Abstract
IκBα serves as a central anchoring molecule in the sequestration of NF-κB transcription factor in the cytoplasm. Ubiquitination-mediated IκBα degradation immediately precedes and is required for NF-κB nuclear translocation and activation. However, the precise mechanism for the deubiquitination of IκBα is still not fully understood. Using a proteomic approach, we have identified Ubiquitin Specific Peptidase 11 (USP11) as an IκBα associated deubiquitinase. Overexpression of USP11 inhibits IκBα ubiquitination. Recombinant USP11 catalyzes deubiquitination of IκBα in vitro. Moreover, knockdown of USP11 expression enhances TNFα-induced IκBα ubiquitination and NF-κB activation. These data demonstrate that USP11 plays an important role in the downregulation of TNFα-mediated NF-κB activation through modulating IκBα stability. In addition, overexpression of a catalytically inactive USP11 mutant partially inhibits TNFα- and IKKβ-induced NF-κB activation, suggesting that USP11 also exerts a non-catalytic function in its negative regulation of TNFα-mediated NF-κB activation. Thus, IκBα ubiquitination and deubiquitination processes function as a Yin-Yang regulatory mechanism on TNFα-induced NF-κB activation.
Keywords: IκBα, NF-κB, USP11, IKKβ, TNFα
1. Introduction
The NF-κB family of transcription factors serves as a critical mediator in inflammation, immunity, development, cell proliferation and apoptosis [1, 2]. Tumor necrosis factor-α (TNFα) is a proinflammatory cytokine that activates NF-κB along with other transcription factors upon binding to its receptor [3]. In unstimulated cells, NF-κB is anchored in the cytoplasm as an inactive complex by its inhibitory proteins, which are members of the IκB family including IκBα, β and ε. IκB proteins bind to NF-κB via ankyrin repeats in the cytoplasm and block its nuclear translocation and activation [4]. However, binding of TNFα to its receptor activates an intracellular signaling pathway that results in phosphorylation of IκB proteins. TNFα-induced phosphorylation of IκB protein is achieved by the activated IκB kinase (IKK) in a complex form including two catalytic subunits, IKKα and IKKβ, and a regulatory subunit, NEMO/IKKγ [5–12]. Gene knockout studies demonstrate that IKKβ subunit is essential for TNFα-mediated IκB phosphorylation [13, 14]. This signal-induced phosphorylation labels IκB for rapid K48-linked polyubiquitination and subsequent degradation through the 26S proteasome [15, 16]. Degradation of the IκB proteins liberates NF-κB and allows its translocation to the nucleus and modulation of NF-κB-dependent gene transcription [17].
Although significant progress has been made on the mechanism of polyubiquitination and degradation of IκB proteins in the TNFα-mediated NF-κB activation, it remains unclear whether deubiquitination plays a role in the regulation of IκB turnover and NF-κB activation. Recently, Ubiquitin Specific Peptidase 15 (USP15), a COP9 signalosome (CSN)-associated deubiquitinylase, has been suggested to be involved in the deubiquitination process of IκBα to inhibit the TNFα-induced NF-κB activation through an inducible interaction of the CSN with IκBα [18]. However, the role of deubiquitination of IκB on inhibiting NF-κB activation has not been fully characterized.
In this report, we used a proteomic approach to identify IκBα-associated deubiquitinase through analyzing IκBα-co-immunoprecipitated proteins by mass spectrometry (MS). We present evidence that USP11 is constitutively associated with IκBα and attenuates IκBα degradation to negatively regulate TNFα-induced NF-κB activation. In this way, IκBα ubiquitination and deubiquitination function as a Yin-Yang regulatory mechanism on TNFα-induced NF-κB activation.
2. Materials and methods
2.1. Identification of IκBα-associated proteins by MS
HEK 293T cells were transfected with the empty vector control and Flag-IκBα vector, then lysed. Flag-IκBα was immunoprecipitated from cell lysates with anti-Flag antibodies after precleaning with normal mouse IgG. The immunoprecipitates were separated on SDS-PAGE and stained with Coomassie blue. Each lane was divided into 12 regions and proteins were identified with MS as described [19].
2.2. Antibodies and reagents
Antibodies against HA epitope, Myc epitope, NF-κB-p65, PCNA (PC-10), Ubiquitin, and IκBα (H-4) (for immunoprecipitation) were purchased from Santa Cruz Biotechnology, Inc. Antibodies against Flag epitope and β-actin were obtained from Sigma-Aldrich Co. Antibodies against Phospho-IKKα/β, IKKβ, Phospho-JNK, JNK, Phospho-Erk, Erk, Phospho-IκBα, and IκBα (for immunoblotting) were from Cell Signaling Technology, Inc. Antibody against USP11 was purchased from Bethyl Laboratories, Inc. Recombinant human TNFα was purchased from the R & D Systems. MG132 was purchased from Sigma-Aldrich Co. FuGene 6 and FuGene HD transfection reagents were obtained from Roche. Cell culture medium was obtained from Invitrogen. Nitrocellulose membrane was obtained from Bio-Rad.
2.3. Expression plasmids and Small Hairpin RNA constructs
The full-length open reading frame of the wild type (WT) human USP11, USP15, USP4 were subcloned in frame into mammalian expression vector pcDNA3.1 with an N-terminal Flag, HA or Myc tag. The USP11 mutant expression constructs were generated by site-directed PCR mutagenesis and verified by DNA sequencing. Mammalian expression vector for Flag-IκBα was obtained from Dr. Paul Chiao. The retrovirus packing vector Pegpam 3e and RDF vectors were obtained from Dr. Gianpietro Dotti. The NF-κB-dependent firefly luciferase reporter plasmid and pCMV promoter-dependent Renilla luciferase reporter plasmid were purchased from Clontech (Mountain View, California). For bacterial expression of USP11 proteins, cDNAs encoding USP11-WT and -C318A mutant were subcloned into a modified pRSET vector to generate the N-terminal His-tagged fusion proteins. A pSuper-retro vector (Ambion) was used to generate sh-RNA plasmids for USP11 by using the following target sequences: 5'-AATGAGAATCAGATCGAGTCC-3' (sh-USP11-1); 5'-AAGGCAGCCTATGTCCTCTTC-3' (sh-USP11-2); 5'-CTGGCATCGGTGTGGATGA-3' (sh-Control). The authenticity of these plasmids was confirmed by sequencing.
2.4. Transfection and luciferase reporter assay
Transfection of plasmids was performed using FuGene 6 and FuGene HD following the manufacturer's instruction. The NF-κB luciferase activity assay was previously described [20].
2.5. Quantitative RT-PCR (qRT-PCR) analyses
Total RNAs were prepared using TriZol reagent (Invitrogen) from HeLa sh-RNA Control and sh-USP11 stable cell lines. The qRT-PCR analysis was carried out as described previously [20]. The primers were designed by using the Primer3.0 software and are as follows: USP11: 5'-GGCTGCATGAGGACCTTAAT-3' and 5'-AGAGGCCGTGGAAAGTGTC-3'; IL-6: 5'-CACACAGACAGCCACTCACC-3' and 5'-TTTTCTGCCAGTGCCTCTTT-3'; GAPDH: 5'-AAGGTGAAGGTCGGAGTCAA-3' and 5'-TGGACTCCACGACGTACTCA-3'.
2.6. Establishment of the stable USP11 knockdown HeLa cell lines
The pSuper sh-Control and sh-USP11 retroviral vectors were transfected into the HEK 293T cells with retrovirus packing vector Pegpam 3e and RDF vector using FuGene 6 transfection reagent according to manufacturer’s instructions. Viral supernatants were collected after 48 and 72 hrs. HeLa cells were incubated with virus-containing medium in the presence of 4 µg/ml polybrene. Stable cell lines were established after 5 days of puromycin (2 µg/ml) selection and knockdown of the target gene was confirmed by qRT-PCR.
2.7. Preparation of nuclear and cytosolic fractions
Nuclear and cytosolic extracts were prepared as described previously [21]. In brief, cells were washed with ice-cold PBS (pH 7.4) and then lysed for 30 min on ice in buffer B (10 mM HEPES buffer, pH 7.9, containing 0.1 mM EDTA, 10 mM KCl, 0.4% (v/v) IGEPAL, 0.5 mM dithiothreitol (DTT), and 1 mM phenylmethylsulfonyl fluoride (PMSF)). Lysates were centrifuged at 15,000 ×g for 15 min. The resulting supernatants constituted cytosolic fractions. The pellets were washed three times with buffer B and then resuspended in buffer C (20 mM HEPES buffer, pH 7.9, 400 mM NaCl, 1 mM EDTA, 1 mM DTT and 1 mM PMSF) and incubated for 20 min on ice, then centrifuged at 15,000 ×g for 15 min. The supernatants were used as nuclear extracts.
2.8. Immunoblotting and immunoprecipitation
Cells were washed with ice-cold PBS (pH 7.4) and then lysed in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% IGEPAL, 0.25% Na-deoxycholate, 1 mM PMSF, 1 mM DTT, 10 µg/mL aprotinin, 10 µg/mL leupeptin, 1 mM Benzamidine, 20 mM disodium p-nitrophenylphosphate (pNPP), 0.1 mM sodium orthovanadate (OV), 10 mM sodium fluoride (NaF), phosphatase inhibitor cocktail A and B (Sigma)). The cell lysates were either subjected directly to 10% SDS-PAGE for immunoblotting analysis or immunoprecipitated 3 hrs with the indicated antibodies. Immune complexes were recovered with protein A-agarose (Santa Cruz Biotechnology) for 3 hrs, then washed three times with wash buffer containing 20 mM HEPES, pH 7.4, 50 mM NaCl, 2.5 mM MgCl2, 0.1 mM EDTA, and 0.05% Triton X-100. For immunoblotting, the immunoprecipitates or 10% whole cell lysates (WCL) were subjected to SDS-PAGE and transferred to nitrocellulose membranes. The membranes were immunoblotted with various antibodies, and the bound antibodies were visualized with horseradish peroxidase-conjugated antibodies against rabbit or mouse IgG using the ECL-Plus Western blotting system following the manufacturer's instruction.
2.9. Purification of His-USP11 recombinant proteins
His-USP11-WT and His-USP11-C318A proteins were expressed in BL-21 E. coli (Invitrogen). After the induction with 0.5 mM IPTG (isopropyl-β-D-thiogalactopyranoside) at 20°C overnight, bacteria were pelleted and lysed with extraction buffer (20 mM Tris-HCl, pH 7.8, 500 mM NaCl, 1 mM DTT, 50 mg/ml lysozyme, 10 µg/ml aprotinin, 10 µg/ml leupeptin, and 1 mM PMSF) for 45 min on ice. The bacteria were sonicated at 4°C in 1% Sarcosyl (Sigma), and then 1% Triton X-100, 5 µg/ml DNase, and 5 µg/ml RNase were added. The lysates were centrifuged at 15,000 ×g for 15 min in a Sovall SS34 rotor and the supernatants containing His fusion protein were collected. A total of 300 µl His-Select™ Nickel Affinity gel (Sigma) was incubated with each bacterial lysated supernatant at 4°C overnight. The beads were washed three times in extraction buffer containing 0.5% Triton X-100, one time in extraction buffer containing 0.1% Triton X-100. Proteins were eluted in elution buffer (250 mM Imidazole, 50 mM Tris-HCl, pH 8.0, 10% glycerol, 300 mM NaCl) and dialyzed in dialyzed buffer (20 mM HEPES, pH 7.9, 150 mM KCl, 0.2 mM EDTA, 20% glycerol). The protein concentrations were determined with a Bradford Protein Assay (Bio-Rad) and proteins were subjected to SDS-PAGE and visualized by Coomassie blue staining of the gel.
2.10. Purification of GST-IκBα fusion proteins
GST plasmids (GST-control and GST-IκBα) were transformed into E. coli BL-21 strain, and then the bacteria were grown in Luria broth at 37°C to an A600=0.6 before induction with 0.1 mM IPTG for 4 hrs at 30°C. Bacteria were pelleted and lysed with extraction buffer (50 mM Tris-HCl, pH 8.5, 100 mM NaCl, 1 mM EDTA, 1 mM DTT, 50 mg/ml lysozyme, 10 µg/ml aprotinin, 10 µg/ml leupeptin, and 1 mM PMSF) 45 min on ice. The bacteria were sonicated at 4°C in 1% Sarcosyl, after which Triton X-100 (1%), 5 µg/ml DNase, and 5 µg/ml RNase were added. The lysates were centrifuged at 15,000 ×g and the supernatants containing GST fusion proteins were collected. Fusion proteins were purified from cell lysates using glutathione-sepharose beads (Sigma Aldrich) overnight at 4°C. The beads were washed three times in extraction buffer containing 0.5% Triton X-100 and one time in extraction buffer containing 0.1% Triton X-100. The protein beads were visualized by 10% SDS-PAGE and Coomassie blue staining of the gel.
2.11. In vitro deubiquitination assay
Deubquitination assays were carried out as follows: Flag-IκBα expression vectors were co-transfected into HEK 293T cells with the vectors encoding the C-terminal V5-His-tagged IKKβ-EE constitutive mutant. Cells were lysed after treatment with MG132 (5 µg/ml) for 3 hrs. Flag-IκBα proteins in the cell lysates were immunoprecipitated with anti-Flag antibodies and co-incubated with purified recombinant His-USP11-WT or -C318A mutant for 2 hrs at 37°C in a final volume of 30 µl of deubiquitnation buffer (30 mM Tris, pH 7.6, 10 mM KCl, 5 mM MgCl2, 5% glycerol, 5 mM DTT and 2 mM ATP), and then analyzed by immunoblotting with the anti-ubiquitin antibodies. The recombinant His-USP11 proteins used in above assays were detected by Coomassie blue staining. HeLa cells were treated with TNFα (10 ng/ml) for 10 min after treatment with MG132 (5 µg/ml) for 3 hrs, and subsequently lysed and evenly divided into three aliquots. Endogenous IκBα proteins were immunoprecipitated from the lysates with anti-IκBα antibodies and co-incubated with purified recombinant His-USP11-WT or -C318A mutant for 2 hrs in the deubiquitnation buffer described above, and then analyzed by immunoblotting with the anti-ubiquitin antibodies. The recombinant His-USP11 proteins used in above assays were detected by Coomassie blue staining.
2.12. In vitro binding assay
GST-Control- and GST-IκBα-bound beads were co-incubated with purified recombinant His-USP11-WT for 3 hrs at 4°C in buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% IGEPAL, 0.25% Na-deoxycholate), and then washed 3 times with wash buffer containing 20 mM HEPES (pH 7.4), 50 mM NaCl, 2.5 mM MgCl2, 0.1 mM EDTA, and 0.05% Triton X-100, and analyzed by immunoblotting with an anti-USP11 antibody. The recombinant His-USP11 proteins and GST-Control and GST-IκBα-bound beads used in above assays were detected by Coomassie blue staining.
2.13. Enzyme-linked Immunosorbent Assay (ELISA)
HeLa cell lines with stable knockdown of USP11 and control cells were treated with or without TNFα (5 ng/ml) and the supernatants were collected at different time points. Human IL-6 concentrations in the medium were determined by ELISA according to the manufacturer's instruction.
3. Results
3.1. USP11 is associated with IκBα
Polyubiquitination and subsequent degradation of IκBα is essential for NF-κB nuclear translocation and activation [4]. Therefore, it is likely that IκBα is associated with a deubiquitinase to attenuate and control the magnitude of NF-κB activation. To test this hypothesis, we immunoprecipitated the Flag-IκBα proteins from the HEK 293T cells transfected with Flag-IκBα expression vectors and identified the co-immunoprecipitated proteins with Flag-IκBα by MS (Fig. 1A). In this assay, we identified 12 peptides from RELA and one peptide from USP11 (LYDTHITVLDAALETGQLIIMETR) as IκBα-associated proteins. USP11 is the only deubiquitinase co-immunoprecipitated with Flag-IκBα (data not shown).
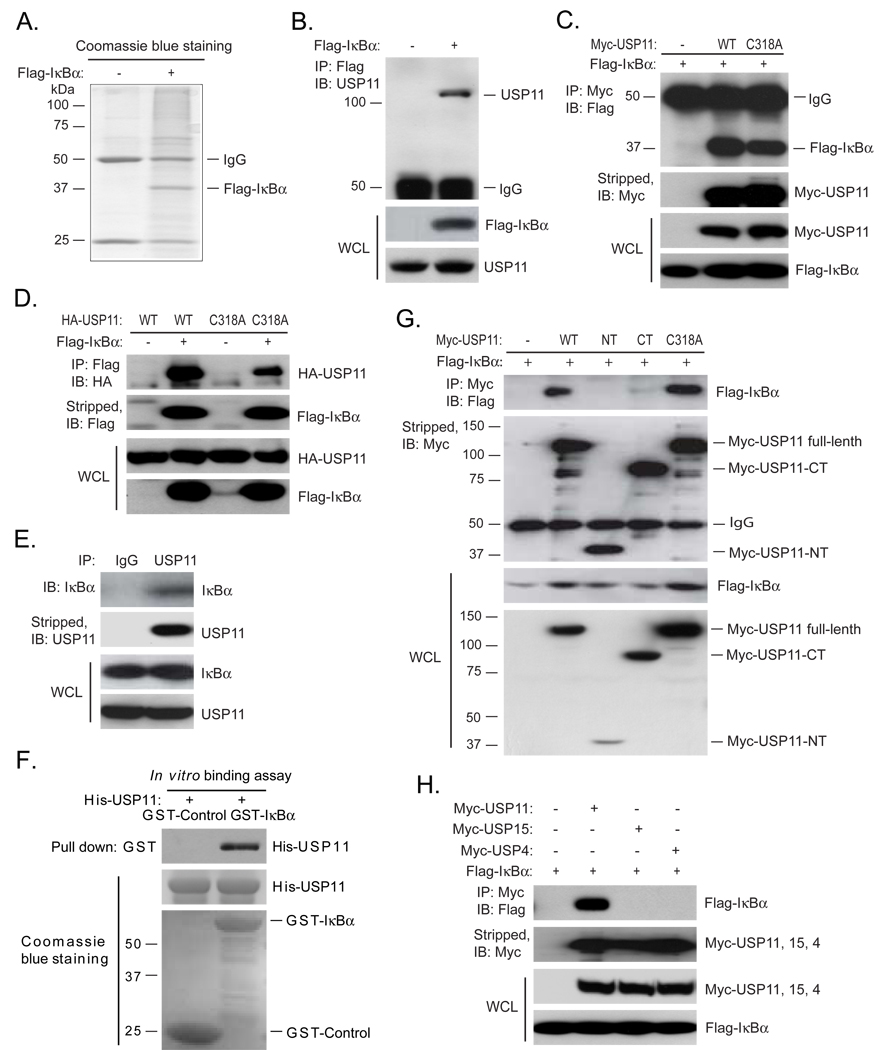
Fig. 1. Identification of USP11 as an IκBα associated deubiquitinase.
(A) Identification of Flag-IκBα-associated proteins by mass spectrometry (MS). HEK 293T cells were transfected with control vectors or expression vectors encoding the N-terminal Flag-tagged IκBα. Cells were lysed after treatment with MG132 for 3 hrs. Flag-IκBα proteins in the cell lysates were immunoprecipitated with anti-Flag antibodies and subjected to 10% SDS-PAGE. Coomassie blue-stained Flag-IκBα proteins were identified by MS. (B) Co-immunoprecipitation of Flag-IκBα and endogenous USP11 proteins. Flag-IκBα proteins in the cell lysates from the transfected HEK 293T cells were immunoprecipitated with anti-Flag antibodies and immunoblotted with anti-USP11 antibodies. (C) Co-immunoprecipitation of Myc-USP11 and Flag-IκBα proteins. Expression vectors encoding Flag-IκBα were co-transfected into HEK 293T cells with control vectors, or expression vectors encoding Myc-USP11 wild type or C318A deubiquitinase-deficient mutant, respectively. Myc-USP11 proteins in the cell lysates were immunoprecipitated with anti-Myc antibodies and immunoblotted with anti-Flag antibodies. (D) Co-immunoprecipitation of Flag-IκBα and HA-USP11 proteins. Expression vectors encoding HA-USP11-WT or -C318A mutant were co-transfected into HEK 293T cells with control vectors, or expression vectors encoding Flag-IκBα. Flag-IκBα proteins in the cell lysates were immunoprecipitated with anti-Flag antibodies and immunoblotted with anti-HA antibodies. (E) Co-immunoprecipitation of endogenous IκBα and USP11 proteins. Endogenous USP11 proteins in the HEK 293T cell lysates were immunoprecipitated with anti-USP11 antibodies and immunoblotted with anti-IκBα antibodies. (F) Recombinant GST-IκBα pulled down recombinant His-USP11. GST-Control and GST-IκBα-bound beads were co-incubated with recombinant His-USP11, co-precipitated His-USP11 were immunoblotted with anti-USP11 antibodies. The recombinant GST-Control, GST-IκBα and His-USP11 proteins were detected by Coomassie blue staining. (G) Co-immunoprecipitation of Flag-IκBα with USP11 full-length but not truncated N-terminal or C-terminal domain. Expression vectors encoding Myc-USP11 full-length (WT and C318A mutant), the truncated Myc-USP11-NT or Myc-USP11-CT were co-transfected into HEK 293T cells with control vectors, or expression vectors encoding Flag-IκBα. Myc-USP11 proteins in the cell lysates were immunoprecipitated with anti-Myc antibodies and immunoblotted with anti-Flag antibodies. (H) Co-immunoprecipitation of Flag-IκBα proteins with Myc-USP11 but not with Myc-USP15 or Myc-USP4 proteins. Expression vectors encoding Flag-IκBα were co-transfected into HEK 293T cells with control vectors, or expression vectors encoding Myc-USP11, Myc-USP15 or Myc-USP4, respectively. Myc-USP11, Myc-USP15 and Myc-USP4 proteins in the cell lysates were immunoprecipitated with anti-Myc antibodies and immunoblotted with anti-Flag antibodies.
To confirm the result from above protein identification analysis by MS, Flag-IκBα proteins in the HEK 293T cells transfected with Flag-IκBα expression vectors were immunoprecipitated from cell lysates with anti-Flag antibodies and immunoblotted with anti-USP11 antibodies. In this assay, we found that endogenous USP11 was pulled down by Flag-IκBα (Fig. 1B). To further confirm this result, we co-transfected either Flag-IκBα with Myc-USP11-WT and deubiquitinase-deficient C318A mutant or Flag-IκBα with HA-USP11-WT and -C318A mutant into HEK 293T cells and found that immunoprecipitation of Myc-USP11 pulled down Flag-IκBα and immunoprecipitation of Flag-IκBα pulled down HA-USP11 (Figs. 1C and D). The association between USP11 and IκBα were also confirmed by co-immunoprecipitation of endogenous IκBα and USP11 in HeLa cells (Fig. 1E). To rule out the possibility that association of USP11 with IκBα is through other proteins, we co-incubated recombinant His-USP11 with either recombinant GST-Control or GST-IκBα in vitro and found that GST-IκBα but not GST-Control pulled down His-USP11 (Fig. 1F). USP11 contains an N-terminal regulatory domain (USP11-NT) and a C-terminal USP domain (USP11-CT). To map out the region in USP11 protein that is required for its binding to IκBα, we co-transfected Flag-IκBα with Myc-USP11 full-length, the truncated Myc-USP11-NT or Myc-USP11-CT into HEK 293T cells and found that only Myc-USP11 full-length could efficiently pull down Flag-IκBα (Fig. 1G). Previous studies suggest that CSN-associated USP15 is involved in the control of IκBα level through deubiquitination of IκBα [18]. Interestingly, USP15 and USP4 are the closest paralogs of USP11 with which it shares 47% amino acid identity while USP15 and USP4 are 58% identical. However, both USP15 and USP4 were not identified to be IκBα-associated proteins in our MS protein identification assay. To further determine the specificity of IκBα association with USP11, we co-transfected Flag-IκBα into HEK 293T cells along with vector control, Myc-USP11, Myc-USP15 or Myc-USP4. Myc-tagged USP11, USP15 and USP4 proteins were immunoprecipitated with anti-Myc antibodies and immunoblotted with an anti-Flag antibody to detect the presence of Flag-IκBα. In this assay, we found that only Myc-USP11 but neither Myc-USP15 nor Myc-USP4 was able to pull down IκBα (Fig. 1H). Together, these results demonstrate that USP11 is specifically and constitutively associated with IκBα in the cells and both USP11 N-terminal regulatory domain and C-terminal USP domain are required for its association with IκBα.
3.2. USP11 is an IκBα deubiquitinase
IKKβ-mediated IκB phosphorylation and ubiquitination is essential in proinflammatory cytokine-induced NF-κB activation [17]. As an IκBα associated deubiquitinase, it is highly likely that USP11 acts as a major IκBα deubiquitinase. To confirm the role of USP11 as an IκBα deubiquitinase, Flag-IκBα was co-transfected into HEK 293T cells with vector control, Myc-USP11-WT, or -C318A mutant. Then cells were lysed after MG132 treatment and Flag-IκBα in the cell lysates was immunoprecipitated and immunoblotted with anti-ubiquitin antibodies. In this assay, we found that USP11-WT but not -C318A mutant abrogated ubiquitination of Flag-IκBα (Fig. 2A). To determine whether the binding of USP11 with IκBα is required for its deubiquitinase activity toward IκBα, we co-transfected Flag-IκBα with Myc-USP11 full-length, Myc-USP11-NT or Myc-USP-CT into HEK 293T cells. Cells were treated with MG132 and Flag-IκBα proteins in the cells were immunoprecipitated with anti-Flag antibodies and immunoblotted with anti-ubiquitinantibodies. In this assay, we found that only USP11 full-length wild type abrogated ubiquitination of Flag-IκBα (Fig. 2B). Since IκBα is associated with both NF-κB and USP11, we further analyzed the role of USP11 in the deubiquitination of IκBα in vitro. In this assay, overexpressed Flag-IκBα in the transfected HEK 293T cells or endogenous IκBα in HeLa cells after TNFα treatment in the presence of MG132 were immunoprecipitated from cell lysates with anti-Flag or anti-IκBα antibodies, and then incubated with recombinant His-USP11-WT or -C318A mutant. The ubiquitination level of immunoprecipitated Flag-IκBα and endogenous IκBα were found to be significantly decreased by co-incubation with recombinant His-USP11-WT but not -C318A mutant proteins (Figs. 2C and D). These results demonstrate that USP11 deubiquitinates IκBα.
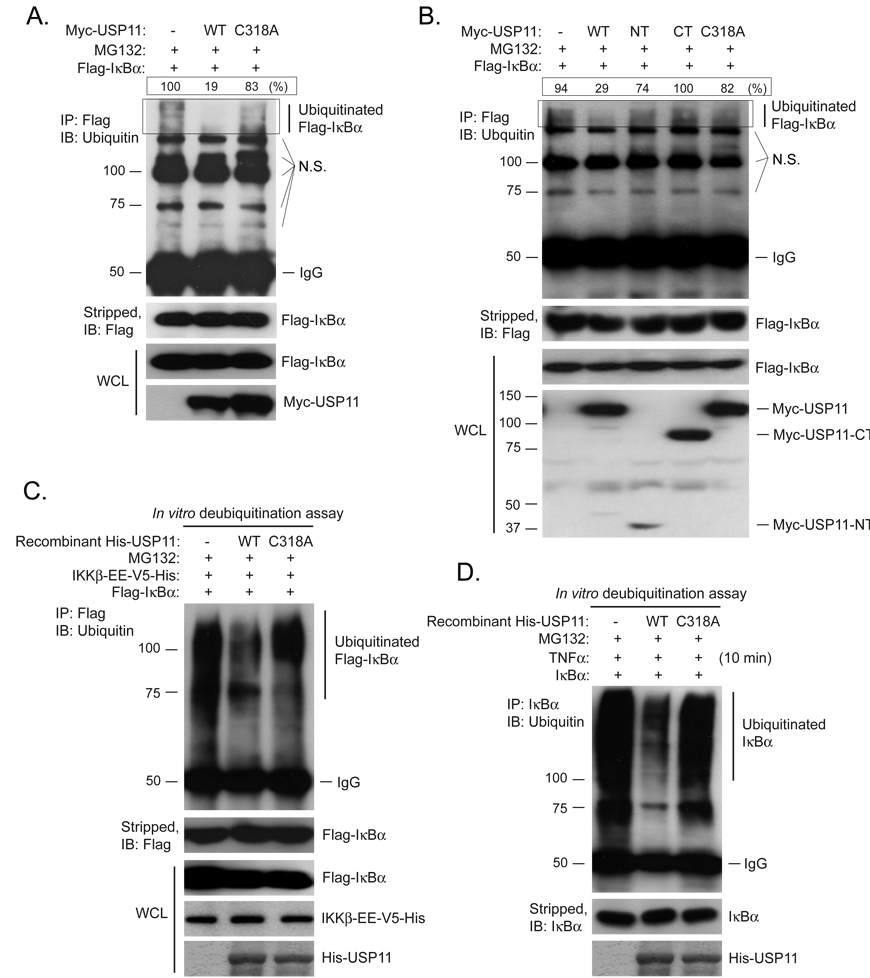
Fig. 2. USP11 is an IκBα deubiquitinase.
(A) USP11 deubiquitinase activity is required for its effect on the Flag-IκBα deubiquitination. Expression vectors encoding Flag-IκBα were co-transfected into HEK 293T cells with control vectors or expression vectors encoding Myc-USP11-WT or -C318A mutant. Cells were lysed after treatment with MG132 for 3 hrs. Flag-IκBα proteins in the cell lysates were immunoprecipitated with anti-Flag antibodies and immunoblotted with anti-ubiquitin antibodies to detect the presence of ubiquitinated Flag-IκBα. The level of polyubiquitinated Flag-IκBα in each lane was quantified from the area within the rectangle. The highest level of polyubiquitination was designated as 100% and the level of the polyubiquitination in other lanes were compared to the highest. N.S. indicates nonspecific bands. (B) Both USP11 N-terminal regulatory domain and C-terminal USP domain are required for its deubiquitinase activity toward IκBα. Expression vectors encoding Myc-USP11 full-length (WT and C318A mutant), the truncated Myc-USP11-NT or Myc-USP11-CT were co-transfected into HEK 293T cells with expression vectors encoding Flag-IκBα. Cells were lysed after treatment with MG132 for 3 hrs. Flag-IκBα poteins in the cell lysates were immunoprecipitated with anti-Flag antibodies and immunoblotted with anti-ubiquitin antibodies. The level of polyubiquitinated Flag-IκBα in each lane was quantified from the area within the rectangle. The highest level of polyubiquitination was designated as 100% and the level of the polyubiquitination in other lanes were compared to the highest. N.S. indicates nonspecific bands. (C) Recombinant USP11 deubiquitinates Flag-IκBα in vitro. HEK 293T cells were transfected with expression vectors encoding Flag-IκBα and the C-terminal V5-His-tagged IKKβ-EE constitutive mutant. Cells were lysed after treatment with MG132 for 3 hrs. Flag-IκBα proteins in the cell lysates were immunoprecipitated with anti-Flag antibodies and co-incubated with purified recombinant His-USP11-WT or -C318A mutant for 2 hrs in the deubiquitnation buffer before being analyzed by immunoblotting with the anti-ubiquitin antibodies. The recombinant His-USP11 proteins used in above assays were detected by Coomassie blue staining. (D) Recombinant USP11 deubiquitinates immunoprecipitated endogenous IκBα in vitro. HeLa cells were treated with TNFα (10 ng/ml) for 10 min in the presence of MG132 and subsequently lysed and evenly divided into three aliquots. Endogenous IκBα proteins then were immunoprecipitated from the cell lysates and co-incubated with purified recombinant His-USP11-WT or -C318A mutant for 2 hrs in the deubiquitnation buffer before being analyzed by immunoblotting with the anti-ubiquitin antibodies. The recombinant His-USP11 proteins used in above assays were detected by Coomassie blue staining.
3.3. Suppression of USP11 expression enhances TNFα-induced IκBα ubiquitination and NF-κB activation
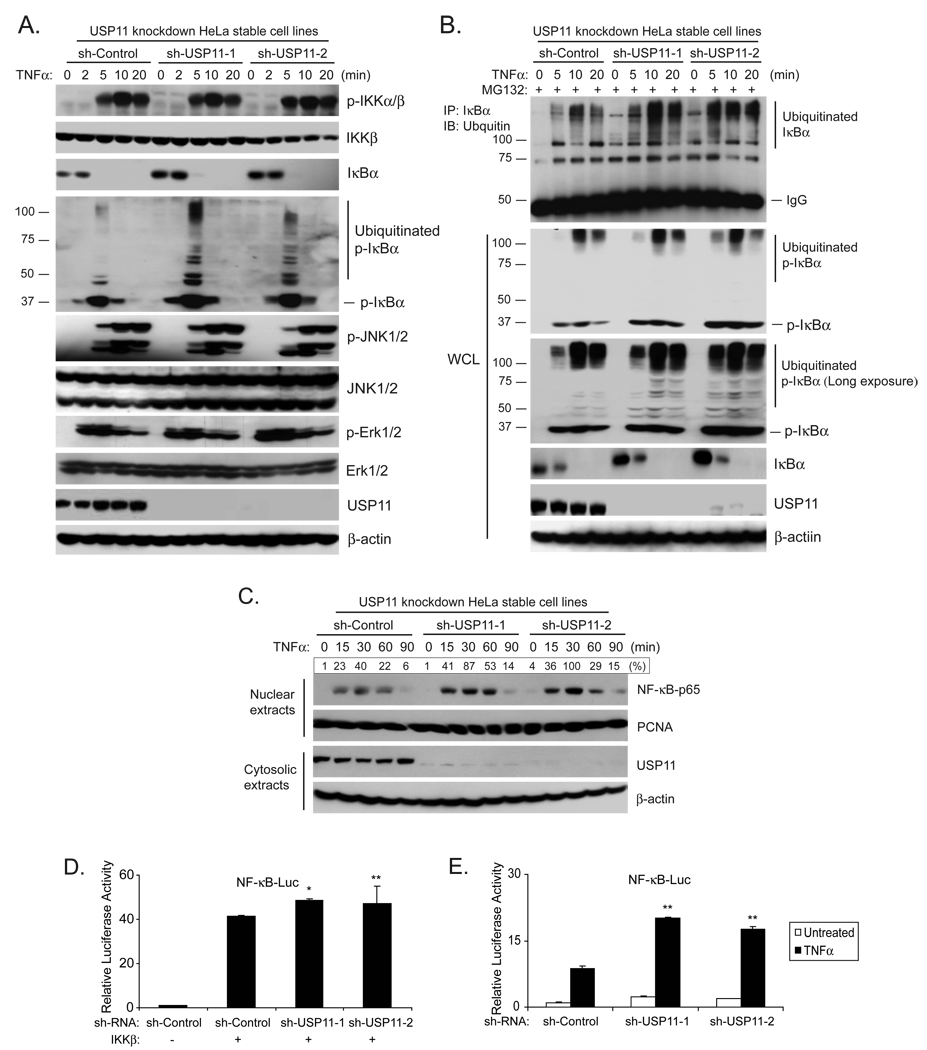
To further assess the role of USP11 in TNFα-mediated NF-κB activation, we generated USP11 knockdown HeLa stable cell lines using a retroviral transduction system. Subsequently we analyzed the effect of USP11 knockdown on the TNFα-induced IκBα ubiquitination and NF-κB activation. In this assay, the control and USP11 knockdown HeLa cells were treated with TNFα at the time periods indicated and subsequently lysed. The cellular extracts from these cells were immunoblotted with the antibodies indicated. In these assays, TNFα induced a stronger IκBα ubiquitination and a stronger NF-κB nuclear translocation in two USP11 knockdown cell lines compared to the control cells whereas TNFα induced a similar level of IKK, JNK and ERK phosphorylation. These results indicate that suppression of USP11 expression leads to an enhanced TNFα-mediated NF-κB activation (Figs. 3A, B and C). Consistent with the above results, knockdown of USP11 expression in HeLa cells resulted in higher IKKβ-and TNFα-induced NF-κB activation in NF-κB-dependent luciferase reporter assays (Figs. 3D and E). Taken together, these results demonstrate that USP11 is involved in the negative regulation of TNFα-induced NF-κB activation by modulating IκBα ubiquitination and turnover in the cells.
Fig. 3. Suppression of USP11 expression enhances TNFα-induced IKKβ-NF-κB activation.
(A) Knockdown of USP11 expression enhances the TNFα-induced IκBα ubiquitination level in the cells. USP11 knockdown HeLa cell lines were first generated after transduction of the HeLa cells with the retrovirus expressing small hairpin RNA against USP11 and selected by puromycin. The knockdown effect of USP11 expression was examined by both qRT-PCR and immunoblotting with anti-USP11 antibodies. Then the sh-Control and two sh-USP11 HeLa cell lines were either untreated or treated with TNFα (10 ng/ml) for the time points indicated and subsequently immunoblotted with the antibodies indicated. β-actin was detected as a loading control. (B) Knockdown of USP11 expression inhibits the IκBα deubiquitination process in the cells. The sh-Control and two sh-USP11 HeLa cell lines were either untreated or treated with TNFα (10 ng/ml) for the time points indicated in the presence of MG132. Endogenous IκBα proteins were immunoprecipitated with anti-IκBα and immunoblotted with anti-ubiquitin antibodies indicated. The WCL were also immunoblotted with antibodies indicated. β-actin was detected as a loading control. (C) Knockdown of USP11 expression enhances TNFα-induced NF-κB nuclear translocation. The sh-Control and two sh-USP11 HeLa cell lines were either untreated or treated with TNFα (10 ng/ml) for the time points indicated and subsequently harvested. The nuclear extracts were prepared and subjected to SDS-PAGE. Nuclear NF-κB-p65 was determined by immunoblotting with an anti-NF-κB-p65 antibody, and PCNA was detected as a loading control. (D) Knockdown of USP11 expression enhances the IKKβ-induced NF-κB activation. IKKβ, NF-κB luciferase reporter and Renilla luciferase vectors were co-transfected into HEK 293T cells with sh-Control or sh-USP11 vectors for 72 hrs. The relative luciferase activity was measured and normalized with the Renilla activity. Error bars indicate ± standard deviation in triplicate experiments. (E) Knockdown of USP11 expression enhances the TNFα-induced NF-κB activation. NF-κB luciferase reporter plasmid and Renilla luciferase plasmid were co-transfected into HEK 293T cells with sh-Control, sh-USP11 vectors for 72 hrs, and then the cells were either untreated or treated with TNFα (1 ng/ml) for 6 hrs. The relative luciferase activity was measured and normalized with the Renilla activity. Error bars indicate ± standard deviation in triplicate experiments. (* p<0.01, ** p<0.05 versus respective control)
3.4. Suppression of USP11 enhances TNFα-induced NF-κB-dependent IL-6 gene expression
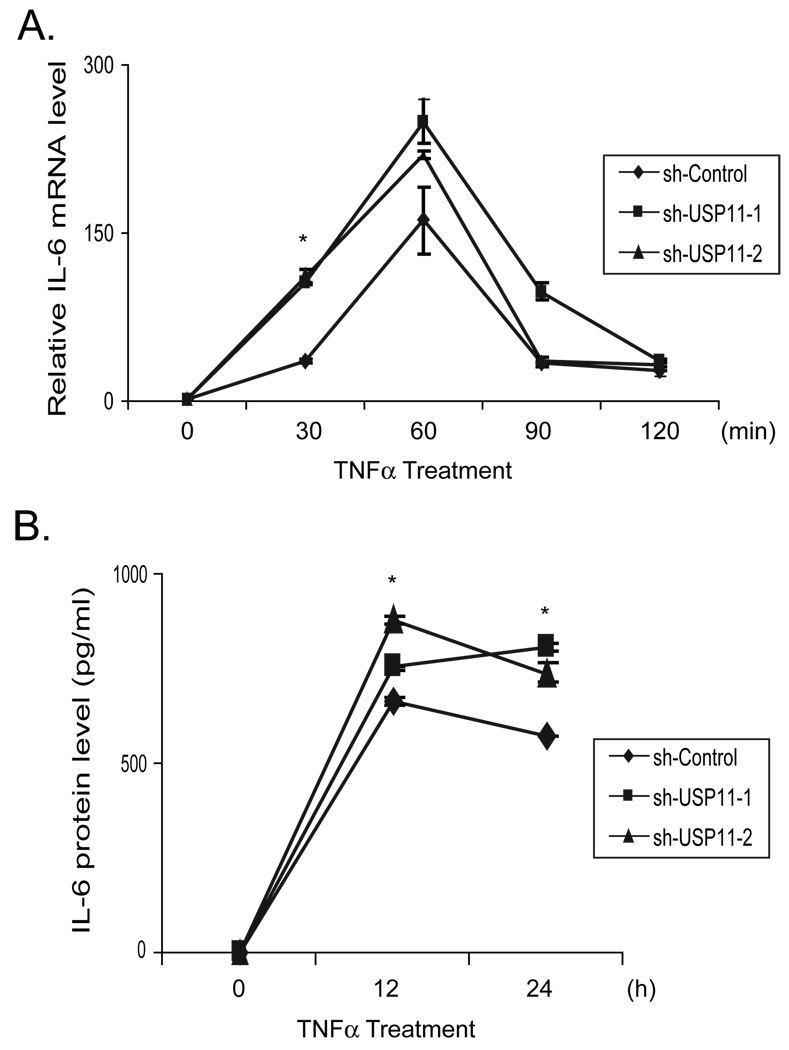
NF-κB activation is required for TNFα-induced IL-6 expression [22–24]. To determine the role of USP11 on the regulation of TNFα-induced IL-6 gene expression, total RNAs were extracted from the control and USP11 knockdown HeLa cell lines treated with TNFα for the time points indicated. Then we performed qRT-PCR to examine the TNFα-induced IL-6 expression levels in the cells. As shown in Figure 4A, TNFα induced a higher level of the IL-6 expression in USP11 knockdown cells compared to the control cells. Consistent with this result, TNFα also induced a higher level of IL-6 protein expression in the cell medium from USP11 knockdown cells compared to the control cells (Fig. 4B). These results suggest that USP11 negatively regulate TNFα-mediated gene expression through inhibiting the TNFα-induced IκBα ubiquitination and degradation.
Fig. 4. USP11 negatively regulates TNFα-mediated IL-6 gene expression.
(A) Knockdown of USP11 expression enhances the TNFα-induced NF-κB-dependent IL-6 gene expression. The sh-Control, sh-USP11 cell lines were either untreated or treated with TNFα (1 ng/ml) for the time points indicated. Total RNAs from these cells were harvested. IL-6 transcript levels in the sh-Control and sh-USP11 cell lines were measured using qRT-PCR normalized to GAPDH. The data is presented as the average of three separate experiments with standard deviation. (B) Knockdown of USP11 expression enhances the TNFα-induced IL-6 production. The sh-Control, sh-USP11 cell lines were either untreated or treated with TNFα (5 ng/ml) for the time points indicated. The supernatants from these cell cultures were collected and subjected to the human IL-6 ELISA analysis according to the manufacturer’s instructions. (* p<0.01 versus respective control)
4. Discussion
Degradation of the ubiquitinated IκBα is an essential step in TNFα-induced NF-κB nuclear translocation and activation. Therefore, stringent control of IκBα protein level is essential for preventing excessive TNFα-mediated cellular responses. Until now, the mechanism and role of IκBα deubiquitination in the negative regulation of the TNFα-induced NF-κB activation has not been completely defined. In this investigation, we took a proteomic approach to identify IκBα associated deubiquitinase and further characterize the mechanism and role of IκBα deubiquitination in the attenuation of TNFα-induced NF-κB activation. We identify that USP11 is an IκBα associated deubiquitinase and acts as IκBα deubiquitinase in vivo and in vitro. We demonstrate that USP11 is involved in the negative regulation of IKKβ-mediated NF-κB activation through targeting on IκBα. Our studies suggest that USP11 plays an important role in maintaining a delicate balance in TNFα-mediated inflammatory responses by being a part of Yin-Yang regulatory mechanism.
In our studies, USP11 acts as an IκBα deubiquitinase in vivo and in vitro. However, we found that overexpression of USP11-C318A mutant only partially rescued the inhibitory effect of USP11-WT on TNFα-induced IκBα ubiquitination (Fig. S1A) as well as TNFα- and IKKβ-induced NF-κB activation (Figs. S1B and C). These results suggest that USP11 exert both catalytic and non-catalytic functions on modulating IκBα stability to negatively regulate TNFα induced NF-κB activation.
USP11 is a member of USP subclass of protein deubiquitinase superfamily that is divided into four subclasses based on their ubiquitin-protease domains in the human genome [25]. The USP subclass represents the majority of the deubiquitinating enzymes encoded in the human genome [25]. USP11 deubiquitinase activity has been reported to be involved in the regulation of RanBPM, BRCA2 and HPV-16E7 turnover [26–28].
Previously, USP15 has been suggested to be involved in the modulation of IκBα deubiquitination through a TNFα-induced interaction with IκBα in the CSN complex [18]. However, in our co-transfection assay, we found that USP11 but not USP15 is associated with IκBα. In addition, we found that only USP domain failed to mediate the association of USP11 with IκBα and deubiquitination of IκBα. These results suggest that binding of USP11 with IκBα is required for USP11-mediated IκBα deubiquitination. Since USP15 is a CSN-associated deubiquitinase, it is possible that USP11 inhibits ubiquitination and degradation of IκBα at the early phase and USP15 fits in at a later time point in the TNFα-induced NF-κB activation. Interestingly, knockdown of both USP15 and USP11 expression leads to an increased basal protein level of IκBα, suggesting that USP11 and USP15 cooperatively modulate IκBα turnover. Therefore, further studies are needed to determine how these two USPs cooperatively downregulate TNFα-induced NF-κB activation.
A recent study on mapping a protein interaction network for TNFα/NF-κB pathway components suggests that USP11 is a RELB-associated protein [29]. Further, USP11 was reported to be involved in controlling the TNFα-mediated IKKα-p53 signaling pathway by modulating IKKα level in the cells [30]. Consistent with our findings, Yamaguchi et al. also found that knockdown of USP11 expression enhances TNFα-induced NF-κB action even though the mechanism of USP11 function is undefined in their study [30]. In our study, we did not find any obvious effect of USP11 knockdown on IKKβ protein level. Furthermore, knockdown of USP11 expression enhanced TNFα-mediated IκBα ubiquitination and NF-κB activation but had no effect on TNFα-mediated MAPK activation. These results indicate that USP11 is only involved in the negative regulation of TNFα-mediated NF-κB activation.
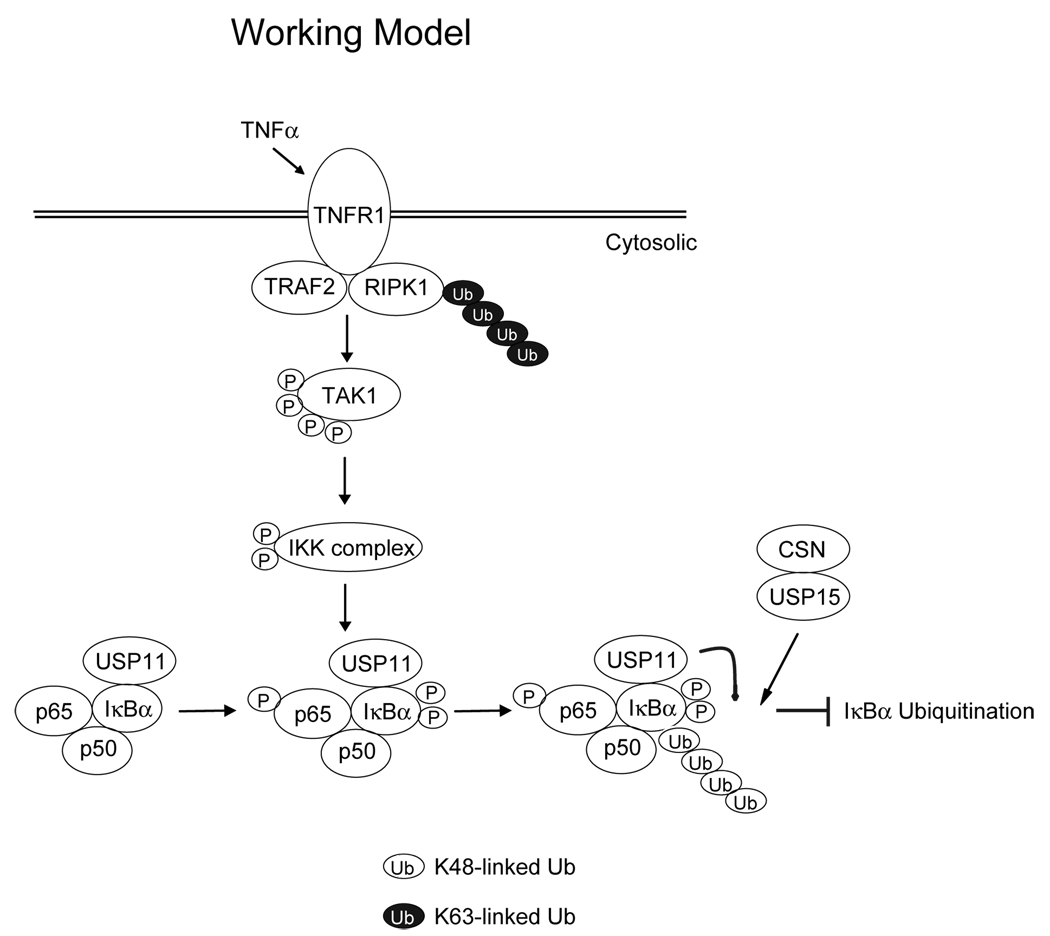
In conclusion, our data provide evidence of the physical and functional interaction between IκBα and USP11. In view of the data presented here and in previous reports, we propose a working model (Fig. 5) in which, upon TNFα-induced TAK1-IKK activation, IκBα is phosphorylated by IKKβ and subsequently ubiquitinated for degradation. Once it is ubiquitinated, IκBα can be deubiquitinated by its associated USP11 in collaboration with USP15 to prevent excessive NF-κB activation induced by TNFα. This is the first report demonstrating that a deubiquitinase is constitutively associated with IκBα and acts as an IκBα deubiquitinase in downregulating TNFα-induced NF-κB activation.
Fig. 5. A working model for the role of USP11 in the negative regulation of TNFα-mediated IκBα ubiquitination and NF-κB activation.
TNFα induces IKKβ-mediated IκBα phosphorylation and ubiquitination. USP11 is constitutively associated with IκBα and acts as an IκBα deubiquitinase to inhibit TNFα-induced IκBα ubiquitination and degradation. USP11 plays a critical role in the downregulation of the NF-κB activation, probably along with USP15.
Supplementary Material
(A) USP11 inhibits TNFα-induced IκBα ubiquitination. Empty vector or expression vectors encoding Myc-USP11-WT or -C318A mutant were transfected into HEK 293T cells. Cells were treated with TNFα (10 ng/ml) for 0, 5, 15 min after treatment with MG132 for 2 hrs, then lysed. IκBα proteins in the cell lysates were immunoprecipitated with anti-IκBα antibodies and immunoblotted with anti-ubiquitin antibodies to detect the presence of ubiquitinated IκBα. (B) USP11 inhibits TNFα-induced NF-κB activation. NF-κB luciferase reporter and control Renilla luciferase reporter vectors were co-transfected into HEK 293T cells with empty vector or expression vectors encoding USP11-WT or -C318A mutant for 42 hrs. Cells were then either untreated or treated with TNFα (1 ng/ml) for 6 hrs. The relative luciferase activity was measured and normalized with the Renilla activity. Error bars indicate ± standard deviation in triplicate experiments. (C) USP11 inhibits IKKβ-induced NF-κB activation. HA-IKKβ expression vectors, NF-κB luciferase reporter and control Renilla luciferase reporter vectors were co-transfected into HEK 293T cells with empty vector or expression vectors encoding USP11-WT or -C318A mutant. The relative luciferase activity was measured 48 hrs later and normalized with the Renilla activity. Error bars indicate ± standard deviation in triplicate experiments.
Acknowledgements
We thank the proteomics core of Diabetes Endocrinology Research Center (DERC) supported by DK079638 for protein identification. We are very grateful to Dr. Paul Chiao for providing Flag-IκBα expression construct, Dr. Xinhua Feng for HA-ubiquitin expression plasmid, and Dr. Zhijian Chen for GST-Control and GST-IκBα plasmids. This work was supported by the grants from the NIH/NCI 1R21CA106513-01A2 (to J.Y.), the American Cancer Society grant RSG-06-070-01-TBE (to J.Y.), the Fleming and Davenport Award (to H.Z.), and the National Basic Research Program (973 Program) of China grant 2007CB511900 (to T. Z.).
Abbreviations used are
- NF-κB
nuclear factor-kappa B
- TNF
tumor necrosis factor
- IKK
IkappaB kinase
- USP
ubiquitin specific peptidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baldwin AS. Jr. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 2.May MJ, Ghosh S. Immunol Today. 1998;19(2):80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- 3.Hayden MS, Ghosh S. Genes Dev. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 4.Rothwarf DM, Karin M. Sci STKE. 1999;1999(5):RE1. doi: 10.1126/stke.1999.5.re1. [DOI] [PubMed] [Google Scholar]
- 5.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. Nature. 1997;388(6642):548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 6.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. Science. 1997;278(5339):860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 7.Regnier CH, Song HY, Gao X, Goeddel DV, Cao Z, Rothe M. Cell. 1997;90(2):373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 8.Rothwarf DM, Zandi E, Natoli G, Karin M. Nature. 1998;395(6699):297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 9.Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV. Science. 1997;278(5339):866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 10.Yamaoka S, Courtois G, Bessia C, Whiteside ST, Weil R, Agou F, Kirk HE, Kay RJ, Israel A. Cell. 1998;93(7):1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 11.Zandi E, Chen Y, Karin M. Science. 1998;281(5381):1360–1363. doi: 10.1126/science.281.5381.1360. [DOI] [PubMed] [Google Scholar]
- 12.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. Cell. 1997;91(2):243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 13.Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. J Exp Med. 1999;189(11):1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka M, Fuentes ME, Yamaguchi K, Durnin MH, Dalrymple SA, Hardy KL, Goeddel DV. Immunity. 1999;10(4):421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- 15.Beg AA, Baldwin AS., Jr Genes Dev. 1993;7(11):2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- 16.Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Genes Dev. 1995;9(22):2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh S, Karin M. Cell. 2002;109 Suppl:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 18.Schweitzer K, Bozko PM, Dubiel W, Naumann M. EMBO J. 2007;26(6):1532–1541. doi: 10.1038/sj.emboj.7601600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mu JJ, Wang Y, Luo H, Leng M, Zhang J, Yang T, Besusso D, Jung SY, Qin J. J Biol Chem. 2007;282(24):17330–17334. doi: 10.1074/jbc.C700079200. [DOI] [PubMed] [Google Scholar]
- 20.Sun W, Yu Y, Dotti G, Shen T, Tan X, Savoldo B, Pass AK, Chu M, Zhang D, Lu X, Fu S, Lin X, Yang J. Cell Signal. 2009;21(1):95–102. doi: 10.1016/j.cellsig.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Y, Ge N, Xie M, Sun W, Burlingame S, Pass AK, Nuchtern JG, Zhang D, Fu S, Schneider MD, Fan J, Yang J. J Biol Chem. 2008;283(36):24497–24505. doi: 10.1074/jbc.M802825200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Libermann TA, Baltimore D. Mol Cell Biol. 1990;10(5):2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimizu H, Mitomo K, Watanabe T, Okamoto S, Yamamoto K. Mol Cell Biol. 1990;10(2):561–568. doi: 10.1128/mcb.10.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang YH, Lin JX, Vilcek J. Mol Cell Biol. 1990;10(7):3818–3823. doi: 10.1128/mcb.10.7.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R. Cell. 2005;123(5):773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Ideguchi H, Ueda A, Tanaka M, Yang J, Tsuji T, Ohno S, Hagiwara E, Aoki A, Ishigatsubo Y. Biochem J. 2002;367(Pt 1):87–95. doi: 10.1042/BJ20011851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin CH, Chang HS, Yu WC. J Biol Chem. 2008;283(23):15681–15688. doi: 10.1074/jbc.M708278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoenfeld AR, Apgar S, Dolios G, Wang R, Aaronson SA. Mol Cell Biol. 2004;24(17):7444–7455. doi: 10.1128/MCB.24.17.7444-7455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouwmeester T, Bauch A, Ruffner H, Angrand PO, Bergamini G, Croughton K, Cruciat C, Eberhard D, Gagneur J, Ghidelli S, Hopf C, Huhse B, Mangano R, Michon AM, Schirle M, Schlegl J, Schwab M, Stein MA, Bauer A, Casari G, Drewes G, Gavin AC, Jackson DB, Joberty G, Neubauer G, Rick J, Kuster B, Superti-Furga G. Nat Cell Biol. 2004;6(2):97–105. doi: 10.1038/ncb1086. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi T, Kimura J, Miki Y, Yoshida K. J Biol Chem. 2007;282(47):33943–33948. doi: 10.1074/jbc.M706282200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) USP11 inhibits TNFα-induced IκBα ubiquitination. Empty vector or expression vectors encoding Myc-USP11-WT or -C318A mutant were transfected into HEK 293T cells. Cells were treated with TNFα (10 ng/ml) for 0, 5, 15 min after treatment with MG132 for 2 hrs, then lysed. IκBα proteins in the cell lysates were immunoprecipitated with anti-IκBα antibodies and immunoblotted with anti-ubiquitin antibodies to detect the presence of ubiquitinated IκBα. (B) USP11 inhibits TNFα-induced NF-κB activation. NF-κB luciferase reporter and control Renilla luciferase reporter vectors were co-transfected into HEK 293T cells with empty vector or expression vectors encoding USP11-WT or -C318A mutant for 42 hrs. Cells were then either untreated or treated with TNFα (1 ng/ml) for 6 hrs. The relative luciferase activity was measured and normalized with the Renilla activity. Error bars indicate ± standard deviation in triplicate experiments. (C) USP11 inhibits IKKβ-induced NF-κB activation. HA-IKKβ expression vectors, NF-κB luciferase reporter and control Renilla luciferase reporter vectors were co-transfected into HEK 293T cells with empty vector or expression vectors encoding USP11-WT or -C318A mutant. The relative luciferase activity was measured 48 hrs later and normalized with the Renilla activity. Error bars indicate ± standard deviation in triplicate experiments.