Abstract
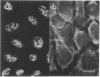
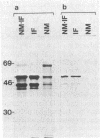
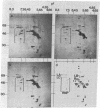
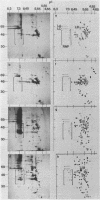
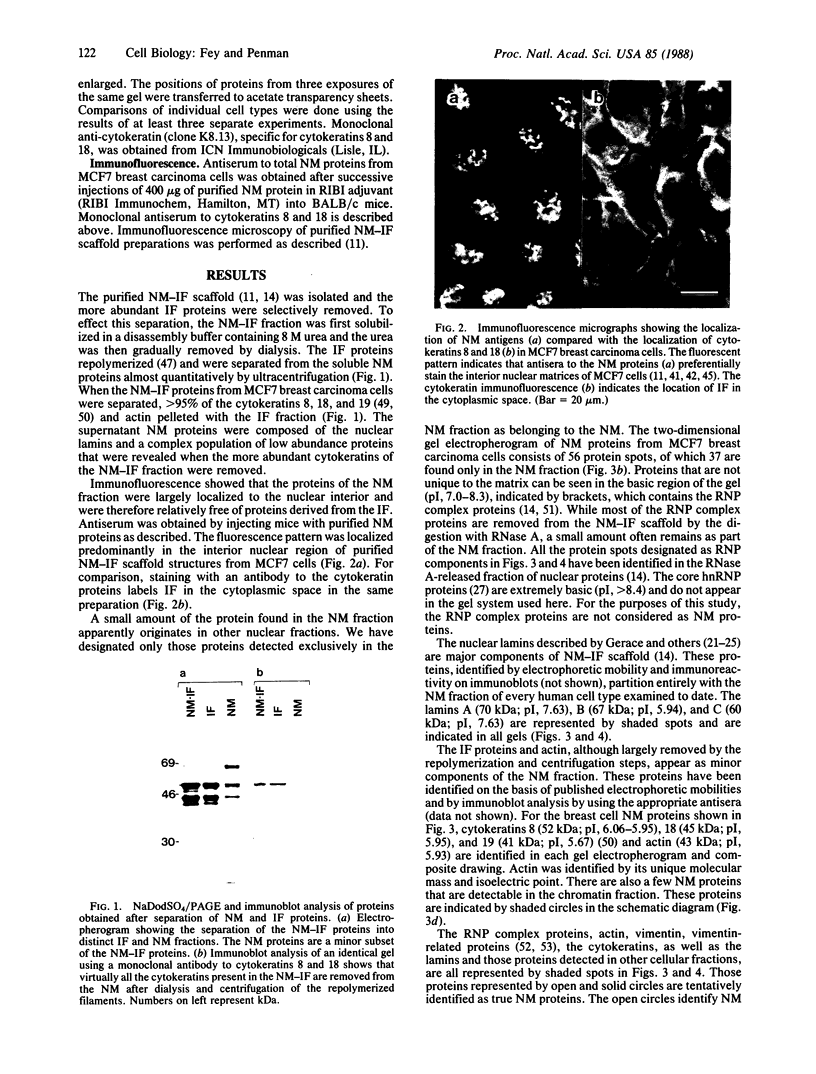
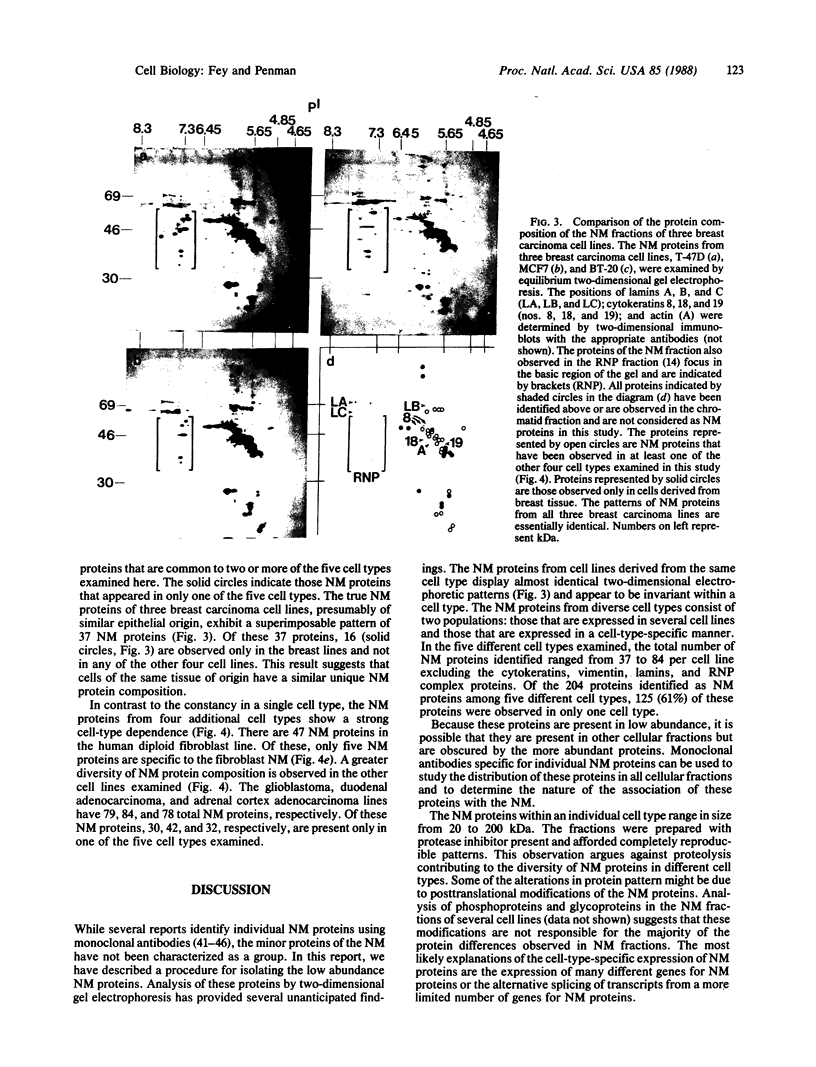
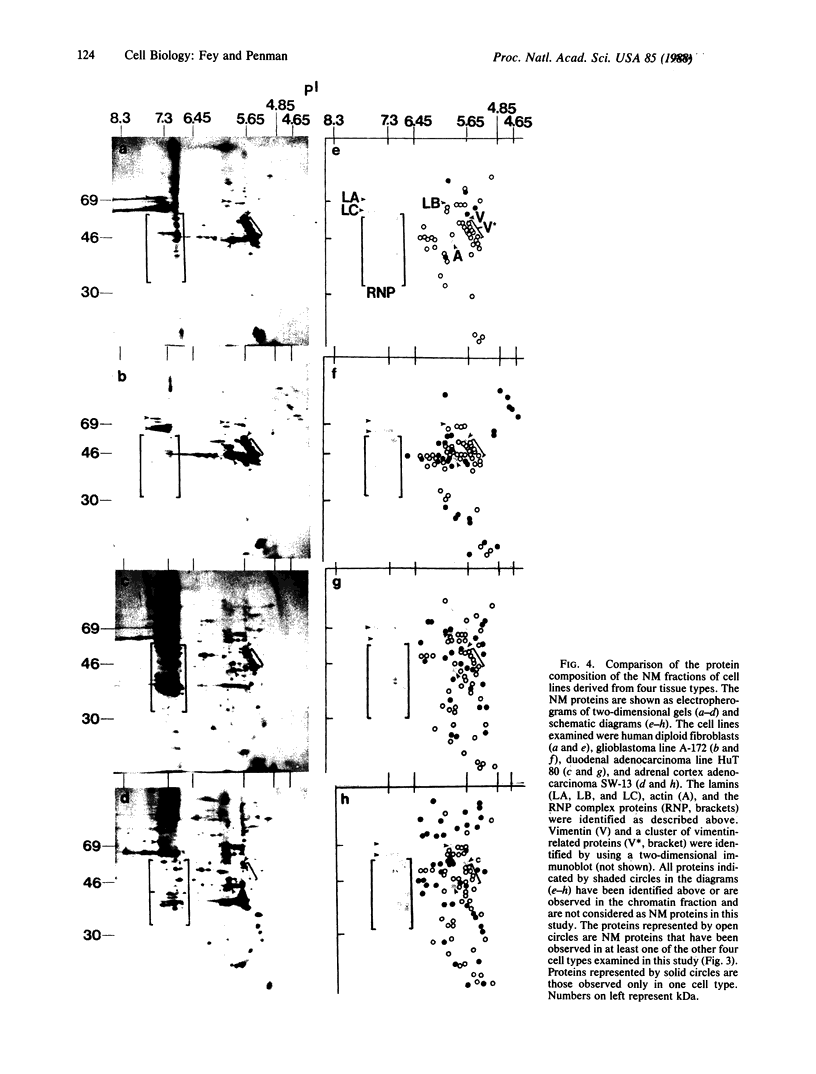
The low abundance proteins of the nuclear matrix (NM) were separated from the intermediate filament (IF) proteins and analyzed by two-dimensional gel electrophoresis. Three human breast carcinoma lines had virtually identical patterns of 37 NM proteins. In contrast, cell lines derived from diverse tissues had qualitatively different NM protein patterns. Together, the five cell types examined here had a total of 205 distinguishable NM proteins with 125 of these proteins unique to a single cell type. The remaining NM proteins were shared among cell types to different degrees. Polyclonal antisera, obtained by immunization with total NM proteins as antigens, preferentially stained the nuclear interior and not the exterior IF. These observations suggest that the NM proteins, localized to the interior of the nucleus, vary in a cell-type-specific manner.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Cohn J., Buhle L., Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986 Oct 9;323(6088):560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Identification of a nuclear protein matrix. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1410–1417. doi: 10.1016/0006-291x(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Nuclear matrix. Isolation and characterization of a framework structure from rat liver nuclei. J Cell Biol. 1977 Jun;73(3):616–637. doi: 10.1083/jcb.73.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezney R. Fractionation of the nuclear matrix. I. Partial separation into matrix protein fibrils and a residual ribonucleoprotein fraction. J Cell Biol. 1980 Jun;85(3):641–650. doi: 10.1083/jcb.85.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer A. L., Christensen M. E., Walker B. W., LeStourgeon W. M. Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell. 1977 May;11(1):127–138. doi: 10.1016/0092-8674(77)90323-3. [DOI] [PubMed] [Google Scholar]
- Bhorjee J. S., Barclay S. L., Wedrychowski A., Smith A. M. Monoclonal antibodies specific for tight-binding human chromatin antigens reveal structural rearrangements within the nucleus during the cell cycle. J Cell Biol. 1983 Aug;97(2):389–396. doi: 10.1083/jcb.97.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blikstad I., Lazarides E. Vimentin filaments are assembled from a soluble precursor in avian erythroid cells. J Cell Biol. 1983 Jun;96(6):1803–1808. doi: 10.1083/jcb.96.6.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasch K. Fine structure and localization of the nuclear matrix in situ. Exp Cell Res. 1982 Jul;140(1):161–171. doi: 10.1016/0014-4827(82)90167-7. [DOI] [PubMed] [Google Scholar]
- Capco D. G., Wan K. M., Penman S. The nuclear matrix: three-dimensional architecture and protein composition. Cell. 1982 Jul;29(3):847–858. doi: 10.1016/0092-8674(82)90446-9. [DOI] [PubMed] [Google Scholar]
- Chaly N., Bladon T., Setterfield G., Little J. E., Kaplan J. G., Brown D. L. Changes in distribution of nuclear matrix antigens during the mitotic cell cycle. J Cell Biol. 1984 Aug;99(2):661–671. doi: 10.1083/jcb.99.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaly N., Little J. E., Brown D. L. Localization of nuclear antigens during preparation of nuclear matrices in situ. Can J Biochem Cell Biol. 1985 Jun;63(6):644–653. doi: 10.1139/o85-082. [DOI] [PubMed] [Google Scholar]
- Chan R., Rossitto P. V., Edwards B. F., Cardiff R. D. Presence of proteolytically processed keratins in the culture medium of MCF-7 cells. Cancer Res. 1986 Dec;46(12 Pt 1):6353–6359. [PubMed] [Google Scholar]
- Ciejek E. M., Nordstrom J. L., Tsai M. J., O'Malley B. W. Ribonucleic acid precursors are associated with the chick oviduct nuclear matrix. Biochemistry. 1982 Sep 28;21(20):4945–4953. doi: 10.1021/bi00263a018. [DOI] [PubMed] [Google Scholar]
- Comings D. E., Okada T. A. Nuclear proteins. III. The fibrillar nature of the nuclear matrix. Exp Cell Res. 1976 Dec;103(2):341–360. doi: 10.1016/0014-4827(76)90271-8. [DOI] [PubMed] [Google Scholar]
- Detke S., Keller J. M. Comparison of the proteins present in HeLa cell interphase nucleoskeletons and metaphase chromosome scaffolds. J Biol Chem. 1982 Apr 10;257(7):3905–3911. [PubMed] [Google Scholar]
- Engelhardt P., Plagens U., Zbarsky I. B., Filatova L. S. Granules 25-30 nm in diameter: basic constituent of the nuclear matrix, chromosome scaffold, and nuclear envelope. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6937–6940. doi: 10.1073/pnas.79.22.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey E. G., Krochmalnic G., Penman S. The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J Cell Biol. 1986 May;102(5):1654–1665. doi: 10.1083/jcb.102.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey E. G., Wan K. M., Penman S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: three-dimensional organization and protein composition. J Cell Biol. 1984 Jun;98(6):1973–1984. doi: 10.1083/jcb.98.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. A., Berrios M., Blobel G. Isolation and characterization of a proteinaceous subnuclear fraction composed of nuclear matrix, peripheral lamina, and nuclear pore complexes from embryos of Drosophila melanogaster. J Cell Biol. 1982 Mar;92(3):674–686. doi: 10.1083/jcb.92.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W. Nuclear lamins and cytoplasmic intermediate filament proteins: a growing multigene family. Cell. 1987 Jan 16;48(1):3–4. doi: 10.1016/0092-8674(87)90345-x. [DOI] [PubMed] [Google Scholar]
- Gallinaro H., Puvion E., Kister L., Jacob M. Nuclear matrix and hnRNP share a common structural constituent associated with premessenger RNA. EMBO J. 1983;2(6):953–960. doi: 10.1002/j.1460-2075.1983.tb01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L., Blum A., Blobel G. Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J Cell Biol. 1978 Nov;79(2 Pt 1):546–566. doi: 10.1083/jcb.79.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R., Weymouth L., Penman S. Heterogeneous nuclear RNA-protein fibers in chromatin-depleted nuclei. J Cell Biol. 1978 Sep;78(3):663–674. doi: 10.1083/jcb.78.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge L. D., Mancini P., Davis F. M., Heywood P. Nuclear matrix of HeLa S3 cells. Polypeptide composition during adenovirus infection and in phases of the cell cycle. J Cell Biol. 1977 Jan;72(1):194–208. doi: 10.1083/jcb.72.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. A., McCready S. J., Cook P. R. RNA is synthesized at the nuclear cage. Nature. 1981 Aug 6;292(5823):552–555. doi: 10.1038/292552a0. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Coffey D. S., Shaper J. H. Considerations in the isolation of rat liver nuclear matrix, nuclear envelope, and pore complex lamina. Exp Cell Res. 1981 Mar;132(1):105–123. doi: 10.1016/0014-4827(81)90088-4. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Shaper J. H. A subset of non-histone nuclear proteins reversibly stabilized by the sulfhydryl cross-linking reagent tetrathionate. Polypeptides of the internal nuclear matrix. Exp Cell Res. 1984 Dec;155(2):477–495. doi: 10.1016/0014-4827(84)90208-8. [DOI] [PubMed] [Google Scholar]
- Kish V. M., Pederson T. Ribonucleoprotein organization of polyadenylate sequences in HeLa cell heterogeneous nuclear RNA. J Mol Biol. 1975 Jun 25;95(2):227–238. doi: 10.1016/0022-2836(75)90392-7. [DOI] [PubMed] [Google Scholar]
- Lehner C. F., Eppenberger H. M., Fakan S., Nigg E. A. Nuclear substructure antigens. Monoclonal antibodies against components of nuclear matrix preparations. Exp Cell Res. 1986 Jan;162(1):205–219. doi: 10.1016/0014-4827(86)90439-8. [DOI] [PubMed] [Google Scholar]
- Long B. H., Huang C. Y., Pogo A. O. Isolation and characterization of the nuclear matrix in Friend erythroleukemia cells: chromatin and hnRNA interactions with the nuclear matrix. Cell. 1979 Dec;18(4):1079–1090. doi: 10.1016/0092-8674(79)90221-6. [DOI] [PubMed] [Google Scholar]
- Maundrell K., Maxwell E. S., Puvion E., Scherrer K. The nuclear matrix of duck erythroblasts is associated with globin mRNA coding sequences but not with the major proteins of 40S nuclear RNP. Exp Cell Res. 1981 Dec;136(2):435–445. doi: 10.1016/0014-4827(81)90023-9. [DOI] [PubMed] [Google Scholar]
- Miller T. E., Huang C. Y., Pogo A. O. Rat liver nuclear skeleton and ribonucleoprotein complexes containing HnRNA. J Cell Biol. 1978 Mar;76(3):675–691. doi: 10.1083/jcb.76.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ottaviano Y., Gerace L. Phosphorylation of the nuclear lamins during interphase and mitosis. J Biol Chem. 1985 Jan 10;260(1):624–632. [PubMed] [Google Scholar]
- Pederson T. Proteins associated with heterogeneous nuclear RNA in eukaryotic cells. J Mol Biol. 1974 Feb 25;83(2):163–183. doi: 10.1016/0022-2836(74)90386-6. [DOI] [PubMed] [Google Scholar]
- Peters K. E., Commings D. E. Two-dimensinal gel electrophoresis of rat liver nuclear washes, nuclear matrix, and hnRNA proteins. J Cell Biol. 1980 Jul;86(1):135–155. doi: 10.1083/jcb.86.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K. E., Okada T. A., Comings D. E. Chinese hamster nuclear proteins. An electrophoretic analysis of interphase, metaphase and nuclear matrix preparations. Eur J Biochem. 1982 Dec;129(1):221–232. doi: 10.1111/j.1432-1033.1982.tb07043.x. [DOI] [PubMed] [Google Scholar]
- Pieck A. C., van der Velden H. M., Rijken A. A., Neis J. M., Wanka F. Protein composition of the chromosomal scaffold and interphase nuclear matrix. Chromosoma. 1985;91(2):137–144. doi: 10.1007/BF00294058. [DOI] [PubMed] [Google Scholar]
- Ross D. A., Yen R. W., Chae C. B. Association of globin ribonucleic acid and its precursors with the chicken erythroblast nuclear matrix. Biochemistry. 1982 Feb 16;21(4):764–771. doi: 10.1021/bi00533a029. [DOI] [PubMed] [Google Scholar]
- STEELE W. J., BUSCH H. STUDIES ON ACIDIC NUCLEAR PROTEINS OF THE WALKER TUMOR AND LIVER. Cancer Res. 1963 Sep;23:1153–1163. [PubMed] [Google Scholar]
- Schmidt W. N., McKusick K. B., Schmidt C. A., Hoffman L. H., Hnilica L. S. Nuclear matrix antigens in azo dye-induced primary rat hepatomas. Cancer Res. 1984 Nov;44(11):5291–5304. [PubMed] [Google Scholar]
- Song M. K., Adolph K. W. Phosphorylation of nonhistone proteins during the HeLa cell cycle. Relationship to DNA synthesis and mitotic chromosome condensation. J Biol Chem. 1983 Mar 10;258(5):3309–3318. [PubMed] [Google Scholar]
- Staufenbiel M., Deppert W. Preparation of nuclear matrices from cultured cells: subfractionation of nuclei in situ. J Cell Biol. 1984 May;98(5):1886–1894. doi: 10.1083/jcb.98.5.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P., Nelson W. J. Occurrence in various mammalian cells and tissues of the Ca 2+ activated protease specific for the intermediate-sized filament proteins vimentin and desmin. Eur J Cell Biol. 1981 Dec;26(1):61–67. [PubMed] [Google Scholar]
- Verheijen R., Kuijpers H., Vooijs P., van Venrooij W., Ramaekers F. Protein composition of nuclear matrix preparations from HeLa cells: an immunochemical approach. J Cell Sci. 1986 Feb;80:103–122. doi: 10.1242/jcs.80.1.103. [DOI] [PubMed] [Google Scholar]
- Werner D., Chanpu S., Müller M., Spiess E., Plagens U. Antibodies to the most tightly bound proteins in eukaryotic DNA. Formation of immuno-complexes with 'nuclear matrix' components. Exp Cell Res. 1984 Apr;151(2):384–395. doi: 10.1016/0014-4827(84)90389-6. [DOI] [PubMed] [Google Scholar]
- Zackroff R. V., Idler W. W., Steinert P. M., Goldman R. D. In vitro reconstitution of intermediate filaments form mammalian neurofilament triplet polypeptides. Proc Natl Acad Sci U S A. 1982 Feb;79(3):754–757. doi: 10.1073/pnas.79.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin S., Parent A., Silverstein S., Efstratiadis A. Pre-mRNA splicing and the nuclear matrix. Mol Cell Biol. 1987 Jan;7(1):111–120. doi: 10.1128/mcb.7.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eekelen C. A., van Venrooij W. J. hnRNA and its attachment to a nuclear protein matrix. J Cell Biol. 1981 Mar;88(3):554–563. doi: 10.1083/jcb.88.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]