Abstract
Background
Positron-emission tomography (PET) imaging using [18F]fluorodeoxyglucose (FDG) is useful for detection, staging, and monitoring a variety of malignancies, including lymphoma, in adults, but its utility in sarcomas, especially soft tissue sarcomas (STS), in children and young adults is not clear.
Procedure
To evaluate the potential utility of FDG PET in the care of STS in children and young adults, we analyzed 46 PET scans in 25 patients acquired over 12 years. Scans were interpreted by two imaging physicians blinded to findings from other imaging studies and clinical information. Results were compared with computed tomography and magnetic resonance imaging, biopsy results, where available, and clinical follow-up of at least 12 months.
Results
For a total of 46 scans in 25 patients, there were 25 true positive scans, 3 false positive scans, 12 true negative scans, and 6 false negative scans. The sensitivity of the PET scan was 86%, specificity was 80%, positive predictive value was 89%, and negative predictive value was 67%.
Conclusion
FDG PET may be a useful imaging modality in the management of children and young adults with STS, although prospective studies are needed to establish its true utility.
Keywords: fluorodeoxyglucose, FDG, PET, Ewing, rhabdomyosarcoma, pediatric
INTRODUCTION
The Ewing sarcoma family of tumors (ESFT) is derived from cells of neural crest origin, probably postganglionic parasympathetic primordial cells, based on their ability to synthesize acetylcholine transferase [1]. The incidence is about 3 per million children younger than 15 years, and these tumors account for about 2% of all childhood malignancies. Soft tissue sarcomas are derived from mesenchymal cells that normally mature into muscle, fat, fibrous tissue, bone, and cartilage [2]. Rhabdomyosarcomas, derived from cells that normally develop into skeletal muscle are the most common form of sarcoma in children and are the third most common extracranial solid tumor in childhood. The incidence is about 4.5 per million children, and there are about 250 new cases per year in the United States. About two thirds occur in children 6 years old or younger.
Positron-emission tomography (PET) using [18F]fluorodeoxyglucose (FDG) is useful for detection, staging, and monitoring a variety of tumors in adults [3], including melanoma, head and neck tumors, bronchogenic carcinoma, lymphoma, colon cancer, and others. As pediatric malignancies are much less common, the experience applying FDG PET in the management of childhood cancers is limited [4–8]. The purpose of this study was to review our 12-year experience using this technique in children and young adults with sarcomas to determine the uptake of FDG in childhood sarcomas and the utility of FDG PET imaging in the staging and restaging of children and young adults with sarcomas.
METHODS
FDG PET scans in 25 patients (46 scans) acquired over 12 years were analyzed retrospectively. Images were analyzed by two reviewers independently without knowledge of clinical details or results of other imaging studies. Final clinical diagnosis was based on biopsy, follow-up imaging, or clinical course. After a transmission scan for attenuation correction, images were acquired beginning 50 minutes after intravenous injection of 7–17 mCi FDG/1.7 m2 of body surface area, depending on the emission acquisition protocol in use at the time of the study. Images were obtained on a Siemens CTI 931, Siemens Exact, or Siemens HR+ PET scanner. Two-dimensional images were acquired, reconstructed by filtered back projection in earlier studies and ordered segmentation in later studies. Sites of abnormal uptake were considered as focal accumulations greater than surrounding background not explained by normal organ uptake. Only SUV max is reported since that value is the most reproducible among the SUV options. SUVs could not be determined on tumors that did not contain elevated concentrations of FDG nor on scans stored on computer systems that are no longer operational.
Patients
There were 9 males and 16 females aged 2–24 years at the time of diagnosis, with a median age of 10 years. Sixteen patients (64%) had ESFT, including 11 with Ewing sarcoma (3 of whom were mentioned in a previous report (4)) and 5 with primitive neuroectodermal tumor. Nine patients (36%) had rhabdomyosarcoma (5 embryonal, 4 alveolar). Nine patients were studied at initial diagnostic staging (staging, 9 scans); 20 patients were studied as part of subsequent restaging (16 patients, 37 scans) which includes scans while still on treatment (11 patients, 23 scans), scans after the end of therapy (7 patients, 13 scans) or scans at the time of relapse (1 patient, 1 scan).
Fifteen patients had 1 scan performed, 5 patients 2 scans, 3 patients 3 scans, 1 patient 4 scans, and 1 patient 8 scans. Follow-up time ranged from 4 to 186 months, with a median follow-up time of 24 months. Scans were obtained between April 1991 and October 2002. Follow up continued until March 2008. Initially, patients were examined at diagnosis to establish the baseline metabolic activity. Subsequently, patients who underwent PET scanning were selected based on clinical indications as determined by the patient’s oncologist.
Sensitivity, specificity, positive predictive value, and negative predictive value were calculated for PET performance based on results of other scans, clinical follow-up, and biopsy results if available. For this analysis, patients had to be at least 12 months from initial diagnosis with concurrent studies that were performed within 1 month of the FDG PET study. Two set of scans were excluded from analyses for not being within 1 month of FDG PT scans.
RESULTS
Initial Staging
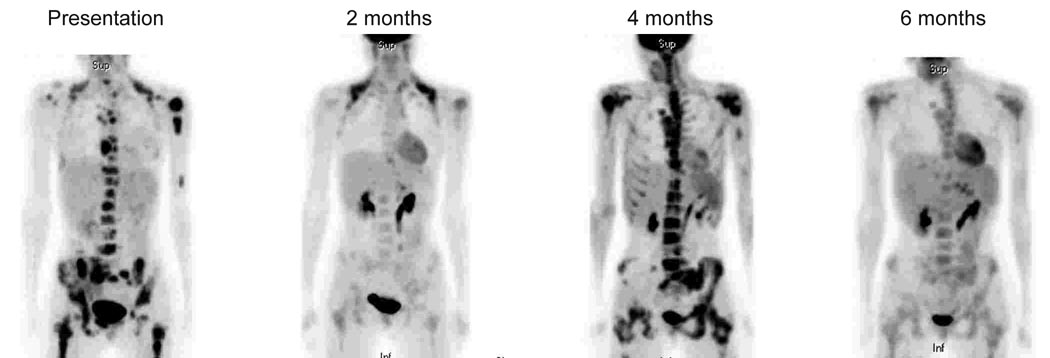
In 9 of 9 patients (7 EWS, 1 PNET and 1 ERMS), FDG PET imaging identified the primary tumor, and in 8 of those, it revealed all known metastases (Table I; Figure 1, Figure 2). In a single patient with EWS, 3-mm lung nodules identified on computed tomography (CT) scanning were not evident on PET scanning, probably because they were too small for the technically capability of this scanning modality (Table I, Patient 2). These nodules resolved after 2 cycles of chemotherapy. No false positive lesions were detected in any patient scans.
Table I.
Patient Summary
| A) Initial Staging: | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient # |
Age in years |
Sex | Dx | Indication | # of scans |
PET blinded | Primary max SUVs |
F/U (months) |
Outcome | Brief performance |
Comments |
| 1 | 7 | F | EWS | Staging | 1 | Positive uptake @ PTS |
3.0 | 186 | CR | TP | NED |
| 2 | 7 | M | EWS | Staging | 1 | Positive uptake @ PTS |
3.5 | 156 | CR | TP | Lack of uptake in multiple 3- mm bilateral lung nodules that resolved on CT, NED |
| 3 | 14 | F | EWS | Staging | 2 | Positive uptake @ PTS |
NA* | 132 | CR | TP | NED |
| 4 | 15 | M | EWS | Staging | 1 | Positive uptake @ PTS |
5.0 | 88 | CR | TP | NED |
| 5 | 8 | F | EWS | Staging | 1 | Positive uptake @ PTS |
8.7 | 60 | CR | TP | NED |
|
B) Restaging: I) Scans During Treatment: | |||||||||||
| 6 | 15 | M | EWS | During treatment |
1 | Positive uptake @ PTS |
2.7 | 15 | DOD | TP | PET remained positive until death |
| 7 | 16 | M | EWS | During treatment |
1 | No FDG-avid disease detected |
NA | 7 | DOD | FN | Known extensive bone metastases not detected by PET |
| 8 | 9 | F | ARMS | During treatment |
2 | Positive uptake @ PTS |
3.3 | 12 | DOD | TP | PET persistently positive, died of PD. |
| 9 | 24 | F | PNET | During treatment |
1 | Positive uptake @ PTS |
3.0 | 13 | NA | TP | NED 1 year after PET |
| 10 | 20 | F | PNET | During treatment |
1 | No FDG-avid disease detected |
NA | 88 | CR | TN | Relapsed twice NED. |
| 11 | 2 | F | PNET | During treatment |
1 | No FDG-avid disease detected |
0.7 | 136 | CR | TN | NED |
| 12 | 4 | F | ERMS | During treatment |
3 | Positive uptake @ PTS & MTS |
1.7 | 3 | DOD | TP | Died of PD |
| 13 | 15 | F | EWS | During treatment |
1 | NO FDG-avid disease detected |
1.9 | 84 | AWD | TN | Late relapse in pelvis and lungs after 12 years, undergoing therapy |
| II) Scans after End of Treatment: | |||||||||||
| 14 | 18 | F | ARMS | After treatment |
1 | Positive uptake @ PTS & MTS |
NA | 4 | DOD | TP | PET detected unexpected lung mets biopsy-proven, died of PD on chemo |
| 15 | 7 | M | ARMS | After treatment |
1 | No FDG-avid disease detected |
NA | 22 | DOD | FN | Died of PD |
| 16 | 11 | F | ERMS | After treatment |
1 | No FDG-avid disease detected |
1.0 | 142 | CR | TN | NED |
| 17 | 9 | M | EWS | After treatment |
1 | Faint positive uptake @ PTS |
1.1 | 72 | CR | FP | Biopsy showed Pseudomonas infection; NED |
| 18 | 4 | M | ERMS | After treatment |
1 | No FDG-avid disease detected |
0.8 | 80 | CR | TN | Long-term survivor, last FU January 2008, NED |
| III) Scans at Relapse: | |||||||||||
| 19 | 9 | F | ARMS | At relapse | 2 | Positive uptake @ MTS |
1.7 | 9 | DOD | TP | Died of PD |
| C) Scans performed at more than one timepoint: | |||||||||||
| 20 | 11 | M | EWS | Staging and after treatment |
2 | Positive uptake @ PTS |
2.7 | 132 | CR | TP / TN | NED |
| 21 | 11 | F | EWS | Staging and during treatment |
3 | Positive uptake @ PTS |
5.8 | 12 | DOD | TP | Died of rapid PD |
| 22 | 17 | F | PNET | Staging and during treatment |
2 | Positive uptake @ PTS & MTS |
NA* | 10 | DOD | TP / FN | Initial scan showed uptake, subsequent scans no uptake but pt died of PD |
| 23 | 2 | F | PNET | During and after treatment |
8 | Positive uptake in left inguinal nodes |
1.2 | 82 | CR | TN / FP | Uptake in left inguinal nodes but pt in clinical CR for 5 years, late relapse locally in left femur |
| 24 | 3 | M | ERMS | During and after treatment |
3 | No FDG-avid disease detected |
3.2 | 27 | DOD | TP/ FN | Initial scan showed uptake, subsequent scans no uptake but pt died of progressive disease |
| 25 | 15 | F | ERMS | Staging and during treatment |
4 | Positive uptake @ PTS & MTS |
NA* | 6 | DOD | TP | Died of PD |
| Total = 46 | |||||||||||
| F = Female | AWD = Alive with disease | TP = True positive | |||||||||
| M = Male | EWS = Ewing Sarcoma |
DOD = Died of disease | TN = True negative | ||||||||
| PNET = Primitive neuroectodermal tumor |
CR = Complete remission | FP = False positive | |||||||||
| ARMS = Alveolar rhabdomyosarcoma |
PD = Progressive disease | FN = False negative | |||||||||
| ERMS = Embryonal rhabdomyosarcoma |
NA = Not available | ||||||||||
| NED = No evidence of disease |
|||||||||||
| PTS = Primary tumor site | SUV = Standard uptake value | ||||||||||
| MTS = Metastatic tumor site | pt = Patient | ||||||||||
| FU = Follow-up | |||||||||||
Figure 1.
Figure 2.
Restaging (During Treatment, After End of Therapy or At Relapse)
These include scans performed while still on treatment or after the end of therapy or at the time of relapse (Figure 2, Figure 3, Figure 4). When patients had scans done during Initial staging and Restaging it is listed as Initial staging and Restaging. There were 37 scans acquired in 20 patients. Table I describes the findings by patient and scan numbers.
Figure 3.
Figure 4.
Positive scans
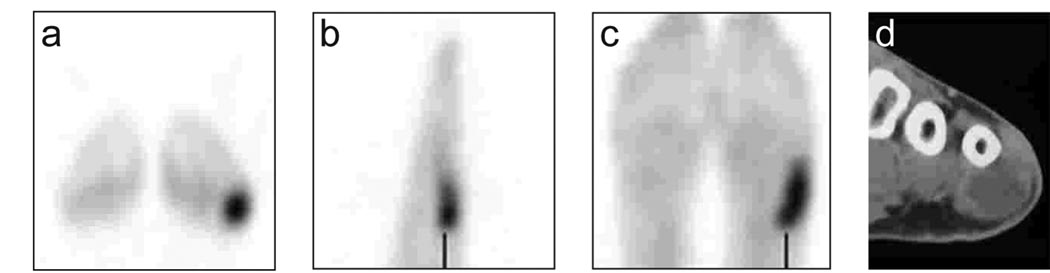
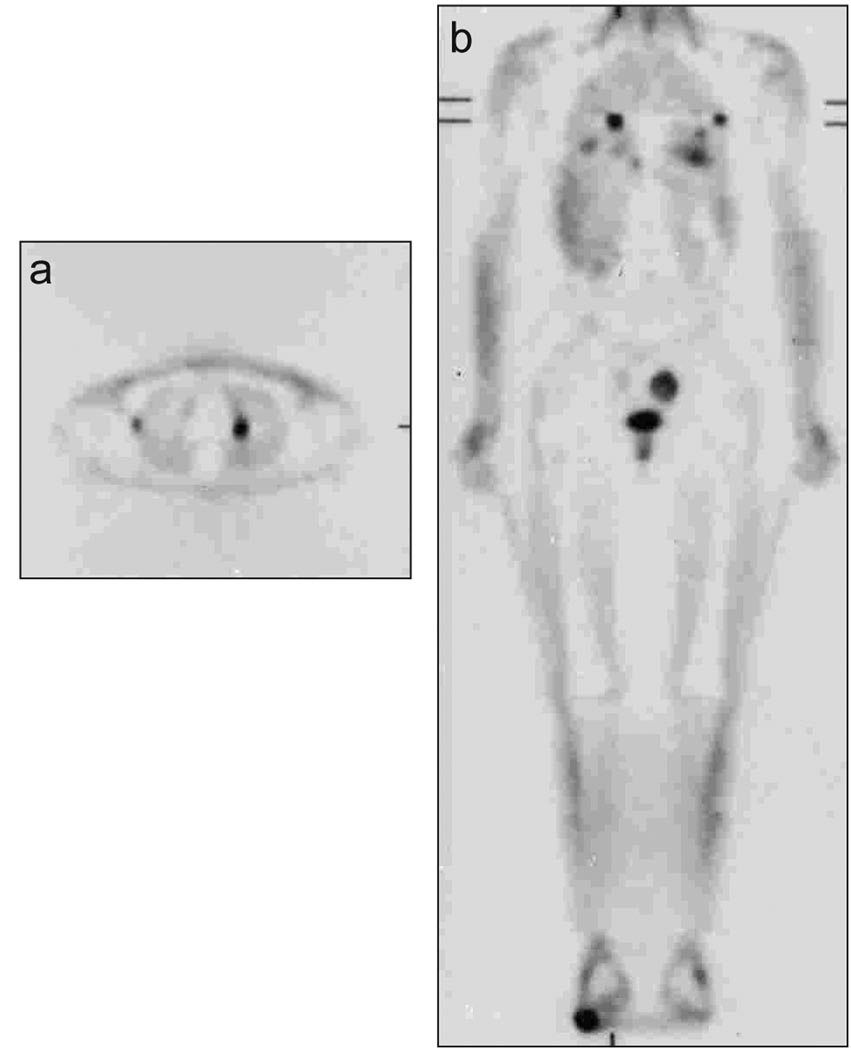
There were 20 positive scans in 10 patients (3 EWS, 2 PNET, 3 ARMS and 2 ERMS). In five patients with EWS/PNET 8 positive scans were recorded, while in five patients with ARMS/ERMS 12 positive scans were recorded, on 3 scans (2 patients), false positive findings were detected. The first patient had a 12-mm nodule in the right lower lobe that was proven on biopsy to be pseudomonas infection. This patient with EWS, remained disease-free 6 years after imaging and pathologic evaluation (Table I, Patient 17). The second patient with PNET had two false positive scans. The scan detected uptake in left inguinal lymph nodes 15 months after completion of therapy (Table I, Patient 23) and remained consecutively positive for 15 months before turning negative. The patient remained in complete clinical remission for 5 years without any symptoms, but subsequently had relapse in the left proximal femur with no inguinal node involvement on the left side. The patient was treated with chemotherapy and surgery and remained in complete remission 27 months after salvage therapy.
Negative scans
There were 17 negative scans in 10 patients (3 EWS, 3 PNET, 3 EMS and 1 ARMS). In six patients with EWS/PNET 12 negative scans were recorded, while in four patients with ARMS/ERMS 5 negative scans were recorded.
False negative scans
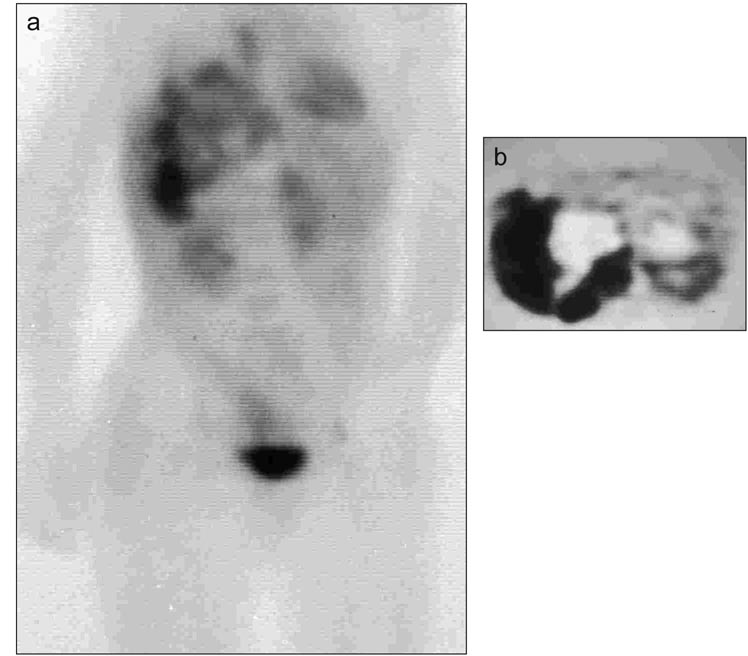
Seven scans in 4 patients were classified as false negative. One patient with EWS showed progression of disease in the femur 3 months after therapy despite lack of FDG uptake (Table I, Patient 7). In the second patient with ERMS, bladder activity interfered with evaluation of a pelvic primary tumor (Table I, Patient 24). In a third patient with PNET, 2-cm inguinal lymph nodes, subsequently excised, did not accumulate FDG (Table I, Patient 22), and in a fourth patient with ARMS, progressive disease in the pelvis did not accumulate FDG (Table I, Patient 15).
True negative scans
Seven patients had 12 true negative scans (2 EWS, 3 PNET and 2 ERMS). In five patients with EWS/PNET 10 true negative scans were recorded, while in two patients with ERMS 2 true negative scans were recorded. These patients remained in CR for 80 to 142 months. One of these patients remained in remission for 84 months after PET scan before relapse in the lungs. This patient with EWS was alive with stable disease 2 months after imaging evaluation (Table I, Patient 13).
Overall Performance of FDG PET for all indications
To evaluate FDG PET performance in pediatric and young adult patients with sarcoma, we compared scanning results to CT or biopsy as available. Off 46 scans in 25 patients, there were 25 true positive scans, 3 false positive scans, 12 true negative scans, and 6 false negative scans. The sensitivity of the PET scan was 86%, specificity 80%, positive predictive value 89%, and negative predictive value 67%.
DISCUSSION
Our findings indicate that FDG PET is a useful imaging modality for the diagnostic evaluation of primary and metastatic sarcomas. In addition, the study provided important diagnostic information that guided the management of individual patients as presented in this report. Importantly, this imaging modality provided important utility for both diagnosis and ongoing tumor monitoring.
In a previous report of FDG PET scanning for the imaging of a variety of pediatric tumors at the time of presentation [4], we suggested a potential role for FDG PET, but conclusions were tempered by patient numbers and the preliminary nature of the findings. This original report included 7 patients with sarcomas: 3 with Ewing sarcoma, and one each with rhabdomyosarcoma, osteosarcoma, hepatic sarcoma, and angiomyosarcoma. The scans indicated that most of the pediatric tumors we studied were quite active metabolically and the information obtained was clinically useful. As a result, we expanded the study, focusing on the more common ESFT and rhabdomyosarcoma. However, not all patients treated at our institution during the early time frame of this study underwent PET scanning due to limited research funds, issues with insurance reimbursement, and scanner availability. Because of this, our focus changed to imaging patients for whom we could subsequently determine the validity of the PET scan findings by either tissue confirmation or sufficient follow-up and for whom a clinical question was posed. As a result, our study represents more than 11 years’ experience using PET in childhood sarcomas.
These findings complement the work of other investigators [8, 9] who have suggested a role for this imaging modality in other cancer settings. In pediatric patients with sarcomas and other tumors, Wegner et al. [8] found that PET led to altered management in 46/175 pediatric oncology patients, including 4 patients with sarcomas. The limitation of this study was that it was conducted by sending a questionnaire to referring physicians, asking them if they thought PET resulted in a change of management in their patients, even though many of these PET scan results were verified. In a second study by Ben Arush et al. [9], FDG PET was determined to be valuable for the detection of local and regional lymph node involvement in children with alveolar rhabdomyosarcoma, though it was a very small study involving only 3 patients. Finally, Hawkins et al. [10], investigating FDG PET in 36 adult and pediatric patients with ESFT, determined that FDG PET uptake correlated with histologic response and that semiquantitative comparison of pre- and post-treatment scans yielded prognostic information.
Our study has important limitations. During the 12-year course of our study, PET equipment and reconstruction algorithms advanced considerably, especially with regard to hybrid imaging using PET CT [11]. As a result, current technology and imaging quality provide more confident distinction of normal sites of uptake from disease and added sensitivity by assisting in the identification of pathology [6, 7, 12–14]. As an example, McCarville et al. [7] found FDG PET CT useful for locating the primary site of a metastatic rhabdomyosarcoma; for identifying unusual and unsuspected metastatic sites; in monitoring response to chemotherapy, radiation therapy, and radiofrequency ablation; and in evaluating tumor resection sites postoperatively. In a recent review investigating the utility of FDG PET CT in the staging and restaging of 53 patients with Ewing sarcoma, it was determined that 21% more lesions were detected using PET CT than with PET alone, yielding higher sensitivity, similar specificity, and higher accuracy for PET CT [15]. Thus, because of the superiority of hybrid imaging over PET alone, our results may underestimate the value of the technique when accompanied by CT scanning for both attenuation correction and lesion localization. In support of this argument, the study by Gyorke et al. [14] determined that FDG PET was less sensitive than CT for detecting pulmonary metastases in ESFT. Similarly, in patients with soft tissue and bone sarcomas, Iagaru et al. [12, 13] showed that FDG PET CT had high sensitivity for the detection of disease but lagged behind dedicated CT for the detection of pulmonary metastases. In fact, our only false negative finding in the 9 patients studied at diagnosis was the lack of detectable FDG uptake in small pulmonary metastases.
Our patients were studied at various times during clinical course on protocols available at the time of presentation. Our image reviewers did not have access to clinical data, other imaging studies, or the indication for the study. Thus, the PET scan was interpreted without the clinical and correlative radiographic information that is available in routine clinical interpretation sessions; thus, this analysis probably underestimates the incremental value provided. To avoid overestimating the value of FDG PET studies during treatment, several studies were classified as false negative, although convincing proof of disease at the time the study was performed was not present. In one patient, diffuse uptake in a femur mimicked bone marrow recovery, although it ultimately proved to be tumor. Magnetic resonance imaging showed multiple lesions that would have resulted in change in interpretation had that been made available to the interpreting physicians. The FDG PET scan of Patient 9 became negative during treatment, and other imaging studies confirmed the response. No further PET studies were obtained, but the patient did suffer progressive disease 4 months later. Patient 8 was studied for the first time during therapy at a time when other studies indicated a therapeutic response. Unfortunately, a distended bladder precluded adequate evaluation of the pelvis. The studies of Patients 15 and 24 were negative, although the patients suffered progressive disease. A false positive finding of FDG uptake in a lower extremity was shown to represent a benign osseous lesion on correlative imaging. Uptake in benign osseous lesions has subsequently been reported in many children undergoing FDG PET CT for evaluation of malignancies [16].
Especially useful is the long-term follow-up of 7 patients with true negative scans, followed up for as long as 12 years. In those patients, the lack of FDG uptake confirmed the eradication of malignancy.
We did not use the semiquantitative standard uptake value (SUV) for analysis either at the time the images were acquired or at the time of this analysis. In several of our patients, SUV could not be determined, either because metabolically inactive foci of tumor could not be identified on PET alone or because tumor was disseminated in the bone marrow and no distinct focal area of abnormal uptake was evident for analysis. Although not applicable to this series, SUV under carefully controlled conditions may have prognostic information and may be useful for determining response to therapy [5, 10, 17, 18]. Nonetheless, SUVs are influenced by many factors, including fasting state, time from injection to imaging, method of reconstruction, and partial volume issues, all of which require standardization for meaningful interpretation [19].
Other less commonly used tracers may also be valuable for investigating the pathophysiology of pediatric sarcomas. These include the cellular proliferation marker FLT, hypoxia markers such as F-MISO, and amino acids including tyrosine [20–23]. Because of the neuroendocrine origin of EFST and their ability to synthesize choline acetyltransferase [1], tracers of the cholinergic nervous system could be valuable [24].
In summary, we have found FDG PET useful in the staging and management of EFST and rhabdomyosarcomas in children. Further studies are currently under way to systematically evaluate the utility of the technique in greater numbers of children and being treated on similar or identical protocols. We anticipate that advances in technology and refinement of FDG PET and FDG PET CT techniques will further aid in the management of children and young adults with ESFT and rhabdomyosarcomas.
ACKNOWLEDGMENTS
Supported in part by NCI CA 54216 (BLS), MO1-RR00042 (Clinical Research Center of the University of Michigan), and by the American Lebanese Syrian Associated Charities (ALSAC). The authors appreciate the secretarial expertise of Sandra Gaither in the preparation of this manuscript. The authors are grateful to David Galloway for scientific editing.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Bernstein M, Kovar H, Paulussen M, et al. Ewing Sarcoma Family of Tumors: Ewing Sarcoma of Bone and Soft Tissue and the Peripheral Primitive Neuroectodermal Tumors. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. Lippincott Williams and Wilkins; 2006. pp. 1003–1032. [Google Scholar]
- 2.Wexler LH, Meyer WH, Helman LJ. Rhabdomyosarcoma and the undifferentiated sarcomas. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. Lippincott Williams and Wilkins; 2006. [Google Scholar]
- 3.Hustinx R, Benard F, Alavi A. Whole-body FDG-PET imaging in the management of patients with cancer. Semin Nucl Med. 2002;32:35–46. doi: 10.1053/snuc.2002.29272. [DOI] [PubMed] [Google Scholar]
- 4.Shulkin BL, Mitchell DS, Ungar DR, et al. Neoplasms in a pediatric population: 2-[F-18]- fluoro-2-deoxy-D-glucose PET studies. Radiology. 1995;194:495–500. doi: 10.1148/radiology.194.2.7824731. [DOI] [PubMed] [Google Scholar]
- 5.Hawkins DS, Rajendran JG, Conrad EU, III, et al. Evaluation of chemotherapy response in pediatric bone sarcomas by [F-18]-fluorodeoxy-D-glucose positron emission tomography. Cancer. 2002;94:3277–3284. doi: 10.1002/cncr.10599. [DOI] [PubMed] [Google Scholar]
- 6.Franzius C, Juergens KU, Vormoor J. PET/CT with diagnostic CT in the evaluation of childhood sarcoma. AJR Am J Roentgenol. 2006;186:581–582. doi: 10.2214/AJR.06.5010. [DOI] [PubMed] [Google Scholar]
- 7.McCarville MB, Christie R, Daw NC, et al. PET/CT in the evaluation of childhood sarcomas. AJR Am J Roentgenol. 2005;184:1293–1304. doi: 10.2214/ajr.184.4.01841293. [DOI] [PubMed] [Google Scholar]
- 8.Wegner EA, Barrington SF, Kingston JE, et al. The impact of PET scanning on management of paediatric oncology patients. Eur J Nucl Med Mol Imaging. 2005;32:23–30. doi: 10.1007/s00259-004-1645-3. [DOI] [PubMed] [Google Scholar]
- 9.Ben Arush MW, Bar SR, Postovsky S, et al. Assessing the use of FDG-PET in the detection of regional and metastatic nodes in alveolar rhabdomyosarcoma of extremities. J Pediatr Hematol Oncol. 2006;28:440–445. doi: 10.1097/01.mph.0000212949.12856.02. [DOI] [PubMed] [Google Scholar]
- 10.Hawkins DS, Schuetze SM, Butrynski JE, et al. [18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol. 2005;23:8828–8834. doi: 10.1200/JCO.2005.01.7079. [DOI] [PubMed] [Google Scholar]
- 11.Blodgett TM, Meltzer CC. Townsend DW. PET/CT: form and function. Radiology. 2007;242:360–385. doi: 10.1148/radiol.2422051113. [DOI] [PubMed] [Google Scholar]
- 12.Iagaru A, Quon A, McDougall IR, Gambhir SS. F-18 FDG PET/CT evaluation of osseous and soft tissue sarcomas. Clin Nucl Med. 2006;31:754–760. doi: 10.1097/01.rlu.0000246846.01492.31. [DOI] [PubMed] [Google Scholar]
- 13.Iagaru A, Chawla S, Menendez L, Conti PS. 18F-FDG PET and PET/CT for detection of pulmonary metastases from musculoskeletal sarcomas. Nucl Med Commun. 2006;27:795–802. doi: 10.1097/01.mnm.0000237986.31597.86. [DOI] [PubMed] [Google Scholar]
- 14.Gyorke T, Zajic T, Lange A, et al. Impact of FDG PET for staging of Ewing sarcomas and primitive neuroectodermal tumours. Nucl Med Commun. 2006;27:17–24. doi: 10.1097/01.mnm.0000186608.12895.69. [DOI] [PubMed] [Google Scholar]
- 15.Gerth HU, Juergens KU, Dirksen U, et al. Significant benefit of multimodal imaging: PET/CT compared with PET alone in staging and follow-up of patients with Ewing tumors. J Nucl Med. 2007;48:1932–1939. doi: 10.2967/jnumed.107.045286. [DOI] [PubMed] [Google Scholar]
- 16.Goodin GS, Shulkin BL, Kaufman RA, McCarville MB. PET/CT characterization of fibroosseous defects in children: 18F-FDG uptake can mimic metastatic disease. AJR Am J Roentgenol. 2006;187:1124–1128. doi: 10.2214/AJR.06.0171. [DOI] [PubMed] [Google Scholar]
- 17.Schuetze SM, Rubin BP, Vernon C, et al. Use of positron emission tomography in localized extremity soft tissue sarcoma treated with neoadjuvant chemotherapy. Cancer. 2005;103:339–348. doi: 10.1002/cncr.20769. [DOI] [PubMed] [Google Scholar]
- 18.Eary JF, O'Sullivan F, Powitan Y, et al. Sarcoma tumor FDG uptake measured by PET and patient outcome: a retrospective analysis. Eur J Nucl Med Mol Imaging. 2002;29:1149–1154. doi: 10.1007/s00259-002-0859-5. [DOI] [PubMed] [Google Scholar]
- 19.Keyes JW., Jr SUV: standard uptake or silly useless value? J Nucl Med. 1995;36:1836–1839. [PubMed] [Google Scholar]
- 20.Cobben DC, Elsinga PH, Suurmeijer AJ, et al. Detection and grading of soft tissue sarcomas of the extremities with (18)F-3' -fluoro-3' -deoxy-L-thymidine. Clin Cancer Res. 2004;10:1685–1690. doi: 10.1158/1078-0432.ccr-03-0040. [DOI] [PubMed] [Google Scholar]
- 21.Been LB, Suurmeijer AJ, Elsinga PH, et al. 18F-fluorodeoxythymidine PET for evaluating the response to hyperthermic isolated limb perfusion for locally advanced soft-tissue sarcomas. J Nucl Med. 2007;48:367–372. [PubMed] [Google Scholar]
- 22.Rajendran JG, Wilson DC, Conrad EU, et al. [(18)F]FMISO and [(18)F]FDG PET imaging in soft tissue sarcomas: correlation of hypoxia, metabolism and VEGF expression. Eur J Nucl Med Mol Imaging. 2003;30:695–704. doi: 10.1007/s00259-002-1096-7. [DOI] [PubMed] [Google Scholar]
- 23.Plaat B, Kole A, Mastik M, et al. Protein synthesis rate measured with L-[1–11C]tyrosine positron emission tomography correlates with mitotic activity and MIB-1 antibody-detected proliferation in human soft tissue sarcomas. Eur J Nucl Med. 1999;26:328–332. doi: 10.1007/s002590050394. [DOI] [PubMed] [Google Scholar]
- 24.Kuhl DE, Minoshima S, Fessler JA, et al. In vivo mapping of cholinergic terminals in normal aging, Alzheimer's disease, and Parkinson's disease. Ann Neurol. 1996;40:399–410. doi: 10.1002/ana.410400309. [DOI] [PubMed] [Google Scholar]