Abstract
CXCR1 and CXCR2 are receptors for CXCL-8 and are differentially expressed on melanoma and endothelial cells. In this study, we determined the functional role of these receptors in melanoma progression. We stably knock-down the expression of CXCR1 and/or CXCR2 in A375-SM (SM; high metastatic) human melanoma cells by short-hairpin RNA (shRNA) transfection. Cell proliferation, migration, invasion, ERK phosphorlyation and cytoskeletal rearrangements were carried out in in vitro. In vivo growth was evaluated using murine subcutaneous xenograft model. Our data demonstrate that knock-down of CXCR1 and/or CXCR2 expression, inhibited melanoma cell proliferation, survival, migration and invasive potential in vitro. Moreover, we also observed inhibition of ERK phosphorylation and cytoskeltal rearrangement in SM-shCXCR1, SM-shCXCR2 and SM-shCXCR1/2 cells. Furthermore, when SM-shCXCR1 or SM-shCXCR2 cells implanted in nude mice, tumor growth, proliferation and microvessel density was significantly inhibited as compared to SM-control cells. In addition, we observed a significant increase in melanoma cell apoptosis in SM-shCXCR1 and SM-shCXCR2 tumors compared to SM-control tumors. Together, these data demonstrate that CXCR1 and CXCR2 expression play a critical role in human melanoma tumor progression and, functional blockade of CXCR1 and CXCR2 could be potential used for future therapeutic intervention in malignant melanoma.
Keywords: Chemokines, CXCR1, CXCR2, Tumor Growth, Melanoma
Introduction
Human melanoma, is the fifth most common cancer in the United States and there will be 8,420 deaths from the disease in 2008 (1). Metastatic melanoma is difficult to treat due to lack of effective treatment modalities. Therefore, improvement in the therapy of metastatic melanoma now depends on improving our understanding of the complex molecular mechanisms governing the progression and aggressiveness of the disease.
Over the last two decades, it has been increasingly recognized that chemokines play an important role in tumor biology (2-5). Chemokines binds particular G-protein-coupled receptors (GPCRs) and are known to mediate leukocyte trafficking, inflammation, angiogenesis, and tumorigenesis (6-8). Interestingly, CXCL-8, a member of the CXC chemokine family has been reported to induce migration, stimulate angiogenesis and promote tumor cell growth in melanoma and other malignancies (9-18). The biological effects of CXCL-8 are mediated through two high affinity receptors: type A CXCL-8 receptor (IL-8RA/IL-8RI or CXCR1) and type B CXCL-8 receptor (IL-8RB/IL-8RII or CXCR2) (19;20). While CXCR2 binds with high affinity to CXCL-8 and other CXC chemokines such as CXCL-6, CXCL-5, CXCL-7 and CXCL-1, CXCR1 is less promiscuous and binds only to CXCL-8 (12;21;22). CXCR1 and CXCR2 share a high degree of sequence similarity (75.8% in amino acid sequence), but differ within the extracellular and intracellular loops and the NH2- and COOH-terminal domains (23). We have previously shown that CXCR1 and CXCR2 are differentially expressed on melanoma and endothelial cells (9;24;25). Several studies have implicated CXCR1 and CXCR2 as important players in tumor progression (3;26;27). Our previous studies demonstrated that CXCR1 and CXCR2 are expressed on melanoma and endothelial cells (9;24;25). We and others have also shown that neutralization of CXCR1 or CXCR2 using small molecule antagonists affects cell proliferation and migration, indicating the involvement of these receptors in altered cellular responses (25;28;29). Thus, involvement of CXCR1 and CXCR2 and their ligand CXCL-8 in different cell process makes this ligand-receptors axis of particular interest in investigating its functional role in melanoma progression.
To examine more directly the role of CXCR1 and CXCR2 in melanoma progression, we employed a gene knock-down strategy to inhibit CXCR1 or CXCR2 expression to modulate cellular phenotypes associated with melanoma growth and invasion. Our results clearly show that down-regulation of CXCR1 or CXCR2 inhibited melanoma tumor growth by increasing apoptosis and decreasing cell proliferation, migration and invasion.
Material and methods
Cell culture
The human melanoma cell line A375-SM (SM; highly metastatic) was maintained in culture as an adherent monolayer in Dulbecco's Modified Eagle Medium (DMEM) (MediaTech, Herndon, VA). Culture medium was supplemented with 5% fetal bovine serum (FBS), 1% L-glutamine (MediaTech), 1% vitamin solution (MediaTech) and gentamycin (Invitrogen, Carlsbad, CA). Cells were grown at 37°C with 5% CO2 in humidified atmosphere.
Generation of shRNA-expression plasmids
Silencing of gene expression was achieved using short hairpin RNA (shRNA) technology. One shRNAs targeting CXCR1 (1sh- 5′CCC GCG TCA CTT GGT CAA GTT TGT), one targeting CXCR2 (2sh- CCC CAA TAC AGC AAA CTG GCG GAT), one targeting both CXCR1/2 (1/2sh- CCC CTT CTA TAG TGG CAT CCT GCT) and scrambled (control) were generated using a CXCR1 or CXCR2 specific sequence with BglII and HindIII overhangs to allow for cloning into the pSuper.neo vector (Oligoengine, seattle, WA). A375-SM cells were stably transfected with pSuper.neo/scrambled (SM-control), pSuper.neo/shCXCR1 (SM-shCXCR1), pSuper.neo/shCXCR2 (SM-shCXCR2) or pSuper.neo/shCXCR1/2 (SM-shCXCR1/2) plasmid using Lipofectamine reagent (Invitrogen, Carlsbad, CA) following the manufacturers protocol. G418-sulphate-resistant colonies were isolated and maintained in medium supplemented with 1000 μg/ml of G418-sulphate (Invitrogen). To avoid clone specific effects, pooled cultures were used for all experiments.
Gene expression analysis
Analysis of gene expression was performed using quantitative RT-PCR as described (24). Briefly, cDNA was synthesized from 5 μg total RNA using SuperScript™ II Reverse Transcriptase (Invitrogen) and oligo(dT) primer. Two micro liter of first strand cDNA (1:10 dilution) was amplified. The following primer sequences were used: CXCR1, 5′-TGG GAA ATG ACA CAG CAA AA-3′ (forward) and 3′,AGT GTA CGC AGG GTG AAT CC-3′ (reverse), CXCR2, 5′-ACT TTT CCG AAG GAC CGT CT-3′ (forward) and 5′-GTA ACA GCA TCC GCC AGT TT-3′ (reverse). For internal control, glyceraldehydes 3-phosphate dehydrogenase (GAPDH), 5′-CGC ATT TGG TCG TAT TGG G-3′ (forward), and 5′-TGA TTT TGG AGG GAT CTC GC-3′ (reverse) was used. Amplified products were resolved through a 1.5% agarose gel containing ethidium bromide and analyzed using an Alpha Imager gel documentation system (AlphaInnotech, San Leandro, CA).
Tumor growth analysis
Female athymic nude (6-8 week old) were purchased from the National Cancer Institute and maintained under specific pathogen-free conditions. All procedures performed were in accordance with institutional guidelines and approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee. To specifically determine the role of CXCR1 or CXCR2 in tumor development SM-control, SM-shCXCR1 or SM-shCXCR2 cells (1 × 106 in 0.1 ml of HBSS) were injected subcutaneously (s.c.) into the right flank region of nude mice. Tumor growth was measured twice weekly and mice were sacrificed on day 45 after tumor implantation. Tumor volume was calculated using the formula π/6 × (smaller diameter)2 × (larger diameter). Tumors recovered from mice were fixed in zinc fixative, embedded in paraffin and processed for histopathological evaluation and immunohistochemistry.
Immunohistochemistry
Immunohistochemical analysis was performed as previously described (30). In brief, 6-μm thick tumor sections were deparaffinized by EZ-Dewax (Biogenex, SanRoman, CA) and blocked for 30 minutes. Tumor sections were incubated overnight in a humid chamber with the following primary antibodies: anti-PCNA (1:40; Santa Cruz Biotechnology, Santa Cruz, CA) or mouse biotinylated anti-GS-IB4 (isolectin from Griffonia simplicifolia; 1:50; Vector Laboratories, Burlingame, CA). For confirming the down-regulation of CXCR1 and/or CXCR2, cells (10,000 cells) were seeded overnight on coverslips, fixed in ice cold 4% formaldehyde, blocked and incubated with following primary antibodies: mouse monoclonal anti-CXCR1 (1:100; R&D systems, Minneapolis, MN) and mouse monoclonal anti-CXCR2 (1:50; R&D systems). Corresponding biotinylated secondary antibody was used (except for GS-IB4) at room temperature. Immunoreactivity was detected using the ABC Elite kit and DAB substrate (Vector Laboratories) per the manufacturer's instructions. A reddish brown precipitate in the cytoplasm indicated a positive reaction. Negative controls had all reagents included except the primary antibody. The number of microvessels was quantitated microscopically with a 5×5 reticle grid (Klarmann Rulings, Litchfield, NH) using 400× objective (250 μm total area).
Apoptotic cells in tumor samples were identified by terminal deoxyribonucleotidyl transferase dUTP nick end fluorescein labeling (TUNEL) assay according to the manufacturer's instructions (In situ Cell Death Detection Kit, Fluorescein; Roche, Indianapolis, IN). The number of apoptotic cells was evaluated by counting the positive (brown-stained) cells in ten arbitrarily selected fields at 200× magnification in a double-blinded manner and expressed as average number of cells per field view.
Cell proliferation and apoptosis assay
Cells were seeded in 96-well plates at low density (1000 cells/well). Following overnight adherence, cells were incubated with media alone or medium containing different serum concentration, with or without CXCL-8 (10ng/ml) for 72 h. Cell proliferation was determined by MTT assay (31;32). Apoptosis was measured using the CaspACE FITC-VAD-FMK in Situ marker (Promega, Madison WI). Cells (1 × 106) were grown in serum free medium for 24 h. Apoptosis was detected by staining the cells with CaspACE FITC-VAD-FMK solution in PBS for 30 min at 37°C. The bound marker was localized by fluorescent detection using a confocal microscope.
Cell motility and invasion assay
To investigate the effect of silencing CXCR1 or CXCR2 expression on cell migration, cells (1 × 106 cells/well) in serum free media were plated in the top chamber of noncoated polyethylene terephthalate membranes (six-well insert; 8 μm pore size; Becton Dickinson, Franklin Lakes, NJ). For invasion, cells (10000 cells/wells) were plated onto Matrigel-coated transwell chambers (24-well insert; 8 μm pore size; Corning Costar Corp., Cambridge, MA) in serum free media. The bottom chamber contained 1.0 ml serum free media with or without CXCL-8 (10 ng/ml). The cells were incubated for 24 h at 37°C and cells that did not pass through the membrane pores were removed. Migrated cells were stained using Hema 3 kit (Fisher Scientific Company L.L.C., Kalamazoo, MI) as per the manufacturer's instructions. Cells were counted in ten random fields (200×) and expressed as the average number of cells per field of view. The data is represented as the average of three independent experiments.
F-actin immunostatining
Cells were grown at low density (10,000 cells) overnight on coverslips, fixed in ice cold 4% formaldehyde, permeabilized in 0.3% Triton X-100 and stained with Texas Red-phalloidin (Molecular Probes, Eugene, OR) for 30 min at room temperature. Cells were further washed with PBS-T (PBS contaning 0.1% Tween 20) and mounted with antifade vectashield mounting medium (Vector Laboratories). The stained cells were analyzed using a confocal microscope (UNMC core facility).
Western blot analysis
Cells were processed for protein extraction and western blotting using standard procedures. Briefly, the cells were washed twice with PBS and scraped in Triton X-100 buffer [1% Triton X-100, 50 mmol/L TBS (pH 7.4), 10mmol/L EDTA with protease inhibitors (Roche Diagnostics, Mannheim, Germany) and phosphatase inhibitors (5mM NaF and 5 mM Na3VO4; Sigma Chemicals, St. Louis, MO)]. Cell lysates were passed through the needle syringe to facilitate the disruption of the cell membranes and were centrifuged at 14,000 rpm for 20 min at 4 °C, and supernatant were collected. The proteins (50 μg) were resolved by electrophoresis on 8% SDS-PAGE. Resolved proteins were transferred onto polyvinylidene difluoride membrane and subjected to standard immunodetection procedure using specific antibodies: anti-CXCR1 and anti-CXCR2 (1:50, mouse monoclonal, R&D systems); anti-pERK1/2, anti-ERK1/2 and anti-GAPDH (1:1000, rabbit polyclonal, Cell Signaling Technology). Secondary antibodies consisted of horseradish peroxidase-conjugated (Santa Cruz Biotechnology, Santa Cruz, CA) and were used at 1:2000 dilution. All the blots were processed with ECL Plus Western Blotting detection kit (GE Healthcare, Piscataway, NJ), and the signal was detected by a Typhoon 9410 Variable Mode Imager.
Statistical analysis
Differences between the groups were compared using the unpaired two-tailed t-test using SPSS software (SPSS Inc., Chicago, Illinois). In vivo analysis was done using the Mann-Whitney U-test. All the values were expressed as mean ± SEM. A p value of equal or less than 0.05 was considered statistically significant.
Results
Knock-down of CXCR1 and CXCR2 resulted in reduced tumor growth and angiogenesis
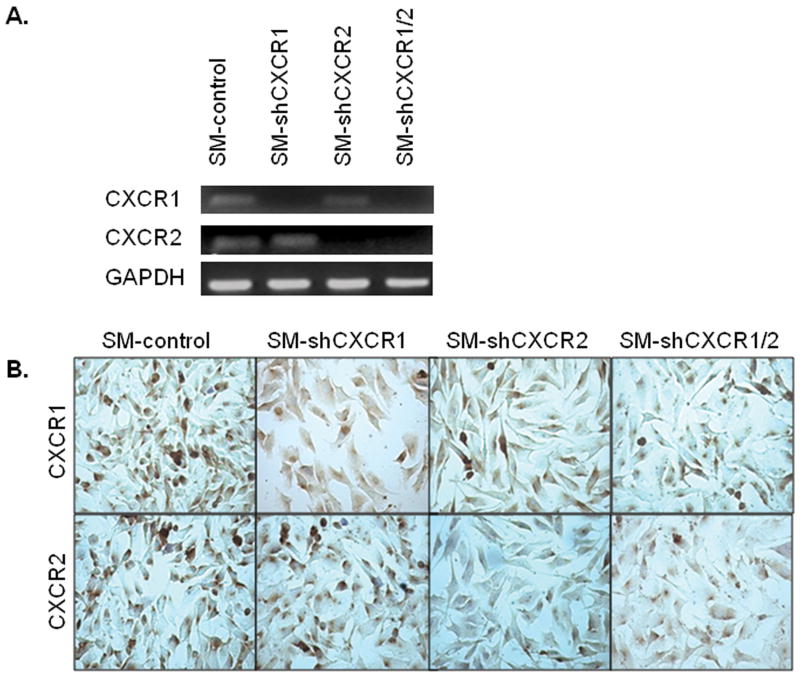
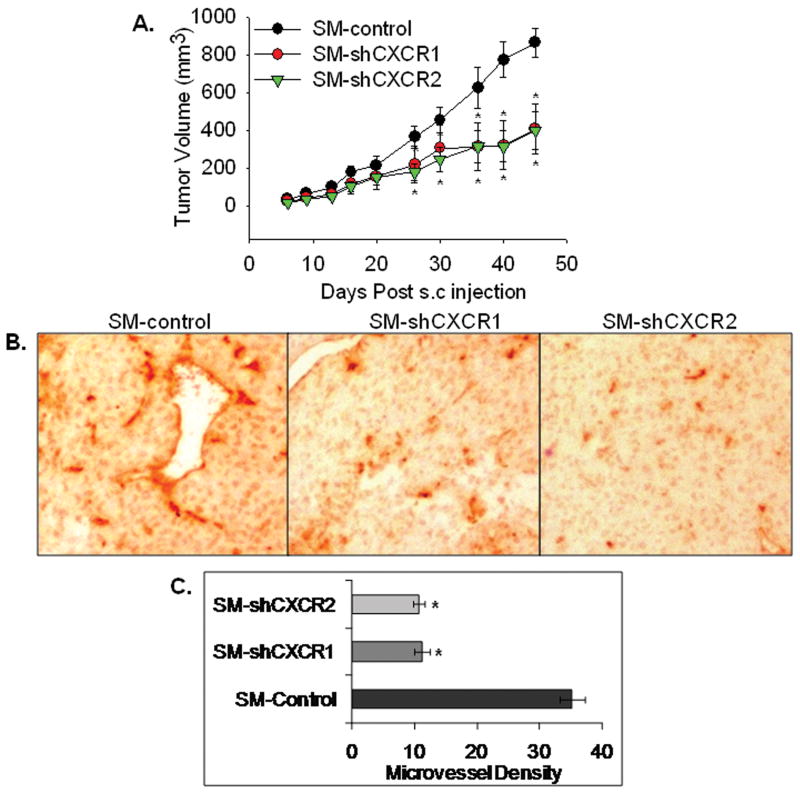
To knock-down CXCR1 or CXCR2 expression, plasmids containing shRNA sequences were transfected into SM melanoma cells (SM-shCXCR1, SM-shCXCR2 or SM-shCXCR1/2) along with SM-control. Expression of CXCR1 and CXCR2 was determined in pooled sublines by RT-PCR (Fig. 1A) and immuncytochemical analyses (Fig. 1B). To specifically determine the role of CXCR1 or CXCR2 in tumor development, SM-shCXCR1, SM-shCXCR2 or SM-control cells were injected into the right flank region of nude mice (n=6/group). Seven weeks post-tumor injection, tumor volumes were measured. Subcutaneous tumors formed from SM-shCXCR1 (2.1 fold) or SM-shCXCR2 (2.2 fold) cells were significantly smaller compared with the SM-control cells (SM-shCXCR1, 407.1 ± 133.7 mm3; SM-shCXCR2, 393.2 ± 99.0 mm3; SM-control, 864.6 ± 78.7 mm3 Fig. 2A). All of the mice injected with SM-shCXCR1, SM-shCXCR2 or SM-control developed tumors.
Figure 1. CXCR1 and/or CXCR2 knock-down in A375SM melanoma cells.
Cells were stably transfected with SM-control, SM-shCXCR1, SM-shCXCR2 or SM-shCXCR1/2 plasmid construct containing scrambled, CXCR1, CXCR2 or CXCR1/2 shRNA. A, RT-PCR shows decreased expression of CXCR1 or CXCR2 mRNA in pooled sublines. GAPDH was used as a loading control. B, Immunocytochemistry showing decreased expression of CXCR1 or CXCR2 in SM-shCXCR1, SM-shCXCR2 and SM-shCXCR1/2 cells. Pictures are representative of at least three experiments.
Figure 2. knock-down of CXCR1 or CXCR2 reduces melanoma tumor growth and decreases microvessel density in vivo.
Melanoma cells (SM-shCXCR1, SM-shCXCR2 or SM-control) were subcutaneously (s.c.) injected into the right flank region of nude mice. Tumor volume was measured twice weekly with a caliper and was calculated by using the formula π/6 × (smaller diameter) 2 × (larger diameter). A, Tumor volume from day 0 to day 45 (n=6). Growth of SM-shCXCR1 and SM-shCXCR2 were significantly reduced (p<0.05) compared with SM-control group. B, Immunohistochemical staining for microvessels with anti-GS-IB4. The representative pictures are shown at 200× C, The values are average number of microvessels ± SEM. Microvessel density was quantitated microscopically with a 5×5 reticle grid at 400× magnification. The values are mean ± standard error of mean (SEM). *Significantly different from SM-control (p<0.05).
One possible mechanism for decreased tumor growth is attenuated neovascularization. In order to determine whether down-regulation of CXCR1 and CXCR2 could potentially disrupt the neovascularization, we examined vascularity in tumors. Staining on SM-shCXCR1 and SM-shCXCR2 tumor sections showed a 3.1-3.3 fold decrease in the number of blood vessels as compared to control tumors (Fig. 2B).
Inhibition of CXCR1 and CXCR2 expression decreased the proliferation and survival both in vitro and in vivo
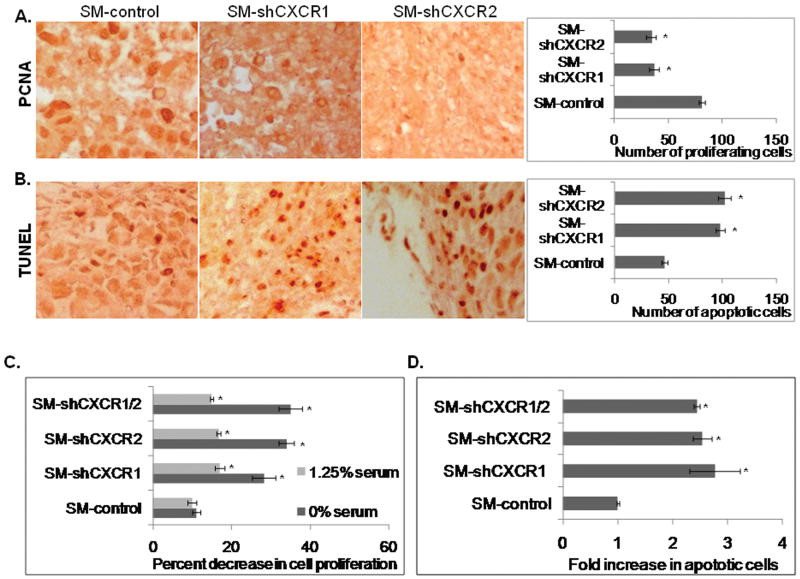
Chemokines are potent regulators of proliferation and survival. Hence, it was of interest to address whether knock down of CXCR1 or CXCR2 may affect melanoma cell proliferation. The average number of PCNA positive-cells by immunohistochemical staining showed a 2.2 fold (p<0.05) decrease for SM-shCXCR1 and 2.3 fold (p<0.05) decrease for SM-shCXCR2 tumors compared with SM-control tumors (Fig. 3A). Additionally, SM-shCXCR1 and SM-shCXCR2 tumors had significantly increased numbers (2.1-2.2 fold, p<0.05) of TUNEL-positive cells as compared with SM-control tumors (Fig. 3B).
Figure 3. Reduced melanoma cell proliferation and apoptosis both in vivo and in vitro.
Immunohistochemical staining of PCNA (A) and TUNEL (B) in the tumor section. Proliferating and apoptotic cells were counted in ten arbitrarily selected fields (200×) in a double-blinded manner. C, In vitro cellular proliferation was determined at 72h by MTT assay (left panel). The values are mean percent inhibition of proliferation ± SEM. D, The frequency of CaspACE-positive cells was determined by counting in ten fields (200×) for each treatment. The values are expressed as average number of cells per field view (right panel). *Significantly different from SM-controls (p<0.05).
Similarly, in an in vitro assay (in the absence of serum), the presence of CXCL-8 did not enhance cell proliferation in SM-shCXCR1, SM-shCXCR2 or SM-shCXCR1/2 melanoma cells as compared to SM-control cells. SM-shCXCR1, SM-shCXCR2 and SM-shCXCR1/2 cells showed a 28-35% decrease in proliferation (Fig. 3C). The in vitro apoptosis assay (Caspase FITC) of SM-shCXCR1, SM-shCXCR2 and SM-shCXCR1/2 cells showed an increase of approximately 1.3-1.7 folds (Fig. 3D), which supports the above findings. Taken together our data suggest a role for CXCR1 and CXCR2 in melanoma cell proliferation and survival.
Reduced CXCR1 and CXCR2 expression also inhibited migration and invasiveness of SM cells
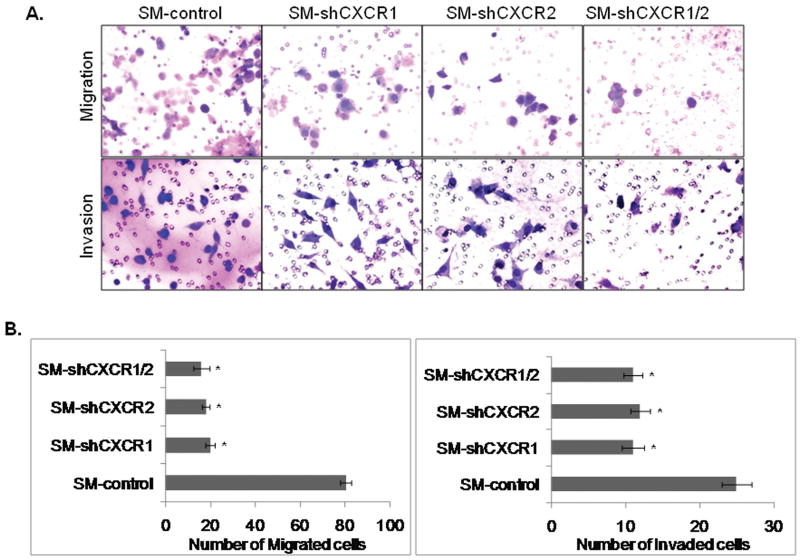
Overall growth of the tumor is determined by the interaction of tumor cells with the host microenvironment. Our recent study has suggested that CXCR1 and CXCR2 signaling not only helps in the migration of melanoma cells and angiogenesis, but may also help in the motility and invasion of melanoma cells (unpublished). Therefore, we investigated whether down-regulation of CXCR1 or CXCR2 would affect tumor cell chemotaxis and invasion. Our data demonstrated a significant (p<0.05) inhibition in CXCL-8-induced chemotaxis of SM-shCXCR1 (4.0 fold), SM-shCXCR2 (5.0 fold) and SM-shCXCR1/2 (5.0 fold) cells as compared to SM-control cells (Fig. 4A-upper panel & 4B-left panel). Similarly, the number of invading cells decreased by 1.8-2.0 fold as compared with control cells in an in vitro Matrigel invasion assay (Fig. 4A-lower panel & 4B-right panel).
Figure 4. Down-regulation of CXCR1 and/or CXCR2 reduces cell motility and invasion.
A, Cells were seeded on non-coated or Matrigel-coated membranes for motility (upper panel) and invasion (lower panel) assays overnight. Migrated cells were stained and photographed at 200× magnification. B, Migrated (left panel) and invaded (right panel) cells were counted in ten random fields (200×) and expressed as the average number of cells per field of view ± SEM. This is a representative of three experiments done in triplicate. *Significantly different from SM-control cells (p<0.05).
Knock-down of CXCR1 and CXCR2 affects CXCL-8-induced actin reorganization and phosphorylation of ERK1/2 in melanoma cells
Actin polymerization stimulates motility of cells, therefore we examined the effect of down-regulation of CXCR1 or CXCR2 on actin polymerization of melanoma cells. The staining of SM-shCXCR1, SM-shCXCR2 and SM-shCXCR1/2 cells showed a reduction in lamellipodial structures as compared to control cells (Fig. 5A). Thus, the decrease in actin polymerization reduced the motility and in turns the invasiveness of the melanoma cells. CXCR1 or CXCR2-associated changes in gene expression indicate their important role in cell signaling. The ERK1/2 MAPK pathway is constitutively activated in most melanomas, and plays a major role in mediating their survival and proliferation (33). Therefore, we investigated the effect of down-regulation of CXCR1 or CXCR2 on ERK1/2 MAP kinase phosphorylation. Figure 5B showed that the levels of ERK1/2 phosphorylation were reduced in SM-shCXCR1, SM-shCXCR2 and SM-shCXCR1/2 cells as compared to SM-control cells. Thus, suggesting the involvement of CXCR1 and CXCR2 mediated signaling in melanoma cells.
Figure 5. Effect of CXCR1 and/or CXCR2 down-regulation on actin reorganization and ERK1/2 phosphorylation.
A, Cells were grown on coverslips and stained with Texas Red phalloidin. Decreased lamellipodial structures were observed in SM-shCXCR1, SM-shCXCR2 and SM-shCXCR1/2 cells compared with the SM-control cells. B, Cell lysates (50ug) were fractionated by SDS-PAGE and subjected to Western blotting using pERK1/2, ERK1/2 and GAPDH antibody.
Discussion
Despite extensive studies on melanoma, the molecular mechanisms of melanoma tumor growth are still not fully understood. Numerous studies have shown that chemokines and chemokine receptors exert a variety of biological functions, including regulation of proliferation, angiogenesis and survival (34;35). In addition, functional chemokine receptors possibly play pivotal role in tumor growth and metastasis of various cancer. Therefore, the expression of chemokines and chemokine receptors give an advantage to tumor cells and may grant them the enhanced ability to proliferate and disseminate. Earlier reports from our group suggest that modulation of CXCL-8 expression in melanoma cells influence the tumor growth and metastasis (32;36). Modulation of chemokine receptors on tumor cells has not been extensively investigated.
Prompted by initial observations, an important question addressed in this study deals with the functional significance of CXCR1 or CXCR2 expression in turn melanoma tumor growth. All the mice injected subcutaneously with SM-shCXCR1 or SM-shCXCR2 cells developed significantly smaller tumors as compared to vector control, demonstrating significance of CXCR1 or CXCR2-dependent signaling. Earlier reports demonstrate that blockade of CXCL-8 activity by neutralizing anti-CXCL-8 antibodies significantly inhibited the growth and metastasis of human melanoma cells in nude mice by suppressing angiogenesis and invasion (37). Our recent data suggest that inhibition of CXCR1 and CXCR2 signaling using small molecule antagonists inhibited melanoma growth and invasion (38). In support, our present study showed that knock-down of both the receptors regulated primary tumor growth by decreasing in cell proliferation, angiogenesis and survival. In addition, we also observed decreased chemotaxis and invasion of CXCR1 or CXCR2 knock-down SM cells. Hence, the results of our study suggest an important association between the expression of the CXCR1 or CXCR2 receptors and melanoma growth and invasion.
Increased cell proliferation and decreased cell death play a pivotal role in tumor progression. Any decreased tumor growth of SM-shCXCR1 and SM-shCXCR2 cells may be due to their decreased response to CXCL-8-induced cell proliferation, motility, survival or invasion. The present in vitro data reveals that down-regulation of CXCR1 or CXCR2 results in a significant decrease in proliferation, especially under serum starvation. Our results are in agreement with our previous observation that neutralization of CXCR1 and CXCR2 inhibited the proliferation of melanoma cells expressing CXCL-8 (25), thus further strengthening that CXCL-8 functions in an autocrine manner by binding to CXCR1 and/or CXCR2.
Because proliferation is important for tumor growth and metastasis, decreased proliferation could influence the apoptosis, migration and invasion of CXCR1 and CXCR2 down-regulated melanoma cells. Interestingly, the numbers of cells migrated and invaded were significantly less than control cells. Cell motility and invasiveness are associated with actin filaments which are organized on the lamellipodia (39). In addition, it has also been shown that CXCR1 and CXCR2 mediate cytoskeletal reorganization of microvascular endothelial cells (40). Therefore, we next chose to focus on the effect of down-regulation of CXCR1 or CXCR2 on actin cytoskeleton organization. Staining of SM-shCXCR1 and SM-shCXCR2 cells showed the presence of fewer lamellipodial structures as compared to control cells. This suggests that down-regulation of CXCR1 or CXCR2 reduces actin reorganization, thereby effecting the motility and, in turn, the invasiveness of melanoma cells. The molecular basis of CXCR1 or CXCR2 induced changes in actin organization is yet to be investigated.
Another important finding of this study was the enhanced apoptosis of SM-shCXCR1 and SM-shCXCR2 cells. An apoptotic property of these cells was observed due to the decrease in the number of proliferating cells both in vitro and in tumor tissues. Additionally, number of TUNEL positive cells in tumors from mice injected with SM-shCXCR1 or SM-shCXCR2 cells was higher as compared to SM-control injected mice. Our results are consistent with the recent report where CXCL-8 mediated chemotaxis of melanoma cells is shown to be mediated mainly through CXCR1 (41). However, our in vitro study did not show any significant difference in CXCL-8 mediated apoptosis, motility and invasion between CXCR1 and/or CXCR2 down-regulated melanoma cells, suggesting the role of both the receptors.
ERK1/2 phosphorylation is an important signaling pathway involved in the control of growth signals, cell survival and invasion (42). Our recent (unpublished) observation along with other studies supports the idea that stimulation of CXCR1 and CXCR2 leads to the activation of MAP kinase (43). Therefore, from our study it is evident that signaling mediated by the CXCR1 or CXCR2 can lead to survival and proliferation of cells.
Angiogenesis is another essential step for tumor growth and metastasis and expression of CXCR1, CXCR2 and VEGF can provide a positive feedback loop (44). Our immunohistochemistry results support that primary tumor vasculature contributed to in vivo differences between SM-shCXCR1, SM-shCXCR2 and control tumors. These results are in agreement with another report where abrogation of CXCL-8-CXCR2 within the tumor markedly inhibited tumor-associated angiogenesis and growth (45). Similarly, attenuation of CXCR2 activity has been shown to inhibit angiogenesis and tumor growth in nude mice (46). In the murine system, CXCL-8 does not exist. The most likely functional murine homologue to human CXCL-8 is murine CXCL1 (KC/Gro1) and CXCL2 (MIP-2/Gro2) (4;47). It has been shown that these murine homologues contribute to tumor growth, angiogenesis and progression (4;47;48).
The role of CXCR2-ligands and CXCR2-dependent signaling during tumor progression is complex. A recent report demonstrates that CXCR2 signaling plays distinct role during early tumorigenesis and late tumor progression (49). These findings suggest that CXCL-8 and CXCL-1 signaling through CXCR2 might limit tumor growth by reinforcing senescence early in tumorigenesis (50). During tumorigenesis and progression CXCR2 and its ligand CXCL-8 and CXCL1 are aberrantly expressed and CXCR2-dependent signaling tipped towards supporting phenotypes associated with progression and invasion (4;50). Our present data demonstrates that loss of CXCR2 in malignant melanoma cells inhibits cell proliferation, survival and invasiveness.
In conclusion, our data provide direct evidence for the role of CXCR1 and CXCR2 in melanoma progression. Knock-down of CXCR1 or CXCR2 modulates cellular phenotypes associated with melanoma tumor growth and angiogenesis, thus indicating CXCR1 and CXCR2 as future therapeutic interventions.
Acknowledgments
This work was supported in part by grants CA72781 (R.K.S.), and Cancer Center Support Grant (P30CA036727) from National Cancer Institute, National Institutes of Health and Nebraska Research Initiative Cancer Glycobiology Program (R.K.S.). We thank Dr. Ajay P Singh, University of Nebraska Medical Center, NE; for careful reading of this manuscript.
Footnotes
Significance: CXCL-8 and its two high affinity receptors, CXCR1 and CXCR2 are important players in cancer progression and metastasis. However, the precise functional role of these receptors in melanoma growth, angiogenesis and invasion remains unclear. Our data provide direct evidence for the role of CXCR1 and CXCR2 in melanoma progression. Knock-down of CXCR1 and/or CXCR2 modulates cellular phenotypes associated with melanoma tumor growth and angiogenesis, thus indicating CXCR1 and CXCR2 as putative targets for future therapeutic interventions.
Reference List
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008 Mar;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Homey B, Muller A, Zlotnik A. Chemokines: agents for the immunotherapy of cancer? Nat Rev Immunol. 2002 Mar;2(3):175–84. doi: 10.1038/nri748. [DOI] [PubMed] [Google Scholar]
- 3.Murphy C, McGurk M, Pettigrew J, Santinelli A, Mazzucchelli R, Johnston PG, Montironi R, Waugh DJ. Nonapical and cytoplasmic expression of interleukin-8, CXCR1, and CXCR2 correlates with cell proliferation and microvessel density in prostate cancer. Clin Cancer Res. 2005 Jun 1;11(11):4117–27. doi: 10.1158/1078-0432.CCR-04-1518. [DOI] [PubMed] [Google Scholar]
- 4.Singh S, Sadanandam A, Singh RK. Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis Rev. 2007 Dec;26(3-4):453–67. doi: 10.1007/s10555-007-9068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang JM, Deng X, Gong W, Su S. Chemokines and their role in tumor growth and metastasis. J Immunol Methods. 1998 Nov 1;220(1-2):1–17. doi: 10.1016/s0022-1759(98)00128-8. [DOI] [PubMed] [Google Scholar]
- 6.Richmond A, Fan GH, Dhawan P, Yang J. How do chemokine/chemokine receptor activations affect tumorigenesis? Novartis Found Symp. 2004;256:74–89. discussion 89-91, 106-11, 266-9.:74-89. [PubMed] [Google Scholar]
- 7.Rollins BJ. If it's Tuesday, this must be a Belgian chemokine. Blood. 2001 Apr 15;97(8):2193. doi: 10.1182/blood.v97.8.2193. [DOI] [PubMed] [Google Scholar]
- 8.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. 217-42. [DOI] [PubMed] [Google Scholar]
- 9.Li A, Dubey S, Varney ML, Singh RK. Interleukin-8-induced proliferation, survival, and MMP production in CXCR1 and CXCR2 expressing human umbilical vein endothelial cells. Microvasc Res. 2002 Nov;64(3):476–81. doi: 10.1006/mvre.2002.2442. [DOI] [PubMed] [Google Scholar]
- 10.Singh RK, Varney ML. IL-8 expression in malignant melanoma: implications in growth and metastasis. Histol Histopathol. 2000 Jul;15(3):843–9. doi: 10.14670/HH-15.843. [DOI] [PubMed] [Google Scholar]
- 11.Matsushima K, Morishita K, Yoshimura T, Lavu S, Kobayashi Y, Lew W, Appella E, Kung HF, Leonard EJ, Oppenheim JJ. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–93. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsushima K, Oppenheim JJ. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989 Nov;1(1):2–13. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- 13.Bar-Eli M. Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology. 1999;67(1):12–8. doi: 10.1159/000028045. [DOI] [PubMed] [Google Scholar]
- 14.Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol. 1993 Sep 1;151(5):2667–75. [PubMed] [Google Scholar]
- 15.Miyamoto M, Shimizu Y, Okada K, Kashii Y, Higuchi K, Watanabe A. Effect of interleukin-8 on production of tumor-associated substances and autocrine growth of human liver and pancreatic cancer cells. Cancer Immunol Immunother. 1998 Sep;47(1):47–57. doi: 10.1007/s002620050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang JM, Taraboletti G, Matsushima K, Van DJ, Mantovani A. Induction of haptotactic migration of melanoma cells by neutrophil activating protein/interleukin-8. Biochem Biophys Res Commun. 1990 May 31;169(1):165–70. doi: 10.1016/0006-291x(90)91449-3. [DOI] [PubMed] [Google Scholar]
- 17.Youngs SJ, Ali SA, Taub DD, Rees RC. Chemokines induce migrational responses in human breast carcinoma cell lines. Int J Cancer. 1997 Apr 10;71(2):257–66. doi: 10.1002/(sici)1097-0215(19970410)71:2<257::aid-ijc22>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 18.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992 Dec 11;258(5089):1798–801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 19.Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991 Sep 13;253(5025):1280–3. doi: 10.1126/science.1891716. [DOI] [PubMed] [Google Scholar]
- 20.Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991 Sep 13;253(5025):1278–80. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- 21.Oppenheim JJ, Zachariae CO, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol. 1991;9:617–48. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 22.Miller MD, Krangel MS. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12(1-2):17–46. [PubMed] [Google Scholar]
- 23.Jones SA, Moser B, Thelen M. A comparison of post-receptor signal transduction events in Jurkat cells transfected with either IL-8R1 or IL-8R2. Chemokine mediated activation of p42/p44 MAP-kinase (ERK-2) FEBS Lett. 1995 May 8;364(2):211–4. doi: 10.1016/0014-5793(95)00397-r. [DOI] [PubMed] [Google Scholar]
- 24.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003 Mar 15;170(6):3369–76. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 25.Varney ML, Li A, Dave BJ, Bucana CD, Johansson SL, Singh RK. Expression of CXCR1 and CXCR2 receptors in malignant melanoma with different metastatic potential and their role in interleukin-8 (CXCL-8)-mediated modulation of metastatic phenotype. Clin Exp Metastasis. 2003;20(8):723–31. doi: 10.1023/b:clin.0000006814.48627.bd. [DOI] [PubMed] [Google Scholar]
- 26.Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005 Apr;7(2):122–33. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kline M, Donovan K, Wellik L, Lust C, Jin W, Moon-Tasson L, Xiong Y, Witzig TE, Kumar S, Rajkumar SV, Lust JA. Cytokine and chemokine profiles in multiple myeloma; significance of stromal interaction and correlation of IL-8 production with disease progression. Leuk Res. 2007 May;31(5):591–8. doi: 10.1016/j.leukres.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Norgauer J, Metzner B, Schraufstatter I. Expression and growth-promoting function of the IL-8 receptor beta in human melanoma cells. J Immunol. 1996 Feb 1;156(3):1132–7. [PubMed] [Google Scholar]
- 29.Wang JM, Chertov O, Proost P, Li JJ, Menton P, Xu L, Sozzani S, Mantovani A, Gong W, Schirrmacher V, Van DJ, Oppenheim JJ. Purification and identification of chemokines potentially involved in kidney-specific metastasis by a murine lymphoma variant: induction of migration and NFkappaB activation. Int J Cancer. 1998 Mar 16;75(6):900–7. doi: 10.1002/(sici)1097-0215(19980316)75:6<900::aid-ijc13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Varney ML, Johansson SL, Singh RK. Distinct expression of CXCL8 and its receptors CXCR1 and CXCR2 and their association with vessel density and aggressiveness in malignant melanoma. Am J Clin Pathol. 2006 Feb;125(2):209–16. doi: 10.1309/VPL5-R3JR-7F1D-6V03. [DOI] [PubMed] [Google Scholar]
- 31.Li A, Varney ML, Singh RK. Expression of interleukin 8 and its receptors in human colon carcinoma cells with different metastatic potentials. Clin Cancer Res. 2001 Oct;7(10):3298–304. [PubMed] [Google Scholar]
- 32.Singh RK, Varney ML. Regulation of interleukin 8 expression in human malignant melanoma cells. Cancer Res. 1998 Apr 1;58(7):1532–7. [PubMed] [Google Scholar]
- 33.Hue J, Kim A, Song H, Choi I, Park H, Kim T, Lee WJ, Kang H, Cho D. IL-18 enhances SCF production of melanoma cells by regulating ROI and p38 MAPK activity. Immunol Lett. 2005 Jan 31;96(2):211–7. doi: 10.1016/j.imlet.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Rollins BJ. Chemokines. Blood. 1997 Aug 1;90(3):909–28. [PubMed] [Google Scholar]
- 35.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004 Jul;4(7):540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 36.Singh RK, Gutman M, Radinsky R, Bucana CD, Fidler IJ. Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res. 1994 Jun 15;54(12):3242–7. [PubMed] [Google Scholar]
- 37.Huang S, Mills L, Mian B, Tellez C, McCarty M, Yang XD, Gudas JM, Bar-Eli M. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol. 2002 Jul;161(1):125–34. doi: 10.1016/S0002-9440(10)64164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh S, Sadanandam A, Nannuru KC, Varney ML, Mayer-Ezell R, Bond R, Singh RK. Small-Molecule Antagonists for CXCR2 and CXCR1 Inhibit Human Melanoma Growth by Decreasing Tumor Cell Proliferation, Survival, and Angiogenesis. Clinical Cancer Research. 2009 Apr 1;15(7):2380–6. doi: 10.1158/1078-0432.CCR-08-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamazaki D, Kurisu S, Takenawa T. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 2005 Jul;96(7):379–86. doi: 10.1111/j.1349-7006.2005.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schraufstatter IU, Chung J, Burger M. IL-8 activates endothelial cell CXCR1 and CXCR2 through Rho and Rac signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2001 Jun;280(6):L1094–L1103. doi: 10.1152/ajplung.2001.280.6.L1094. [DOI] [PubMed] [Google Scholar]
- 41.Ramjeesingh R, Leung R, Siu CH. Interleukin-8 secreted by endothelial cells induces chemotaxis of melanoma cells through the chemokine receptor CXCR1. FASEB J. 2003 Jul;17(10):1292–4. doi: 10.1096/fj.02-0560fje. [DOI] [PubMed] [Google Scholar]
- 42.Smalley KS. A pivotal role for ERK in the oncogenic behaviour of malignant melanoma? Int J Cancer. 2003 May 1;104(5):527–32. doi: 10.1002/ijc.10978. [DOI] [PubMed] [Google Scholar]
- 43.Venkatakrishnan G, Salgia R, Groopman JE. Chemokine receptors CXCR-1/2 activate mitogen-activated protein kinase via the epidermal growth factor receptor in ovarian cancer cells. J Biol Chem. 2000 Mar 10;275(10):6868–75. doi: 10.1074/jbc.275.10.6868. [DOI] [PubMed] [Google Scholar]
- 44.Folkman J. Angiogenesis and apoptosis. Semin Cancer Biol. 2003 Apr;13(2):159–67. doi: 10.1016/s1044-579x(02)00133-5. [DOI] [PubMed] [Google Scholar]
- 45.Mestas J, Burdick MD, Reckamp K, Pantuck A, Figlin RA, Strieter RM. The role of CXCR2/CXCR2 ligand biological axis in renal cell carcinoma. J Immunol. 2005 Oct 15;175(8):5351–7. doi: 10.4049/jimmunol.175.8.5351. [DOI] [PubMed] [Google Scholar]
- 46.Keane MP, Belperio JA, Xue YY, Burdick MD, Strieter RM. Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. J Immunol. 2004 Mar 1;172(5):2853–60. doi: 10.4049/jimmunol.172.5.2853. [DOI] [PubMed] [Google Scholar]
- 47.Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002 Jun;118(6):915–22. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]
- 48.Son DS, Parl AK, Rice VM, Khabele D. Keratinocyte chemoattractant (KC)/human growth-regulated oncogene (GRO) chemokines and pro-inflammatory chemokine networks in mouse and human ovarian epithelial cancer cells. Cancer Biol Ther. 2007 Aug;6(8):1302–12. doi: 10.4161/cbt.6.8.4506. [DOI] [PubMed] [Google Scholar]
- 49.Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, Takatsu Y, Melamed J, et al. Chemokine Signaling via the CXCR2 Receptor Reinforces Senescence. Cell. 2008 Jun 13;133(6):1006–18. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 50.Acosta JC, Gil J. A Role for CXCR2 in Senescence, but What about in Cancer? Cancer Res. 2009 Mar 15;69(6):2167–70. doi: 10.1158/0008-5472.CAN-08-3772. [DOI] [PubMed] [Google Scholar]