Abstract
Excess glutamate release and stimulation of post-synaptic glutamatergic receptors have been implicated in the pathophysiology of many neurological diseases. The hippocampus, and the pyramidal cell layer of the cornu ammonus 1 (CA1) region in particular, has been noted for its selective sensitivity to excitotoxic insults. The current studies examined the role of N-methyl-D-aspartate (NMDA) receptor subunit composition and sensitivity to stimulatory effects of the polyamine spermidine, an allosteric modulator of NMDA NR2 subunit activity, in hippocampal CA1 region sensitivity to excitotoxic insult. Organotypic hippocampal slice cultures of 8 day-old neonatal rat were obtained and maintained in vitro for 5 days. At this time, immunohistochemical analysis of mature neuron density (NeuN); microtubule associated protein-2(a,b) density (MAP-2); and NMDA receptor NR1 and NR2B subunit density in the primary cell layers of the dentate gyrus (DG), CA3, and CA1 regions, was conducted. Further, autoradiographic analysis of NMDA receptor distribution and density (i.e. [125I]MK-801 binding) and spermidine (100 μM)-potentiated [125I]MK-801 binding in the primary cell layers of these regions was examined. A final series of studies examined effects of prolonged exposure to NMDA (0.1–10 μM) on neurodegeneration in the primary cell layers of the DG, CA3, and CA1 regions, in the absence and presence of spermidine (100 μM) or ifenprodil (100 μM), an allosteric inhibitor of NR2B polypeptide subunit activity. The pyramidal cell layer of the CA1 region demonstrated significantly greater density of mature neurons, MAP-2, NR1 and NR2B subunits, and [125I]MK-801 binding than the CA3 region or DG. Twenty-four hour NMDA (10 μM) exposure produced marked neurodegeneration (~350% of control cultures) in the CA1 pyramidal cell region that was significantly reduced by co-exposure to ifenprodil or APV. The addition of spermidine significantly potentiated [125I]MK-801 binding and neurodegeneration induced by exposure to a non-toxic concentration of NMDA, exclusively in the CA1 region. This neurodegeneration was markedly reduced with co-exposure to ifenprodil. These data suggest that selective sensitivity of the CA1 region to excitotoxic stimuli may be attributable to the density of mature neurons expressing polyamine-sensitive NR2B polypeptide subunits.
Keywords: glutamate, calcium, head injury, amino acid, spermidine
INTRODUCTION
Glutamate is the most abundant excitatory amino acid in the brain, activating metabotropic (mGluR) and ionotropic glutamate receptors. Glutamatergic soma and receptor fields demonstrate widespread distribution in the CNS, with peak density observed in the pyramidal cell layers of the hippocampus proper (Monaghan & Cotman, 1985). Dysfunction of glutamatergic signaling has been implicated in the pathophysiology of a variety of neurological diseases, including Parkinson's disease, Alzheimer's disease, Huntington's disease, ischemia, epilepsy (reviewed by Gardoni & Di Luca, 2006), and amyotrophic lateral sclerosis (reviewed by Heath & Shaw, 2002). Additionally, a variety of animal models of human diseases employing both in vivo and in vitro models of stroke and neurotrauma support the role of an overactive glutamatergic system in neurodegeneration (Arundine & Tymianksi, 2004). During periods of neural hyperexcitability, synaptic glutamate concentrations are elevated to near mM concentrations (Silverstein et al., 1991; Gibson et al., 2003), allowing for overactivation of Ca2+-permeable N-methyl-D-aspartate (NMDA) receptors. Excess synaptic glutamate contributes to neurodegeneration, in part, because of NMDA-mediated influx of extracellular Ca2+ (Choi, 1987) and release of intracellular Ca2+ from endoplasmic reticulum (Pelletier et al., 1999), which can be attenuated by NMDA receptor antagonism or allosteric modulation of NMDA receptor activity by mGluR5 agonists, in a phospholipase C-dependent manner (Choi et al. 1988; Baskys et al., 2005).
This Ca2+-dependent excitotoxic cascade is further characterized by many processes that disrupt the functional and structural integrity of neurons. Excess glutamtergic activity results in pathological overactivation of proteases, lipases, phosphatases, and endonucleases (Arundine & Tymianksi, 2004). In particular, activation of the Ca2+-dependent cysteine protease calpain is a consequence of glutamate receptor overactivity, leading to breakdown in the cytoskeletal proteins α-spectrin, actin, and microtubule-associated protein-2 (MAP-2) (Siman & Noszek, 1988; Halpain et al., 1998; Gerencser et al., 2009). Further, short term NMDA exposure and resulting Ca2+ influx into neurons may result in formation of the mitochondrial permeability transition pore and release of cytochrome c, leading to activation of caspase 9- and 3-dependent neurodegeneration (Budd & Nicholls, 1996; Atlante et al., 1997; Castilho et al., 1999; Pivovarova et al., 2004; Schuh et al., 2008).
In concert with the high density of glutamatergic soma and receptors in the hippocampus, this brain region has been noted to be particularly vulnerable to many different types of excitotoxic insult (Walsh & Emerich, 1988; Stoltenburg-Didinger, 1994; Prendergast et al., 2004; Cater et al., 2007). The organotypic hippocampal cell culture model has proven useful for modeling the role of glutamate-associated excitotoxic neurodegeneration as it relates to several insults, including oxygen glucose deprivation (Scartabelli et al., 2008), human immunodeficiency virus-1 infection (Self et al., 2004), ethanol withdrawal (Prendergast et al., 2004), and traumatic brain injury (Cater et al., 2007). Organotypic hippocampal cultures taken from 6–9 day old rat pups maintain structural and functional integrity over time, and maintain trisynaptic circuitry despite the lack of afferent input to the dentate gyrus (Gutierrez and Heinemann, 1999). Additionally, Martens and Wree (2001) have demonstrated the lasting integrity of hippocampal glutamate receptors (NMDArs and AMPArs) taken from rat pups on post-natal day (PND) 6 and allowed to age in vitro, as they do not differ markedly in distribution or electrophysiological properties (Gutierrez and Heinemann 1999) from PND 30 rat brain.
Data obtained employing organotypic cultures and other models of hippocampal injury, including those discussed above, suggest that pyramidal cells of the CA1 region of explants are selectively injured in a manner reflecting the importance of the glutamatergic system as a final common pathway mediating neuronal damage (Prendergast et al., 2004; Bonde et al., 2005; Cater et al., 2007). In contrast, hippocampal bipolar and stellate cells are markedly resistant to glutamate receptor-mediated neurodegeneration (Mattson & Kater, 1989). Little is known of factors that may contribute to the selective sensitivity of CA1 pyramidal cells to excitotoxic insult, however, endogenous polyamines (e.g., putrescine, spermidine and spermine) are small aliphatic molecules that have been suggested to be associated with neuronal injury following traumatic brain injury (Henley et al., 1996); oxygen-glucose deprivation (Kim et al., 2009); ethanol withdrawal (Prendergast et al. 2000; Gibson et al., 2003); and possibly, epileptiform activity (Laschet et al., 1999). Importantly, polyamines function as allosteric activators of NMDA receptors via N-terminal actions on NR2 subunits of NMDA receptors (Williams et al., 1991, 1994). NMDA receptors are particularly implicated in mediating glutamatergic signaling after excitotoxic insult. All NMDA receptors are tetrameric ion channels formed by an obligatory NR1 subunit and NR2(A–D) subunits. NMDA receptor activation requires glutamate binding on the NR2 subunit and co-agonism by glycine binding on the NR1 subunit. Further, the NR2 subunit contains multiple binding domains associated with allosteric modulation of channel opening probability (reviewed by Yamakura & Shimoji, 1999). Thus, the present studies were designed to examine the potential role that NMDA receptor subunit composition and sensitivity to allosteric activation of NR2-containing NMDA receptors by the polyamine spermidine may have in selective CA1 region sensitivity to excitotoxic insult.
EXPERIMENTAL METHODS
Organotypic Hippocampal Slice Culture Preparation
Eight-day old male and female Sprague Dawley rat pups (Harlan Laboratories, Indianapolis, IN, USA) were humanely euthanized for aseptic whole brain removal. Brains were immediately transferred into frozen dissecting medium made of Minimum Essential Medium containing Hanks' salts and L-glutamine (MEM; Gibco, Gaithersburg, MD, USA), 25 mM HEPES (Sigma-Aldrich Co., St. Louis, MO, USA), and 50 μM Penicillin/Streptomycin (Gibco). Bilateral hippocampi were removed, cleaned of extra tissue under a dissecting microscope, and placed into chilled culture medium, composed of dissecting medium with the addition of: 36 mM glucose (Fisher Scientific, USA), 25% (v/v) Hanks' balanced salt solution (Gibco), 25% heat-inactivated horse serum (Sigma-Aldrich Co.), and 0.05% Penicillin/Streptomycin. Hippocampi were then sectioned coronally at 200 μm using the McIllwain Tissue Chopper (Mickle Laboratory Engineering Co. Ltd., Gomshall, UK) and placed into fresh culture medium. Morphologically intact slices were selected under a dissecting microscope and placed onto porous Teflon membrane inserts (Millicell-CM 0.4 μm; Millipore, Marlborough, MA). Inserts were pre-incubated in a 35 mm 6-well culture plate with 1 ml of culture medium beneath the insert in an incubator environment of 37°C, 5% CO2/95% air. Three slices were placed onto each insert, yielding 18 slices per plate. Excess medium from the top of the membrane insert was aspirated to allow the slices exposure to the incubator atmosphere. Slices were allowed five days in vitro (DIV) to attach to the insert membrane before any experiments were conducted. This method was adapted from Stoppini et al., 1991. Care of animals was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23, revised 1996), as well as the University of Kentucky's Institutional Animal Care and Use Committee. All experiments discussed below were replicated using different rat litters.
Immunoreactivity of Neuron Specific Nuclear Protein (NeuN) and Microtubule Associated Protein-2a,b (MAP-2)
Immunohistochemical labeling with neuron specific nuclear protein (NeuN) was conducted to compare the density of mature neurons in the primary cell layers of the dentate gyrus (DG), cornu ammonis (CA3), and CA1 hippocampal regions (after Wilkins et al. 2006). Additionally, immunoreactivity of microtubule associated protein-2 (MAP-2), found in dendrites and cell bodies, was measured in the CA1, CA3, and DG hippocampal regions (after Prendergast et al. 2007). After 5 DIV, cultures were removed from culture medium for fixation with 10% formalin. Formalin was applied to the bottom and top of cultures for 30 minutes followed by two washes in 1x PBS and storage at 4°C until immunohistochemistry was conducted. At the initiation of the immunohistochemistry procedure, cultures were placed into a PBS buffer containing 0.005% bovine serum albumin and 0.1% Triton-X (Sigma-Aldrich Co.) for 45 minutes to permeabilize cell membranes. Following this incubation period, cultures were transferred into fresh culture plates with 1 ml of 1x PBS on the bottom of the plate, and 1 ml of buffer containing either the mouse anti-NeuN monoclonal primary antibody (1:200; Chemicon, Temecula, CA) or mouse anti-bovine MAP-2 (2a & 2b; (Sigma-Aldrich) monoclonal primary antibody added slowly to the top of the culture membrane. Following 24 hour incubation with primary antibody at 4°C, cultures were washed twice in 1x PBS and placed into fresh culture plates with 1 ml of 1x PBS on the bottom of the plate, and 1 ml of buffer containing tetramethylrhodamine isothiocynate (TRITC)-conjugated secondary antibody for NeuN labeled cultures (1:200; produced in goat; Sigma-Aldrich Co.), and fluorescein isothiocynate (FITC)-conjugated secondary antibody for MAP-2 labeled cultures (1:200; produced in mouse), slowly added to the top of the culture membrane. After plates were stored at 4°C for 24 hours, cultures were washed twice in 1x PBS and fluorescent images were taken. TRITC fluorescence was elicited using a band-pass filter that excites wavelengths between 515 and 560 nm (visual range ~620 nm), and FITC fluorescence was elicited using a band-pass filter that excites wavelengths of approximately 495 nm (emission wavelength = 520 nm). Images were taken using a 5× objective lens, as described in more detail below. N = 20–22 for each hippocampal region for NeuN, and N = 6–9 for each hippocampal region for MAP-2.
NR1 and NR2B Subunit Immunoreactivity
Immunohistochemistry was also conducted to compare the density of the NMDA NR1 subunit and the NMDA NR2B subunit in the primary cell layers of the DG, CA3, and CA1 hippocampal regions. After the 45 minute 0.1% Triton-X incubation period, cultures were transferred into fresh culture plates with 1 ml of 1x PBS on the bottom of the plate, with 1 ml of buffer containing either the mouse anti-NMDA NR1 monoclonal primary antibody (1:200; BD Biosciences, San Jose, CA) or rabbit anti-NMDA NR2B monoclonal primary antibody (1:200; Millipore) added slowly to the top of the culture membrane. Following 24 hour incubation with the primary antibody at 4°C, cultures were washed twice in 1x PBS and placed into fresh culture plates with 1 ml of 1x PBS on the bottom of the plate and 1 ml of buffer containing either FITC-or TRITC -conjugated secondary antibody (1:200) added slowly to the top of the culture membrane. After twenty-four hour incubation with the secondary antibody, FITC fluorescence was elicited using a band-pass filter that excites wavelengths of approximately 495 nm (emission wavelength = 520 nm), or TRITC fluorescence was excited using a band-pass filter exciting wavelengths between 515–560 nm (emission wavelength = 615 nm). Fluorescent images were taken using a 5× objective for analysis. N = 12 for each hippocampal region for both NR1 and NR2B analyses.
[125I]MK-801 Autoradiography
At 5 DIV, a portion of cultures were formalin-fixed for autoradiographic analysis of [125I]MK-801 (PerkinElmer, Boston MA) with or without spermidine to determine density of polyamine-sensitive NMDA receptors. MK-801 is a non-competitive NMDA receptor antagonist, binding within the ion channel. Spermidine is an endogenous polyamine that positively modulates NMDA receptor channel activity at NR2 subunits. Cultures were stored at −80°C until experiments were conducted. At the initiation of the autoradiography protocol, cultures were brought to room temperature and incubated in room temperature 50 mM Tris acetate buffer (pH = 7.4) for 90 minutes. Cultures were then incubated with 100 pM [125I]MK-801 for 90 minutes, with a subset of cultures co-exposed to [125I]MK-801 and 100 μM spermidine. Cultures were then washed in double-distilled H2O before placement into clean, empty culture plates, in which slices were dried under a fan. Dried slices were placed into a dessicator for 24 hours, after which time they were exposed to x-ray radiographic film for 96 hours at room temperature. Films (Kodak BioMax®; Sigma-Aldrich) were developed in Kodak D-19 developer for 5 minutes, followed by 30 second exposure to an indicator stop bath, and 4 minutes in Kodak rapid fixer. N = 14–17 for each hippocampal region.
PI Uptake in NMDA-Exposed Cultures
At 5 DIV, separate cultures were exposed to a range of NMDA concentrations (0, 0.1, 1, or 10 μM) for twenty-four hours (N=28–53/group). Additional cultures were co-exposed to NMDA (10 μM) and the competitive NMDA receptor antagonist DL-2-Amino-5-phosphonopentanoic acid (APV; 20 μM) or the NMDA receptor NR2B subunit polyamine site antagonist ifenprodil (+)-tartrate salt (Sigma-Aldrich Co.; 100 μM). Cultures were exposed to compounds dissolved in culture medium and placed on the bottom of new six-well culture plates. Additional cultures were co-exposed to a non-toxic concentration of NMDA (5 μM) and the polyamine spermidine (100 μM), exposed to spermidine alone, or co-exposed to NMDA, spermidine, and the NR2B-specific polyamine site antagonist ifenprodil (100 μM). Propidium iodide (PI; 3.74 μM) was present in the medium at the initiation of drug treatment for visualization of cytotoxicity. Cellular uptake of PI was visualized using fluorescent microscopy. Images were taken using SPOT Advanced version 4.0.9 software for Windows (W. Nuhsbaum Inc., McHenry, IL, USA) with a 5× objective on an inverted Leica DMIRB microscope (W. Nuhsbaum Inc.) fitted for fluorescence detection (mercury-arc lamp) and connected to a personal computer via a SPOT 7.2 color mosaic camera (W. Nuhsbaum Inc.). Propidium iodide has a maximum excitation wavelength of 536 nm and was excited using a band-pass filter that excites wavelengths between 515 and 560 nm. The emission of PI in the visual range is 620 nm.
Data Analysis
For PI and immunohistochemistry studies, fluorescent intensity (arbitrary optical units) was analyzed by densitometry using Image J software (National Institutes of Health, Bethesda, MD, USA). Similarly, for autoradiographic studies, binding density was analyzed by densitometry. Hippocampal regions analyzed included the granule cell layer of the dentate gyrus (DG) and the pyramidal cell layers of the CA3 and CA1 regions. For each slice, background intensity was subtracted from each region's measurement prior to statistical analysis. For immunohistochemical studies (NeuN, MAP-2, NR1, and NR2B), one-way ANOVAs were conducted (factor: hippocampal region). For studies of NMDA toxicity measured by PI uptake, two-way ANOVAs were conducted (treatment × region) with Tukey's post hoc tests interpreted when appropriate. For autoradiographic analysis, a two-way ANOVA (treatment × region) was conducted to determine the effect of spermidine on [125I]MK-801 binding between hippocampal regions, and Tukey's post-hoc tests were interpreted when appropriate.
RESULTS
Immunoreactivity of Neuron Specific Nuclear Protein (NeuN) and Microtubule-Associated Protein-2a,b (MAP-2)
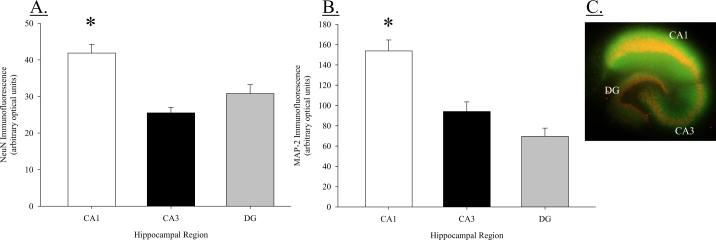
Mature neuron density (NeuN immunoreactivity) was analyzed using densitometry in the granule cell layer of the DG and the pyramidal cell layers of the CA3 and CA1 regions. A one-way ANOVA indicated a significant difference in NeuN immunofluorescence among hippocampal regions (F(2, 63) = 15.105, P < 0.001; Figure 1A). Neuronal density was greatest in the CA1 region compared to both the CA3 region (post hoc P < 0.001) and DG (post hoc P < 0.001). Mean immunofluorescence (in optical units) in the CA1 region reached values of 41.85 ± 2.33, as compared to 25.50 ± 1.49 in the CA3 region and 30.78 ± 2.44 in the DG. No significant difference was detected in NeuN fluorescence between the CA3 region and DG (post hoc P = 0.079).
Figure 1.
(A) NeuN Immunoreactivity in the CA1, CA3, and dentate gyrus regions of organotypic hippocampal slice cultures. Density of mature neurons is significantly greater in the pyramidal cell layer of the CA1 region as compared to the pyramidal cell layer of the CA3 region or the granule cell layer of the dentate gyrus; *P < 0.001 vs. DG and CA3. (B) Immunoreactivity of microtubule associated protein-2(a,b) (MAP-2) in the CA1, CA3, and dentate gyrus. MAP-2 immunoreactivity is significantly greater in the pyramidal cell layer of the CA1 region as compared to the pyramidal cell layer of the CA3 region or the granule cell layer of the DG; *P < 0.001 vs. DG and CA3. (C) Representative image of an organotypic hippocampal slice dual-labeled for NeuN (TRITC) and MAP-2 (FITC) immunoreactivity.
MAP-2 immunofluorescence was also analyzed using densitometry in the granule cell layer of the DG and the pyramidal cell layers of the CA3 and CA1 regions. A one-way ANOVA indicated a significant difference in MAP-2 immunofluorescence among hippocampal regions (F(2, 23) = 22.095, P < 0.001; Figure 1B). MAP-2 immunoreactivity was greater in the CA1 region compared to either the CA3 region (post hoc P < 0.001) or the DG (post hoc P < 0.001). No difference was present between the CA3 region and DG. A representative dual-labeled, merged image of NeuN and MAP-2 immunofluorescence is presented in Figure 1C.
NR1 and NR2B Subunit Immunoreactivity
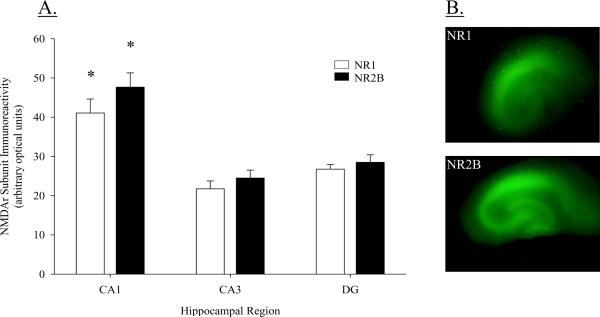
Densitometry was also used to analyze NR1 and NR2B subunit immunoreactivity in the granule cell layer of the DG and the pyramidal cell layers of the CA3 and CA1 regions. Separate one-way ANOVAs were conducted for NR1 and NR2B immunoreactivity. Analysis revealed a significant regional difference for both NR1 and NR2B immunoreactivity (NR1: F(2, 35) = 16.537, P < 0.001; NR2B: F(2, 35) = 21.985, P < 0.001; Figure 2A). Subunit density did not differ between the DG and CA3 regions for NR1 or NR2B (post hoc P = 0.161 for NR1; post hoc P = 0.292 for NR2B). However, subunit density of NR1 and NR2B subunit was significantly greater in the CA1 region compared to both the DG and the CA3 region (post hoc Ps < 0.001). Mean immunoreactivity for the NR1 subunit in the CA1 resulted in optical fluorescent values of 41.07 (± 3.56) and were nearly 50% greater than mean values for the CA3 region (21.73 ± 2.02) or the DG (26.74 ± 1.22). Similarly, mean values for the NR2B subunit were markedly elevated in the CA1 region (47.65 ± 3.63) compared to the CA3 region (24.50 ± 2.02) or the DG (28.50 ± 1.91). Representative images of NR1 and NR2B immunoreactivity are presented in Figure 2B.
Figure 2.
(A) Immunoreactivity of NMDA receptor NR1 and NR2B subunits in the CA1, CA3, and DG hipppocampal regions. Density of both NR1 and NR2B receptor subunits is greatest in the pyramidal cell layer of the CA1 region as compared to the CA3 region or DG; *P < 0.001 vs. DG & CA3. (B) Representative images of NMDA NR1 and NR2B subunit immunoreactivity in the pyramidal cell layer of the CA1 and CA3 regions, as well as, the granule cell layer of the DG.
[125I]MK-801 Autoradiography
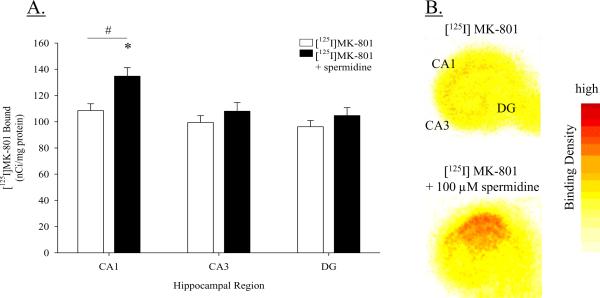
Autoradiographic analysis of [125I]MK-801 binding density with or without the addition of spermidine was conducted in the primary cell layers of the DG, CA3, and CA1 regions. A two-way ANOVA revealed a significant main effect of treatment (F(1, 92) = 8.776, P < 0.01) and region (F(2, 92) = 7.258, P < 0.01), but not a significant interaction (F(2, 92) = 1.464, P = 0.237); Figure 3A. Regarding the main effect of region, overall [125I]MK-801 binding was significantly greater in the CA1 region as compared to either the CA3 region or the DG (post hoc P < 0.05). With respect to the factor of treatment, the presence of spermidine significantly elevated [125I]MK-801 binding in the hippocampus (post hoc P < 0.05). Subsequent one-way ANOVAs within each hippocampal region (factor: treatment) showed that spermidine significantly elevated [125I]MK-801 binding only in the CA1 region (post hoc P < 0.05), showing a 26% increase in binding compared to cultures not exposed to spermidine. These data indicate greater polyamine-potentiated [125I]MK-801 binding in the CA1 region, as well as higher basal [125I]MK-801 binding in the CA1 region. Representative autoradiographic images are presented in Figure 3B.
Figure 3.
(A) Autoradiographic measurement of [125I]MK-801 binding in the absence or presence of spermidine (100 μM) in the CA1, CA3, and DG hippocampal regions. The addition of the polyamine spermidine significantly potentiated [125I]MK-801 binding only in the CA1 region. *P < 0.05 vs. CA1 [125I]MK-801. #P < 0.05 vs. CA3 region and DG, collapsed across treatment groups. (B) Representative autoradiographic images of [125I]MK-801 binding and spermidine-potentiated [125I]MK-801 binding.
PI Uptake in NMDA-Exposed Cultures
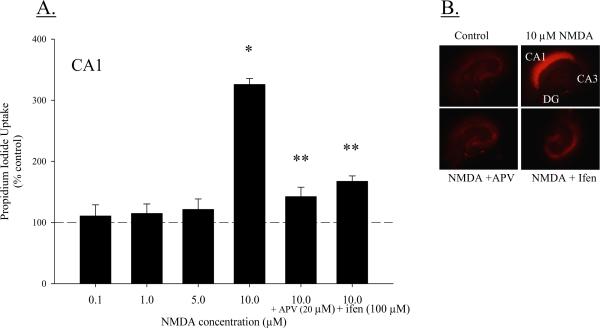
At 5 DIV, cultures were exposed to NMDA (0.1, 1, 5 or 10 μM) or co-exposed to NMDA (10 μM) and APV (20 μM) or ifenprodil (100 μM) for twenty-four hours. A two-way ANOVA (treatment × region) indicated a significant interaction (F(8, 434) = 108.991; P < 0.001; Figure 4A). Within the CA1 region, exposure to 10 μM NMDA produced significantly greater toxicity compared to control cultures (~350%; post hoc P < 0.001), and cultures exposed to 0.1 μM or 1 μM NMDA (post hoc P < 0.001 for both comparisons). Further, the toxicity observed in the CA1 region with exposure to 10 μM NMDA was greater than toxicity produced by 10 μM NMDA in either the CA3 region or DG (post hoc P < 0.001 for both comparisons). In the CA1, neither 0.1 μM nor 1 μM NMDA exposure produced toxicity above control values, and the NMDAr antagonist APV significantly reduced toxicity produced by 10 μM NMDA (post hoc P < 0.001). Similarly, a significant treatment × region interaction was detected (F(6, 575) = 39.358, P < 0.001; Figure 4) in additional studies in which cultures were co-exposed to NMDA and ifenprodil. Again, 10 μM NMDA produced toxicity only in the CA1 region (post hoc P < 0.0001) and toxicity was significantly reduced with co-exposure to the NR2B-specific polyamine-site antagonist ifenprodil (P < 0.001). Toxicity was not observed after 24 hour exposure to any concentration of NMDA or ifenprodil in the CA3 region or DG. Representative images of PI uptake are presented in Figure 4B.
Figure 4.
(A) Propidium Iodide (PI) uptake following 24 hour exposure to NMDA (0.1, 1, 5, or 10 μM) or co-exposure to NMDA (10 μM) and APV (20 μM) or ifenprodil (100 μM) in the CA1, CA3, and DG hippocampal regions. Exposure to 10 μM NMDA produced marked toxicity in the CA1 region that was reduced with co-exposure to APV or ifenprodil. * P < 0.05 vs. control (dashed line) and NMDA-treated cultures; **P < 0.001 vs. 10 μM NMDA. (B) Representative images of PI fluorescence following 24hrs of NMDA exposure.
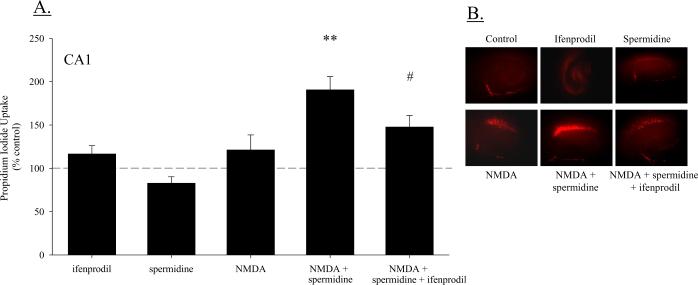
Additional cultures were exposed to a sub-toxic concentration of NMDA (5 μM) or co-exposed to NMDA and the polyamine spermidine, or NMDA and spermidine and ifenprodil. A two-way ANOVA (treatment × region) revealed a significant interaction (F(8, 269) = 3.98; P < 0.001; Figure 5A), such that toxicity was significantly greater in the CA1 region compared to either the CA3 region (post hoc P < 0.001) or the DG (post hoc P < 0.001). Though 5 μM NMDA did not produce toxicity in any hippocampal region, co-exposure to NMDA and spermidine increased PI uptake modestly in the DG above NMDA-treated cultures (post hoc P < 0.05; data not shown), and produced a marked increase in PI uptake in the CA1 pyramidal cell region, as compared to NMDA-treated cultures not exposed to spermidine (post hoc P < 0.001). This increase in PI uptake produced by co-exposure to NMDA and spermidine, to nearly 200% of control levels in the CA1 region, was significantly reduced by the addition of ifenprofil (post hoc P < 0.01). Exposure to spermidine in the absence of NMDA did not produce toxicity in any hippocampal region. Representative images of PI uptake in these cultures are presented in Figure 5B.
Figure 5.
(A) Co-exposure to a non-toxic concentration of NMDA (5 μM) and the polyamine spermidine (100 μM) produced significant toxicity in the CA1 region of hippocampal slice cultures. This toxicity was significantly reduced in cultures additionally exposed the NR2B-polyamine site specific antagonist ifenprodil (100 μM); **P < 0.05 vs. NMDA; #P < 0.05 vs. NMDA and spermidine. (B) Representative images of PI fluorescence following co-exposure to NMDA and spermidine or NMDA, spermidine and ifenprodil.
DISCUSSION
The present findings suggest a role for both increased neuronal density and increased abundance of polyamine-sensitive NMDA receptors in the selective sensitivity of CA1 pyramidal cells to excitotoxic insult, as compared to cells of the CA3 and dentate regions. Not only does the CA1 region contain the greatest abundance of mature neurons and dendritic processes as reflected in NeuN and MAP-2 immunoreactivity, respectively, but the CA1 region also contains the greatest density of NR1 and NR2B subunits of NMDA receptors. These findings of polypeptide subunit distribution differences are of functional importance, as spermidine, an allosteric modulator of the N-terminal polyamine binding site on NR2 subunits, potentiated [125I]MK-801 binding in the CA1 region. This suggests a greater density of polyamine-sensitive NMDA receptors in the CA1 pyramidal cell region, which is further supported by spermidine-potentiated NMDA toxicity exclusively in the CA1 region of organotypic hippocampal slice cultures. Antagonism of NMDA toxicity and spermidinepotentiated NMDA neurodegeneration in the CA1 pyramidal cell layer with co-exposure to ifenprodil strongly suggests a role for NR2B subunit function in the selective sensitivity of CA1 pyramidal cells to excitotoxic insult. Spermidine and other polyamines do not demonstrate selectivity for NMDA receptor NR2 subunits, as they bind to and modulate activity of inward rectifying K+ channels (Yan et al. 2005), L-type Ca2+ channels (Herman et al. 1993) and may even may bind to an extracellular glutamate-binding core on NR1-1b subunits (Stoll et al. 2007). Thus, use of the NR2B-selective allosteric inhibitor ifenprodil is confirmatory of a role for NMDA receptors expressing this subunit in the neurodegeneration observed. Together, these data support selective vulnerability of CA1 pyramidal cells to NMDA-induced cell death, in large part because of increased density of NMDA receptors and, in particular, polyamine-sensitive/NR2B-containing NMDA receptors.
It has also been suggested that the CA1 region in hippocampal explants may be particularly vulnerable to NMDA-induced neurodegeneration, in part, as a consequence of the network excitability that culminates from mossy fiber and Schaffer collateral excitability terminating in the CA1 pyramidal cell region of hippocampal explants (Mulholland and Prendergast 2003; Prendergast et al., 2004). These reports demonstrated that surgical transection of CA1 afferents nearly abolished excitotoxic insults to CA1 pyramidal cells, while transection of CA1 efferents had no effect. Similarly, Lahtinen et al. (2001) demonstrated that application of glutamate produced delayed CA1 region pyramidal cell degeneration that required post-insult, Na+-channel-dependent propagation of network excitability in intact hippocampal explants. In contrast, Ikegaya and Matsuki (2002) reported that surgical isolation of the CA1 region from efferent stimulation did not markedly alter NMDA-induced neurodegeneration, suggesting an inherent susceptibility of CA1 pyramidal cells to excitotoxicity. However, significant methodological differences between these studies are apparent, most notably, the age of tissue in vitro (5 vs 10 days in vitro, after Mulholland & Prendergast, 2003 and Ikegaya & Matsuki, 2002, respectively). Previous work employing dissociated hippocampi has demonstrated that in vitro aging (as early as 0–8 days in culture) is associated with increased sensitivity of hippocampal pyramidal cells to NMDA-induced excitability (Mattson et al., 1991). Thus, the differences described above with regard to the necessity of tri-synaptic hippocampal circuitry for the expression of CA1 pyramidal cell injury may be related to the in vitro aging.
The NMDA receptor is composed of an obligatory NR1 subunit, which shows further diversity by the existence of eight splice variants (Zukin & Bennett, 1995). NR1 subunits form functional heteromers with various NR2(A–D) subunits that regulate properties of the ion channel, including resistance to Mg2+ blockade, agonist-specific deactivation time (offset decays), and antagonist affinity (Hollmann and Heinemann, 1994; Janssen et al., 2005). The NR2A and NR2B receptor subunits are the predominant NR2 subtypes that form heteromers with the NR1 subunit in the hippocampus. It has been shown that NR1/NR2B containing receptors allow greater time for intracellular Ca2+ entry as compared to NR1/NR2A containing receptors (Chen et al., 1999). In addition, NR2-containing receptors contain a modulatory binding site for endogenous polyamines (spermine, spermidine, putrescine), which act to potentiate NMDA receptor activity under physiologically-similar conditions (Williams et al., 1994). Polyamines are endogenous constituents of all cells, and are important in cell growth and differentiation. In the CNS, spermine and spermidine have been shown to have both stimulatory and inhibitory actions on NMDA receptor currents. However, stimulatory effects at NMDA receptors expressed in oocytes are mediated by NR1A/NR2B heteromers, but not by NR1 homomers or NR1A/NR2A or NR1/NR2C heteromers (Williams et al., 1994). Further, polyamine release has been reported to contribute to hippocampal neurodegeneration via multiple possible mechanisms, including allosteric activation of NMDA receptor function, following traumatic brain injury (Henley et al., 1996); oxygen-glucose deprivation (Kim et al., 2009); ethanol withdrawal (Gibson et al., 2003; Prendergast et al., 2000); and possibly, epileptiform activity (Laschett et al., 1999).
In CA1 pyramidal cells, NMDA receptors are located synaptically and extrasynaptically, with a similar expression of NR2B subunits in each compartment (Thomas et al., 2006; Harris & Pettit, 2007). In addition to diversity in NMDA receptor function that is conferred by different subunit heteromers, some data suggest that cellular localization (i.e. synaptic versus extrasynaptic) determines whether NMDA receptor stimulation is excitotoxic or neurotrophic. Hardingham et al. (2002) reported that selective activation of hippocampal synaptic NMDA receptors increased CREB activity and BDNF gene expression, whereas bath application of glutamate and concomitant activation of synaptic and extrasynaptic NMDA receptors resulted in disruption of the mitochondrial membrane potential and cell death. However, disruption of CREB signaling via extrasynaptic NMDA receptor activation is a developmentally regulated process, as this effect is apparent in hippocampal neurons cultured for 12, but not 7, days (Hardingham & Bading, 2002). Further, examination of excitatory synaptic activity in hippocampal neurons has refuted differential roles of synaptic and extrasynaptic NMDA receptors, as no change in toxicity was observed after application of glutamate during preferential inhibition of either synaptic or extrasynaptic NMDA receptor signaling (Sattler et al., 2000). Thus, findings in this regard are equivocal and conclusions cannot be made with the current dataset on the potential contribution of cellular NMDA receptor localization to excitotoxic injury.
Expression of NR2 subunits is developmentally regulated. NR2B subunit mRNA is detectable as early as embryonic day 14, and persists beyond birth, in contrast with NR2A mRNA that is not present until birth (Monyer et al., 1994). Postnatally, NR2B subunit expression is abundant early in development, followed by a progressive decline in NR2B subunit density and progressive increase in NR2A subunit density beginning 7–10 days postnatally (Laurie et al., 1997). The modulatory effect of polyamines on NMDA receptor activity and potency for the NMDA receptor has also been demonstrated to change throughout development in rat forebrain (Williams et al., 1991). For instance, potency of spermine in potentiating [125I]MK-801 binding in cortex progressively increases from PND 3 and reaches adult-levels by PND 10. This may be of direct relevance for the current data, as the cultures utilized in the current preparation are prepared from PND 8 rat hippocampi and allowed 5 DIV before experimental manipulation. Together this suggests that the current culture system may appropriately model polyaminepotentiation of NMDA receptor-mediated effects in the developing and adult brain.
It is well established, and the current data support, that greater NMDA-mediated excitotoxicity occurs in the CA1 region of the hippocampus. However, it must be noted that some excitotoxic stimuli reliably produce the most marked hippocampal toxicity in the CA3 region. For example, both in vitro and intracerebroventricular administration of kainic acid produce greater cell death in the CA3 pyramidal cell layer compared to other hippocampal regions (Nadler & Cuthbertson, 1980; Casaccia-Bonnefil et al., 1993; Holopainen et al., 2004). Also, the excitotoxin kainic acid produces spontaneous firing in the CA3 region and has been widely used as preclinical model for temporal lobe epilepsy (Bragin et al., 1999). Increased neuronal excitability upon administration and toxicity may be, in part, because the CA3 hippocampal region contains the greatest abundance of [3H]kainate-labeled receptors in both immature rat brain grown in culture for several weeks and in rat brain taken from rats at PND 30 (Martens & Wree, 2001). Though it is not clear whether degeneration of CA3 neurons after kainic acid administration is exclusively mediated by apoptotic (Pollard et al., 1994) or necrotic (Holopainen et al., 2004) processes, it does appear to depend on mossy fiber input from the dentate gyrus (Nadler & Cuthbertson, 1980; Casaccia-Bonnefil et al., 1993). Dentate gyrus granule cells are also preferentially vulnerable to cell death by toxins such as the microtubule-assembly inhibitor colchicine and binge ethanol exposure (Obernier et al., 2002; Kristensen et al., 2003), though these toxins are not excitatory in nature.
In sum, the present findings demonstrate significant topographical diversity of mature neuronal density and NMDA receptor subunit density throughout the developing hippocampus, which likely contributes to selective sensitivity of CA1 region pyramidal cells to excitotoxic insult. Topographical studies demonstrated that polyamine-sensitive NMDA receptors are preferentially expressed in the CA1 region pyramidal cell layer. Further, selective activation of these receptors may contribute to the CA1 region pyramidal degeneration observed with exposure to several different forms of excitotoxic insult. It will be of importance in future studies to examine these hypotheses employing in vivo models of hippocampal injury. These data may be particularly relevant to examination of novel pharmacological interventions for the treatment of hippocampal neurodegeneration given evidence that allosteric inhibitors of NR2B subunit activation are well-tolerated by humans and demonstrate clinical efficacy in the treatment of some neurological disorders (for review, see Chizh et al. 2005).
Acknowledgements
The authors acknowledge the support of AA103561, AA013388, AA015676, and AA014771.
Abbreviations
- AMPAr
α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor
- ANOVA
analysis of variance
- APV
DL-2-Amino-5-phosphonopentanoic acid
- BDNF
brain derived neurotrophic factor
- CA
cornu ammonus
- Ca2+
calcium
- CNS
central nervous system
- CREB
cAMP reponse element binding protein
- DG
dentate gyrus
- DIV
days in vitro
- FITC
fluorescein isothiocynate
- MAP-2
microtubule associated protein-2
- Mg2+
magnesium
- MEM
minimum essential medium
- mGluR
metabotropic glutamate receptor
- Na+
sodium
- NeuN
neuron specific nuclear protein
- NMDA
N-methyl-D-aspartate
- PBS
phosphate-buffered saline
- PI
propidium iodide
- PND
postnatal day
- TRITC
tetramethylrhodamine isothiocynate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004;61:657–668. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlante A, Gagliardi S, Minervini GM, Ciotti MT, Marra E, Calissano P. Glutamate neurotoxicity in rat cerebellar granule cells: A major role for xanthine oxidase in oxygen radical formation. J Neurochem. 1997;68:2038–2045. doi: 10.1046/j.1471-4159.1997.68052038.x. [DOI] [PubMed] [Google Scholar]
- Baskys A, Bayazitov I, Fang L, Blaabjerg M, Poulsen FR, Zimmer J. Group I metabotropic glutamate receptors reduce excitotoxic injury and may facilitate neurogenesis. Neuropharmacology. 2005;49(Suppl 1):146–156. doi: 10.1016/j.neuropharm.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Bonde C, Noraberg J, Noer H, Zimmer J. Ionotropic glutamate receptors and glutamate transporters are involved in necrotic neuronal cell death induced by oxygen-glucose deprivation of hippocampal slice cultures. Neurosci. 2005;136:779–794. doi: 10.1016/j.neuroscience.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Vizentin E, Mathern GW. Electrophysiologic analysis of a chronic seizure model after unilateral hippocampal KA injection. Epilepsia. 1999;40:1210–1221. doi: 10.1111/j.1528-1157.1999.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Budd SL, Nicholls DG. Mitochondria, calcium regulation, and acute glutamate excitotoxicity in cultured cerebellar granule cells. J Neurochem. 1996;67:2282–2291. doi: 10.1046/j.1471-4159.1996.67062282.x. [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Benedikz E, Rai R, Bergold PJ. Excitatory and inhibitory pathways modulate kainite excitotoxicity in hippocampal slice cultures. Neurosci Lett. 1993;154:5–8. doi: 10.1016/0304-3940(93)90157-g. [DOI] [PubMed] [Google Scholar]
- Castilho RF, Ward MW, Nicholls DG. Oxidative stress, mitochondrial function, and acute glutamate excitotoxicity in cultured cerebellar granule cells. J Neurochem. 1999;72:1394–1401. doi: 10.1046/j.1471-4159.1999.721394.x. [DOI] [PubMed] [Google Scholar]
- Cater HL, Gitterman D, Davis SM, Benham CD, Morrison B, 3rd, Sundstrom LE. Stretch-induced injury in organotypic hippocampal slice cultures reproduces in vivo post-traumatic neurodegeneration: Role of glutamate receptors and voltage-dependent calcium channels. J Neurochem. 2007;101:434–447. doi: 10.1111/j.1471-4159.2006.04379.x. [DOI] [PubMed] [Google Scholar]
- Chen N, Luo T, Raymond LA. Subtype-dependence of NMDA receptor channel open probability. J Neurosci. 1999;19:6844–6854. doi: 10.1523/JNEUROSCI.19-16-06844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizh BA, Headley PM. NMDA antagonists and neuropathic pain—multiple drug targets and multiple uses. Curr Pharm Des. 2005;11:2977–2994. doi: 10.2174/1381612054865082. [DOI] [PubMed] [Google Scholar]
- Choi DW. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987;7:369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, Koh JY, Peter S. Pharmacology of glutamate neurotoxicity in cortical cell culture: Attenuation by NMDA antagonists. J Neurosci. 1988;8:185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardoni F, Di Luca M. New targets for pharmacological intervention in the glutamatergic synapse. Eur J Pharmacol. 2006;545:2–10. doi: 10.1016/j.ejphar.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Gerencser AA, Mark KA, Hubbard AE, Divakaruni AS, Mehrabian Z, Nicholls DG, Polster BM. Real-time visualization of cytoplasmic calpain activation and calcium deregulation in acute glutamate excitotoxicity. J Neurochem. 2009;110:990–1004. doi: 10.1111/j.1471-4159.2009.06194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DA, Harris BR, Prendergast MA, Hart SR, Blanchard JA, 2nd, Holley RC, Pedigo NW, Littleton JM. Polyamines contribute to ethanol withdrawal-induced neurotoxicity in rat hippocampal slice cultures through interactions with the NMDA receptor. Alcohol Clin Exp Res. 2003;27:1099–1106. doi: 10.1097/01.ALC.0000075824.10502.DD. [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Heinemann U. Synaptic reorganization in explanted cultures of rat hippocampus. Brain Res. 1999;815:304–316. doi: 10.1016/s0006-8993(98)01101-9. [DOI] [PubMed] [Google Scholar]
- Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability indendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998;18:9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Coupling of extrasynaptic NMDA receptors to a CREB shut-off pathway is developmentally regulated. Biochim Biophys Acta. 2002;1600:148–153. doi: 10.1016/s1570-9639(02)00455-7. [DOI] [PubMed] [Google Scholar]
- Harris AZ, Pettit DL. Extrasynaptic and synaptic NMDA receptors form stable and uniform pools in rat hippocampal slices. J Physiol. 2007;584:509–519. doi: 10.1113/jphysiol.2007.137679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath PR, Shaw PJ. Update on the glutamatergic neurotransmitter system and the role of excitotoxicity in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:438–458. doi: 10.1002/mus.10186. [DOI] [PubMed] [Google Scholar]
- Henley CM, Muszynski C, Cherian L, Robertson CS. Activation of ornithe decarboxylase and accumulation of putrescine after traumatic brain injury. J Neurotrauma. 1996;13:487–496. doi: 10.1089/neu.1996.13.487. [DOI] [PubMed] [Google Scholar]
- Herman MD, Reuveny E, Narahashi T. The effect of polyamines on voltage-activated calcium channels in mouse neuroblastoma cells. J Physiol. 1993;462:645–660. doi: 10.1113/jphysiol.1993.sp019574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annual Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Holopainen IE, Jarvela J, Lopez-Picon FR, Pelliniemi LJ, Kukko-Lukjanov TK. Mechanisms of kainite-induced region-specific neuronal death in immature organotypic hippocampal slice cultures. Neurochem Int. 2004;45:1–10. doi: 10.1016/j.neuint.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Matsuki N. Regionally selective neurotoxicity of NMDA and colchicines is independent of hippocampal neural circuitry. Neuroscience. 2002;113:253–256. doi: 10.1016/s0306-4522(02)00217-8. [DOI] [PubMed] [Google Scholar]
- Janssen WG, Vissavajjhala P, Andrews G, Moran T, Hof PR, Morrison JH. Cellular and synaptic distribution of NR2A and NR2B in macaque monkey and rat hippocampus as visualized with subunit-specific monoclonal antibodies. Exp Neurol. 2005;191(Suppl 1):S28–44. doi: 10.1016/j.expneurol.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Kim GH, Komotar RJ, McCullough-Hicks ME, Otten ML, Starke RM, Kellner CP, Garrett MC, Merkow MB, Rynkowski M, Dash KA, Connolly S. The role of polyamine metabolism in neuronal injury following cerebral ischemia. Can J Neurol Sci. 2009;36:14–19. doi: 10.1017/s0317167100006247. [DOI] [PubMed] [Google Scholar]
- Kristensen BW, Noer H, Gramsbergen JB, Zimmer J, Noraberg J. Colchicine induces apoptosis in organotypic hippocampal slice cultures. Brain Res. 2003;964:264–278. doi: 10.1016/s0006-8993(02)04080-5. [DOI] [PubMed] [Google Scholar]
- Lahtinen H, Autere AM, Paalasmaa P, Lauri SE, Kaila K. Post-insult activity is a major cause of delayed neuronal death in organotypic hippocampal slices exposed to glutamate. Neurosci. 2001;105:131–137. doi: 10.1016/s0306-4522(01)00168-3. [DOI] [PubMed] [Google Scholar]
- Laschet J, Trottier S, Leviel V, Guibert B, Bansard JY, Chauvel P, Bureau M. Heterogeneous distribution of polyamines in temporal lobe epilepsy. Epilepsy Res. 1999;35:161–172. doi: 10.1016/s0920-1211(99)00009-1. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Bartke I, Schoepfer R, Naujoks K, Seeburg PH. Regional, developmental, and interspecies expression of the four NMDAR2 subunits, examined using monoclonal antibodies. Brain Res., Mol Brain Res. 1997;51:23–32. doi: 10.1016/s0169-328x(97)00206-4. [DOI] [PubMed] [Google Scholar]
- Martens U, Wree A. Distribution of [3H]MK-801, [3H]AMPA and [3H]Kainate binding sites in rat hippocampal long-term slice cultures isolated from external afferents. Anat Embryol (Berl) 2001;203:491–500. doi: 10.1007/s004290100174. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Kater SB. Development and selective neurodegeneration in cell cultures from different hippocampal regions. Brain Res. 1989;490:110–125. doi: 10.1016/0006-8993(89)90436-8. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Wang H, Michaelis EK. Developmental expression, compartmentalization, and possible role in excitotoxicity of a putative NMDA receptor protein in cultured hippocampal neurons. Brain Res. 1991;565:94–108. doi: 10.1016/0006-8993(91)91740-r. [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Cotman CW. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985;5:2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ, Prendergast MA. Transection of intrinsic polysynaptic pathways reduces N-methyl-D-aspartate neurotoxicity in hippocampal slice cultures. Neurosci Res. 2003;46:369–376. doi: 10.1016/s0168-0102(03)00102-0. [DOI] [PubMed] [Google Scholar]
- Nadler JV, Cuthbertson GJ. Kainic acid neurotoxicity toward hippocampal formation: Dependence on specific excitatory pathways. Brain Res. 1980;195:47–56. doi: 10.1016/0006-8993(80)90865-3. [DOI] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res. 2002;26:547–557. [PubMed] [Google Scholar]
- Pelletier MR, Wadia JS, Mills LR, Carlen PL. Seizure-induced cell death produced by repeated titanic stimulation in vitro: Possible role of endoplasmic reticulum calcium stores. J Neurophysiol. 1999;81:3054–3064. doi: 10.1152/jn.1999.81.6.3054. [DOI] [PubMed] [Google Scholar]
- Pivovarova NB, Nguyen HV, Winters CA, Brantner CA, Smith CL, Andrews SB. Excitotoxic calcium overload in a subpopulation of mitochondria triggers delayed death in hippocampal neurons. J Neurosci. 2004;24:5611–5622. doi: 10.1523/JNEUROSCI.0531-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard H, Charriaut-Marlangue C, Cantagrel S, Represa A, Robain O, Moreau J, Ben-Ari Y. Kainate-induced apoptotic cell death in hippocampal neurons. Neurosci. 1994;63:7–18. doi: 10.1016/0306-4522(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Harris BR, Blanchard JA, 2nd, Mayer S, Gibson DA, Littleton JM. In vitro effects of ethanol withdrawal and spermidine on viability of hippocampus from male and female rat. Alcohol Clin Exp Res. 2000;24:1855–1861. [PubMed] [Google Scholar]
- Prendergast MA, Harris BR, Mulholland PJ, Blanchard JA, 2nd, Gibson DA, Holley RC, Littleton JM. Hippocampal CA1 region neurodegeneration produced by ethanol withdrawal requires activation of intrinsic polysynaptic hippocampal pathways and function of N-methyl-D-aspartate receptors. Neurosci. 2004;124:869–877. doi: 10.1016/j.neuroscience.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Self RL, Smith KJ, Ghayoumi L, Mullins MM, Butler TR, Buccafusco JJ, Gearhart DA, Terry AV., Jr Microtubule-associated targets in chlorpyrifos oxon hippocampal neurotoxicity. Neuroscience. 2007;146:330–339. doi: 10.1016/j.neuroscience.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler R, Xiong Z, Lu WY, MacDonald JF, Tymianski M. Distinct roles of synaptic and extrasynaptic NMDA receptors in excitotoxicity. J Neurosci. 2000;20:22–33. doi: 10.1523/JNEUROSCI.20-01-00022.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scartabelli T, Gerace E, Landucci E, Moroni F, Pellegrini-Giampietro DE. Neuroprotection by group I mGlu receptors in a rat hippocampal slice model of cerebral ischemia is associated with the PI3K-Akt signaling pathway: A novel postconditioning strategy? Neuropharmacology. 2008;55:509–516. doi: 10.1016/j.neuropharm.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Schuh RA, Matthews CC, Fishman PS. Interaction of mitochondrial respiratory inhibitors and excitotoxins potentiates cell death in hippocampal slice cultures. J Neurosci Res. 2008;86:3306–3313. doi: 10.1002/jnr.21772. [DOI] [PubMed] [Google Scholar]
- Self RL, Mulholland PJ, Harris BR, Nath A, Prendergast MA. The human immunodeficiency virus type-1 transcription factor Tat produces elevations in intracellular Ca2+ that require function of an N-methyl-D-aspartate receptor polyamine-sensitive site. Brain Res. 2004;995:39–45. doi: 10.1016/j.brainres.2003.09.052. [DOI] [PubMed] [Google Scholar]
- Silverstein FS, Naik B, Simpson J. Hypoxia-ischemia stimulates hippocampal glutamate efflux in perinatal rat brain: An in vivo microdialysis study. Pediatr Res. 1991;30:587–590. doi: 10.1203/00006450-199112000-00021. [DOI] [PubMed] [Google Scholar]
- Siman R, Noszek JC. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988;1:279–287. doi: 10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Stoll L, Hall J, Van Buren N, Hall A, Knight L, Morgan A, Zuger S, Van Deusen H, Gentile L. Differential regulation of ionotrophic glutamate receptors. Biophys J. 2007;92(4):1343–1349. doi: 10.1529/biophysj.106.089896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenburg-Didinger G. Neuropathology of the hippocampus and its susceptibility to neurotoxic insult. Neurotoxicology. 1994;15:445–450. [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–82. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95:1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Emerich DF. The hippocampus as a common target of neurotoxic agents. Toxicology. 1988;49:137–140. doi: 10.1016/0300-483x(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Wilkins LH, Jr, Prendergast MA, Blanchard JA, Holley RC, Chambers ER, Littleton JM. Potential value of changes in cell markers in organotypic hippocampal cultures associated with chronic EtOH exposure and withdrawal: comparison with NMDA-induced changes. Alcohol Clin Exp Res. 2006;30:1768–1780. doi: 10.1111/j.1530-0277.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Williams K, Hanna JL, Molinoff PB. Developmental changes in the sensitivity of the N-methyl-D-aspartate receptor to polyamines. Mol Pharmacol. 1991;40:774–782. [PubMed] [Google Scholar]
- Williams K, Zappia AM, Pritchett DB, Shen YM, Molinoff PB. Sensitivity of N-methyl-D-aspartate receptor to polyamines is controlled by NR2 subunits. Mol Pharmacol. 1994;45:803–809. [PubMed] [Google Scholar]
- Yamakura T, Shimoji K. Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog Neurobiol. 1999;59:279–298. doi: 10.1016/s0301-0082(99)00007-6. [DOI] [PubMed] [Google Scholar]
- Yan DH, Nishimura K, Yoshida K, Nakahira K, Ehara T, Igarashi K, Ishihara K. Differential intracellular polyamine concentrations underlie the difference in the inward rectifier K(+) currents in atria and ventricles of the guinea-pig heart. J Physiol. 2005;563(3):713–724. doi: 10.1113/jphysiol.2004.077677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukin RS, Bennett MV. Alternatively spliced isoforms of the NMDAR1 receptor subunit. Trends Neurosci. 1995;18:306–313. doi: 10.1016/0166-2236(95)93920-s. [DOI] [PubMed] [Google Scholar]