Abstract
The present study tested whether the topical application of a local anesthetic (Lidocaine) to the vaginocervical region altered the pattern of paced mating behavior displayed by gonadectomized, hormone-primed female rats. Both rats receiving Lidocaine and rats receiving vehicle exhibited the expected lengthening of contact-return latency as the intensity of the mating stimuli increased (mount < intromission < ejaculation). Although rats given Lidocaine versus vehicle received a greater number of intromissions, no other group differences were observed. The present study found no evidence for a change in behavioral responsiveness following the vaginocervical application of Lidocaine.
Keywords: local anesthetic, sexual behavior, vagina, cervix, genitosensory, sexual motivation
1. Introduction
Topically applied local anesthestics, such as Lidocaine and Xylocaine, have been used to desensitize genital tissues in studies examining the contributions of sensory input to the display of copulatory behaviors [1-9]. These local anesthetics block voltage-gated sodium channels thereby inhibiting the stimulation-induced excitability of afferent fibers [10-11]. Vaginal application of Lidocaine in women is an effective local analgesic [12-13] and can prevent increases in vaginal wall pressure induced by clitoral stimulation [14]. A well-known behavioral study conducted in rats measured the effect of vaginal Lidocaine treatment on the display of an operant response (bar-press) that gave a female rat access to a sexually active male rat [4]. The latency for the female rat to bar-press to re-gain access to the male following the receipt of an intromission or ejaculation was shortened after application of Lidocaine [4]. A subsequent study evaluating approach responses to different stimulus animals housed in three separate compartments observed no effect of the intravaginal application of Lidocaine on the latency of female rats to approach sexually active male rats [5].
More recently, a number of researchers have adopted the paced mating paradigm to examine the approach and withdrawal behaviors exhibited by a female rat during a sexual encounter with a male rat [15]. The display of paced mating behavior varies as a function of the intensity of sexual stimulation received from the male, such that the contact-return latency and the percentage of exits increase after the receipt of more intense stimulations (mount < intromission < ejaculation) [16]. The transmission of sensory information from the vagina and cervix is necessary for the normal display of paced mating behavior; rats with pelvic nerve transection return to the male more quickly following intromissions than rats with sham transection [17]. The present experiment tested the effects of Lidocaine on the approach and withdrawal behaviors exhibited during paced mating behavior. Contrary to the findings of Bermant and Westbrook [4], the pattern of approach and withdrawal from the male was not altered in rats receiving vaginocervical Lidocaine.
2. Methods
Twenty-four virgin female Long-Evans rats weighing approximately 200 g were obtained from Harlan (Indianapolis, IN). Rats were housed individually in hanging metal cages in a light-(12:12, lights off at 1000 h) and temperature-controlled vivarium. Commercial rat food pellets and water were available ad lib. Rats were gonadectomized under ketamine/xylazine anesthesia (50 mg/kg; Henry Schein, Indianapolis, IN) approximately 7-10 days before the start of behavioral testing. Sexually experienced male Long-Evans rats, aged 3-4 months, were used as stimulus rats. Experimental female rats received 10 ug estradiol benzoate (EB, Sigma, St. Louis, MO) 48 h and 1 mg progesterone (P, Sigma) 4 h prior to mating tests. Hormones were administered s.c. in a sesame oil vehicle. Vaginocervical application of vehicle (petroleum jelly, Vaseline, Unilever) or Lidocaine ointment (5%, Henry Schein, Indianapolis, IN) occurred as described below. Behavioral testing took place under dim red illumination. The Institutional Animal Care and Use Committee at Dartmouth College approved the use of rats in these studies and all procedures were conducted in accordance with NIH guidelines.
Paced mating behavior was measured as described previously [18]. Briefly, tests were conducted in a clear Plexiglas arena divided into three equally sized compartments using two clear partitions with a 5-cm hole in each bottom corner. Opaque Plexiglas partitions adjacent to the clear partition separated the experimental female rat from the male rats prior to the start of behavioral testing. Two tests for paced mating were conducted approximately 1 week apart; a baseline test followed by a treatment test. The treatments (vehicle or Lidocaine) were administered according to the procedures outlined in Bermant and Westbrook [4]. Specifically, a 1 ml syringe containing 0.15 ml of the treatment (vehicle or Lidocaine) was introduced into the vagina until contact was made with the cervix; the treatment was then delivered into the vagina and a cotton swab was used to distribute the vehicle or Lidocaine over the vaginal walls and cervix for a 1 min-period. Following the initial treatment the rat was situated in a holding chamber for 1 min and then the treatment was repeated. At the conclusion of the second treatment, the rats were acclimated to the paced mating arena for 5 min before the start of the paced mating test.
Tests for paced mating behavior began when the opaque partition was removed allowing the female rat access to the male compartment; during the test, the female rat had access to only one male rat at a time. When ejaculations were received, the experimental rat was allowed to leave the male rat’s compartment and subsequently return, at which point the test timer was stopped, the opaque partition was replaced and the experimental rat was once again confined to the center compartment. The test was resumed immediately upon removal of the other opaque partition, allowing the experimental rat access to a different male rat. The test was concluded when the female had received 5 ejaculations, or 60 min elapsed, whichever came first [4].
The following measures were recorded by experimenters: number and timing of mounts, intromissions, and ejaculations as well as sexual receptivity (lordosis quotient (LQ) defined as the number of lordosis responses of 2 or 3 divided by the number of mounts × 100, and lordosis response (LR) defined as the sum of lordosis responses divided by the number of mounts), test duration, activity, defined as the rate of entries/exits per minute from the stimulus animal compartments and the rate per minute of proceptive (hops, darts and ear wiggling) and rejection (kicks and defensive postures) behaviors [16,19-21]. The contact-return latency was defined as the length of time the female withdrew from the male compartment following each type of sexual stimulation and the percentage of exits was defined as the rate of withdrawal from the male following each type of sexual stimulation [16].
Data from the baseline test were used to match statistically the treatment groups on all behavioral measures: therefore, neither the data nor statistical analyses (F values) are reported for the baseline. A treatment x type of sexual stimulation (mount, intromission, ejaculation) analysis of variance (ANOVA) was conducted collapsing the data across the 5 ejaculatory series. Contact-return latencies and percentage of exits were analyzed separately. A second analysis examined the effect of treatment on the display of mating behaviors across the 5 ejaculatory series using a treatment x type of sexual stimulation (intromission and ejaculation; no rat received a mount without either intromission or ejaculation on all five ejaculatory series) ANOVA with repeated measures on ejaculatory series. One-way ANOVAs were conducted on lordosis quotients and lordosis responses, rate of proceptive and rejection behaviors, number of mating stimulations (mounts, intromissions and ejaculations), test duration, and activity. Rats were excluded from the analysis if they did not receive 5 ejaculations within the time limits of the test (n = 2) or if they differed by more than 2 standard deviations from the mean on baseline measures of paced mating behavior (n = 3) resulting in final group assignments of vehicle, n = 8 and Lidocaine, n = 11. The alpha level was set at 0.05.
3. Results
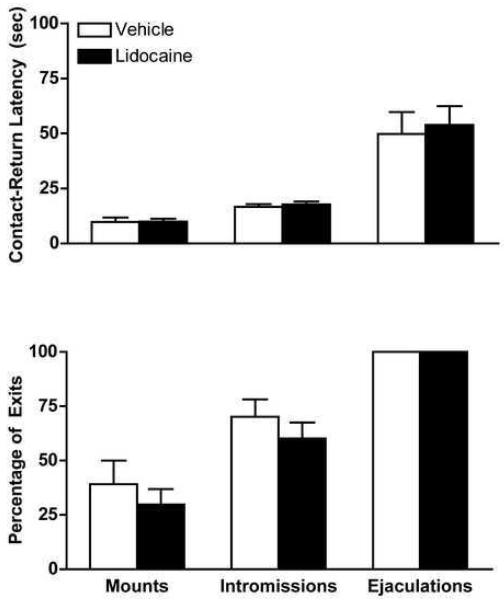
Rats in both treatment groups exhibited maximal levels of receptivity and there were no effects of treatment on receptivity. The pattern of contact-return latencies and percentage of exits did not differ as a function of treatment group (Figure 1). There was a significant effect of type of stimulation for contact-return latencies (F(2,48) = 30.3), and for percentage of exits (F(2,51) = 46.9) in the absence of a treatment effect or type of stimulation x treatment interaction. The second analysis, examining contract-return latencies across the five ejaculatory series, showed that contact-return latencies were longer following ejaculations than intromissions and lengthened following ejaculations over the course of the five ejaculatory series, consistent with published reports [21-24] (Figure 2). There was a significant overall effect of type of stimulation (F(1,30) = 22.8) and ejaculatory series (F(4,120) = 2.96) on contact-return latency but no effect of treatment or interactions. Follow-up analyses showed that the effect of ejaculatory series was significant for contact-return latencies following ejaculations (F(4,68) = 3.4).
Fig. 1.
Mean ± SEM contact-return latencies (top) and percentage of exits (bottom) for mounts, intromissions and ejaculations in rats tested for paced mating behavior observed following vaginocervical application of vehicle (white bars, n = 8) or Lidocaine (black bars, n = 11). No group differences were observed.
Fig. 2.
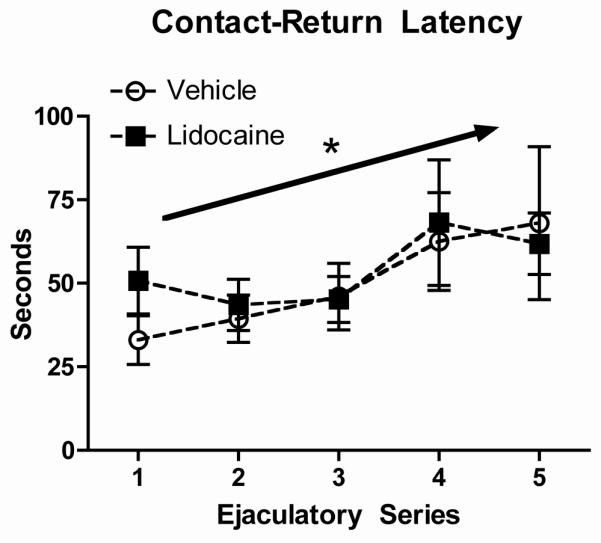
Mean ± SEM contact-return latencies following ejaculations across five ejaculatory series are shown for female rats treated with vehicle (open circles, n = 8) or Lidocaine (closed squares, n = 11). Arrow with asterisk indicates that for both groups contact-return latencies following ejaculations increased significantly across the five ejaculatory series.
Rats treated with Lidocaine received significantly more intromissions than rats receiving vehicle (Mean ± SEM, vehicle, 29.1 ± 1.64; Lidocaine, 35.7 ± 1.71, F(1,17) = 7.3). The greater number of intromissions received by the Lidocaine group may have occurred because the Lidocaine-treated rats received slightly, but not significantly, more intromissions per visit to the male rat (data not shown). The rate of proceptive or rejection behaviors, activity or test duration (approximately 20 mins) did not differ between the groups (data not shown).
4. Discussion
The normal “stair-step” pattern of responses to varied intensities of mating stimuli in tests of paced mating behavior was exhibited by rats treated with vehicle and Lidocaine. In addition, latencies to return to the male following mating stimulations were comparable in both treatment groups. Contact-return latencies after ejaculations lengthened across the five ejaculatory series, in agreement with previous reports [22-25]. We observed that rats treated with Lidocaine received significantly more intromissions compared to rats treated with vehicle. Taken together the evidence from the present experiment indicates that the vaginocervical application of Lidocaine does not disrupt the signals required to execute paced mating behavior.
Afferent fibers innervating the vagina and cervix are responsive to various types of mechanical stimulation from light touch to intense pressure [26-27]. Our working hypothesis was that Lidocaine treatment would diminish the activation of vaginocervical targets in response to mating stimulation leading to the abbreviation of contact-return latencies as reported by Bermant and Westbrook [4]. Other studies from our laboratory have shown that information conveyed via the pelvic nerve, which innervates the vagina and cervix [28], modulates the display of paced mating behavior [17; see also 29]. Shorter contact-return latencies are observed in female rats with pelvic nerve transection compared to intact rats, presumably due to a dampening in vaginocervical sensitivity [17]. We were surprised that Lidocaine treatment did not alter the display of paced mating behavior. However, the present data join several other studies in failing to find an effect of Lidocaine or Xylocaine treatment on the display of rodent female sexual behavior [5,8] in contrast to the effect of pelvic neurectomy which has a pronounced effect on the pattern of paced mating behavior [17,29]. Lidocaine may attenuate, but not eliminate, the relay of somatosensory information received during mating [7]. The residual sensory stimulation appears to be sufficient for the display of paced mating behavior in female rats.
The number of intromissions received during a paced mating test was influenced by Lidocaine treatment, consistent with the report of a trend towards increased number of intromissions and ejaculations received by female rats treated with Xylocaine [5]. Further analysis revealed that rats treated with Lidocaine received slightly more intromissions per visit to the male rat than vehicle-treated rats. It is known that the number of intromissions that male rats require to reach ejaculation is reduced when the inter-intromission interval is shorter rather than longer [30]. Therefore, because the male rats mated with Lidocaine-treated female rats could receive intromissions close to each other in time, they achieved more intromissions prior to ejaculation.
In conclusion, although the sensory cues required for paced mating behaviors are relayed via the genitosensory nerves, vaginocervical treatment with a local anesthetic is not sufficient to disrupt the pattern of paced mating behavior.
Acknowledgements
This work was supported by HD050726 to Ann S. Clark.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Adler N, Bermant G. Sexual behavior of male rats: effects of reduced sensory feedback. J Comp Physiol Psychol. 1966;61:240–243. doi: 10.1037/h0023151. [DOI] [PubMed] [Google Scholar]
- [2].Baum MJ, Everitt BJ. Increased expression of c-fos in the medial preoptic area after mating in male rats: role of afferent inputs from the medial amygdala and midbrain central tegmental field. Neurosci. 1992;50:627–646. doi: 10.1016/0306-4522(92)90452-8. [DOI] [PubMed] [Google Scholar]
- [3].Baum MJ, Sodersten P, Vreeburg JTM. Mounting and receptive behavior in the ovariectomized female rat: Influence of estradiol, dihydrotestosterone, and genital anesthetization. Horm Behav. 1974;5:175–190. doi: 10.1016/0018-506x(74)90042-7. [DOI] [PubMed] [Google Scholar]
- [4].Bermant G, Westbrook WH. Peripheral factors in the regulation of sexual contact by female rats. J Comp Physiol Psychol. 1966;61:244–50. doi: 10.1037/h0023152. [DOI] [PubMed] [Google Scholar]
- [5].Emery DE, Whitney JF. Effects of vagino-cervical stimulation upon sociosexual behaviors in female rats. Behav Neural Biol. 1985;43:199–205. doi: 10.1016/s0163-1047(85)91367-6. [DOI] [PubMed] [Google Scholar]
- [6].Kohlert JG, Olexa N. The role of vaginal stimulation for the acquisition of conditioned place preference in female Syrian hamsters. Physiol Behav. 2005;84:135–139. doi: 10.1016/j.physbeh.2004.10.020. [DOI] [PubMed] [Google Scholar]
- [7].Oboh AM, Paredes RG, Baum MJ. A sex comparison of increments in FOS immunoreactivity in forebrain neurons of gonadectomized, testosterone-treated rats after mounting an estrous female. Neurobiol Learn Mem. 1995;63:66–73. doi: 10.1006/nlme.1995.1006. [DOI] [PubMed] [Google Scholar]
- [8].Peretti PO. Effects of external genital sensory feedback on copulatory behavior of rats. J Psychol. 1973;84:81–88. doi: 10.1080/00223980.1973.9915633. [DOI] [PubMed] [Google Scholar]
- [9].Taziaux M, Keller M, Ball GF, Balthazart J. Site-specific effects of anosmia and cloacal gland anesthesia on Fos expression induced in male quail brain by sexual behavior. Beh Brain Res. 2008;194:52–65. doi: 10.1016/j.bbr.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Butterworth JF, 4th, Strichartz GR. Molecular mechanisms of local anesthesia: a review. Anesthesiol. 1990;72:711–734. doi: 10.1097/00000542-199004000-00022. [DOI] [PubMed] [Google Scholar]
- [11].Sawnyok J. Topical and peripherally acting analgesics. Pharm Rev. 2003;55:1–20. doi: 10.1124/pr.55.1.1. [DOI] [PubMed] [Google Scholar]
- [12].Zilbert A. Topical anesthesia for minor gynecological procedures: a review. Obstet Gynecol Surv. 2002;57:171–178. doi: 10.1097/00006254-200203000-00022. [DOI] [PubMed] [Google Scholar]
- [13].Rylander E, Sjoberg I, Lillieborg S, Stockman O. Local anesthesia of the genital mucosa with a Lidocaine/Prilocaine cream (EMLA) for laser treatment of condylomata acuminata: a placebo controlled study. Obstet Gynecol. 1990;75:302–306. [PubMed] [Google Scholar]
- [14].Shafik A, El Sibai O, Shafik AA. Vaginal response to clitoral stimulation: identification of the clitorovaginal reflex. J Reprod Med. 2008;53:111–116. [PubMed] [Google Scholar]
- [15].Erskine MS, Lehmann ML, Cameron NM, Polston EK. Co-regulation of female sexual behavior and pregnancy induction: an exploratory synthesis. Behav Brain Res. 2004;153:295–315. doi: 10.1016/j.bbr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- [16].Erskine MS. Solicitation behavior in the estrous female rat: a review. Horm Behav. 1989;23:473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- [17].Meerts SH, Clark AS. Conditioned place preference for mating is preserved in rats with pelvic nerve transection. Behav Neurosci. 2009;123:539–546. doi: 10.1037/a0015267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Clark AS, Guarraci FA, Megroz AB, Porter DM, Henderson LP. The display of sexual behaviors by female rats administered ICI 182,780. Horm Behav. 2003;43:454–464. doi: 10.1016/s0018-506x(03)00029-1. [DOI] [PubMed] [Google Scholar]
- [19].Beach FA. Sexual attractivity, proceptivity, and receptivity in female mammals. Horm Behav. 1976;7:105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- [20].Hardy DF, Debold JF. Effects of mounts without intromission upon the behavior of female rats during the onset of estrogen-induced heat. Physiol Behav. 1971;7:643–645. doi: 10.1016/0031-9384(71)90120-x. [DOI] [PubMed] [Google Scholar]
- [21].Madlafousek J, Hlinak Z. Sexual behavior of the female laboratory rat: inventory, patterning and measurement. Behav. 1977;63:129–174. [Google Scholar]
- [22].Agmo A, Turi AL, Ellingsen E, Kaspersen H. Preclinical models of sexual desire: conceptual and behavioral analyses. Pharmacol Biochem Behav. 2004;78:379–404. doi: 10.1016/j.pbb.2004.04.013. [DOI] [PubMed] [Google Scholar]
- [23].Coopersmith C, Candurra C, Erskine MS. Effects of paced mating and intromissive stimulation on feminine sexual behavior and estrus termination in the cycling rat. J Comp Psychol. 1996;110:176–186. doi: 10.1037/0735-7036.110.2.176. [DOI] [PubMed] [Google Scholar]
- [24].Yang L, Clemens LG. Relation of intromissions to the female’s post-ejaculatory refractory period in rats. Physiol Behav. 1996;60:1505–1511. doi: 10.1016/s0031-9384(96)00314-9. [DOI] [PubMed] [Google Scholar]
- [25].Yang L, Clemens LG. Function of intromissions on intromission-return latency of female rats during paced sexual behavior. Physiol Behav. 1997;61:889–894. doi: 10.1016/s0031-9384(96)00614-2. [DOI] [PubMed] [Google Scholar]
- [26].Berkley KJ, Hotta H, Robbins A, Sato Y. Functional properties of afferent fibers supplying reproductive and other pelvic organs in pelvic nerve of female rat. J Neurophysiol. 1990;63:256–272. doi: 10.1152/jn.1990.63.2.256. [DOI] [PubMed] [Google Scholar]
- [27].Berkley KJ, Robbins A, Sato Y. Afferent fibers supplying the uterus in the rat. J Neurophysiol. 1988;59:142–163. doi: 10.1152/jn.1988.59.1.142. [DOI] [PubMed] [Google Scholar]
- [28].Peters LC, Kristal MB, Komisaruk BR. Sensory innervation of the external and internal genitalia of the female rat. Brain Res. 1987;408:199–204. doi: 10.1016/0006-8993(87)90372-6. [DOI] [PubMed] [Google Scholar]
- [29].Erskine MS. Pelvic and pudendal nerves influence the display of paced mating behavior in response to estrogen and progesterone in the female rat. Behav Neurosci. 1992;106:690–697. doi: 10.1037//0735-7044.106.4.690. [DOI] [PubMed] [Google Scholar]
- [30].Bermant G. Effects of single and multiple enforced intercopulatory intervals on the sexual behavior of male rats. J Comp Physiol Psych. 1964;57:398–403. doi: 10.1037/h0043537. [DOI] [PubMed] [Google Scholar]