19981215)12:23<1957::AID-RCM418>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 34.Hofstadler SA, Kristin ASL, Richard HG. Rapid Commun Mass Spectrom. 1999;13:1971–1979. doi: 10.1002/(SICI)1097-0231(19991030)13:20<1971::AID-RCM740>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 35.Witt M, Fuchser J, Baykut G. J Am Soc Mass Spectrom. 2002;13:308–317. doi: 10.1016/S1044-0305(01)00367-1. [DOI] [PubMed] [Google Scholar]
- 36.Cheng X, Fenselau C. Int J Mass Spectrom Ion Processes. 1992;122:109–119. [Google Scholar]
- 37.Campbell S, Rodgers MT, Marzluff EM, Beauchamp JL. J Am Chem Soc. 1994;116:9765–9766. [Google Scholar]
- 38.Lifshitz C. Int J Mass Spectrom. 2004;234:63–70. [Google Scholar]
- 39.Ranasinghe A, Cooks GR, Sethi KSK. Org Mass Spectrom. 1992;27:77–88. [Google Scholar]
- 40.Ausloos P, Lias SG. J Am Chem Soc. 1981;103:3641–3647. [Google Scholar]
- 41.Valentine SJ, Clemmer DE. J Am Chem Soc. 1997;119:3558. [Google Scholar]
- 42.Myung S, Badman ER, Lee YJ, Clemmer DE. J Phys Chem A. 2002;106:9976–9982. [Google Scholar]
- 43.Valentine SJ, Counterman AE, Clemmer DE. J Am Soc Mass Spectrom. 1997;8:954–961. doi: 10.1016/S1044-0305(99)00079-3. [DOI] [PubMed] [Google Scholar]
- 44.Clemmer DE, Hudgins RR, Jarrold MF. J Am Chem Soc. 1995;117:10141–10142. [Google Scholar]
- 45.Wright PJ, Zhang J, Douglas DJ. J Am Soc Mass Spectrom. 2008;19:1906–1913. doi: 10.1016/j.jasms.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 46.McLafferty FW, Guan ZQ, Haupts U, Wood TD, Kelleher NL. J Am Chem Soc. 1998;120:4732–4740. [Google Scholar]
- 47.Geller O, Lifshitz C. Int J Mass Spectrom. 2004;233:125–129. [Google Scholar]
- 48.Reuben BG, Ritov Y, Geller O, McFarland MA, Marshall AG, Lifshitz C. Chem Phys Lett. 2003;380:88–94. [Google Scholar]
- 49.Geller O, Lifshitz C. J Phys Chem A. 2005;109:2217–2222. doi: 10.1021/jp044737c. [DOI] [PubMed] [Google Scholar]
- 50.Campbell S, Rodgers MT, Marzluff EM, Beauchamp JL. J Am Chem Soc. 1995;117:12840–12854. [Google Scholar]
- 51.Squires RR, Bierbaum VM, Grabowski JJ, DePuy CH. J Am Chem Soc. 1983;105:5185–5192. [Google Scholar]
- 52.Sullivan PA, Axelsson J, Altmann S, Quist AP, Sunqvist BUR, Reimann CT. J Am Soc Mass Spectrom. 1996;7:329–341. doi: 10.1016/1044-0305(95)00702-4. [DOI] [PubMed] [Google Scholar]
- 53.Reimann CT, Sullivan PA, Axelsson J, Quist AP, Altmann S, Roepstorff P, Velazquez I, Tapia O. J Am Chem Soc. 1998;120:7608–7616. [Google Scholar]
- 54.Hemling ME, Conboy JJ, Bean MF, Mentzer M, Carr SA. J Am Soc Mass Spectrom. 1994;5:434–442. doi: 10.1016/1044-0305(94)85059-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.