Abstract
Background
Outcome of patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS) with chromosome 5 and 7 abnormalities [excluding del 5(q)] has been poor with fewer than 10% of patients alive at 2 years.
Methods
We investigated whether treatment with hypomethylating agents (5-azacytidine/decitabine) leads to an improved outcome. Between January 2004 and December 2007, 81 patients [37 (46%) with AML (≥ 20% blast); 44 (54%) with high-risk MDS] with chromosome 5 and 7 abnormalities were treated with hypomethylating agents as their initial therapy. These included 68 patients with complex (≥ 3) abnormalities and 13 with less than 3 aberrations. During the same period, 151 patients (126 with AML, 25 with MDS) with chromosome 5 and 7 abnormalities (128 complex, 23 non-complex) were treated with intensive chemotherapy (including cytarabine based regimens in 72% and other in 28%).
Results
Median ages for the two groups were 66 and 61 years, respectively [(ranges (37–85) and (19–89)]. Thirty three (41%) patients in the hypomethylating group achieved CR versus 53 (35%) in the chemotherapy group (p=0.395). With a median follow up of 51 weeks (range 12 – 101) and 40 weeks (range, 5–128), 22/33 patients in the hypomethylating group and 33/53 patients in the chemotherapy group have relapsed. The median CR duration was 45 weeks and 23 weeks, respectively (p=0.153). The overall survival was superior for the hypomethylating group compared to the chemotherapy group (p=0.019).
Conclusion
Treatment with hypomethylating agents may be superior to chemotherapy in patients with chromosome 5 and 7 abnormalities.
Keywords: Acute myeloid leukemia, high-risk myelodysplastic syndrome, decitabine, 5-azacytidine, chemotherapy
INTRODUCTION
The importance of karyotype at diagnosis in predicting outcome in patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) is well established.1–5 These studies have clearly demonstrated that the outcome for patients with chromosome 5 and 7 abnormalities [including monosomy 5 and 7 (−5, −7) and deletion of long arm of these chromosomes (5q-, 7q-)] have an inferior leukemia-free survival and overall survival when traditional cytotoxic chemotherapy, including combinations of cytarabine and anthracyclines, are used.2–4 Such inferior outcome is also seen even with the use of allogeneic stem cell transplantation in first complete remission (CR1).5. Other studies have shown that the prognosis of patients with refractory anemia with excess blasts (RAEB) or refractory anemia with excess blast in transformation (RAEB-T) with chromosome 5 and 7 abnormalities who are given AML-type chemotherapy is the same as that for patients with AML and these cytogenetic abnormalities.6 Therefore, new strategies are needed for these patients.
The use of hypomethylating agents, 5-azacytidine (5AZA) and 2’ deoxyazacytidine (decitabine, DAC), has been clearly associated with an improved outcome in patients with MDS. These agents are now approved for use in this indication.7,8 Treatment with 5AZA was shown to be superior to conventional care strategies including traditional AML-type chemotherapy in 358 patients with MDS.9 Of interest, patients with ≥ 20% blasts (diagnosed as AML by WHO) treated with hypomethylating agents in these trials had favorable responses raising the possibility that these agents may be beneficial in patients with AML, particularly those who are unable to tolerate traditional chemotherapy.8–10
In this study, we examined whether the use of hypomethylating agents, 5AZA and DAC was associated with a better outcome than traditional cytotoxic chemotherapy regimens in patients with AML and high risk MDS who are typically resistant to the latter.
METHODS
Patient characteristics
Between January 2004 and December 2007, 81 patients [including 37 (46%) with AML (≥ 20% blast, as defined by the WHO criteria) and 44 (54%) with high-risk MDS (Intermediate-2 and high-risk by IPSS)] with chromosome 5 and 7 abnormalities were treated with hypomethylating agents as their initial therapy. These included 68 patients with complex abnormalities (≥ 3 abnormalities) and 13 with less than 3 aberrations. During the same period, 151 patients (126 AML, 25 MDS) with chromosome 5 and 7 abnormalities (128 complex, 23 noncomplex) were treated with intensive cytotoxic chemotherapy (Table 1). All patients were treated on clinical trials conducted in the department during that period; all signed an institutional review board approved consent form to participate in these studies. The median age for the two groups was 66 and 61 years, respectively (ranges 37 to 85 years, and 19 to 89 years). Patients with advanced age in the chemotherapy group received single agent clofarabine or similar treatments. Overall, 52 (22%) patients were considered as having de novo disease, whereas 88 (38%) and 92 (40%) had either therapy-related disease or history of antecedent hematological disorder (AHD). Other patient characteristics are shown in Table 2.
Table 1.
| Chemotherapy Regimens | N |
|---|---|
| Clofarabine + high dose cytarabine13 | 4 |
| Clofarabine +/− low dose cytarabine12 | 44 |
| DCTER (Daunorubicin + cytarabine + 6-Thioguanine + Etoposide14 | 2 |
| Daunorubicin + cytarabine + PKC41215 | 2 |
| Idarubicin + cytarabine + Pravastatin16 | 7 |
| Idarubicin + cytarabine + Zarnestra17 | 18 |
| Idarubicin + cytarabine | 41 |
| Fludarabine + cytarabine ± idarubicin18 | 13 |
| Cloretazine19 | 20 |
| Hypomethylating Regimens | |
| Decitabine20 | 56 |
| Decitabine + valproic acid21 | 9 |
| Decitabine + vorinostat22 | 2 |
| 5-Azacytidine10 | 3 |
| 5-Azacytidine + valproic acid + ATRA23 | 11 |
Table 2.
Patient characteristics
| Characteristic | Hypomethylating group, N (%) |
Chemotherapy group, N (%) |
P value |
|---|---|---|---|
| Number | 81 | 151 | |
| AML | 37 (46) | 126 (83) | < 0.001 |
| High risk MDS | 44 (54) | 25 (17) | |
| Median age in years (range) | 66 (37 – 85) | 61 (19 – 89) | 0.015 |
| Gender F/M | 25 (31)/56 (69) | 72 (48)/79 (52) | 0.014 |
| Median WBC at presentation x | 2.3 (1.0 – 43.1) | 3.5 (0.3 – 433.0) | 0.059 |
| 109/L (range) | |||
| Cytogenetics: | |||
| −5 alone | 0 | 0 | |
| −7 alone | 2 (2) | 8 (5) | |
| −5 with one other abnormality | 0 | 0 | |
| −7 with one other abnormality | 4 (5) | 5 (3) | |
| 5q- with one other abnormality | 2 (2) | 5 (3) | |
| 7q- alone | 3 (4) | 2 (1) | |
| 7q- with one other abnormality | 2 (2) | 2 (1) | |
| Complex (≥ 3 abnormalities) | 68 (84) | 129 (86) | NS |
| De novo | 10 (12) | 42 (28) | 0.025 |
| Therapy related | 36 (44) | 52 (34) | |
| Secondary | 35 (43) | 57 (38) | |
| Better prognosis 5 and 7* | 13 (16) | 34 (23) | 0.242 |
Legends - AML: acute myeloid leukemia; MDS: myelodysplastic syndrome; WBC: white blood cell
Estey E, et al.11
The two groups were comparable with regards to presenting WBC (p=0.059), and proportion of patients with various cytogenetic categories (Table 2). The two groups did differ with respect to their median age (66 versus 61 for the hypomethylating and chemotherapy groups, respectively). Significantly more patients in the chemotherapy group had AML. However, a higher proportion of the hypomethylating group had secondary or therapy-related AML or MDS (p=0.025). Furthermore, the proportion of patients with better prognosis 5 and 7 disease as described by Estey et al was similar in the two cohorts (16% vs 23%; Table 2).11
Definitions
Cytogenetic analysis on bone marrow specimens was performed at the institutional cytogenetics laboratory using standard criteria. The recommendations of the International System for Human Cytogenetics Nomenclature (ISCN) were used to describe a cytogenetic clone. At least 3 metaphases with the specific cytogenetic abnormality were needed to include the patient in the study. Patients with 5q- as the sole abnormality were not included in this study due to their expected favorable prognosis. Patients with an additional favorable abnormality such as t(8;21) or inv(16) are considered to have the better prognosis associated with the favorable abnormality, and were not included in this study.
Treatment regimens
The details of the regimens have been previously published (Table 1). Three and 56 patients were treated with 5AZA and DAC alone respectively. Twenty two (eleven each) patients received either 5AZA or DAC in combination with another agent, typically a histone deacetylase inhibitor such as valproic acid or vorinostat.
In the chemotherapy group, 66 patients received combination of high-dose cytarabine and idarubicin, 20 patients had cytarabine plus another agent, 48 patients had clofarabine-based regimens, and 17 patients had other miscellaneous regimens. The various treatment categories administered and the responses to them are summarized in Table 3.
Table 3.
Treatment regimens and responses
| Patients | Response, n (%) |
||||
|---|---|---|---|---|---|
| treated, n (%) |
CR | PR/CRp | Resistan t |
ED | |
| Hypomethylating | 33 (41) | 1/2 (1)/(2) | 31 (38) | 14 (17) | |
| regimens, n=81 | |||||
| 5AZA alone | 3 (4) | 1 (1) | −/− | 2 (2) | − |
| 5AZA + another agent | 11 (14) | 4 (5) | −/− | 4 (5) | 2 (2) |
| DAC alone | 56 (69) | 23 (28) | −/2 (2) | 22 (27) | 9 (11) |
| DAC + another agent | 11 (14) | 5 (6) | 1 (1)/ − | 3 (4) | 2 (2) |
| Chemotherapy | 53 (35) | 1/15 (1)/(10) | 57 (38) | 25 (17) | |
| regimens, n=151 | |||||
| Ara-C + anthracycline | 66 (44) | 32 (21) | −/5 (6) | 23 (15) | 6 (4) |
| Ara-C + other agents | 20 (13) | 2 (1) | −/4 (3) | 10 (7) | 4 (3) |
| Clofarabine based | 48 (32) | 16 (11) | −/4 (3) | 17 (11) | 11 (7) |
| Miscellaneous | 17 (11) | 3 (2) | 1/2 (1)/(1) | 7(5) | 4 (3) |
Legends – CR: complete remission; PR: partial remission; CRp: complete remission without platelet recovery; ED: early death; 5AZA: 5-azacytidine; DAC; 2’ deoxy 5-azacytidine
Statistical methods
Survival curves were plotted by the Kaplan-Meier method and compared using the log-rank test. Differences in subgroups by different covariates were evaluated using the chi-square test for nominal values, and the Mann-Whitney U for continuous variables.
RESULTS
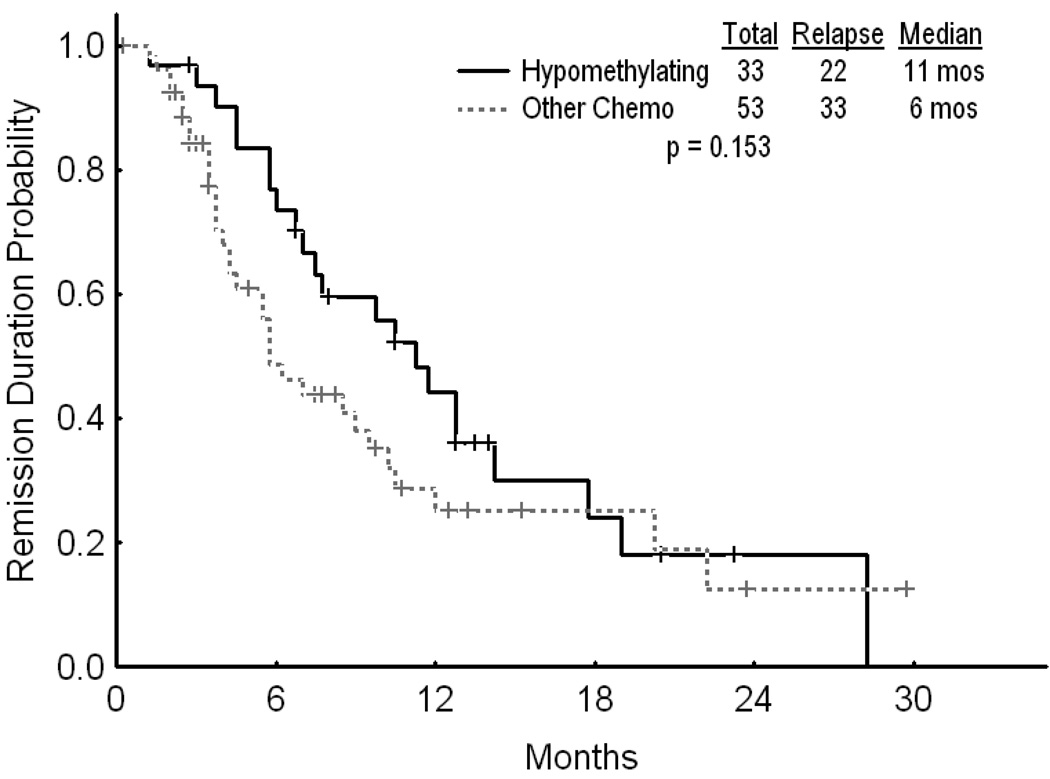
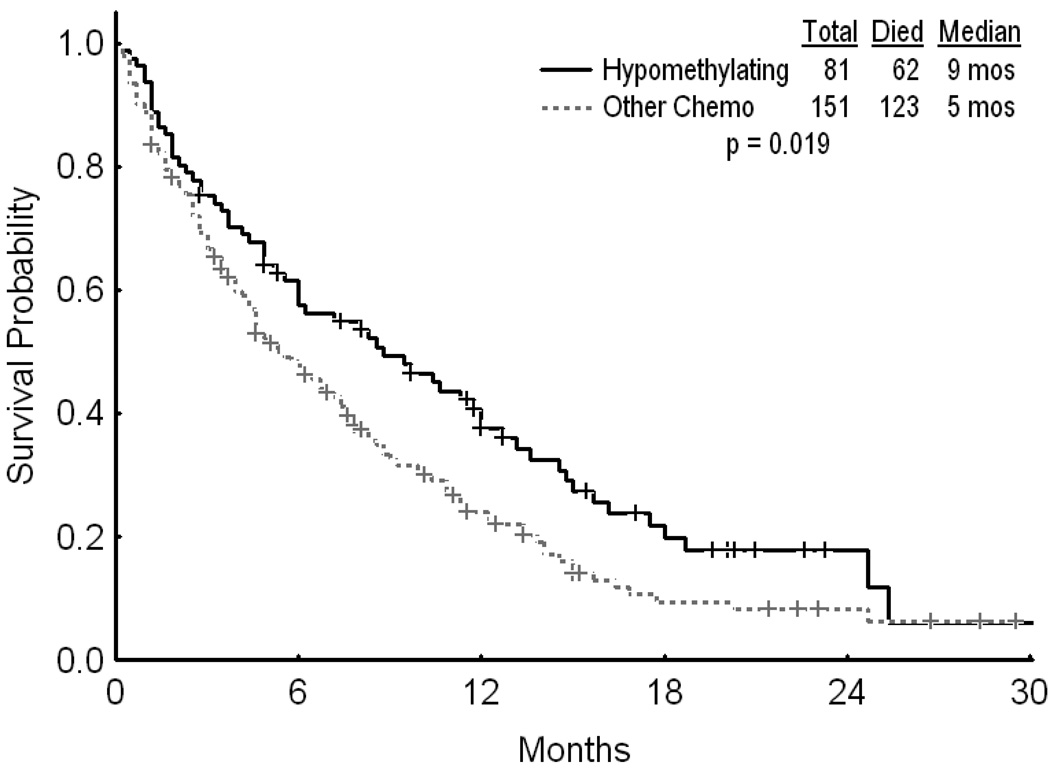
Two hundred and thirty two patients were included in the analysis. These included 81 patients (35%) who received hypomethylating agents as their initial induction regimen, and 151 patients (65%) treated with various chemotherapy regimens. Thirty three (41%) patients in the hypomethylating group achieved CR versus 53 (35%) patients in the chemotherapy group (p=0.395). Two (2%) and 15 (10%) patients in each group achieved a CR without platelet recovery (CRp). The corresponding numbers of patients with primary resistant disease for the hypomethylating and chemotherapy strategies were 31 (38%) and 57 (38%), respectively. Similarly, 14 (17%) patients in the hypomethylating cohort and 25 (17%) patients in the chemotherapy group died in the first 4 weeks of treatment.(Table 3) With a median follow-up of 51 weeks (range 12 – 101) and 40 weeks (range, 5–128), 22 of 33 (66%) patients in the hypomethylating group and 33 of 53 (62%) patients in the chemotherapy group have relapsed. The median CR duration was 45 weeks for the hypomethylating group and 23 weeks for the chemotherapy group (p=0.153) (Figure 1). The overall survival was superior for the hypomethylating group compared to the chemotherapy group (p=0.019) (Figure 2). The median survival was similar when comparing patients with AML who received hypomethylating agents with those who received chemotherapy (21 vs. 24 weeks, respectively; p=0.4). Similarly, the median survival for patients with MDS treated by the two strategies was 46 weeks and 19 weeks (p=0.11). The median survival was significantly longer for patients with lower presenting white cell count when treated with hypomethylating agents as compared to chemotherapy (192 vs. 40 weeks for patients with WBC < 10 × 109/L, respectively, p=0.017)
Figure 1.
CR duration by treatment strategy
Figure 2.
Overall survival by treatment strategy
DISCUSSION
Patients with AML and high risk MDS with chromosome 5 and 7 abnormalities have a poor prognosis. Estey et al showed that it is possible to identify subgroups of patients with slightly better outcome.11 Among the 400 patients with chromosome 5 and 7 abnormalities, those with detectable normal metaphases in addition to the abnormal clone, and those with no AHD had significantly better survival. Patients with simple or complex abnormalities had similar CR rates but disease-free survival in CR was significantly better for those with simple chromosome 5 and 7 abnormalities. Both CR rates and disease-free survival in CR were better for patients without AHD and those with detectable normal metaphases, They were able to identify a subgroup, with simple abnormality, no AHD and detectable normal metaphases that had a significantly better survival than all other patients with chromosome 5 and 7 abnormalities, which was comparable with patients with normal cytogenetics treated during the same period.
The poor outcome of patients with chromosome 5 and 7 abnormalities is likely due to inherent resistance to cytotoxic chemotherapy. Although CR rates particularly in patients with non-complex disease, can approach those seen in patients with intermediate risk disease, the majority of patients with these abnormalities relapse rapidly. It is likely that the small proportion of patients with a better prognosis as described above have fewer cellular abnormalities, rendering them more sensitive to cytotoxic agents. However, it is clear that alternative treatment strategies are needed for this subgroup of patients with AML and MDS. Alternative agents such as clofarabine with or without low dose subcutaneous cytarabine are being evaluated in older patients with AML that comprise a significant proportion of these patients.12
Although our data is not indicative of a better response to hypomethylating agents, the significantly better survival is suggestive of superiority of these drugs in treating these patients. It is important to note that the two groups were not matched. However, the cohort treated with hypomethylating agents had a higher median age and larger proportion of patients with secondary or therapy-related AML/MDS, both of which are expected to adversely affect the outcome. When examining the AML and MDS groups separately, there was no significant difference in the outcome using the two strategies within each group. However, there was a significantly better outcome for hypomethylating therapy for patients with a presenting WBC < 10 × 109/L. This was also true for other cut-off values including the median and the 75% value of presenting WBC. This may be a reflection of the time required to achieve a response using the hypomethylating agents (typically several courses or months) and the lower efficacy of these agents in patients with proliferative disease.
It is possible that the better outcome with hypomethylating agents is at least partly attributable to their lower toxicity and potentially less early mortality in this population of typically older patients, who are less able to tolerate cytotoxic agents. However, such improvement based on reduced early mortality would only be realized if hypomethylating therapy had at least a similar efficacy to cytotoxic agents. Prospective studies are necessary to clearly demonstrate whether this strategy is truly superior to chemotherapy-based regimens in this subset of patients and to evaluate whether such non-cross resistant approaches of cytotoxic chemotherapy combined with hypomethylating agents used simultaneously or sequentially would further improve the clinical outcome..
Acknowledgments
This work was supported in part by NCI grant 5P01 CA108631
REFERENCE
- 1.Keating MJ, Cork A, Broach Y, et al. Toward a clinically relevant cytogenetic classification of acute myelogenous leukemia. Leuk Res. 1987;11:119–133. doi: 10.1016/0145-2126(87)90017-8. [DOI] [PubMed] [Google Scholar]
- 2.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 3.Grimwade D, Walker H, Harrison G, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1312–1320. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 4.Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 5.Ferrant A, Labopin M, Frassoni F, et al. Karyotype in acute myeloblastic leukemia: prognostic significance for bone marrow transplantation in first remission: a European Group for Blood and Marrow Transplantation study. Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Blood. 1997;90:2931–2938. [PubMed] [Google Scholar]
- 6.Estey E, Thall P, Beran M, et al. Effect of diagnosis (refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, or acute myeloid leukemia [AML]) on outcome of AML-type chemotherapy. Blood. 1997;90:2969–2977. [PubMed] [Google Scholar]
- 7.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 9.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009 doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverman LR, McKenzie DR, Peterson BL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24:3895–3903. doi: 10.1200/JCO.2005.05.4346. [DOI] [PubMed] [Google Scholar]
- 11.Estey EH, Pierce S, Keating MJ. Identification of a group of AML/MDS patients with a relatively favorable prognosis who have chromosome 5 and/or 7 abnormalities. Haematologica. 2000;85:246–249. [PubMed] [Google Scholar]
- 12.Faderl S, Ravandi F, Huang X, et al. A randomized study of clofarabine versus clofarabine plus low-dose cytarabine as front-line therapy for patients aged 60 years and older with acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2008;112:1638–1645. doi: 10.1182/blood-2007-11-124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faderl S, Verstovsek S, Cortes J, et al. Clofarabine and cytarabine combination as induction therapy for acute myeloid leukemia (AML) in patients 50 years of age or older. Blood. 2006;108:45–51. doi: 10.1182/blood-2005-08-3294. [DOI] [PubMed] [Google Scholar]
- 14.Rytting M, Verstovsek S, Garcia-Manero G, et al. Intensively Timed Induction (ITI) Chemotherapy in Adults with Acute Myelogenous Leukemia (AML) Blood. 2007;110 [Google Scholar]
- 15.Stone RM, Fischer T, Paquette R, et al. Phase IB Study of PKC412, an Oral FLT3 Kinase Inhibitor, in Sequential and Simultaneous Combinations with Daunorubicin and Cytarabine (DA) Induction and High-Dose Cytarabine Consolidation in Newly Diagnosed Adult Patients (pts) with Acute Myeloid Leukemia (AML) under Age 61. Blood. 2006;108 [Google Scholar]
- 16.Kornblau SM, Banker DE, Stirewalt D, et al. Blockade of adaptive defensive changes in cholesterol uptake and synthesis in AML by the addition of pravastatin to idarubicin + high-dose Ara-C: a phase 1 study. Blood. 2007;109:2999–3006. doi: 10.1182/blood-2006-08-044446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delmonte J, Kantarjian HM, Garcia-Manero G, et al. Final Update of Phase I-II Study of the Farnesyltransferase Inhibitor Tipifarnib in Combination with Idarubicin and Cytarabine for Patients with Newly Diagnosed Acute Myeloid Leukemia or High-Risk Myelodysplastic Syndrome. Blood. 2007;110 [Google Scholar]
- 18.Estey EH, Thall PF, Pierce S, et al. Randomized phase II study of fludarabine + cytosine arabinoside + idarubicin +/− all-trans retinoic acid +/− granulocyte colony-stimulating factor in poor prognosis newly diagnosed acute myeloid leukemia and myelodysplastic syndrome. Blood. 1999;93:2478–2484. [PubMed] [Google Scholar]
- 19.Giles F, Rizzieri D, Karp J, et al. Cloretazine (VNP40101M), a novel sulfonylhydrazine alkylating agent, in patients age 60 years or older with previously untreated acute myeloid leukemia. J Clin Oncol. 2007;25:25–31. doi: 10.1200/JCO.2006.07.0961. [DOI] [PubMed] [Google Scholar]
- 20.Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–57. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, et al. Phase 1/2 study of the combination of 5-aza-2'-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108:3271–3279. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravandi R, Faderl S, Thomas D, et al. Phase I Study of Suberoylanilide Hydroxamic Acid (SAHA) and Decitabine in Patients with Relapsed, Refractory or Poor Prognosis Leukemia. Blood. 2007;110 [Google Scholar]
- 23.Soriano AO, Yang H, Faderl S, et al. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood. 2007;110:2302–2308. doi: 10.1182/blood-2007-03-078576. [DOI] [PubMed] [Google Scholar]