Abstract
Purpose
In the classic view of bladder development, the trigone originates from the mesoderm-derived Wolffian ducts while the remainder of the bladder originates from the endoderm-derived urogenital sinus. Recent molecular developmental studies have questioned the veracity of this received wisdom, suggesting an endodermal origin for the trigone. To shed further light on this issue we observed mesenchymalepithelial interactions between trigone epithelium and fetal urogenital sinus mesenchyme (UGM) to infer the germ layer of origin of the trigone.
Materials and methods
Mouse trigone epithelium was recombined with fetal rat UGM in tissue recombinant grafts that were placed beneath the renal capsule of athymic mouse hosts. Grafts were harvested at 4 weeks. Control grafts with bladder dome and ureteral epithelium were also examined. Tissues were evaluated with hematoxylin / eosin and Hoechst dye 33258 (to confirm cell species origin). Immunohistochemistry to androgen receptor, broad spectrum uroplakin, dorsal lateral prostate secretions and seminal vesicle secretions were used to differentiate prostatic and seminal vesicle differentiation.
Results
Grafts of mouse trigone epithelium with fetal rat UGM yielded epithelial tissue which stained for dorsal lateral prostate secretions but not for seminal vesicle secretions. Control grafts of bladder dome epithelium yielded the expected endodermal prostate differentiation. Control grafts of ureteral epithelium yielded the expected mesodermal seminal vesicle differentiation.
Conclusions
The consistent finding of prostatic epithelium in tissue recombinants of trigone epithelium and fetal UGM reinforces the hypothesis that the trigone is derived from the endoderm and not the mesoderm as commonly accepted.
Keywords: urinary bladder, mesoderm, endoderm, embryonic and fetal development
INTRODUCTION
The classic view of bladder trigone development, based upon anatomic observation, proposes that the embryologic origin of the trigone differs from that of the remainder of the bladder urothelium. During early embryonic development, the cloaca divides into an anterior urogenital sinus and a posterior anorectal canal. The paired Wolffian ducts fuse with the cloaca and remain with the urogenital sinus. In the classic view, the trigone forms from the mesoderm-derived Wolffian ducts while the remainder of the bladder forms from the endoderm-derived urogenital sinus. 1 More recent studies2 3 have suggested that the trigonal epithelium is, in fact, endodermal in origin and that the mesodermal urothelial cells of the ureters do not persist in the bladder but rather are removed by apoptosis as the common nephric duct joins the fetal bladder.
Tissue recombination and the study of mesenchymal-epithelial interactions have been widely applied to the developmental biology of the urogenital tract including development of the prostate (reviewed in Cunha et al. 4), urethra5 and genitalia.6, 7 The epithelial germ layer of origin has been found to limit the influence of inductive fetal mesenchyme. For example, under the influence of fetal urogenital sinus mesenchyme (UGM), endoderm-derived adult epithelium from the prostate, bladder, urethra or vagina generates prostatic tissue. In contrast, in the presence of the same inductive influences, mesoderm-derived adult epithelium from the vas deferens, ureter, or seminal vesicle forms seminal vesicle epithelium.8
Our goal was to infer the embryologic origin of the bladder trigone epithelium using tissue recombination methods. Based on previous tissue recombination studies, we hypothesized that if trigone epithelium were derived from mesoderm as proposed in the classic view, fetal UGM would induce adult trigone epithelium to differentiate to seminal vesicle epithelium. Alternatively, if the trigone were of endodermal origin as suggested by more recent studies, the recombinants would form prostatic tissue.
MATERIALS AND METHODS
Tissue recombination grafts with rat urogenital sinus mesenchyme and mouse trigone epithelium
All animal experiments were approved by the Institutional Animal Care and Use Committee at Vanderbilt University. Pregnant Sprague-Dawley rats (Harlan Laboratories, Inc., Indianapolis, IN) were sacrificed with anesthetic overdose followed by cervical dislocation at embryonic day 18 (plug day = 0). The embryos were isolated, and their urogenital sinus tissue was removed. Rat UGM was isolated from urogenital sinus tissue by chemical digestion with 10 mg/ml trypsin 1:250 at 4°C for 90 minutes (Sigma Chemical Co., St. Louis, MO) followed by mechanical separation under a dissecting microscope. UGM was further reduced to a single cell suspension with 187 U/ml collagenase at 37°C for 90 minutes (Gibco-BRL, Grand Island, NY). 9
Trigone epithelium was obtained from adult male and female CD-1 mice (Charles River Laboratories Inc., Wilmington, MA). In order to identify the trigone, saline was infused into each renal pelvis through a 27-gauge needle. The bladder was opened, and ureteral orifices were visualized. A section of posterior bladder wall incorporating both ureteral orifices was removed. The trigone sample included approximately 1mm of bladder tissue superior and lateral to the ureteral orifices and 1 mm of bladder tissue distal to the ureteral orifices. Remaining ureteral tissue was removed from the trigone sample. Trigone epithelium was similarly harvested from pups as young as 5 days old to study epithelium at an earlier stage of development. Additional tissue from the ureters and bladder dome of adult mice were harvested for the control grafts.
Epithelium was isolated from bladder samples by incubation with 20 mM EDTA in calcium and magnesium-free Hank’s solution at room temperature for 60 minutes followed by mechanical separation of the epithelium and stroma under a dissecting microscope.10 Similarly, epithelium was isolated from ureteral samples by incubation with 20mM EDTA for 30 minutes followed by mechanical separation.
Athymic Nude Mouse Host Xenografting
Tissue recombinants were constructed of mouse bladder trigone epithelium and rat UGM. Nineteen trigone recombinant grafts were evaluated for this study. Additional control recombinants were constructed of mouse bladder dome epithelium with rat UGM or mouse ureteral epithelium with rat UGM. Three of each type of control recombinant was evaluated.
The tissue recombinants were grafted beneath the renal capsule of a rodent host as previously described.11 Briefly, using tribromoethanol general anesthesia, tissue recombinant grafts were placed beneath the renal capsule of an adult athymic CD-1 nude mouse. The hosts were sacrificed 4 weeks post grafting by anesthetic overdose followed by cervical dislocation. Whole kidneys with the tissue recombinant grafts were removed and placed in neutral buffered formalin for 24 hours. After fixation, grafts were excised from the kidney, processed and embedded in paraffin. Mouse prostate and seminal vesicles were similarly processed to serve as controls for immunohistochemistry.
Staining and immunohistochemistry
Sections (5 μm) were cut from paraffin embedded samples. Samples were stained with hematoxylin / eosin to demonstrate overall histologic appearance. Hoechst dye 33258 (Sigma-Aldrich, St. Louis, MO) staining was also used as previously described to determine species origin of cells in the tissue recombinant grafts.12 Hoechst dye 33258 sections were evaluated by fluorescence microscopy.
Immunohistochemistry was performed using the Vectastain avidin-biotin complex kit (Vector Laboratories Inc., Burlington, CA) according to the manufacturer’s protocol. Briefly, sections were deparaffinized and hydrated through a graded series of ethanol. Sections were incubated overnight at 4°C in a humidified chamber with primary antibodies to: androgen receptor N-20 (AR) (Santa Cruz Biotechnology, Santa Cruz, CA), broad spectrum uroplakin (gift from Dr. Tung-Tien Sun, New York University), mouse dorsal lateral prostate secretions mDLP (gift from Dr. Gerald Cunha, University of California San Francisco)13 and mouse seminal vesicle secretions (mSV) (gift from Dr. Cory Abate-Shen, Columbia University). After overnight incubation, slides were incubated with biotinylated secondary antibody followed by Vectastain Elite ABC reagent. Visualization of immunoreactivity was achieved by incubating sections with the substrate 3,3′-diaminobenzidine (DAB). Normal mouse prostate and seminal vesicle tissue were processed in parallel and used as controls for immunohistochemistry.
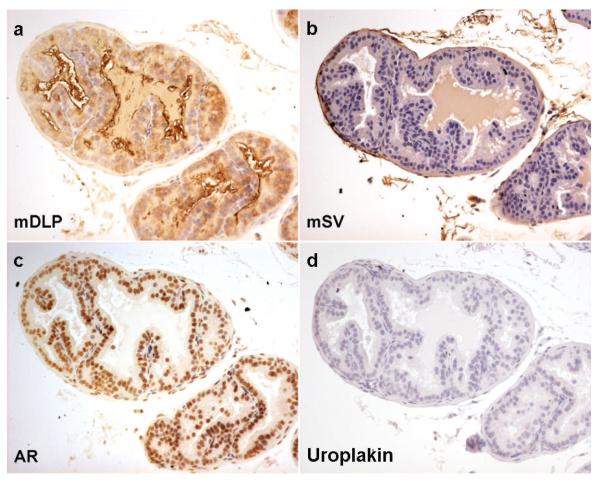
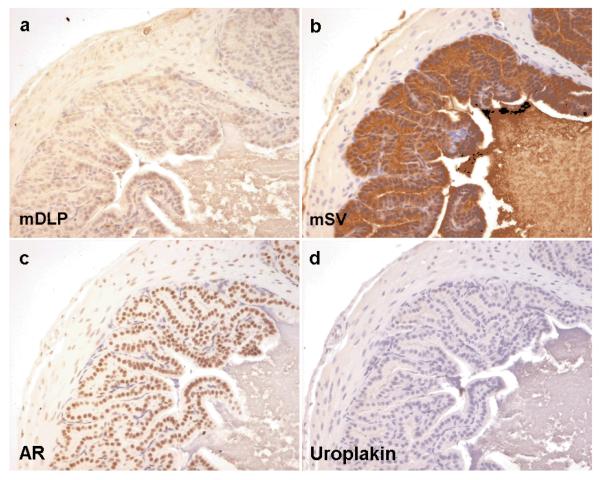
Figures 1 and 2 show the immunohistochemical staining profile for control mouse prostate and seminal vesicle, respectively. Although both tissues stain for androgen receptor, they can be differentiated by their immunoreactivity to mDLP and mSV. Neither tissue type stains positively for the urothelial marker uroplakin.
Figure 1. Expression of markers in mouse dorsolateral prostate (DLP).
Control mouse seminal vesicle immunohistochemistry for dorsal lateral prostate secretions (mDLP), seminal vesicle secretions (mSV), androgen receptor (AR) and uroplakin. As expected mouse DLP shows cytoplasmic brown staining in the luminal epithelial cells with antibodies raised against mDLP secretions (a) but not with those raised against mouse seminal vesicle secretions (b) or uroplakin (d). Nuclear brown staining is seen in both epithelial and some stromal cells when antibodies against AR were used (c)
Figure 2. Expression of markers in mouse seminal vesicle.
Control mouse seminal vesicle immunohistochemistry for dorsal lateral prostate secretions (mDLP), seminal vesicle secretions (mSV), androgen receptor (AR) and uroplakin. As expected mouse seminal vesicle shows cytoplasmic brown staining in the luminal epithelial cells with antibodies raised against mSV secretions (b) but not with those raised against mDLP (a) or uroplakin (d). Nuclear brown staining is seen in both epithelial and some stromal cells when antibodies against AR were used (c)
RESULTS
After 4 weeks of in vivo incubation, the grafts appeared as discrete, raised white patches on the surface of the kidney. There was no evidence of invasion into the renal capsule or parenchyma.
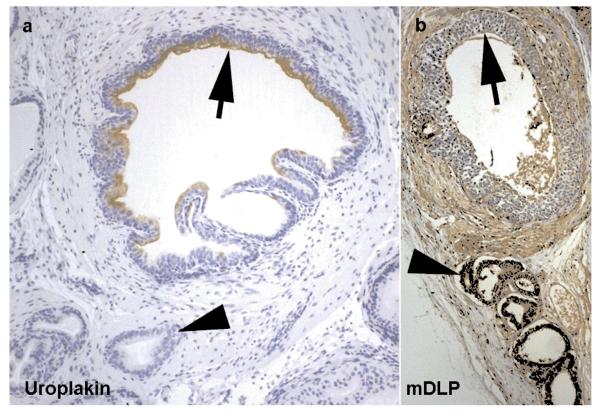
Grafts of mouse trigone epithelium and rat UGM demonstrated both bladder and prostate structures. As previously noted in similar recombinants, fragments of the initially grafted urothelium were found in each graft with multilayered uroplakin-staining transitional epithelium juxtaposed with smaller secretory glands with scant fibromuscular stroma. The secretory glands expressed the prostate epithelial marker mDLP (Figure 3).
Figure 3. Development of prostatic tissue.
Recombinant graft of trigone epithelium and urogenital sinus mesenchyme demonstrating the development of prostatic structure from urothelium under the influence of urogenital sinus mesenchyme. Fragments of the grafted urothelium can be seen (arrows)continuing to express uroplakin (a). More characteristic prostatic glandular epithelium grows from these structures (arrowheads) under the influence of urogenital sinus mesenchyme (as previously described) these structures show positive staining using antibodies targeting dorsal lateral prostate secretions (mDLP) (b).
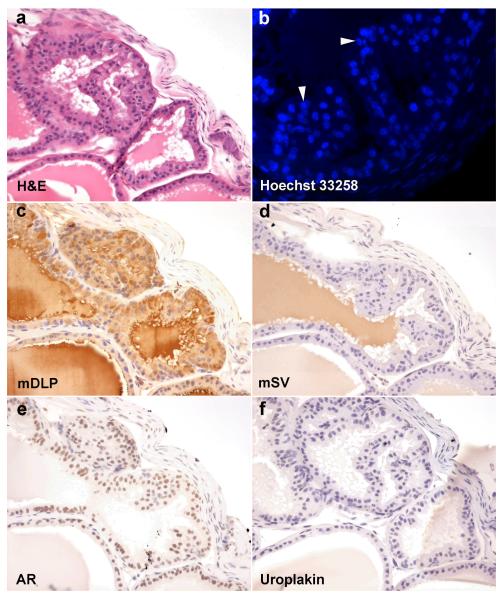
At higher power magnification, these smaller glands consisted of a mixed epithelium with secretory columnar to cuboidal cells and a basal epithelial layer (Figure 4A). Under fluorescence microscopy with Hoechst 33258 staining, the epithelial cells contained small discrete regions with high focal intensity resulting in the “speckled nuclei” classically ascribed to cells of murine origin 12(Figure 4B) and therefore differentiated from the mouse trigone epithelium. The fetal rat UGM induced the mouse trigone epithelium to differentiate to glandular structures with the same immunohistochemical staining pattern (Figures 4C-F) as control mouse prostate. Secretory epithelium expressing mSV was never generated.
Figure 4. Tissue recombinant composed of mouse trigone epithelium and rat urogenital sinus mesenchyme.
Recombinant graft of trigone epithelium and urogenital sinus mesenchyme stained with H&E (a), Hoechst 33258 (b), mDLP (c), mSV (d), AR (e), and uroplakin (f). Clear glandular epithelial differentiation with columnar secretory luminal cells is seen. Epithelial nuclei stained with Hoechst 33258 (b) demonstrates the speckled nuclear patterning characteristic of mouse chromatin packaging (white arrowheads) in epithelial cells while the stromal cells show the more diffuse staining characteristic of rat nuclei. Recombinant glandular structures show brown cytoplasmic staining in luminal epithelial cells for mDLP (c) not mSV (d) or uroplakin (f). Brown nuclear staining is seen using antibodies against AR (e).
Control grafts of endoderm-derived bladder dome epithelium and UGM yielded the expected result of prostate structures as previously described.9, 14, 15 These grafts had glandular structures with epithelial cells that exhibited immunoreactivity to mDLP but not to mSV or uroplakin. In contrast, control grafts of mesoderm-derived ureteral epithelium and UGM yielded the expected result of seminal vesicle structures. These grafts showed glands with tall columnar epithelium with basally-located nuclei. The epithelial cells showed immunoreactivity to mSV but not to mDLP or uroplakin.
DISCUSSION
The bladder trigone is a triangular shaped region at the base of the bladder defined by the two ureteral orifices and the internal urethral meatus. Edges of thickened muscle between the ureteral orifices (the interureteric crest) and between each ureteral orifice and the internal urethral meatus (Bell’s muscle) demarcate the trigone from the remainder of the bladder. Although the majority of the bladder is thought to develop from the endodermal urogenital sinus, the germ layer of origin of the trigone was historically debated largely because of its unique appearance. Wesson1 in 1920 proposed that the trigone developed from the mesodermal Wolffian ducts. Ultimately multiple detailed anatomic dissections of human and animal embryos consolidated this accepted classic view. 14-16
The Wolffian ducts are paired tubes that derive from the mesoderm. Ureteric buds branch from each Wolffian duct on the 28th day of gestation. In the classic view of trigone development, the common nephric ducts, the portion of the Wolffian ducts distal to the ureteric buds, dilate and incorporate into the urogenital sinus wall. The superior urogenital sinus cephalad to the insertion of common nephric ducts forms the remainder of the bladder. The classic view proposed that the trigone is formed from the mesoderm-derived common nephric ducts while the remainder of the bladder is formed from the endoderm-derived urogenital sinus. 17
In recent years modern biologic techniques have advanced developmental biology. Tissue recombination experiments have shown that mesenchymal-epithelial interactions play a critical role in the development of the urogenital tract.18 Fetal mesenchyme provides powerful inductive signals to induce epithelial differentiation. For example, adult bladder epithelium can be induced by fetal urogenital sinus mesenchyme to give rise to prostatic epithelium.19 Fetal UGM has been shown to induce prostatic differentiation in other endodermally-derived epithelia including urethral epithelium19 and sinus-derived vaginal epithelium.20 Prostatic differentiation, however, could not be induced in mesodermal Wolffian duct derived epithelium. Instead, under the influence of fetal seminal vesicle mesenchyme, fetal epithelium from the upper, middle and lower Wolffian duct differentiated to seminal vesicle epithelium.21 Similar results were seen when epithelium from Wolffian duct derivatives such as the vas deferens and ureter differentiated to seminal vesicle epithelium under mesenchymal influences.22 Consistent with these observations seminal vesicle mesenchyme induced prostatic differentiation in endodermally-derived urogenital tract epithelia 23 suggesting that the restriction on differentiation profile rested in the germ layer orgin of the epithelium.
These tissue recombination experiments demonstrated that adult epithelial cells retain plasticity for differentiation; only endodermal derivatives of the urogenital tract could differentiate to form prostate; and only mesodermal derivatives of the urogenital tract could differentiate to form seminal vesicle. The underlying hypothesis of the present study was that the germ layer of origin of trigone epithelium could be inferred from the results of tissue recombination experiments. In our experiments we found that trigone epithelium repeatedly differentiated to prostate, and not seminal vesicle, epithelium in tissue recombinant grafts with fetal UGM. Our results suggest that the trigone does not originate from the mesoderm.
Another potential explanation for our results is that the trigone is of mesoderm origin but its epithelium is replaced by endodermal epithelium early during development. Gyllensten24 in 1949 observed in embryonic sections that proliferating sinus epithelium grew over the epithelium of the common nephric duct. To test this possibility we performed parallel experiments using trigonal epithelium from pups as young as 5 days old. The results of these experiments were consistent with those using adult epithelium in that they failed to yield any seminal vesicle epithelium indicating that no mesodermally-derived epithelium was present even at that early stage.
Our results complement recently published work which concluded that bladder trigone does not differentiate from the mesodermally-derived common nephric duct but instead forms from urogenital sinus. Batourina et al.2 used cell lineage analysis in a transgenic mouse line in which Wolffian duct cells and their daughter cells were labeled. In early embryonic stages, cell labeling was present in the ureters, Wolffian ducts and common nephric duct. In later embryonic stages, cell labeling persisted in the ureters and Wolffian duct derivatives but was decreased in the common nephric duct. After birth, cell labeling again persisted in the ureters and Wolffian duct derivatives but not in the trigonal region. Common nephric duct cells, instead of fusing to form the trigone, underwent apoptosis in the region of contact with the urogenital sinus. Viana et al. 3 similarly followed the fate of both mesonephric mesenchymal cells and urogenital sinus mesenchymal cells and found that the stromal cells underlying the trigone are formed primarily from urogenital sinus mesenchymal cells with only a limited contribution from ureteral fibers.
In this present study we used an entirely different methodology from cell lineage analysis to refute the accepted belief that the trigone originated from the mesoderm-derived Wolffian duct. The results of our study and the previously published cell lineage analysis studies2, 3 are consistent with each other and at odds with accepted teaching based upon anatomic observations dating back close to 100 years. In the presence of fetal UGM, we expected that trigone epithelium should differentiate to seminal vesicle epithelium if its origin was mesodermal. In all our recombinant grafts, trigone epithelium differentiated to prostatic epithelium in the presence of UGM. From these results, we infer that trigone epithelium has an endodermal origin.
CONCLUSIONS
The consistent finding of prostatic epithelium in tissue recombinants of trigone epithelium and fetal UGM reinforces the hypothesis that the trigone is derived from the endoderm. While some prostatic epithelium could be ascribed to contamination from non-trigonal isolates the absence of any evidence of seminal vesicle differentiation suggests that this is not an issue. This study provides independent confirmation of recently published work that refutes the classically accepted view of trigone development from the mesoderm.
ACKNOWLEDGMENTS
The authors wish to thank Suzanne Fernandez and Harold Love for technical support for this project. Supported by National Institutes of Health Grants DK067049 (SWH) and DK068593 (JCP).
REFERENCES
- 1.Wesson MB. Anatomical, embryological and physiological studies of the trigone and neck of the bladder. Journal of Urology. 1920;4:279. [Google Scholar]
- 2.Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, et al. Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet. 2005;37:1082. doi: 10.1038/ng1645. [DOI] [PubMed] [Google Scholar]
- 3.Viana R, Batourina E, Huang H, Dressler GR, Kobayashi A, Behringer RR, et al. The development of the bladder trigone, the center of the anti-reflux mechanism. Development. 2007;134:3763. doi: 10.1242/dev.011270. [DOI] [PubMed] [Google Scholar]
- 4.Cunha GR, Ricke W, Thomson A, Marker PC, Risbridger G, Hayward SW, et al. Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J Steroid Biochem Mol Biol. 2004;92:221. doi: 10.1016/j.jsbmb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Kurzrock EA, Baskin LS, Cunha GR. Ontogeny of the male urethra: theory of endodermal differentiation. Differentiation. 1999;64:115. doi: 10.1046/j.1432-0436.1999.6420115.x. [DOI] [PubMed] [Google Scholar]
- 6.Boutin EL, Battle E, Cunha GR. The germ layer origin of mouse vaginal epithelium restricts its responsiveness to mesenchymal inductors: uterine induction. Differentiation. 1992;49:101. doi: 10.1111/j.1432-0436.1992.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 7.Kurzrock EA, Baskin LS, Li Y, Cunha GR. Epithelial-mesenchymal interactions in development of the mouse fetal genital tubercle. Cells Tissues Organs. 1999;164:125. doi: 10.1159/000016650. [DOI] [PubMed] [Google Scholar]
- 8.Hayward SW. Approaches to modeling stromal-epithelial interactions. J Urol. 2002;168:1165. doi: 10.1016/S0022-5347(05)64620-4. [DOI] [PubMed] [Google Scholar]
- 9.Hayward SW, Haughney PC, Lopes ES, Danielpour D, Cunha GR. The rat prostatic epithelial cell line NRP-152 can differentiate in vivo in response to its stromal environment. Prostate. 1999;39:205. doi: 10.1002/(sici)1097-0045(19990515)39:3<205::aid-pros9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Cunha GR, Fujii H, Neubauer BL, Shannon JM, Sawyer LM, Reese BA. Epithelial-mesenchymal interactions in prostatic development. I. Morphological observations of prostatic induction by urogenital sinus mesenchyme in epithelium of the adult rodent urinary bladder. J. Cell Biol. 1983;96:1662. doi: 10.1083/jcb.96.6.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brody J YP, Cunha G. Renal capsule grafting. 1998.
- 12.Cunha GR, Vanderslice KD. Identification in histological sections of species origin of cells from mouse, rat and human. Stain Technol. 1984;59:7. doi: 10.3109/10520298409113823. [DOI] [PubMed] [Google Scholar]
- 13.Donjacour AA, Rosales A, Higgins SJ, Cunha GR. Characterization of antibodies to androgen-dependent secretory proteins of the mouse dorsolateral prostate. Endocrinology. 1990;126:1343. doi: 10.1210/endo-126-3-1343. [DOI] [PubMed] [Google Scholar]
- 14.Tanagho EA, Pugh RC. The anatomy and function of the ureterovesical junction. Br J Urol. 1963;35:151. doi: 10.1111/j.1464-410x.1963.tb02610.x. [DOI] [PubMed] [Google Scholar]
- 15.Woodburne RT. The Ureter, Ureterovesical Junction, and Vesical Trigone. Anat Rec. 1965;151:243. doi: 10.1002/ar.1091510305. [DOI] [PubMed] [Google Scholar]
- 16.Tejedo-Mateu A, Vilanova-Trias J, Ruano-Gil D. Contribution to the study of the development of the terminal portion of the Wolffian duct and the ureter. Eur Urol. 1975;1:41. [PubMed] [Google Scholar]
- 17.Larsen WJ. Human Embryology. 3rd ed Churchill Livingstone; New York: 2001. [Google Scholar]
- 18.Cunha GR, Alarid ET, Turner T, Donjacour AA, Boutin EL, Foster BA. Normal and abnormal development of the male urogenital tract. Role of androgens, mesenchymal-epithelial interactions, and growth factors. J Androl. 1992;13:465. [PubMed] [Google Scholar]
- 19.Donjacour AA, Cunha GR. Assessment of prostatic protein secretion in tissue recombinants made of urogenital sinus mesenchyme and urothelium from normal or androgen-insensitive mice. Endocrinology. 1993;132:2342. doi: 10.1210/endo.132.6.7684975. [DOI] [PubMed] [Google Scholar]
- 20.Boutin EL, Battle E, Cunha GR. The response of female urogenital tract epithelia to mesenchymal inductors is restricted by the germ layer origin of the epithelium: prostatic inductions. Differentiation. 1991;48:99. doi: 10.1111/j.1432-0436.1991.tb00248.x. [DOI] [PubMed] [Google Scholar]
- 21.Higgins SJ, Young P, Cunha GR. Induction of functional cytodifferentiation in the epithelium of tissue recombinants. II. Instructive induction of Wolffian duct epithelia by neonatal seminal vesicle mesenchyme. Development. 1989;106:235. doi: 10.1242/dev.106.2.235. [DOI] [PubMed] [Google Scholar]
- 22.Cunha GR, Young P, Higgins SJ, Cooke PS. Neonatal seminal vesicle mesenchyme induces a new morphological and functional phenotype in the epithelia of adult ureter and ductus deferens. Development. 1991;111:145. doi: 10.1242/dev.111.1.145. [DOI] [PubMed] [Google Scholar]
- 23.Donjacour AA, Cunha GR. Induction of prostatic morphology and secretion in urothelium by seminal vesicle mesenchyme. Development. 1995;121:2199. doi: 10.1242/dev.121.7.2199. [DOI] [PubMed] [Google Scholar]
- 24.Gyllensten L. Contributions to the embryology of the urinary bladder. Acta Anat (Basel) 1949;7:305. doi: 10.1159/000140392. [DOI] [PubMed] [Google Scholar]