Abstract
Declines in neural processing speed have been proposed to underlie a broad range of cognitive deficits in older adults. However, the impact of delays in neural processing during stimulus encoding on working memory (WM) performance is not well understood. In the current study, we assessed the influence of aging on the relationship between neural measures of processing speed and WM performance during a selective delayed-recognition task for color and motion stimuli, while electroencephalography (EEG) was recorded in young and older adults. A latency delay was observed for the selection negativity (SN) and alpha band activity (measures of attentional allocation) in older adults during WM encoding of both motion and color stimuli, with the latency and magnitude of the SN predicting subsequent recognition performance. Furthermore, an age-related delay in the N1 latency occurred specifically during the encoding of color stimuli. These results suggest that the presence of both generalized feature-based and feature-specific deficits in the speed of selective encoding of information contributes to WM performance deficits in older adults.
Keywords: working memory, selective attention, aging, EEG, processing speed delay
1. Introduction
A large body of research has documented performance deficits in the cognitive abilities of older adults. Typically affected are working memory (WM), episodic memory and attention (Craik & Salthouse, 2000; Greenwood, 2000). Within the WM domain, age-related deficits in performance have been reported for numerous stimuli, including letters, words, digits, spatial position, pattern discrimination and complex stimuli (for review, see T.A. Salthouse, 1994). In an attempt to generate an underlying account of such diverse deficits, the findings of many behavioral studies on older adults have given rise to the processing speed hypothesis of cognitive aging (T. A. Salthouse, 1996). This hypothesis attributes age-related cognitive decline to a general slowing of information processes, and is based on the premise that if less computational processing is completed in a set amount of time, then less information is available to higher-level functions. For example, time-accuracy functions indicate that older adults require a longer stimulus presentation time during encoding in order to achieve recognition accuracies comparable to younger adults (e.g. Kliegl, Mayr, & Krampe, 1994). The majority of evidence supporting this hypothesis is derived from meta-analysis studies that have revealed the statistical control of response time measurements reduces age-cognition correlations (T. A. Salthouse, 1996). Although such behavioral research and statistical analysis has provided many important insights to the field of cognitive aging, they are intrinsically limited in their ability to directly address the neural underpinnings of age-related decline. Thus, the neural correlates of changes in processing speed during memory encoding and its influence on recognition performance have yet to be established.
Research on experimental animals has generated data to support a neural basis for the processing speed hypothesis. It has revealed that age-related cognitive decline in non-human primates is not due to loss of significant numbers of neurons (Alan Peters, 2002), but rather is associated with axonal myelin integrity, thus providing anatomical evidence suggestive of diminished information transfer in the aging brain (Peters, et al., 1996). Related to this, in humans, processing speed was shown to be closely related to the structural integrity of white matter tracts (Rabbitt, Lunn, et al., 2007; Turken, et al., 2008). Additionally, carotid and basilar artery blood flow and age-associated losses of brain volume have been correlated with white matter lesions as well as information processing speed (Rabbitt, Mogapi, et al., 2007).
Electrophysiological studies in humans are a direct manner in which to address neural correlates of the processing speed deficit hypothesis and its impact on cognition. Electroencephalography (EEG) allows us to measure electrical correlates of neural activity during a behavioral task with high temporal resolution (i.e., milliseconds), and is thus an ideal tool to explore neural processing speed delays with aging. Most neural data supporting the processing speed hypothesis has focused on changes in the peak latency of the P300 component of the event-related potential (ERP). The P300 is a positive deflection that occurs 300–600 ms post-stimulus onset, and is thought to reflect processing involved in attention and memory operations; it is typically evoked by infrequent, random targets (i.e. oddballs; Sutton, Braren, Zubin, & John, 1965) and an increased memory load results in an increased latency (for review, see Kok, 1997). There is extensive evidence that the P300 latency is delayed in older adults, thereby providing evidence of neural slowing during cognitive operations (for reviews, see Kok, 2000; Kugler, Taghavy, & Platt, 1993; Polich, 1996). EEG studies have also shown that earlier ERP markers of visual processing exhibit slowing in older adults. In a cued, two-choice discrimination task, Curran et al. (2001) examined neural correlates of selective attention in aging and reported slowing of the P1 and N1 components of the visual ERP in older adults. The P1 is a positive deflection in the ERP, peaking around 100 ms post-stimulus onset, whereas the N1 is a negative deflection peaking approximately 170 ms after stimulus onset. Comparably, Gazzaley et al. (2008) reported that during a face/scene WM task, older adults exhibited a delayed N1 suppression index and a delayed P300 peak latency while encoding face stimuli. However, to our knowledge, the relationship between the latency of neural measures to lower-level encoded stimuli and WM recognition has not yet been assessed in an aging study. The advantage of utilizing lower-level stimuli, such as simple feature detection, is that individual perceptual differences can be controlled for. This is important when evaluating the nature of changes in processing speed on WM, since it has been suggested that discriminability differences may account for visual WM deficits in older adults (Sara & Faubert, 2000).
The current study aimed to identify age-related changes in neural measures of processing speed during the selective encoding of lower-level visual features (i.e. color hue and motion direction) and assess its influence on WM performance. To achieve this, younger (18–35 years old) and older (60–80 years old) adults performed delayed-recognition WM tasks that required selective attention while 64-channel EEG was recorded. Prior to the main experiment all participants went through visual acuity correction, as well as a thresholding procedure to adjust for feature discriminability differences between individuals in color and motion perception. Furthermore, the perception of brightness for all stimuli was equated for each participant. By minimizing these influences, we can interpret neural delays as being the result of changes in endogenous influences such as WM encoding or selective attention. Based on the processing speed hypothesis, we hypothesized that delays in neural processing during encoding would be associated with declines in WM performance.
The neural measures analyzed for age-related differences include the P1 and N1 of the ERP, the selection negativity and time-frequency (spectral) measures to cue stimuli. The P1 and the N1, which reflect early stages of visual processing for stimulus representation generated in extrastriate cortex (for review, see Herrmann & Knight, 2001) and are modulated by attention (Gazzaley, et al., 2008; S. A. Hillyard, Vogel, & Luck, 1998; Rugg, Milner, Lines, & Phalp, 1987; Valdes-Sosa, Bobes, Rodriguez, & Pinilla, 1998). The selection negativity (SN), which is calculated from the ERP difference wave between attended and ignored stimuli, begins between 140–180 ms post stimulus onset and may persist for more than 200 ms (Harter & Aine, 1984). The SN has been shown to reflect the timing of feature selections and is influenced by attention (AnlloVento & Hillyard, 1996; Kenemans, Smulders, & Kok, 1995). Further exploration of age-related neural changes during stimulus encoding focused on spectral activity between 4–50 Hz, as attentional allocation has been linked to modulation in the theta (Jensen & Tesche, 2002; Schacter, 1977), alpha (Muller & Keil, 2004) and gamma bands (Gruber, Muller, Keil, & Elbert, 1999).
2. Methods
2.1 Participants
Twenty-two healthy young adults (mean age 24.0 years; range 18–29 years; 8 males) and twenty-one older adults (mean age 70.1 years; range 60–83 years; 10 males) gave informed consent to participate in the study approved by the Committee on Human Research at the University of California in San Francisco. All participants had normal or corrected to normal vision and were screened to ensure they were healthy, had no history of neurological, psychiatric, or vascular disease, were not depressed, and were not taking any psychotropic or hypertensive medications. Visual acuity was checked for each participant using a Snellen chart and corrective lenses were utilized as necessary to achieve 20/40 vision or better. Additionally, all participants were required to have 12 years minimum education.
2.2 Neuropsychological testing
To ensure older adults were “normal” relative to their age-matched peers, participants in the older age group were required to score within two standard deviations of control values on 13 neuropsychological tests. The neuropsychological evaluation consisted of tests designed to assess general intellectual function (MMSE; Folstein, Folstein, & McHugh, 1975), verbal learning (CVLT-II; Delis, Kramer, Kaplan, & Ober, 2000), geriatric depression (GDS; Yesavage, et al., 1982), visual-spatial function (copy of a modified Rey-Osterrieth figure), visual-episodic memory (memory for details of a modified Rey-Osterrieth figure), visual-motor sequencing (trail making test A and B; Reitan, 1958; Tombaugh, 2004), phonemic fluency (words beginning with the letter ‘D’), semantic fluency (animals), calculation ability (arithmetic), executive functioning (Stroop interference test; Stroop, 1935), working memory and incidental recall (backward digit span and digit symbol, WAIS-R; Wechsler, 1981). All neuropsychological test scores are summarized in table 1.
Table 1.
Neuropsychological scores averaged over all participants. Each participant scored within 2 standard deviations of their age-matched normative value.
| Neuropsychological Test | Mean | S.E.M. |
|---|---|---|
| Mini-mental state examination | 28.9 | 1.2 |
| Geriatric depression scale | 2.1 | 2.0 |
| CVLT: Trial 5 recall | 12.5 | 2.9 |
| CVLT: Short delay free recall | 10.8 | 4.1 |
| CVLT: Short delay cued recall | 12.3 | 3.3 |
| CVLT: Long delay free recall | 11.4 | 3.6 |
| CVLT: Long delay cued recall | 12.3 | 3.4 |
| Memory for modified Rey | 12.3 | 2.7 |
| Calculation ability (out of 5) | 4.9 | 0.3 |
| WAIS-R: Backward digit span | 5.6 | 1.6 |
| WAIR-R: Digit symbol | 48.9 | 10.1 |
| Trail making test: A | 34.6 sec. | 8.5 sec. |
| Trail making test: B | 88.2 sec. | 37.4 sec. |
| Stroop: Color naming | 82.9 | 15.3 |
| Stroop: Color-word naming | 48.6 | 16.8 |
| Semantic fluency | 20.3 | 5.5 |
| Phonemic fluency | 15.8 | 5.2 |
2.3 Stimuli
The stimuli consisted of a circular aperture of 290 dots (0.08° × 0.08° each) that subtended 8° of visual angle at a 75 cm viewing distance and were centered at the fovea. Two types of dots were used during the experiment: 1) gray and moving coherently at 10° per second or 2) stationary and colored along the tritan axis. All colored and gray dots were equated for brightness by minimizing heterochromatic flicker in tests carried out prior to the experiment for each participant (for details on color generation and flicker photometry, see Hardy, Delahunt, Okajima, & Werner, 2005). Stimuli were presented with a gray fixation cross in the center of the circular aperture and a black background with a luminance level of 0.32 cm/m2. Stimuli were presented through E-Prime software (Psychology Software Tools, Inc.) run on a Dell Optiplex GX620 and a ViewSonic G220fb CRT monitor.
2.4 Thresholding
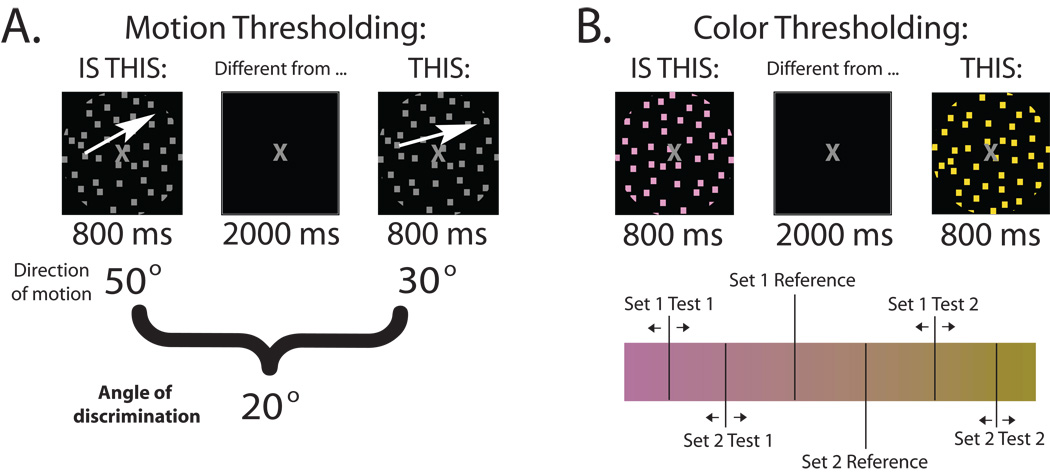
After all stimuli were equated for brightness, participants went through two thresholding tests (one for motion, one for color) in order to minimize discriminability differences. A stair-step procedure required participants to determine whether two stimuli (directions of motion or colors) were different from each other. The two stimuli were presented for 800 ms each and separated by 2000 ms. The procedure continued until a “just 100%” level of performance was reached, meaning that if the stimuli were any more similar, performance would drop below 100% as determined by 10 out of 10 correct responses. An angle of discrimination was identified as the difference between the two directions of motion at the just 100% level of performance (Fig. 1a). All four quadrants except the cardinal directions (up, down, left, right) were used during the thresholding procedure, as well as the experimental task conditions.
Figure 1.
Thresholding procedure. a. An angle of discrimination is determined to be the closest angle two directions of motion may be when separated by 2000 ms and retain a just 100% level of performance. b. Two sets of three colors are determined for the experimental stimulus set. A set of three colors consists of a reference (the same for each subject) and one color on either side of it (along the axis) determined as the closest colors to the reference that retains a just 100% level of performance.
The tritan color axis was divided into 40 linearly separated hues. Two sets of three colors (hues) were identified by using two reference colors (one for each set) near the middle of the tritan axis (Fig. 1b). The stair-step procedure determined how close the test colors could be to the reference (one on either side) at the just 100% level of performance (same as motion thresholding). The two reference colors were the same for each participant. It did not matter whether participants could discriminate between the two reference colors because only one set was used per trial during the experiment.
2.5 Experimental Procedure
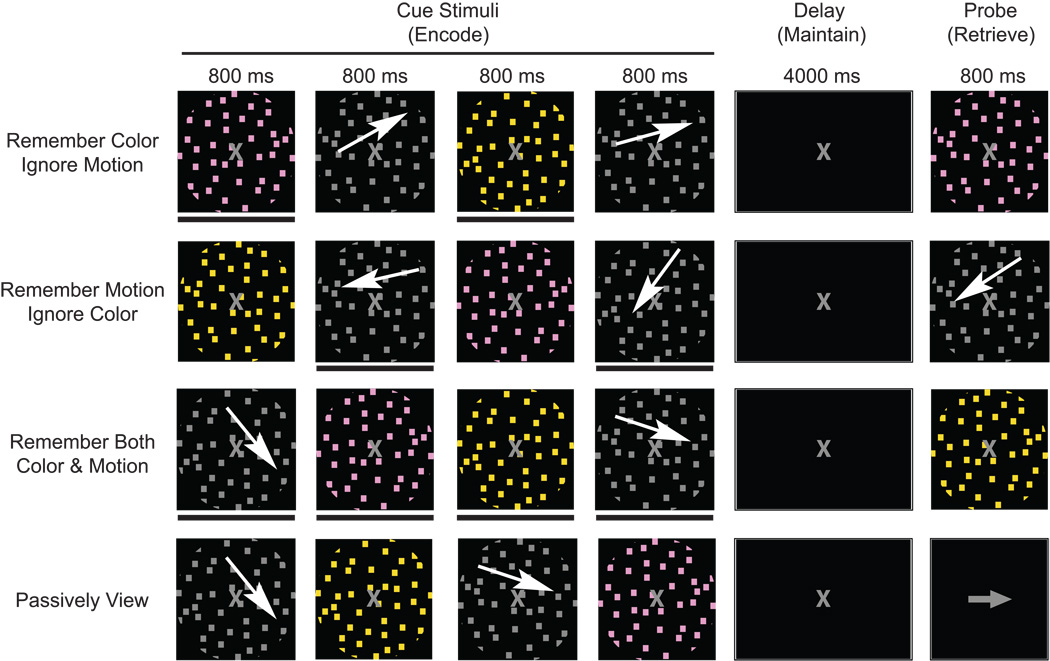
All conditions required viewing four sequentially presented images: two differently colored stimuli and two different directions of motion. Every image was presented for 800 ms with an 800 ms inter-stimulus-interval. After the four images were presented, there was a four second delay followed by a probe stimulus (800 ms duration) when participants must determine whether the probe matched any of the items held in memory. Participants were presented with four different conditions, 3 WM and one passive view condition (Fig. 2). The three WM conditions required participants to either 1) remember the two directions of motion (ignore the two colors), 2) remember the two colors (ignore motion) or 3) remember both colors and both directions of motion. The fourth condition instructed participants to passively view all stimuli. The features of the four sequentially presented images cue participants whether it is to be remembered or ignored by placing it in context of the task instructions. Each condition was presented in two blocks, and the eight blocks were randomized across the experiment. Prior to beginning each block, participants were given task instructions for the subsequent condition. Additionally, a brief (1 sec.) task reminder was provided at the start of each trial.
Figure 2.
Experimental procedure. Four cue stimuli are presented for 800 ms each with an 800 ms inter-stimulus-interval followed by a four second delay and an 800 ms probe. Three working memory conditions require participants to hold relevant information in memory, ignore irrelevant information and determine if the probe matches any item stored in memory. The fourth condition instructed participants to passively view the stimuli and respond to an arrow as the probe.
Motion stimuli consisted of 12 different directions of motion (3 per quadrant). Within each quadrant, the 3 directions of motion are separated by the participant’s angle of discrimination. For example, if the angle of discrimination were 20 degrees, the 3 directions of motion for quadrant one would be 30°, 50° and 70°. Only one quadrant was used per trial during the experiment and was randomly selected. The two moving cue stimuli were randomly chosen from the three possibilities within the previously determined quadrant. Color stimuli consisted of two sets of three different hues (six different hues total). Within each set, the three hues were separated by the participant’s color threshold. During each trial, one of the two sets of colors was randomly selected, and the two colored cue stimuli were randomly chosen from the three possible hues within the previously determined set of colors. Probe stimuli matched an attended cue stimulus on half the trials. In the event a probe did not match a cue stimulus, the probe was selected as the third possibility belonging to the quadrant or color set for that trial. During the conditions when participants were instructed to remember motion (ignore color) or remember color (ignore motion), probe stimuli never matched to-be-ignored stimuli (i.e. motion probes never appeared when participants were instructed to remember the color and ignore motion). Participants responded by pressing one of two buttons. One half of the probe stimuli matched a previously attended object. During the passive view condition, the probe was either a left or right arrow and participants had to respond whether it was left or right. Participants were instructed to respond as quickly as possible and yet retain accuracy during all conditions. Prior to beginning the experiment, participants were given 12 practice trials for each of the four conditions, split into two blocks (6 trials each). Following each trial during practice and the experiment, participants received accuracy and response time feedback.
2.6 Data Acquisition
Participants sat in an armchair in a dark, sound-attenuated room and were monitored by camera during all tasks. Data was recorded during 8 blocks lasting approximately 6 minutes each and a total of 60 trials per condition, yielding 120 epochs of data during the encoding period. Electrophysiological signals were recorded with a BioSemi ActiveTwo 64-channel EEG acquisition system in conjunction with BioSemi ActiView software (CortechSolutions, LLC). Signals were amplified and digitized at 1024 Hz with a 24-bit resolution. All electrode offsets were maintained between +/−20 mV.
2.7 Data Analysis
Raw EEG data were referenced to the average off-line. Eye artifacts were removed through an independent component analysis by excluding components consistent with topographies for blinks and eye movements and the electrooculogram time-series. Data was segmented into epochs beginning 200 ms pre-stimulus onset and ending 1000 ms post-stimulus onset. Each trial contained four epochs, two attended and two ignored, whereas all four epochs were attended in the ‘remember both’ condition. Thus, over the 60 trials, 120 epochs were acquired for each event-related potential (ERP) of interest. This preprocessing was conducted in Brain Vision Analyzer (Cortech Solutions, LLC) and exported to Matlab (The Mathworks, Inc.) for all subsequent analyses. To minimize spurious peak ERP measures, epochs were band-pass filtered from 1–30 Hz. Epochs that exceeded a voltage threshold of +/−50 µV were rejected. A 200 ms pre-stimulus baseline was subtracted from each epoch prior to calculating the ERP. Peak P1 values were chosen as the largest local peak amplitude between 50–150 ms post-stimulus onset, and the N1 was identified as the most negative local peak amplitude between 120–220 ms. The selection negativity was calculated by subtracting the ignored stimuli ERP from attended stimuli and identified as the largest local minimum following the N1 between 200–400 ms. Mean amplitudes for the P1, N1 and SN were measured by averaging +/− 5 ms around the peak prior to statistical analysis. Eight lateralized, posterior-occipital electrodes (four from each hemisphere; left: P5, P7, P9, PO7; right: P6, P8, P10, PO8) were chosen for ERP analysis, consistent with the topographical distribution of the visual ERP. The P1 and N1 amplitudes and latencies were subjected to a repeated measures analysis of variance (ANOVA) with age (younger, older), task (attend, ignore, passive view, remember both) and electrode (eight from lateral posterior/occipital areas) as factors whereas the SN amplitude and latency utilized only age and electrode as factors. A Geisser-Greenhouse correction was applied when appropriate. Post-hoc analysis consisted of the Scheffe test with a Bonferroni correction for multiple comparisons. A time-frequency decomposition was also applied to the epochs from the encoding period, except the data was not band-pass filtered prior to its calculation in order to resolve frequencies 4–50 Hz. Time-frequency data was acquired via complex Morlet wavelets and converted to decibels relative to pre-stimulus activity as implemented through EEGLAB (Delorme & Makeig, 2004). Contrasts between conditions utilized t-tests at each time-frequency point with a false-discovery rate (FDR; Benjamini & Hochberg, 1995) correction for multiple comparisons.
3. Results
3.1 Behavior
Each participant performed a perceptual thresholding task to identify individual differences in motion direction and color discrimination, prior to the main delayed-recognition WM experiment. This information was then used to guide the selection of stimuli for the WM task, so that each participant performed the main experiment with stimulus parameters based on their own perceptual abilities. Older participants yielded larger angles of discrimination for the motion stimuli (younger = 26°, s.e.m. = 2°; older = 34°, s.e.m. = 1°; p < 0.005) and a wider span of color (younger mean distance from reference 1 = 11/−6 hues, s.e.m. = 1/1 hue, and reference 2 = 7/−9 hues, s.e.m. = 1/1 hue; older mean distance from reference 1 = 14/−10 hues, s.e.m. = 1/1 hue, and reference 2 = 11/−13 hues, s.e.m. = 1/1 hue; p < 0.05 for all comparisons), indicating an impairment in perceptual discrimination of these features with age.
A control condition utilized in the main experiment was a passive viewing task, used to generate baseline neural measures, as well as an index of general motoric slowing with aging (i.e., participants were required to press a button as rapidly as possible to indicate the direction of an arrow). Unpaired t-tests revealed that older participants responded significantly slower during the passive view condition (young response time (RT) = 444 ms, s.e.m. = 24 ms; older RT = 722 ms, s.e.m. = 86 ms; p < 0.01), indicating a decline in response speed even for simple detection. To evaluate changes in WM performance, the passive view condition RT was subtracted from the WM condition RT for each participant, as general slowing is thought to have a linear trend (Lima, Hale, & Myerson, 1991; T. A. Salthouse, 1993). Therefore, for the WM RT results, correction was made for both age-related changes in motor and basic perceptual processing, permitting the interpretation of the results to be more specific for age-related changes in WM and attention processes.
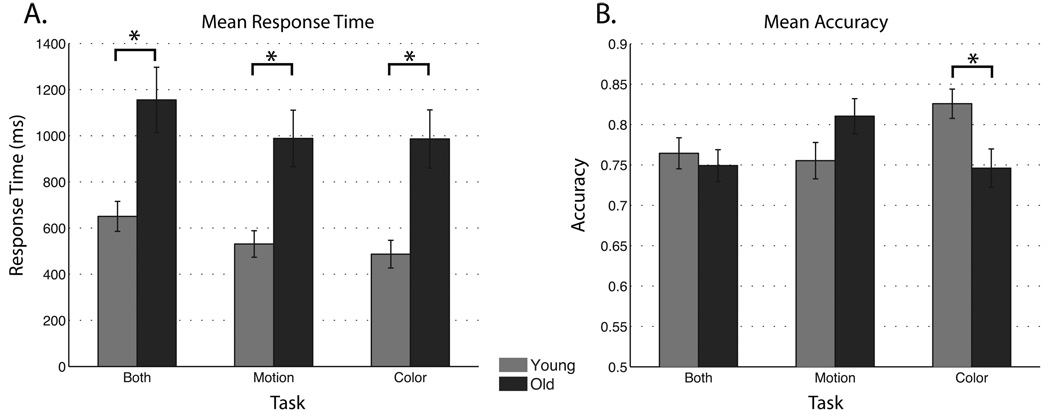
For WM RT (corrected by passive RT), a 2 × 3 repeated measures ANOVA was conducted with age (younger, older) and task (remember motion, remember color, remember both) as factors. A main effect for task (F(2,82) = 6.81; p < 0.005) revealed longer RTs occurred with a greater WM load, such that recognition of stimuli from the ‘remember both’ task took longer than either the ‘remember motion’ or ‘remember color’ tasks (each comparison p < 0.005). Moreover, a main effect for age indicated that older participants responded slower during all of the WM tasks (Fig. 3a; F(1,41) = 14.06, p < 0.001). No interaction between age and task was observed.
Figure 3.
Behavioral results compared between age groups for a. response time (corrected by passive view) and b. accuracy data.
For WM accuracy, a 2 × 3 repeated measures ANOVA was conducted with age (younger, older) and task (remember motion, remember color, remember both) as factors. No significant main effects were observed (age: F(1,41) = 0.34, p > 0.05; task: F(2,82) = 1.94, p > 0.05), however, an age by task interaction (F(2,82) = 8.54, p < 0.001) revealed enhanced accuracy in younger participants during the ‘color’ task compared to their performance on the ‘motion’ and ‘both’ tasks (each comparison p < 0.01). Older participants displayed higher WM accuracy during the ‘motion’ task compared to their performance on the ‘color’ and ‘both’ tasks (each comparison p < 0.01). Moreover, across-group analysis younger participants were more accurate than older participants on the ‘color’ task (Fig. 3b; p < 0.05), with no significant age-related difference on either the ‘motion’ or ‘both’ tasks. In summary, older adults display a lower WM accuracy to color stimuli and a slowed RT during all tasks even when motoric and perceptual differences were taken into account.
3.2 Neural Measures to Cue Stimuli
To address if changes in WM performance are associated with neural processing delays and/or neural response magnitude changes, analyses focused on the following ERP amplitude and latency measures, as well as spectral measures, for the cue stimuli. Analysis of the P300 component was not included in this study, as it was not consistently identified, presumably because the P300 is typically evoked by infrequent target stimuli embedded within many non-targets.
3.2.1 P1 modulation
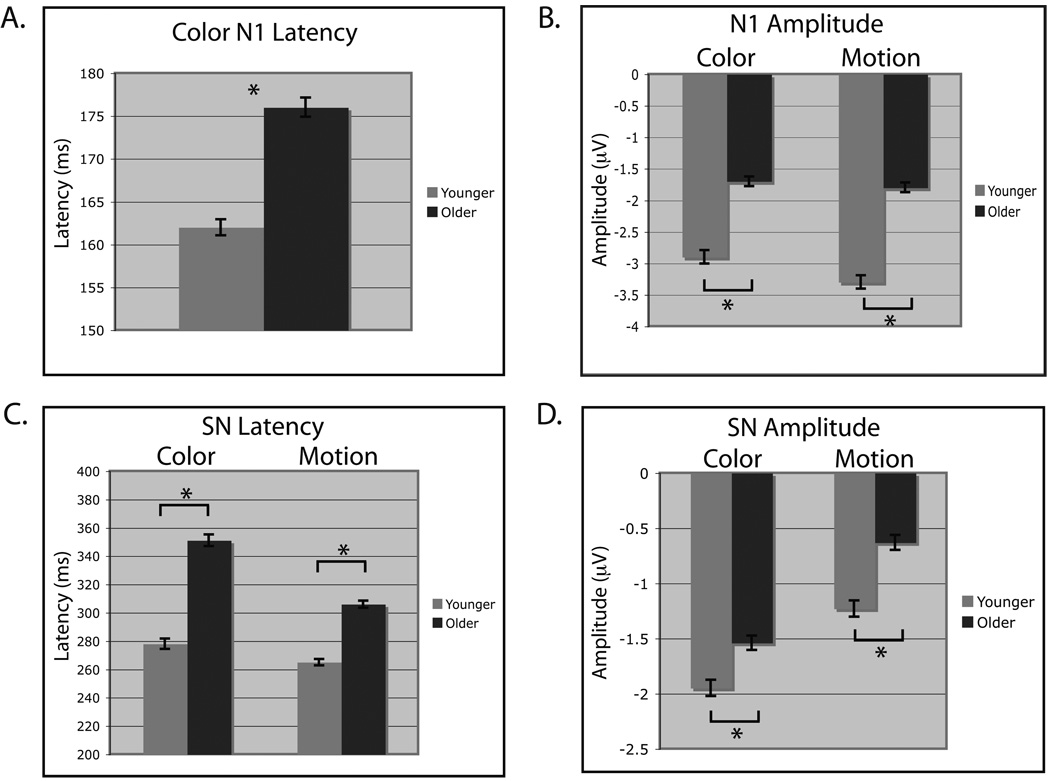
P1 latency measures to cue stimuli were subjected to separate analysis of variance (ANOVA) for motion and color stimuli with factors of age (younger, older), task (attend, ignore, passive view, remember both) and electrode (eight from lateral posterior/occipital areas). The P1 latency to motion stimuli displayed a main effect of electrode (F(7,287) = 4.99, p < 0.005), such that P6, P8, PO7 and PO8 peaked earlier than the other electrodes (p < 0.05 for each post-hoc comparison). No task or age main effects or interactions were observed for the motion P1 latency. The P1 latency to color stimuli showed a subtle but significant main effect of task (F(3,123) = 3.18, p < 0.05), such that attended and passively viewed stimuli peaked earlier than ignored stimuli or color stimuli in the remember both task (attend = 97 ms, s.e.m. = 5 ms; ignore = 99 ms, s.e.m. = 5 ms; both = 99 ms, s.e.m. = 5 ms; passive = 97 ms, s.e.m. = 5 ms; p < 0.05 for each significant difference described). No other color P1 latency main effects or interactions were observed.
The P1 amplitudes were submitted to the same ANOVAs performed for the latency analysis. The P1 amplitude to motion stimuli displayed a main effect for electrode (F(7,287) = 17.83, p < 0.001) and post-hoc analysis indicated a larger response in the right hemisphere. A main effect of task was also observed for the motion P1 amplitude (F(3,123) = 17.42, p < 0.001; attend = 3.70 mV, s.e.m. = 0.20 mV, ignore = 3.35 mV, s.e.m. = 0.18 mV, passive = 3.13 mV, s.e.m. = 0.17 mV, both = 3.76 mV, s.e.m. = 0.20 mV). Post-hoc analysis showed amplitude attentional enhancement for the ‘attend’ and ‘both’ tasks relative to passively viewed and ignored stimuli (p < 0.001, all four comparisons). Moreover, ignored stimuli displayed greater amplitude relative to passively viewed stimuli (p < 0.05). No age effect or interactions were observed for the motion P1 amplitude. The P1 amplitude to color stimuli displayed main effects for electrode (F(7,287) = 16.83, p < 0.001) and task (F(3,123) = 9.76, p < 0.001; attend = 2.8 mV, s.e.m. = 0.1 mV; ignore = 2.9 mV, s.e.m. = 0.1 mV; both = 2.9 mV, s.e.m. = 0.1 mV; passive = 2.4 mV, s.e.m. = 0.1 mV) and an age by task interaction (F(3,123) = 3.26, p < 0.05). Post-hoc analysis identified the passive view P1 to color was smaller than all other tasks (p < 0.005 for all three comparisons), largest at PO8 (p < 0.01 for all seven comparisons) and the age by task interaction was driven by a diminished P1 in older participants during the passive view task (p < 0.01).
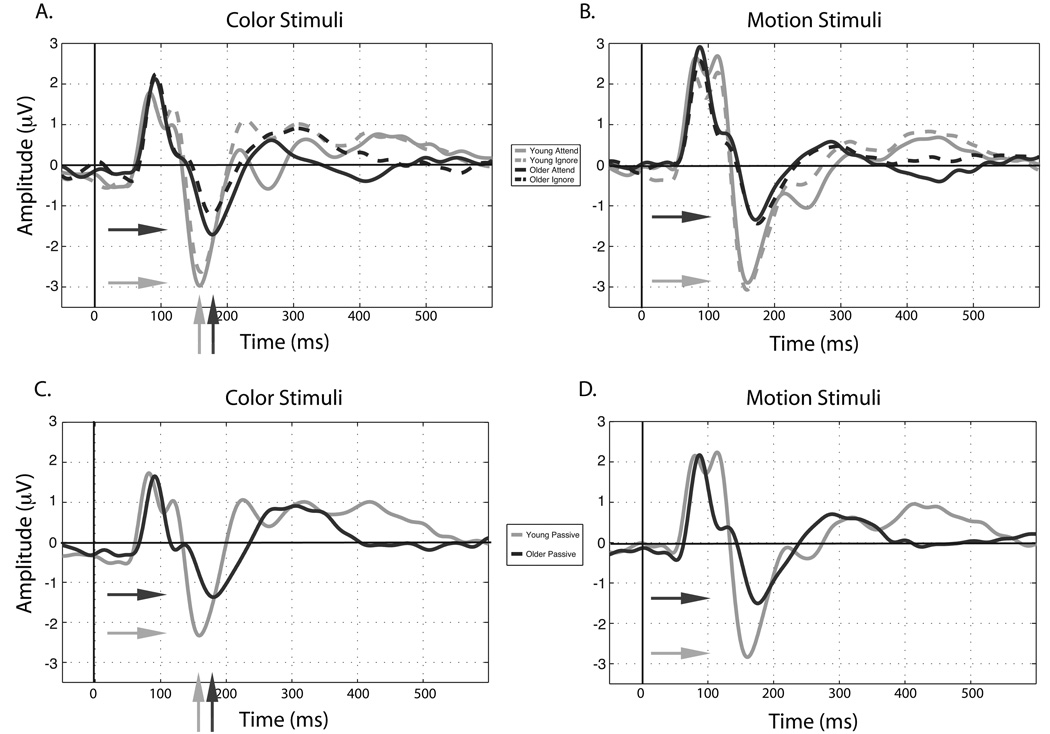
In summary, the P1 data reveals overall attentional modulation of the P1 latency for color (earlier for attend than ignore) and the P1 amplitude for motion (Fig. 4b; greater for attend than ignore). No main effects of age were observed for motion or color stimuli at the P1, indicating that in this study the earliest stage of attentional modulation of stimulus features are not affected by age.
Figure 4.
ERPs averaged over subjects and electrodes of interest for attended and ignored a. color and b. motion stimuli. Waveforms from the passive view task are displayed for c. color and d. motion stimuli. Horizontal arrows indicate larger N1 amplitudes in younger adults while vertical arrows indicate the N1 latency is delayed in older adults.
3.2.2 N1 modulation
N1 latency and amplitude measures were subjected to the same ANOVAs as the P1 analysis. For N1 latency to motion stimuli, no significant effects or interactions were observed. However, the N1 latency to color stimuli yielded main effects for electrode (F(7,287) = 8.25, p < 0.001) and age (F(1,41) = 12.49, p < 0.005). Post-hoc analysis revealed the N1 to peak first at PO8 (p < 0.05 compared to P7, P8, P9, P10) and that older participants displayed a longer N1 latency when collapsed across conditions and electrodes (Fig. 4a (vertical arrows) & Fig. 5a). No task main effect or interactions were observed for the color N1 latency.
Figure 5.
Significant attentional and aging effects for the P1 and N1. a. Amplitude of the P1 to motion stimuli elicits attentional enhancement when collapsed across age. b. The color N1 latency is delayed in older adults when collapsed across tasks. c. Amplitude at the color N1 is enhanced by attending and d. is smaller in older participants.
The N1 amplitude to motion stimuli displayed main effects for electrode (F(7,287) = 6.50, p < 0.001) and age (Fig. 4b & Fig. 5b; F(1,41) = 7.17, p < 0.05), such that the amplitude is greater (more negative) in younger adults (young = −3.3 mV, s.e.m. = −0.1 mV; older = −1.8 mV, s.e.m. = −0.1 mV: Fig. 4b (horizontal arrows) & Fig. 5b) and is largest at PO7 (p < 0.05 compared to P5, P6, P7, P8). The N1 amplitude to color stimuli produced main effects for electrode (F(7,287) = 6.81, p < 0.001), task (F(3,123) = 6.34, p < 0.001; attend = −2.62 mV, s.e.m. = 0.14 mV, ignore = −2.21 mV, s.e.m. = 0.12 mV, passive = −2.12 mV, s.e.m. = 0.11 mV, both = −2.31 mV, s.e.m. = 0.12 mV) and age (Fig. 4a (horizontal arrows) & Fig. 5a; F(1,41) = 5.18, p < 0.05) such that younger participants yield larger (i.e. more negative) N1 amplitudes. Post-hoc analysis showed enhancement of the attended stimuli to be larger than all other tasks (p < 0.05 for all three comparisons).
In summary, main effects of age were observed for the N1 amplitude to both color and motion stimuli, such that the amplitude decreases with age (Fig. 4 – horizontal arrows, and Fig. 5a & b). Additionally, color-selective changes at the N1 by attention were observed in both age groups and the N1 latency was delayed in older adults (Fig. 4a - vertical arrows, and Fig. 5a & Fig. 6a). Due to the absence of an age by task interaction at the N1, an age-related decline in the N1 amplitude is observed regardless whether the color is attended, ignored or passively viewed. This indicates that the N1 may partially reflect a lower-level, feature selective processing stage and that this feature selection process is slowed in older adults specifically for colored stimuli.
Figure 6.
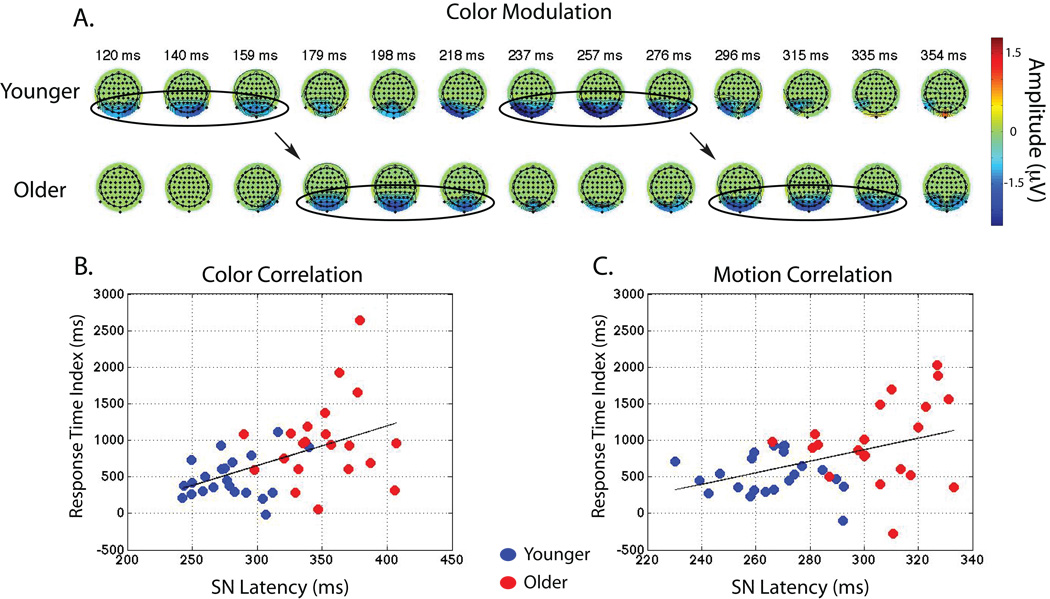
Main effects for the SN delay. a. Topographies depicting difference waves (attend-ignore) show a delay in the N1 as well as the SN in older adults. Color represents significant attentional modulation from paired t-tests at each time-point and was conducted for display purposes only. b. Correlation between the SN latency and response time during the ‘color’ and c. ‘motion’ tasks.
3.2.3 Attentional modulation: Difference waves
To explore other age-related changes in attentional influences on stimulus representation during WM encoding, the difference between attended and ignored stimuli were compared by submitting amplitude and latency measures obtained from the selection negativity (SN), to separate ANOVAs for color and motion stimuli, with electrode and age as factors.
The SN latency to color stimuli elicited main effects for electrode (F(7,287) = 3.89, p < 0.005) and age (Fig. 5c; F(1,41) = 69.32, p < 0.001), such that the color SN peaked earliest at PO7 (p < 0.05 compared to P5–P10) and was delayed in older adults. The SN latency to motion also displayed a main effect for age (Fig. 5c; F(1,41) = 56.64, p < 0.001), replicating longer latencies in older participants.
The SN amplitude to color stimuli displayed main effects for electrode (F(7,287) = 6.83, p < 0.001) and age (Fig. 5d; F(1,41) = 5.02, p < 0.05), such that the amplitude was largest at P9 (p < 0.01 compared to P5, P6, P8, P10) and smaller in older adults. Similarly, the SN amplitude elicited by motion produced a main effect for age (Fig. 5d; F(1,41) = 8.53, p < 0.01) and was smaller in older adults. The SN amplitude and latency were replicated using another analysis approach, 50% fractional area latency.
Given that both the N1 and the SN for color stimuli are smaller and delayed in older adults, a regression analysis was conducted between these two component amplitudes and latencies to determine whether early neural differences correlate with changes at later stages of processing. Results indicated that there was no relationship between the N1 and the SN amplitude or latency (p > 0.05, both comparisons), suggesting that the N1 and the SN reflect distinct neural processing stages that can be differentially affected by age.
To assess whether the SN reflects attentional selection or WM encoding/maintenance, the amplitude of the SN was compared between the first and second encoded stimulus. Paired t-tests were conducted separately for each age group and for each task (motion and color). Uncorrected for multiple comparisons, no WM load effects were observed (p > 0.05, all comparisons). Thus, the SN most likely principally represents the selection of targets, as opposed to WM encoding-related activity, which has been shown to be influenced by the number of items in WM (Vogel & Machizawa, 2004). However, this finding does not definitively exclude the possibility that early WM encoding processes are involved, as attention and memory encoding are intimately related.
These results show that attentional modulation to motion and color stimuli as evaluated by the SN was reduced in magnitude and occurred later in older adults (for example, see Fig. 6a). The similarities at the SN for motion and color processing suggest generalized age-related differences at this later stage of selective WM encoding.
3.2.4 Spectral Analysis
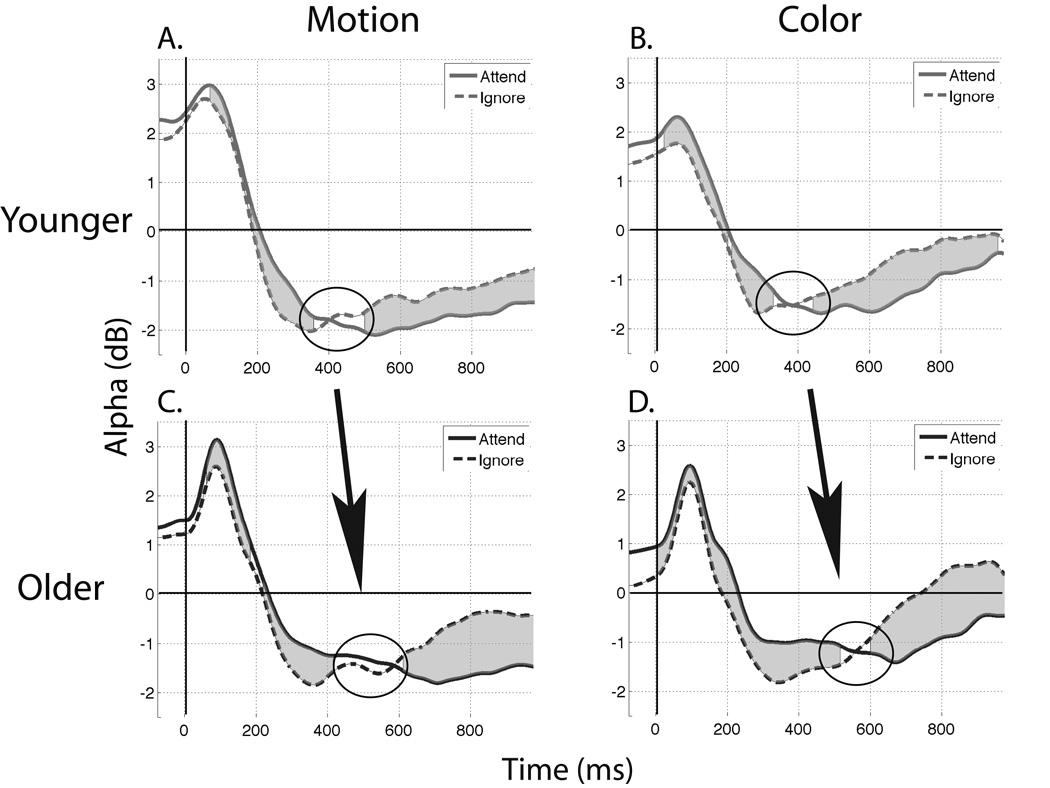
Paired t-tests were conducted within each age group between the attended and ignored stimuli at every posterior electrode to identify space-time-frequency measures of attentional modulation. Within-group contrasts displayed attentional modulation focused in the alpha band (8–12 Hz) for both motion and color stimuli (p < 0.05, FDR corrected) in posterior-occipital electrodes, including the electrodes of interest from the ERP analyses, although the effect was more pronounced centrally. Other frequency bands did not display significant modulation and were excluded from further analysis. To identify the time-course of attentional modulation in the alpha band, data were averaged over frequencies from 8–12 Hz and eight medial posterior-occipital electrodes (POZ, PO3/4, PO7/8, OZ, O1/2) for each task and stimulus type. T-tests were conducted at each time-point with an FDR correction. Both age groups displayed an early positive alpha burst at stimulus onset followed by a decrease in alpha activity below the pre-stimulus baseline (Fig. 7). Additionally, the relationship between the “attend’ and ‘ignore’ stimuli shifted, such that for motion and color stimuli in both age groups, the attended stimuli were more positive in the first half of the time course and more negative in the second half (p < 0.05 FDR corrected, at each shaded time-point from Fig. 7). Observation of the modulation time-course shows the latency at which alpha power flips between ‘attend’ and ‘ignore’ is delayed in older participants. To confirm this observation, unpaired t-tests were conducted on the latency by picking the first time-point where attended alpha equals ignored alpha in a window 300–700 ms post-stimulus onset. Results confirm an age-related delay in the alpha-band attentional modulation for both motion and color stimuli (motion: younger = 388 ms, s.e.m. = 13 ms; older = 466 ms, s.e.m. = 20 ms; color: younger = 387 ms, s.e.m. = 15 ms; older = 461 ms, s.e.m. = 27 ms; both comparisons p < 0.05).
Figure 7.
Average alpha (8–12 Hz) activity. Gray shaded areas indicate a significant difference between the two waveforms. a,b. Motion and color stimuli display an attention-related drop in alpha activity from the young participants c,d. that is delayed in older adults.
In summary, alpha activity between 8–12 Hz was shown to be modulated by attention in younger and older adults such that attention to relevant stimuli synchronizes alpha activity at early stages of processing followed by alpha desynchronization at later stages. However, the point where attended stimuli became more desynchronized than ignored stimuli was delayed in older adults. The delay in attentional evolution of the alpha band time-course provides more evidence for slowed attentional modulation in aging for motion and color feature processing.
3.3 Neural Measures to Probe Stimuli
The N1 to cue stimuli was the earliest ERP component to exhibit an aging effect, such that the amplitude was attenuated in older adults. Additionally, the N1 latency was delayed in older adults selectively for color stimuli. To identify whether these age-related changes were due to encoding-specific changes or were the result of a more generalized delay in feature processing, the N1 to motion and color stimuli were compared between the encoding and probe stages, using a repeated measures ANOVA with electrode, age and stage (cue or probe) as factors.
The N1 latency for motion probe stimuli displayed no main effects or interactions. However, the N1 latency to color stimuli elicited a main effect for age (F(1,41) = 8.16, p < 0.01; younger = 165 ms, s.e.m. = 9 ms; older = 174 ms, s.e.m. = 9ms) and an age by stage interaction (F(1,41) = 7.89, p < 0.01). Post-hoc analysis of the interaction reveals that the N1 latency was earlier for young adults to cue stimuli (161 ms, s.e.m. = 12 ms) compared to older adults response to cue stimuli (as reported above; 177 ms, s.e.m. = 14 ms). However, no age-difference was observed at probe (younger = 170 ms, s.e.m. = 13 ms; older = 171 ms, s.e.m. = 13 ms; p > 0.05). Thus, age-related neural slowing is observed only during cue stimuli, indicating that the N1 delay in aging to color stimuli is likely due to an encoding difference and not solely due to a change in feature selection.
The N1 amplitude to motion stimuli yields main effects for electrode (F(7,287) = 8.12, p < 0.001), age group (F(1,41) = 7.66, p < 0.01) and stage (F(1,41) = 7.79, p < 0.01) indicating that peak amplitude was left lateralized, larger in young adults and more negative during cue stimuli compared to the probe. Additionally, a two-way interaction was observed for electrode by stage (F(7,287) = 20.28, p < 0.001), as well as a three-way interaction for electrode by stage by age (F(7,287) = 3.24, p < 0.05). Post-hoc analysis revealed that the N1 amplitude to cue stimuli was more negative than the probe at PO7, whereas the probe was more negative than the cue at P7. Additionally, activity at both P7 and PO7 were larger in young adults (p < 0.05 for all comparisons). The N1 amplitude to color stimuli also yields main effects for electrode (F(7,287) = 8.36, p < 0.001), age (F(1,41) = 5.41, p < 0.05) and stage (F(1,41) = 11.31, p < 0.005). Similar to motion stimuli, the N1 amplitude main effects to color stimuli indicate that peak amplitude is left lateralized, larger in young adults and more negative to cue than probe stimuli. An electrode by type interaction (F(7,287) = 26.88, p < 0.001) reveals that the N1 amplitude to cue stimuli was more negative than the probe at PO7, whereas the probe was more negative than the cue at P7 (p < 0.05 for all comparisons).
In summary, young adults elicit a larger N1 response to cue and probe stimuli than older adults. Interestingly, different time-courses between cue and probe stimuli were observed as well as a differential locus of activity between the topographies, which indicate that distinct neural processes are occurring at the N1 during the cue and probe. This supports the interpretation that the age-related delay in the N1 latency for color cue stimuli may be attributed to encoding specific factors.
3.4 Neural-behavioral correlations
To better elucidate the role attentional modulation may play in subsequent WM performance and age-related changes, amplitudes and latencies to the cue stimuli P1, N1 and SN that displayed main effects of task or age were subjected to linear regression analysis with WM accuracy and RT (corrected by passive view RT) across all participants. An FDR correction was applied for multiple comparisons.
The P1 and N1 measures did not correlate with either accuracy or RT and the SN did not display a relationship to WM accuracy. However, the latency of the SN during encoding predicted subsequent WM RT for both color (Fig. 6b; amplitude: r = 0.33, p < 0.05, Spearman-Brown coefficient = 0.50; latency: r = 0.49, p < 0.001, Spearman-Brown coefficient = 0.66) and motion stimuli (Fig. 6c; amplitude: r = 0.38, p < 0.05, Spearman-Brown coefficient = 0.55; latency: r = 0.43, p < 0.005, Spearman-Brown coefficient = 0.61), such that a decrease in latency (earlier SN) predicted faster RTs at the recognition stage.
Given the observed correlations between the SN and RT, the younger and older adult groups were split in half by their RT and unpaired t-tests were conducted to determine whether the fastest responding older adults elicit similar neural activity at the SN compared to the slower responding younger adults. Indeed, the SN amplitude and latency for both color and motion stimuli was similar between the high-performing older adults and low-performing younger adults ( p > 0.05, all comparisons).
Thus, early ERP measures (P1/N1) did not correlate with WM performance, whereas later stages of attentional modulation (SN) did predict WM response time for color and motion stimuli. While it is recognized that some variance is unaccounted for by the regression, this finding suggests that later stages of attentional modulation declines with age and contributes to changes in WM performance.
4. Discussion
Age-related differences in WM recognition performance and neural measures of WM encoding for lower-level stimulus features (motion direction and color) exist even after correction for individual differences in visual acuity, perceptual discrimination, and motor response speed. During the encoding of both motion and color information there was a diminished N1 and SN amplitude, delayed SN latency and alpha band modulation in older participants. Importantly, the SN amplitude and latency predicted the RT during subsequent WM recognition. Color-specific differences were also present, such that older adults were less accurate in WM recognition and exhibited a delay in the N1 latency, a measure that was an early ERP marker of attentional modulation.
4.1 Perceptual Discrimination
In this study, to rigorously evaluate the neural changes that specifically underlie WM performance impairments with aging, we minimized alternative influences by equilibrating for individual differences in visual acuity and perceptual discriminability. Adjustments were made in the stimulus presentation parameters used in the WM task to account for the older participant’s decreased perceptual sensitivity to motion and color stimuli, as has been observed in previous reports (e.g. Ball & Sekuler, 1986; Knoblauch, Vital-Durand, & Barbur, 2001). It should also be noted that the thresholding procedure included a minor WM component, since the stimuli were separated by a two-second delay (necessary to obtain stimulus parameters in which the main WM task is not too difficult). Thus, any age-related differences in WM performance observed during the main experimental tasks were sufficiently large enough to overcome this equilibration procedure, strengthening our conclusions. Additionally, we corrected for differences in overall motor response speed by indexing all WM RT measures with the RT measures obtained in the passive view task (simple detection task of the direction of an arrow). Therefore, any observed age-related performance differences on the main WM task were due to alterations in aspects of attention and WM processes, and not secondary to differences in sensory abilities or motor speed.
Nonetheless, the adjustments made in the current experiment to equate sensory abilities do not exclude the possibility that age-related changes exist at basic visual processing stages. All visual information is derived from color and luminance contrast (Faubert, 2002). Therefore, color and luminance defined motion are considered first-order visual processes and begin cortical analyses in layer 4C of V1, the striate cortex (Merigan & Maunsell, 1993). As monkeys age, V1 synapses and myelinated fibers degrade (A. Peters, 2002), which is thought to increase the latency of information transfer in V1 neurons (Wang, Zhou, Ma, & Leventhal, 2005). This may provide a physiological basis for delays observed in early ERPs to color and luminance contrast (Fiorentini, Porciatti, Morrone, & Burr, 1996), faces (Gazzaley, et al., 2008) and shapes (Curran, et al., 2001), thereby lending credence to the idea that processing speed deficits begin at early stages of vision. The current data shows decreased N1 amplitude in older adults for motion and colored stimuli and delayed latency for colored stimuli, regardless of whether the stimuli were attended, ignored or passively viewed. While this provides some evidence that basic visual processing stages may change with age, the P1 was modulated by attention and did not display any age-related differences. Thus, the earliest stage of visual processing explored here was not affected by age.
4.2 Working Memory
In general, it has been documented that performance variability increases with age, such that not all older adults display deficits in WM accuracy or RT, and some perform as well, if not better, than younger adults (Christensen, et al., 1999; Morse, 1993). It has been proposed that once perceptual differences and generalized slowing in baseline measures (as was seen in the passive view RT) are accounted for, no additional age-related differences in WM performance or selective attention exist (Faubert, 2002; Verhaeghen & Cerella, 2002). However, both aforementioned studies find age-related WM differences do occur with increased stimulus or task complexity. The current study equilibrated for visual, perceptual and motor differences, and still observed that older participants displayed slower recognition RTs than younger adults on both color and motion WM tasks, as well as decreased recognition accuracy on the color task. It should be considered if older adults performed more slowly in order to boost their accuracy (speed-accuracy tradeoff), particularly on the remember motion task where accuracy was not impaired. Although this might have been a factor, response times of the older adults did not differ between the motion and color tasks, while WM accuracy declined only for the color task. If older adults were using a speed-accuracy tradeoff strategy in this study, we would expect different response times between the tasks, such that response time would be longer on the color task in an attempt to maintain accuracy. Given that RT is a sensitive indicator of WM performance, this finding provides evidence for age-related WM performance impairment for low-level visual features.
The age-related differences in early visual ERPs were limited to main effects and not changes in attentional modulation. The delayed N1 processing in older adults for color stimuli may help explain the selective deficit in the WM accuracy for color with age. However, this supposition remains tentative because no direct correlation was observed between the N1 latency and WM accuracy. Nonetheless, previous research may support the possibility. The P1 and N1 have been suggested to act as a sensory gain mechanism that enhances perceptual sensitivity to encode elementary features of incoming stimuli (S.A. Hillyard, Mangun, Woldorff, & Luck, 1995). Moreover, the N1 has been dissociated from the P1’s role in sensory selection as an orienting of attention to a task-relevant stimulus (Luck, Heinze, Mangun, & Hillyard, 1990). The current results show that the N1 amplitude to color stimuli is modulated by attention equally in both age groups, whereas the latency is delayed in older adults regardless of whether the color is attended, ignored or passively viewed. This would indicate that the N1 plays a role in feature selection. However, the N1 latency was not delayed at the probe in older adults, indicating that encoding color into WM contributed to the delay observed at the N1. Taken together, these results suggest that the N1 to color stimuli most likely reflects the intersection between feature selection and WM encoding. Because attention and memory are so intertwined, early stages of WM encoding may be inseparable from feature selection.
The N1 latency delay at this early stage of visual processing is the only age-related neural change that we identified that might account for the information loss that resulted in the color-specific decline in recognition accuracy with age. This interpretation that the N1 latency delay is related to changes in WM accuracy is in contrast to the conclusions of a spatial selective attention study in which physiological slowing was not linked to information loss (Curran, et al., 2001). Curran et al. (2001) utilized Posner’s spatial cueing paradigm to show that older adults display delayed P1 and N1 latencies to target stimuli, but no age-related reduction in stimulus detection accuracy was observed. The discrepant results may be due to different selective attention mechanisms for allocating attention in space versus features (Treisman & Gormican, 1988) or even time (Griffin, Miniussi, & Nobre, 2002). Additionally, stimulus or task complexity/demands may not have been great enough to observe age-related performance differences in the Curran et al. study, as accuracy for both age groups were at ceiling.
ERPs time-locked to both motion and color probe stimuli showed no age-related neural slowing of the N1 latency. This finding, coupled with a comparison of the N1 between the encoding and recognition stages that revealed differential time-courses and topographies, suggests that the color-specific neural delay observed at the N1 in older adults to cue stimuli was more likely due to changes in stimulus encoding than the result of age-related changes in feature processing. This interpretation is consistent with findings from a recent study that revealed encoding deficiencies in aging were more critical than differences at the retrieval stage in understanding the causes of episodic memory decline (Friedman, Nessler, & Johnson, 2007).
Given the difference in age-related performance between the color and motion tasks (accuracy decline only for color), it may be hypothesized that feature-specific deficits occur at lower-levels of visual WM encoding. This idea may be strengthened by virtue of the separate networks identified for color and motion processing (for reviews, see Born & Bradley, 2005; Merigan & Maunsell, 1993). In his review on the neural basis of visual deficits during aging, Spear (1993) suggests some psychophysical deficits may be due to specific changes in the magnocellular or parvocellular pathways. Indeed, within motion processing, recent research has begun to show specialized neuronal networks differ in their vulnerability to physiological changes during aging (Billino, Bremmer, & Gegenfurtner, 2008). Therefore, selective network deficits in WM encoding may supplement general aging hypotheses to account for unaffected cognitive domains.
4.3 Attentional modulation
Another explanation for the age-related WM performance deficits may lie in a difference in allocation of attention to relevant and irrelevant information (i.e., the presence of distractors in the ‘color’ and ‘motion’ tasks), as others have suggested (Gazzaley, et al., 2008; Gazzaley, Cooney, Rissman, & D'Esposito, 2005; Hasher & Zacks, 1988). Based on previous findings using the same paradigm utilized in the current study, but with face and scene stimuli (Gazzaley, et al., 2008; Gazzaley, Cooney, McEvoy, Knight, & D'Esposito, 2005), we hypothesized that while both age groups would display enhanced neural activity for early EEG measures to relevant stimuli (color and motion), only the younger participants would significantly suppress irrelevant stimuli relative to the passive baseline. Such a finding would reveal the generalization of an age-related selective suppression deficit across stimuli of varying complexity. However, the current analysis did not reveal significant suppression of irrelevant stimuli below passive view for either age group, and so unlike more complex stimuli, there were no markers to evaluate the presence or absence of a suppression deficit in older adults for lower-level stimuli. We speculate that the lack of suppression relative to passive view may be due to low-level visual stimuli, such as colored and moving dots, eliciting a strong enough bottom-up response to override top-down suppression signals. Alternatively, the lack of suppression in both age groups may be due to a large bottom-up response during passive view that results in an elevated baseline level of activity. This explanation seems unlikely, as the passively viewed P1 amplitude to color and motion stimuli were smaller than the P1 to ignored stimuli. A third alternative may be that top-down suppression does not exist at this stage of neural processing.
Despite the absence of significant suppression below passive view, significant levels of overall attentional modulation (attend > ignore) were observed as early as 100 ms post-stimulus onset for both age groups. The P1 amplitude to motion stimuli, the N1 amplitude to color stimuli, and the P1 latency to color stimuli were all modulated by attention in both age groups. None of these early markers of attentional modulation significantly changed with aging. Given that the P1 is often attributed to early sensory selection (e.g. Heinze, Luck, Mangun, & Hillyard, 1990), it may be surmised that the thresholding procedure successfully equated age groups for perceptual processing differences at the P1 to motion and color stimuli.
Following the N1 in time is the SN, a negative deflection in the ERP difference waveform (attended minus ignored stimuli) with a posterior scalp distribution that can last over 200 ms (S. A. Hillyard & Anllo-Vento, 1998). The SN has been shown to reflect selection of stimulus features such as color and motion direction (AnlloVento & Hillyard, 1996; Schoenfeld, et al., 2007). In the current study, the magnitude and timing of this attentional modulation index was reduced and delayed in older participants for both color and motion stimuli. These findings replicate previous results reporting diminished and delayed posterior SN with aging, where the SN was interpreted to reflect late strategic processes, such as updating memory traces (Kenemans, et al., 1995). Although the SN is defined by attentional modulation, the current results appear to support the view that selectively attending to a stimulus is intimately related to encoding that information into WM (Zanto & Gazzaley, 2009). Contrary to the current results, a speeded forced-choice RT task showed that the SN was not delayed in aging and the amplitude was enhanced in older adults (Talsma, Kok, & Ridderinkhof, 2006). This finding was interpreted as reflecting compensatory mechanisms in aging because no RT differences were observed between the age groups. Taken together, we conclude that the simple RT task did not tax cognitive resources, unlike the task used in the current study. The age-related delay in the SN is related to a slowing of selective WM encoding in older adults, and serves as a neural correlate for the processing speed hypothesis of cognitive aging (T. A. Salthouse, 1996).
Alpha band (8–12 Hz) activity also displayed attentional modulation in both age groups, but the latency of the transition point that defines when in the time course alpha becomes desynchronized for attended relative to ignored stimuli is delayed with aging. Increased and decreased alpha activity are thought to reflect inhibitory and excitatory brain processes, respectively (for review, see Klimesch, Sauseng, & Hanslmayr, 2007). It has been suggested that alpha band desynchronization may represent encoding stimuli into working memory (Gomarus, Althaus, Wijers, & Minderaa, 2006; Stipacek, Grabner, Neuper, Fink, & Neubauer, 2003) and, less alpha desynchronization has been related to impaired memory performance in older adults with mild cognitive impairment (van der Hiele, et al., 2007). Moreover, greater alpha desynchronization has been linked to enhanced memory performance (Doppelmayr, Klimesch, Hodlmoser, Sauseng, & Gruber, 2005; Klimesch, Doppelmayr, Pachinger, & Ripper, 1997). Therefore, the current results of an age-related delay in the relative timing of the onset of alpha desynchronization is further evidence for delayed attentional modulation during WM encoding in normal aging.
Although the P300 has been attributed to attention and memory processes and that it is delayed in older adults (for reviews, see Kok, 2000; Kugler, et al., 1993; Polich, 1996), the current experimental paradigm did not elicit a reliable P300 component in younger or older adults. This may be due to the fact that the P300 is typically elicited by oddball or go/no-go paradigms, which require stimulus detection and involves minimal WM processes. Given the current results, selective attention to visual features in the context of a challenging WM paradigm may not elicit a reliable P300 response.
4.4 Attention changes and WM impairment
Neuroimaging studies have suggested that compensatory neural mechanisms may be responsible for retaining high performance levels in a subset of older adults (Cabeza, 2002; Reuter-Lorenz, et al., 2000), which may reflect maintained cognitive efficiency (Rypma, Berger, & D'Esposito, 2002). Recent functional magnetic resonance imaging (fMRI) data has provided insight into why some older adults may not exhibit age-related differences (Gazzaley, Cooney, Rissman, et al., 2005). It was shown that the magnitude of neural suppression to irrelevant stimuli in older adults predicted subsequent WM performance. Older adults that experienced a suppression deficit also displayed decreased WM accuracy and longer response times, whereas those who did not show a suppression deficit performed as well as younger participants. Although the current study did not yield neural markers to compare suppression, the correlation of the SN (an attentional index) with subsequent WM performance shows that older participants whose processing speed (i.e., latency of the SN) was comparable to that of young adults, elicited comparable recognition RTs. This result underscores the heterogeneity found within each age group and that cognitive decline affects some older adults more than others - if at all.
It is possible that the observed WM impairment and changes in attentional modulation in older adults may reflect aspects of a general age-related decline in context processing (Braver, et al., 2001). Here, the term context refers to an internal representation of goals that biases processing in task-related neural pathways. Context representations are hypothesized to influence multiple stages of processing, including early stages such as interpretive or attentional processes, including inhibition, as well as later stages of WM maintenance and updating of contextual information. An age-related decline in context processing has been linked to disturbance in prefrontal function (Braver & Barch, 2002; West & Schwarb, 2006). Moreover, attentional modulation during visual feature selection as reflected by the selection negativity has been shown to be influenced by prefrontal damage (Yago, Duarte, Wong, Barcelo, & Knight, 2004). Given the prevalence of white matter lesions in older adults and their relationship to slowed processing speed (Rabbitt, Lunn, et al., 2007; Rabbitt, Mogapi, et al., 2007), a decline in attentional modulation and the delayed latency at the SN may be due to deficits in long-range prefrontal modulation of visual processing, which in turn contributes to decreased WM performance.
5. Conclusion
Using psychophysiological techniques in humans, we reveal age-related neural differences that directly support the processing speed hypothesis of cognitive aging and offers neural correlates of reported behavioral changes. These new findings extend previous research by showing that neural delays early in the visual processing system can be attributed to delayed WM encoding in response to first-order visual features. The first neural delay in aging was observed at the N1 only to color stimuli, suggesting a feature-specific decline in WM encoding. As this was the only stimulus-specific neural change observed in aging, the N1 latency delay may reflect the loss of information that results in a WM accuracy decline for color stimuli in older adults. The N1 delay was followed by a delay in attentional measures of selective encoding, the SN, for both types of stimuli. This provides evidence for a more generalized age-related change in attentional modulation and further highlights the interaction of selective attention and WM encoding. Furthermore, the SN was predictive of recognition RT, which serves as a direct link between encoding processing speed, attentional modulation and WM performance. Later in the time course, after the SN, alpha activity desynchronization (relationship between attended and ignored stimuli) was delayed in older participants for both motion and color stimuli, further suggesting a delay in the selective encoding of features into working memory.
Cognitive decline was evident in older adults as indexed by slower RT on WM tasks and a decreased accuracy during color WM recognition. These results were found even after equating for perceptual differences via thresholding and correcting RTs for motoric slowing and basic visual processing. Neural data suggests older adults may exhibit early stimulus-specific declines as well as later more general deficits in the selective encoding of relevant features.
Acknowledgements
This research was supported by the National Institute of Health Grants K08-AG025221 and R01-AG30395, the Ellison Medical Foundation and the American Federation for Aging Research. A special thanks to Ezequiel Morsella, and the members of the Gazzaley Lab for their insightful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AnlloVento L, Hillyard SA. Selective attention to the color and direction of moving stimuli: Electrophysiological correlates of hierarchical feature selection. [Article] Perception & Psychophysics. 1996;58(2):191–206. doi: 10.3758/bf03211875. [DOI] [PubMed] [Google Scholar]
- Ball K, Sekuler R. Improving Visual-Perception in Older Observers. [Article] Journals of Gerontology. 1986;41(2):176–182. doi: 10.1093/geronj/41.2.176. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. [Article] Journal of the Royal Statistical Society Series B-Methodological. 1995;57(1):289–300. [Google Scholar]
- Billino J, Bremmer F, Gegenfurtner KR. Differential aging of motion processing mechanisms: Evidence against general perceptual decline. [Article] Vision Research. 2008;48(10):1254–1261. doi: 10.1016/j.visres.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Born RT, Bradley DC. Structure and function of visual area MT. [Review] Annual Review of Neuroscience. 2005;28:157–189. doi: 10.1146/annurev.neuro.26.041002.131052. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DA. A theory of cognitive control, aging cognition, and neuromodulation. 2002 doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Keys BA, Carter CS, Cohen JD, Kaye JA, et al. Context processing in older adults: Evidence for a theory relating cognitive control to neurobiology in healthy aging. 2001 [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Christensen H, Mackinnon AJ, Korten AE, Jorm AF, Henderson AS, Jacomb P, et al. An analysis of diversity in the cognitive performance of elderly community dwellers: individual differences in change scores as a function of age. Psychol Aging. 1999;14(3):365–379. doi: 10.1037//0882-7974.14.3.365. [DOI] [PubMed] [Google Scholar]
- Craik FI, Salthouse TA. Handbook of Aging and Cogntion II. Mahwah, NJ: Erlbaum; 2000. [Google Scholar]
- Curran T, Hills A, Patterson MB, Strauss ME. Effects of aging on visuospatial attention: an ERP study. Neuropsychologia. 2001;39(3):288–301. doi: 10.1016/s0028-3932(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. Second ed. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M, Klimesch W, Hodlmoser K, Sauseng P, Gruber W. Intelligence related upper alpha desynchronization in a semantic memory task. [Article] Brain Research Bulletin. 2005;66(2):171–177. doi: 10.1016/j.brainresbull.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Faubert J. Visual perception and aging. [Article] Canadian Journal of Experimental Psychology-Revue Canadienne De Psychologie Experimentale. 2002;56(3):164–176. doi: 10.1037/h0087394. [DOI] [PubMed] [Google Scholar]
- Fiorentini A, Porciatti V, Morrone MC, Burr DC. Visual ageing: Unspecific decline of the responses to luminance and colour. [Article] Vision Research. 1996;36(21):3557–3566. doi: 10.1016/0042-6989(96)00032-6. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedman D, Nessler D, Johnson R. Memory encoding and retrieval in the aging brain. [Article] Clinical Eeg and Neuroscience. 2007;38(1):2–7. doi: 10.1177/155005940703800105. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight R, D'Esposito M. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proceedings of the National Academy of Science USA. 2008;105(35):13122–13126. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, McEvoy K, Knight RT, D'Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci. 2005;17(3):507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8(10):1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gomarus HK, Althaus M, Wijers AA, Minderaa RB. The effects of memory load and stimulus relevance on the EEG during a visual selective memory search task: An ERP and ERD/ERS study. [Article] Clinical Neurophysiology. 2006;117(4):871–884. doi: 10.1016/j.clinph.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Greenwood PM. The frontal aging hypothesis evaluated. J Int Neuropsychol Soc. 2000;6(6):705–726. doi: 10.1017/s1355617700666092. [DOI] [PubMed] [Google Scholar]
- Griffin IC, Miniussi C, Nobre AC. Multiple mechanisms of selective attention: differential modulation of stimulus processing by attention to space or time. [Article] Neuropsychologia. 2002;40(13):2325–2340. doi: 10.1016/s0028-3932(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Gruber T, Muller MM, Keil A, Elbert T. Selective visual-spatial attention alters induced gamma band responses in the human EEG. Clin Neurophysiol. 1999;110(12):2074–2085. doi: 10.1016/s1388-2457(99)00176-5. [DOI] [PubMed] [Google Scholar]
- Hardy JL, Delahunt PB, Okajima K, Werner JS. Senescence of spatial chromatic contrast sensitivity. I. Detection under conditions controlling for optical factors. Optical Society of America A. 2005;22(1):49–59. doi: 10.1364/josaa.22.000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter MR, Aine CJ. Brain mechanisms of visual selective attention. In: Parasuraman R, Davies DR, editors. Varieties of Attention. New York: Academic Press; 1984. pp. 293–321. [Google Scholar]
- Hasher L, Zacks RT. Working Memory, comprehension and aging: A review and a new view. In: Bower GH, editor. The Psychology of Learning and Motivation. Vol. 22. New York, NY: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Heinze HJ, Luck SJ, Mangun GR, Hillyard SA. Visual Event-Related Potentials Index Focused Attention within Bilateral Stimulus Arrays .1. Evidence for Early Selection. [Article] Electroencephalography and Clinical Neurophysiology. 1990;75(6):511–527. doi: 10.1016/0013-4694(90)90138-a. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Knight RT. Mechanisms of human attention: event-related potentials and oscillations. Neuroscience and Biobehavioral Reviews. 2001;25(6):465–476. doi: 10.1016/s0149-7634(01)00027-6. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc Natl Acad Sci U S A. 1998;95(3):781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Mangun GR, Woldorff MG, Luck SJ. Neural systems mediating selective attention. In: Gazzaniga MS, editor. The Cognitive Neurosciences. Cambridge, MA: MIT Press; 1995. pp. 665–681. [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philos Trans R Soc Lond B Biol Sci. 1998;353(1373):1257–1270. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;18(8):1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Kenemans JL, Smulders FTY, Kok A. Selective Processing of 2-Dimensional Visual-Stimuli in Young and Old Subjects - Electrophysiological Analysis. [Article] Psychophysiology. 1995;32(10):108–120. doi: 10.1111/j.1469-8986.1995.tb03302.x. [DOI] [PubMed] [Google Scholar]
- Kliegl R, Mayr U, Krampe RT. Time Accuracy Functions for Determining Process and Person Differences - an Application to Cognitive Aging. [Article] Cognitive Psychology. 1994;26(2):134–164. doi: 10.1006/cogp.1994.1005. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Pachinger T, Ripper B. Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. [Article] Neuroscience Letters. 1997;238(1–2):9–12. doi: 10.1016/s0304-3940(97)00771-4. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: The inhibition-timing hypothesis. [Review] Brain Research Reviews. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Knoblauch K, Vital-Durand F, Barbur JL. Variation of chromatic sensitivity across the life span. [Article] Vision Research. 2001;41(1):23–36. doi: 10.1016/s0042-6989(00)00205-4. [DOI] [PubMed] [Google Scholar]
- Kok A. Event-related-potential (ERP) reflections of mental resources: A review and synthesis. [Article] Biological Psychology. 1997;45(1–3):19–56. doi: 10.1016/s0301-0511(96)05221-0. [DOI] [PubMed] [Google Scholar]
- Kok A. Age-related changes in involuntary and voluntary attention as reflected in components of the event-related potential (ERP) Biol Psychol. 2000;54(1–3):107–143. doi: 10.1016/s0301-0511(00)00054-5. [DOI] [PubMed] [Google Scholar]
- Kugler CFA, Taghavy A, Platt D. The Event-Related P300 Potential Analysis of Cognitive Human Brain Aging - a Review. [Review] Gerontology. 1993;39(5):280–303. doi: 10.1159/000213544. [DOI] [PubMed] [Google Scholar]
- Lima SD, Hale S, Myerson J. How General Is General Slowing - Evidence from the Lexical Domain. [Article] Psychology and Aging. 1991;6(3):416–425. doi: 10.1037//0882-7974.6.3.416. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Heinze HJ, Mangun GR, Hillyard SA. Visual Event-Related Potentials Index Focused Attention within Bilateral Stimulus Arrays .2. Functional Dissociation of P1 and N1 Components. Electroencephalography and Clinical Neurophysiology. 1990;75(6):528–542. doi: 10.1016/0013-4694(90)90139-b. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JHR. How Parallel Are the Primate Visual Pathways. [Review] Annual Review of Neuroscience. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Morse CK. Does Variability Increase with Age - an Archival Study of Cognitive Measures. [Article] Psychology and Aging. 1993;8(2):156–164. doi: 10.1037//0882-7974.8.2.156. [DOI] [PubMed] [Google Scholar]
- Muller MM, Keil A. Neuronal synchronization and selective color processing in the human brain. J Cogn Neurosci. 2004;16(3):503–522. doi: 10.1162/089892904322926827. [DOI] [PubMed] [Google Scholar]
- Peters A. Structural changes that occur during normal aging of primate cerebral hemispheres. Neuroscience & Biobehavioral Reviews. 2002;26(7):733–741. doi: 10.1016/s0149-7634(02)00060-x. [DOI] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: A review. [Review] Journal of Neurocytology. 2002;31(8–9):581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Tigges J, et al. Neurobiological bases of age-related cognitive decline in the rhesus monkey. [Review] Journal of Neuropathology and Experimental Neurology. 1996;55(8):861–874. doi: 10.1097/00005072-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Polich J. Meta-analysis of P300 normative aging studies. Psychophysiology. 1996;33(4):334–353. doi: 10.1111/j.1469-8986.1996.tb01058.x. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Lunn M, Pendleton N, Horan M, Scott M, Thacker N, et al. White matter lesions account for all age-related declines in speed but not in intelligence. [Article] Neuropsychology. 2007;21(3):363–370. doi: 10.1037/0894-4105.21.3.363. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Mogapi O, Scott M, Thacker N, Lowe C, Horan M, et al. Effects of global atrophy, white matter lesions, and cerebral blood flow on age-related changes in speed, memory, intelligence, vocabulary, and frontal function. [Article] Neuropsychology. 2007;21(6):684–695. doi: 10.1037/0894-4105.21.6.684. [DOI] [PubMed] [Google Scholar]
- Reitan R. Validity of the trail making test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12(1):174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Milner AD, Lines CR, Phalp R. Modulation of Visual Event-Related Potentials by Spatial and Nonspatial Visual Selective Attention. Neuropsychologia. 1987;25(1A):85–96. doi: 10.1016/0028-3932(87)90045-5. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, D'Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. [Article] Journal of Cognitive Neuroscience. 2002;14(5):721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Influence of Working-Memory on Adult Age-Differences in Matrix Reasoning. [Article] British Journal of Psychology. 1993;84:171–199. doi: 10.1111/j.2044-8295.1993.tb02472.x. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The aging of working memory. Neuropsychology. 1994;8(4):535–543. [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Sara M, Faubert J. Aging, perception, and visual short-term memory for luminance-defined form. [Article] Ophthalmic and Physiological Optics. 2000;20(4):314–322. [PubMed] [Google Scholar]
- Schacter DL. EEG theta waves and psychological phenomena: a review and analysis. Biol Psychol. 1977;5(1):47–82. doi: 10.1016/0301-0511(77)90028-x. [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Hopf JM, Martinez A, Mai HM, Sattler C, Gasde A, et al. Spatio-temporal analysis of feature-based attention. Cerebral Cortex. 2007;17(10):2468–2477. doi: 10.1093/cercor/bhl154. [DOI] [PubMed] [Google Scholar]
- Spear PD. Neural Bases of Visual Deficits During Aging. [Review] Vision Research. 1993;33(18):2589–2609. doi: 10.1016/0042-6989(93)90218-l. [DOI] [PubMed] [Google Scholar]
- Stipacek A, Grabner RH, Neuper C, Fink A, Neubauer AC. Sensitivity of human EEG alpha band desynchronization to different working memory components and increasing levels of memory load. [Article] Neuroscience Letters. 2003;353(3):193–196. doi: 10.1016/j.neulet.2003.09.044. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643. [Google Scholar]
- Sutton S, Braren M, Zubin J, John ER. Evoked-Potential Correlates of Stimulus Uncertainty. [Article] Science. 1965;150(3700):1187. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Talsma D, Kok A, Ridderinkhof KR. Selective attention to spatial and non-spatial visual stimuli is affected differentially by age: Effects on event-related brain potentials and performance data. [Article] International Journal of Psychophysiology. 2006;62(2):249–261. doi: 10.1016/j.ijpsycho.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN. Trail Making Test A and B: Normative data stratified by age and education. [Article] Archives of Clinical Neuropsychology. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Treisman A, Gormican S. Feature Analysis in Early Vision - Evidence from Search Asymmetries. [Review] Psychological Review. 1988;95(1):15–48. doi: 10.1037/0033-295x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Turken AU, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JDE. Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. [Article] Neuroimage. 2008;42(2):1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes-Sosa M, Bobes MA, Rodriguez V, Pinilla T. Switching attention without shifting the spotlight: Object-based attentional modulation of brain potentials. Journal of Cognitive Neuroscience. 1988;10(1):137–151. doi: 10.1162/089892998563743. [DOI] [PubMed] [Google Scholar]
- van der Hiele K, Vein AA, Kramer CGS, Reijntjes R, van Buchem MA, Westendorp RGJ, et al. Memory activation enhances EEG abnormality in mild cognitive impairment. [Article] Neurobiology of Aging. 2007;28(1):85–90. doi: 10.1016/j.neurobiolaging.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J. Aging, executive control, and attention: a review of meta-analyses. Neuroscience and Biobehavioral Reviews. 2002;26(7):849–857. doi: 10.1016/s0149-7634(02)00071-4. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428(6984):748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Wang YC, Zhou YF, Ma YY, Leventhal AG. Degradation of signal timing in cortical areas V1 and V2 of senescent monkeys. [Article] Cerebral Cortex. 2005;15(4):403–408. doi: 10.1093/cercor/bhh143. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised Manual. New York: The Psychological Corporation; 1981. [Google Scholar]
- West R, Schwarb H. The influence of aging and frontal function on the neural correlates of regulative and evaluative aspects of cognitive control. [Article] Neuropsychology. 2006;20(4):468–481. doi: 10.1037/0894-4105.20.4.468. [DOI] [PubMed] [Google Scholar]
- Yago E, Duarte A, Wong T, Barcelo F, Knight RT. Temporal kinetics of prefrontal modulation of the extrastriate cortex during visual attention. [Article] Cognitive Affective & Behavioral Neuroscience. 2004;4(4):609–617. doi: 10.3758/cabn.4.4.609. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. Journal of Neuroscience. 2009;29(10):3059–3066. doi: 10.1523/JNEUROSCI.4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]