Abstract
Weight gain and adiposity are often attributed to the overconsumption of unbalanced, high-fat diets however, the pattern of consumption can also contribute to associated body weight and compositional changes. The present study explored the rapid alterations in meal patterns of normal-weight rats given continuous access to high-fat diet and examined body weight and composition changes compared to chow fed controls. Ten Long-Evans rats were implanted with subcutaneous microchips for meal pattern analysis. Animals were body weight-matched and separated into two groups: high-fat or chow fed. Each group was maintained on their assigned diet for nine days and monitored for 22-hours each day for meal pattern behavior. Body weight was evaluated every other day, and body composition measures were taken prior and following diet exposure. High-fat fed animals gained more weight and adipose tissue than chow fed controls and displayed a reduced meal frequency and increased meal size. Furthermore, meal size was significantly correlated with the gain of adipose tissue. Together, these results suggest that consumption of a high-fat diet can rapidly alter meal patterns, which in turn contribute to the development of adiposity.
Keywords: meal patterns, high-fat diet, body weight, body composition
Introduction
Overweight and obesity are chronic global health issues [1, 2] associated with heart disease, diabetes, fatty liver, kidney disease, certain cancers, disability and mortality [3]. The increased incidence of obesity is often attributed to increased intake of dietary fats and decreased energy expenditure [1, 4].
Like humans, rodents also show a preference for high-fat (HF) diet [5, 6] resulting in similar metabolic consequences [4]. Specifically, rats with access to HF diet defend a higher body weight [7] and continue to over-consume HF diet even when palatability and energy density are kept constant [8]. This indicates that HF diet has orosensory and palatability characteristics as well as postingestive effects that contribute to overconsumption which, in turn, lead to increased weight gain and adiposity [9, 10].
However, the way in which foods are consumed, or their pattern of consumption, also has implications on body weight and composition [11]. For example, consuming many small meals throughout the day decreases body weight relative to consuming the same number of calories in a few large meals [12], whereas reducing meal frequency by as little as one meal per day has been shown to increase body adiposity [13]. Meal patterns are affected by a variety of physiological and environmental factors including food deprivation or restriction [14, 15], eating disorders [16], stress [17, 18], pharmacological treatments [19, 20], exercise [14, 21], social situations [22, 23], time of day [24, 25], macronutrients [25, 26], and hormones [27, 28]. The pattern of consumption ultimately determines total caloric intake; thus, examination of feeding behavior on a meal-to-meal basis provides insight into the microstructure of food intake, which can specifically determine the characteristics of ingestive behavior that influence changes in physiology. For example, prior to the development of obesity and as early as 2 days of age OLETF rats, which lack cholecystokinin-1 receptors, consume larger meals. Similarly, outbred diet-induced obese (DIO) rats display disrupted feeding patterns in the preobese state [21, 29, 30]. These animal models of obesity as well as normal-weight rats will take larger meals when given limited access to HF diet [24, 31, 32]. This suggests that taking larger meals promotes the gain of adipose tissue, but also that exposure to a HF diet provokes the taking of larger meals that may contribute to the development and/or maintenance of increased body weight and obesity. Previous studies of meal patterns and dietary manipulations have included diets of different forms (liquid, snacks, pellets), short exposure periods, the use of multiple testing apparati, and animals known to be prone to obesity [32–37] however, few have examined the meal patterns of normal-weight, non-genetically altered animals presented with continuous access to HF diet to determine how ingestive behavior changes relate to the gain of body weight and adipose tissue.
The current study assessed the development of meal patterns differences in normal-weight rats fed a HF or chow diet ad libitum over a nine-day period to test the hypothesis that access to HF diet alters the pattern of feeding, with HF fed animals consistently consuming larger meals than chow fed conspecifics, and that these animals, not known to be prone to obesity, would gain more weight and adipose tissue.
Methods
Animals
Ninety-day old male Long-Evans rats (Harlan; Indianapolis, IN) were individually housed in DietMax-ID monitoring cages (#45-DMCD2R, Accuscan Instruments; Columbus, OH). The animal room was temperature- and humidity-controlled on a 12-hr light:dark cycle. Animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals [38]. All protocols, animal handling and treatment were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati.
Animals were implanted with a subcutaneous microchip (Trovan, Electronic Identification Devices, LTD; Santa Barbara, CA), providing each animal with a unique identification number. Each DietMax-ID cage was equipped to monitor an individual animal’s food intake using a microchip-scale system. Scales were located outside of the cage with a food cup resting on top. Food tunnels were connected to the cage and are positioned above the food cup and scale allowing the animal’s head to enter the tunnel and reach the food cup. Tunnels were activated when the animal’s head entered and broke an infrared beam triggering the microchip reader. Microchip readers and scales were individually connected to a central analyzer, which recorded time of entry, duration of entry and changes in food cup weight.
Following habituation to the DietMax cages, animals were body weight matched and divided into two groups (N=5 per group). One remained on standard laboratory chow (5% fat, 3.46 kcals/g; Teklad Lab Animal Diets-#7012, Indianapolis, IN) that was powdered and the other group was given HF powdered diet (20% fat, 4.54 kcals/g; Research Diets Inc, New Brunswick, NJ); each diet was available ad libitum. Food intake was monitored for 9-days, 22-hours per day, leaving 2 hours for animal care, cage maintenance and body weight measures.
Body Weight and Composition
Body weight was recorded every-other day throughout the study. Body composition was assessed before initiation and following completion of the study using a whole body NMR machine (Echo-MRI, Waco, TX). Animals were placed into a clear Plexiglas tube and NMR-scanned for less than one minute, minimizing stress to the animal. Change in adipose and lean tissue was determined by calculating the difference of the pre- and post-study measurements.
Meal Patterns
Meal patterns were determined using data obtained from the DietMax-ID system extracted in text format recorded each day of the experiment. The extracted data included the entire set of data from a single scale stored as one line per reading read at 0.1s intervals while activated, followed by the entire set of data from that scale’s associated chip reader. A computer algorithm was established to combine both sets of data such that they would be time-stamp matched creating a behavioral food intake profile for each animal. The computer program was implemented in the C++ object-oriented programming language using the Microsoft Visual.Net Integrated Development Environment, 2005. The algorithm generates doubly linked-lists of “scale events” and “microchip events”. The scale event list was stepped through, reading by reading, to find the start of each meal event and the associated time-stamp. The time-stamp was used to index the microchip event list. Due to noise in scale readings, the starting weight of the meal event was found by averaging the scale readings 5 timestamps before the microchip reading, which indicates the initiation of a potential meal event. The ending weight of the meal event was determined in a similar manner by averaging the 5 timestamps after the microchip reading had ended. An animal’s meal size was determined to be the difference between the averaged starting scale weight and the end scale weight for a bout of eating. The inter-meal interval (Inter-MI) was established as the time between microchip recordings.
Meal Pattern Criteria
Meals were defined as having a consumption rate (CR: grams consumed per minute) of less than 0.50 g/min (chow) or 1.2 g/min (HF) as this was determined to be the maximum rate at which an adult male rat is able to consume powdered chow or HF powdered diet based on previous behavioral analyses (data not shown). Feeding events that exceeded this criterion were discarded. Feeding events were combined into a single meal if the Inter-MI, or time between meal events, was 5-min or less. The use of these criteria accounted for greater than 95% of the food consumed in a given test day. Total food intake was calculated by summing the size of each determined meal. Meal number was calculated after the criteria were applied to the data and includes the number of meals taken in the 22-hour testing period. Meal duration includes the length of a meal event (time eating + Inter-MI if it was less than 5-min), and Intra-MI was the time during a meal in which the animal is not engaged in active eating.
Food intake and meal pattern data consisted of measurements from the first 8 days of housing, as the animals were removed on the ninth day for final body weight and body composition analysis. Day 6 food intake and meal pattern data was omitted due to a computer failure, which caused the loss of up to 7 hours of data. Average meal size was not effected, therefore we expect no differences in behavior during the hours lost.
Statistics
Statistical analyses were preformed using SigmaStat v3.1. T-tests and Pearson Product moment correlation analysis were applied to the data. Data was considered significant when p<0.05. Any data outside of three standard deviations from the mean was discarded from analysis.
Results
Exposure to HF diet resulted in both weight gain and body composition changes. Animals fed the HF diet began to gain more weight than chow fed controls by day 5 and the difference reached statistical significance on day 9 (t(8)=2.457, p<0.04) (Figure 1A). During exposure to the HF diet animals gained more adipose tissue than chow fed controls (t(8)=5.275, p<0.001). Both groups gained lean tissue and there was no statistical difference between groups in this measure (Figure 1 B&C).
Figure 1.
Body weight and composition. A) Percent body weight change. Animals consuming HF diet gained significantly more weight than chow fed controls by day 9. B) Animals fed a HF diet put on more weight as adipose tissue than chow fed controls. C) There were no differences in the amount of lean tissue gained during the study between groups. *p<0.05 vs. chow fed controls, **p<0.001 vs. chow fed controls.
Animals consuming a HF diet had an overall increase in caloric consumption (t(7)=4.016, p<0.01) which occurred immediately upon diet exposure (t(7)=5.657, p<0.001) (Figure 2 C&D). However, HF fed animals consumed fewer grams of food overall (t(8)=−2.453, p<0.04), which was significant by the end of the testing period (t(6)= −3.042, p<0.02) (Figure 2 A&B).
Figure 2.
Food Intake. A) HF fed animals consumed fewer grams on days 5 and 8. B) HF fed animals consumed fewer grams of food overall. C) Animals consuming a HF diet took more calories on days 1–3. D) HF fed animals had an overall increase in average caloric intake. *p<0.05 vs. chow fed controls, **p<0.001 vs. chow fed controls.
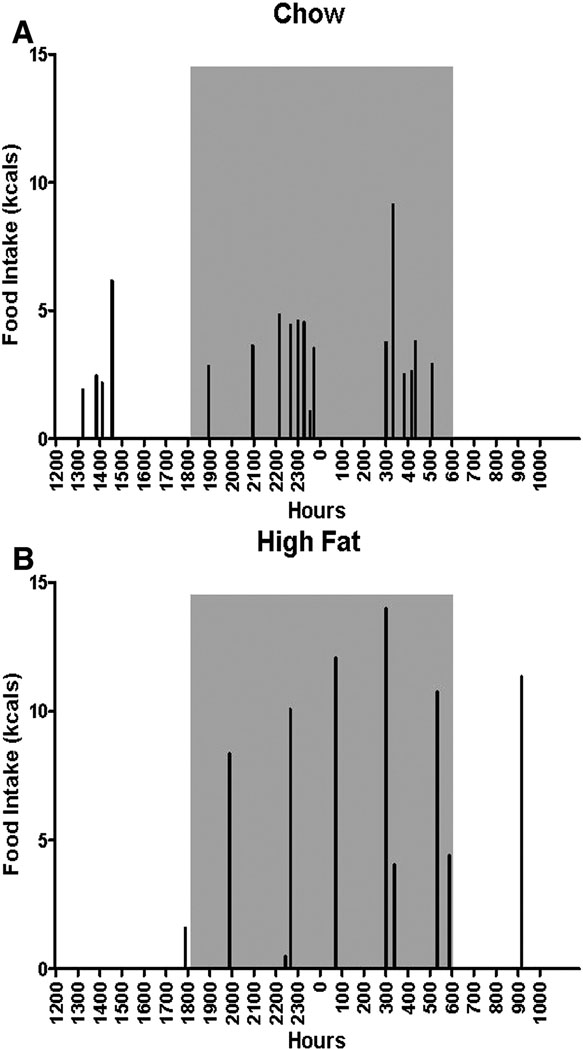
Meal patterns differed between groups depending on the type of diet. Figure 3 depicts a representative temporal pattern of food intake of one HF fed animal (upper panel) and one chow fed animal (lower panel) on day 5 of the experiment, a day in which average overall caloric food intake was similar.
Figure 3.
Representative histograms of food intake (kcals) on Day 5 in an animal fed HF diet and another fed chow. Gray boxes indicate dark cycle.
HF fed animals had an average overall decrease in meal frequency (t(7)=2.902, p<0.02), which developed by the fourth day of diet exposure (t(7)= −2.460, p<0.04) (Figure 4 A&B). However, average overall meal size was greater in animals consuming a HF diet (t(7)=3.106, p<0.02), a behavioral change that was observed at the initiation of HF diet exposure (t(7)=2.993, p<0.02) (Figure 4 C&D).
Figure 4.
Meal number and meal size. A) Chow fed controls took significantly more meals on days 4, 5 and 7. B) HF fed animals took fewer meals overall. C) Animals consuming a HF diet ate larger meals on days 1–5. D) HF fed animals consumed larger meals overall. *p<0.05 vs. chow fed controls.
Average overall meal duration, Intra-MI and Inter-MI were not different between groups (data not shown), however HF fed animals had a lower average overall satiety ratio (Inter-MI/meal size) than chow fed controls (t(7)= −2.464, p<0.04) (Figure 5A).
Figure 5.
Average satiety ratio and consumption rate. A) Animals consuming a HF diet have a significantly lower satiety ratio than chow fed controls. B) The consumption rate was significantly greater in HF versus chow fed animals. *p<0.05 vs. chow fed controls, **p<0.001 vs. chow fed controls.
Male Long-Evans rats are able to consume a powdered HF diet faster than a powdered chow diet ([39], data not shown), and in an ad lib fed state both groups spontaneously fed at a rate which was half of their maximum ability. Therefore, the average consumption rate (g/min) of all meals was greater in HF fed animals (t(7)=6.215, p<0.001) (Figure 5B).
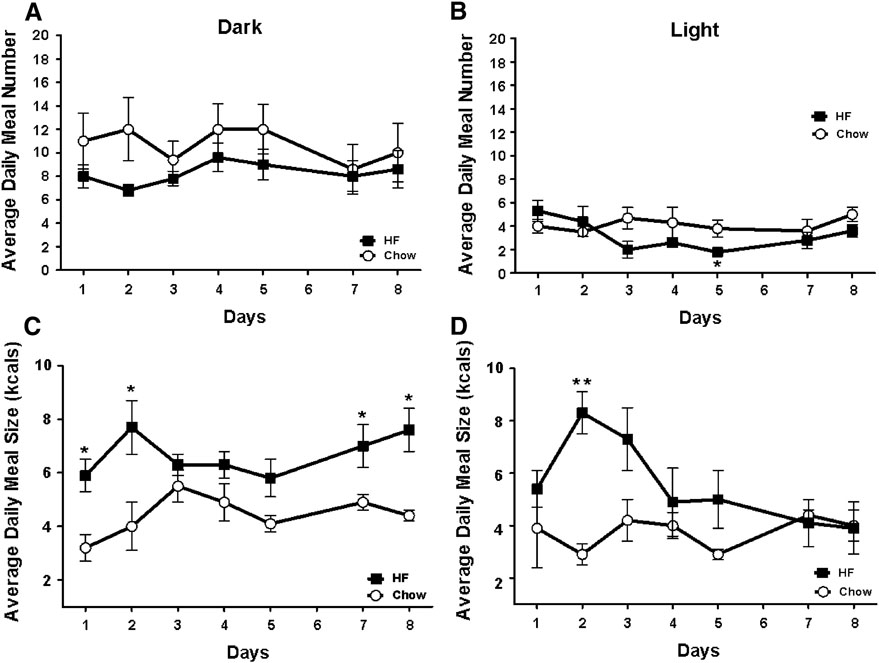
Animals consumed a similar number of meals during the dark and light cycle (Figure 6 A&B), however HF fed animals consumed larger meals during both the light and dark cycle initially and continued to take larger meals in the dark cycle compared to chow fed controls at the end of the experimental period (t(7)=3.243, p<0.01) (Figure 6 C&D).
Figure 6.
Meal patterns during the dark and light cycle. A&B) Average meal number was not significantly different during the dark or light phase. C&D) HF fed animals consumed larger meals during the dark phase on days 1, 2, 6, 7, and 8 and during the light phase on day 2. *p<0.05 vs. chow fed controls
Taken together, animals consuming a HF diet consumed more calories faster and in fewer, larger bouts than chow fed controls. Often increased caloric intake is implicated for the gain of adiposity. However, this study suggests that meal size has greater predictive value on changes in adiposity than overall food intake as larger meals positively correlated with a gain in adipose tissue (r=0.849, p<0.005) (Figure 7).
Figure 7.
The effect of meal size and food intake on the gain of adipose tissue. Each data point represents an animal’s average overall meal size/food intake versus the same animal’s change in adiposity. A) Meal size is strongly, positively correlated with the gain of adipose tissue (r=0.849, p=0.0038). B) Food intake does not have the same influence as meal size in predicting the gain in adipose tissue (r=0.662, p=0.0523).
Discussion
The present study characterized the changes in meal patterns that occur during continuous access to HF diet in normal-weight rats and related the possible differences in ingestive behavior to changes in body weight and composition. Consistent with previous studies, animals fed a HF diet consumed more calories via larger, yet fewer, meals [24, 40–42]. Furthermore, despite only an acute exposure to the HF diet (9 days), these animals gained more weight and adipose tissue than chow fed controls.
Consuming a HF diet increases adipocyte size and number [43, 44] and changes fat deposition compared to a balanced meal [45] but the way in which the food is consumed may be of equal importance to the development of adipose tissue. Meal size was immediately affected by exposure to HF diet. These animals consumed larger meals through day 5 and had a significantly larger average meal size. Some of this effect is likely driven by an increased palatability and novelty of HF diet. However, meal size remained significantly larger in this group on days 6–8 during the dark cycle, a time when these animals took the majority of their meals. It is possible that the immediate hyperphagia in the HF fed group altered signals which control meal termination, thus impairing the rats ability to appropriately end a meal as evidence by their increased meal size in their active phase. In fact, exposure to HF diet has been shown to change the sensitivity of satiety and satiation signals [46]. Satiation occurs during feeding and is measured by the size and length of a meal, where satiety is the state an animal is in following consumption and is indicated by the Inter-MI [46, 47]. It has been reported that rats maintained on an isocaloric low-fat or HF diet showed no differences in overall caloric intake or body weight gain. However, when rats previously exposed to the isocaloric HF diet were presented with a HF, high-calorie test diet they consumed more calories suggesting that prior exposure to HF diet, independent of caloric value, altered their satiety signaling and rendered them unresponsive to these factors [48].
HF fed rats from the current study initially had an increase in caloric consumption but later recovered intake to chow fed animals despite continuing to take larger meals. It is possible that exposure to HF diet initially caused impairment in satiety signaling, but continued to have lasting effects on satiation as meal size was larger but not overall caloric intake. Despite this, animals fed a HF diet had a decreased satiety ratio after just 9 days of HF food intake. However, this also may be a reflection of impaired satiation signaling. Meal initiation is not completely biologically controlled, as environmental, cultural and social factors can affect meal patterns and often override homeostatic cues [14, 22, 23, 49–51]. Meal termination, however, is largely dependent on biological signals and can be altered by diet composition [52–54]. HF fed animals have intact meal initiation control and satiety signals based on a reduced meal frequency and similar Inter-MI to chow fed animals, but satiation is impaired based on an increased meal size and possibly dietary composition which drives the overall decrease in the satiety ratio.
During the active phase, when HF fed animals took larger meals compared to chow fed controls, caloric intake was similar between groups. However, HF fed rats had increased body weight and adiposity compared with chow fed animals. Larger meals are correlated with increased retroperitoneal depot weight and fat cell number, and not with total food intake [43]. Similarly, in the present study meal size positively correlated with adiposity, but caloric intake did not, suggesting that meal size may be the best predictor of adiposity. Consistent with this, human studies indicate that caloric overconsumption in smaller, more frequent meals can prevent hyperphagic weight gain and can reduce serum lipid and cholesterol levels [12].
HF fed animals spent less time consuming food, likely because HF powdered diet can be, and was, consumed at a faster rate than powdered chow diet (data not shown, [39]). It is clear that the HF diet is consumed in a different manner than standard laboratory chow. Figure 3 illustrates this difference suggesting chow fed animals consume their meals in clusters, or in close proximity to one another leaving long periods without feeding behavior, whereas HF fed animals eat larger meals in discrete sessions, and are not temporally linked.
The cluster-like consumption pattern along with the smaller meal size could be considered nibbling in the chow fed group, where the large meals observed in HF fed animals could represent a gorging behavior. Gorging and nibbling behavior have been described in the feeding patterns of lean and obese rats [12, 31, 32] and animals exposed to a HF diet [43]. In the current study, HF fed rats behaved similarly to that of gorgers by taking half of their meals of a large size (>2g). On the other hand, 51% of the meals taken by chow fed controls were of medium size (1–2g) (data not shown). Gorging and nibbling have effects on body composition, such that nibblers gain less weight and adipose tissue even when consuming the same overall calories as gorgers [55]. Additionally, others have speculated that satiation signals respond to volume of intake, not the caloric value, which could lead to overconsumption and/or gorging of HF diet as more calories could be consumed before a similar volume of chow diet could be reached [46]. Caloric intake was similar between groups on days 4–8 and HF fed animals consumed fewer grams of food overall suggesting that these animals were able to regulate their caloric intake on a daily basis, but that their meal termination signals were altered as they continued to take larger meals.
It is well established that consuming a HF, high-energy diet stimulates weight gain, accruement of adipose tissue and a range of metabolic disruptions ultimately increasing the risk of developing life-altering and threatening diseases and disorders. Although macronutrient composition contributes to the changes in body weight and composition, this study indicates that meal patterns are a co-contributor to the physiological consequences of HF diet consumption and that these changes can occur rapidly. Studies using animal models of obesity have indicated that meal size is increased even before the onset of obesity and if these animals are pair-fed to lean controls or given an agent that reduces meal size, such as amylin or a melanin-concentrating hormone-1 receptor antagonist, obese animals reduce their body weight [30, 56–59]. This along with the current study suggests that reducing meal size may prevent the gain of weight and adipose tissue.
This study illustrates the importance of measuring meal patterns in ingestive behavior as differences may exist in meal patterns which can effect body weight, composition and physiology despite similar caloric consumption. Many food intake studies provide a snapshot of ingestive behavior following some experimental treatment, however the way in which meal patterns are potentially altered over time may provide additional evidence for how the experimental treatment is affecting ingestive behavior. The meal pattern analysis program developed and used in this current study allows for these types of experimental manipulations as it has flexible algorithms that allow investigators to account for changes in food availability, caloric content and palatability while continuing to provide continuous meal pattern information.
Ultimately, studies in which meal patterns of HF fed animals are controlled to mimic those of chow fed animals will help determine if pattern of ingestion can ameliorate some of the detrimental effects of HF diet consumption. However, the current study along with others suggests that increased meal size and decreased meal frequency, perhaps due to impaired satiation signals following HF diet consumption, contributes to weight gain and adipose tissue accumulation [31, 32, 34, 35, 60].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogden CL, et al. The epidemiology of obesity. Gastroenterology. 2007;132(6):2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM. Epidemiologic aspects of overweight and obesity in the United States. Physiol Behav. 2005;86(5):599–602. doi: 10.1016/j.physbeh.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Metabolic complications of obesity. Endocrine. 2000;13(2):155–165. doi: 10.1385/ENDO:13:2:155. [DOI] [PubMed] [Google Scholar]
- 4.Woods SC, et al. Consumption of a high-fat diet alters the homeostatic regulation of energy balance. Physiol Behav. 2004;83(4):573–578. doi: 10.1016/j.physbeh.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 5.Castonguay TW, Dallman MF, Stern JS. Some metabolic and behavioral effects of adrenalectomy on obese Zucker rats. Am J Physiol. 1986;251(5 Pt 2):R923–R933. doi: 10.1152/ajpregu.1986.251.5.R923. [DOI] [PubMed] [Google Scholar]
- 6.Shor-Posner G, et al. Meal patterns of macronutrient intake in rats with particular dietary preferences. Am J Physiol. 1994 4 Pt 2;266:R1395–R1402. doi: 10.1152/ajpregu.1994.266.4.R1395. [DOI] [PubMed] [Google Scholar]
- 7.Peck JW. Rats defend different body weights depending on palatability and accessibility of their food. J Comp Physiol Psychol. 1978;92(3):555–570. doi: 10.1037/h0077474. [DOI] [PubMed] [Google Scholar]
- 8.Warwick ZS, Weingarten HP. Determinants of high-fat diet hyperphagia: experimental dissection of orosensory and postingestive effects. Am J Physiol. 1995;269(1 Pt 2):R30–R37. doi: 10.1152/ajpregu.1995.269.1.R30. [DOI] [PubMed] [Google Scholar]
- 9.Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81(5):773–779. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg D, Smith GP. The controls of fat intake. Psychosom Med. 1996;58(6):559–569. doi: 10.1097/00006842-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Nicklas TA, et al. Eating patterns, dietary quality and obesity. J Am Coll Nutr. 2001;20(6):599–608. doi: 10.1080/07315724.2001.10719064. [DOI] [PubMed] [Google Scholar]
- 12.Fabry P, Tepperman J. Meal frequency--a possible factor in human pathology. Am J Clin Nutr. 1970;23(8):1059–1068. doi: 10.1093/ajcn/23.8.1059. [DOI] [PubMed] [Google Scholar]
- 13.Chapelot D, et al. Consequence of omitting or adding a meal in man on body composition, food intake, and metabolism. Obesity (Silver Spring) 2006;14(2):215–227. doi: 10.1038/oby.2006.28. [DOI] [PubMed] [Google Scholar]
- 14.Levitsky DA. Feeding patterns of rats in response to fasts and changes in environmental conditions. Physiol Behav. 1970;5(3):291–300. doi: 10.1016/0031-9384(70)90101-0. [DOI] [PubMed] [Google Scholar]
- 15.Larue-Achagiotis C, Le Magnen J. Changes of meal patterns induced by food deprivation: metabolic correlates. Neurosci Biobehav Rev. 1980;4 Suppl 1:25–27. doi: 10.1016/0149-7634(80)90043-3. [DOI] [PubMed] [Google Scholar]
- 16.Boggiano MM, et al. High intake of palatable food predicts binge-eating independent of susceptibility to obesity: an animal model of lean vs obese binge-eating and obesity with and without binge-eating. Int J Obes (Lond) 2007;31(9):1357–1367. doi: 10.1038/sj.ijo.0803614. [DOI] [PubMed] [Google Scholar]
- 17.Varma M, et al. Effect of operative stress on food intake and feeding pattern in female rats. Nutrition. 1999;15(5):365–372. doi: 10.1016/s0899-9007(99)00033-7. [DOI] [PubMed] [Google Scholar]
- 18.Morgan CM, et al. Loss of control over eating, adiposity, and psychopathology in overweight children. Int J Eat Disord. 2002;31(4):430–441. doi: 10.1002/eat.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davoodi N, et al. Hyperphagia and increased meal size are responsible for weight gain in rats treated sub-chronically with olanzapine. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1415-1. [DOI] [PubMed] [Google Scholar]
- 20.Leibowitz SF, et al. Effects of serotonin and the serotonin blocker metergoline on meal patterns and macronutrient selection. Pharmacol Biochem Behav. 1993;45(1):185–194. doi: 10.1016/0091-3057(93)90103-z. [DOI] [PubMed] [Google Scholar]
- 21.Moran TH. Unraveling the obesity of OLETF rats. Physiol Behav. 2008;94(1):71–78. doi: 10.1016/j.physbeh.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Castro JM, de Castro ES. Spontaneous meal patterns of humans: influence of the presence of other people. Am J Clin Nutr. 1989;50(2):237–247. doi: 10.1093/ajcn/50.2.237. [DOI] [PubMed] [Google Scholar]
- 23.de Castro JM. Genes, the environment and the control of food intake. Br J Nutr. 2004;92 Suppl 1:S59–S62. doi: 10.1079/bjn20041143. [DOI] [PubMed] [Google Scholar]
- 24.Farley C, et al. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res. 2003;11(7):845–851. doi: 10.1038/oby.2003.116. [DOI] [PubMed] [Google Scholar]
- 25.Tempel DL, et al. Nocturnal patterns of macronutrient intake in freely feeding and food-deprived rats. Am J Physiol. 1989;256(2 Pt 2):R541–R548. doi: 10.1152/ajpregu.1989.256.2.R541. [DOI] [PubMed] [Google Scholar]
- 26.Burton-Freeman B, Gietzen Dw, Schneeman BO. Meal pattern analysis to investigate the satiating potential of fat, carbohydrate, and protein in rats. Am J Physiol. 1997;273(6 Pt 2):R1916–R1922. doi: 10.1152/ajpregu.1997.273.6.R1916. [DOI] [PubMed] [Google Scholar]
- 27.Lutz TA, et al. Amylin decreases meal size in rats. Physiol Behav. 1995;58(6):1197–1202. doi: 10.1016/0031-9384(95)02067-5. [DOI] [PubMed] [Google Scholar]
- 28.Zorrilla EP, et al. Leptin and post-prandial satiety: acute central leptin more potently reduces meal frequency than meal size in the rat. Psychopharmacology (Berl) 2005;177(3):324–335. doi: 10.1007/s00213-004-1952-1. [DOI] [PubMed] [Google Scholar]
- 29.Cottone P, et al. Feeding microstructure in diet-induced obesity susceptible versus resistant rats: central effects of urocortin 2. J Physiol. 2007;583(Pt 2):487–504. doi: 10.1113/jphysiol.2007.138867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blumberg S, et al. Independent ingestion and microstructure of feeding patterns in infant rats lacking CCK-1 receptors. Am J Physiol Regul Integr Comp Physiol. 2006;290(1):R208–R218. doi: 10.1152/ajpregu.00379.2005. [DOI] [PubMed] [Google Scholar]
- 31.Becker EE, Grinker JA. Meal patterns in the genetically obese Zucker rat. Physiol Behav. 1977;18(4):685–692. doi: 10.1016/0031-9384(77)90067-1. [DOI] [PubMed] [Google Scholar]
- 32.Castonguay TW, et al. Meal patterns in the genetically obese Zucker rat: a reexamination. Physiol Behav. 1982;28(5):911–916. doi: 10.1016/0031-9384(82)90213-x. [DOI] [PubMed] [Google Scholar]
- 33.Wellman PJ, et al. An inexpensive food cup for use in a commercially available food monitoring system. Physiol Behav. 2004;83(3):525–530. doi: 10.1016/j.physbeh.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Smith JC. Microstructure of the rat's intake of food, sucrose and saccharin in 24-hour tests. Neurosci Biobehav Rev. 2000;24(2):199–212. doi: 10.1016/s0149-7634(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 35.Zorrilla EP, et al. Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol. 2005;288(6):R1450–R1467. doi: 10.1152/ajpregu.00175.2004. [DOI] [PubMed] [Google Scholar]
- 36.Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci. 1992;106(1):217–228. [PubMed] [Google Scholar]
- 37.Levitsky DA. Feeding conditions and intermeal relationships. Physiol Behav. 1974;12(5):779–787. doi: 10.1016/0031-9384(74)90014-6. [DOI] [PubMed] [Google Scholar]
- 38.C.o.L.S. Institute of Laboratory Animal Resources, Editor. Washington D.C.: National Academy Press; 1996. Guide for the Care and use of Laboratory Animals. [Google Scholar]
- 39.Miller GD, et al. Rats on a macronutrient self-selection diet eat most meals from a single food cup. Appetite. 1994;23(1):67–78. doi: 10.1006/appe.1994.1035. [DOI] [PubMed] [Google Scholar]
- 40.Synowski SJ, Smart AB, Warwick ZS. Meal size of high-fat food is reliably greater than high-carbohydrate food across externally-evoked single-meal tests and long-term spontaneous feeding in rat. Appetite. 2005;45(2):191–194. doi: 10.1016/j.appet.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Warwick ZS, et al. Independent effects of diet palatability and fat content on bout size and daily intake in rats. Physiol Behav. 2003;80(2–3):253–258. doi: 10.1016/j.physbeh.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Warwick ZS, et al. Behavioral components of high-fat diet hyperphagia: meal size and postprandial satiety. Am J Physiol Regul Integr Comp Physiol. 2000;278(1):R196–R200. doi: 10.1152/ajpregu.2000.278.1.R196. [DOI] [PubMed] [Google Scholar]
- 43.Drewnowski A, et al. Meal-taking behavior is related to predisposition to dietary obesity in the rat. Physiol Behav. 1984;32(1):61–67. doi: 10.1016/0031-9384(84)90071-4. [DOI] [PubMed] [Google Scholar]
- 44.Braun T, Fabry P. Adaptation to the pattern of food intake: changes in adipose tissue. Adv Enzyme Regul. 1969;7:49–55. doi: 10.1016/0065-2571(69)90009-0. [DOI] [PubMed] [Google Scholar]
- 45.Votruba SB, et al. Meal fatty acid uptake in visceral fat in women. Diabetes. 2007;56(10):2589–2597. doi: 10.2337/db07-0439. [DOI] [PubMed] [Google Scholar]
- 46.Blundell JE, MacDiarmid JI. Fat as a risk factor for overconsumption: satiation, satiety, and patterns of eating. J Am Diet Assoc. 1997;97(7 Suppl):S63–S69. doi: 10.1016/s0002-8223(97)00733-5. [DOI] [PubMed] [Google Scholar]
- 47.Bensaid A, et al. A high-protein diet enhances satiety without conditioned taste aversion in the rat. Physiol Behav. 2003;78(2):311–320. doi: 10.1016/s0031-9384(02)00977-0. [DOI] [PubMed] [Google Scholar]
- 48.Savastano DM, Covasa M. Adaptation to a high-fat diet leads to hyperphagia and diminished sensitivity to cholecystokinin in rats. J Nutr. 2005;135(8):1953–1959. doi: 10.1093/jn/135.8.1953. [DOI] [PubMed] [Google Scholar]
- 49.de Castro JM. Physiological, environmental, and subjective determinants of food intake in humans: a meal pattern analysis. Physiol Behav. 1988;44(4–5):651–659. doi: 10.1016/0031-9384(88)90331-9. [DOI] [PubMed] [Google Scholar]
- 50.Popkin BM, Duffey K, Gordon-Larsen P. Environmental influences on food choice, physical activity and energy balance. Physiol Behav. 2005;86(5):603–613. doi: 10.1016/j.physbeh.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 51.Woods SC, Ramsay DS. Pavlovian influences over food and drug intake. Behav Brain Res. 2000;110(1–2):175–182. doi: 10.1016/s0166-4328(99)00194-1. [DOI] [PubMed] [Google Scholar]
- 52.Karhunen LJ, et al. Effect of protein, fat, carbohydrate and fibre on gastrointestinal peptide release in humans. Regul Pept. 2008;149(1–3):70–78. doi: 10.1016/j.regpep.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Paulino G, et al. Adaptation of lipid-induced satiation is not dependent on caloric density in rats. Physiol Behav. 2008;93(4–5):930–936. doi: 10.1016/j.physbeh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D'Alessio D. Intestinal hormones and regulation of satiety: the case for CCK, GLP-1, PYY, and Apo A-IV. JPEN J Parenter Enteral Nutr. 2008;32(5):567–568. doi: 10.1177/0148607108322401. [DOI] [PubMed] [Google Scholar]
- 55.Wheeler J, et al. Weight cycling in female rats subjected to varying meal patterns. Am J Physiol. 1990;258(1 Pt 2):R124–R129. doi: 10.1152/ajpregu.1990.258.1.R124. [DOI] [PubMed] [Google Scholar]
- 56.Roth JD, et al. Antiobesity effects of the beta-cell hormone amylin in combination with phentermine or sibutramine in diet-induced obese rats. Int J Obes (Lond) 2008;32(8):1201–1210. doi: 10.1038/ijo.2008.91. [DOI] [PubMed] [Google Scholar]
- 57.Furnes MW, Zhao CM, Chen D. Development of Obesity is Associated with Increased Calories per Meal Rather than per Day. A Study of High-Fat Diet- Induced Obesity in Young Rats. Obes Surg. 2009 doi: 10.1007/s11695-009-9863-1. [DOI] [PubMed] [Google Scholar]
- 58.Kowalski TJ, et al. Melanin-concentrating hormone-1 receptor antagonism decreases feeding by reducing meal size. Eur J Pharmacol. 2004;497(1):41–47. doi: 10.1016/j.ejphar.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 59.Bi S, et al. A role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2001;281(1):R254–R260. doi: 10.1152/ajpregu.2001.281.1.R254. [DOI] [PubMed] [Google Scholar]
- 60.Castonguay TW, Kaiser LL, Stern JS. Meal pattern analysis: artifacts, assumptions and implications. Brain Res Bull. 1986;17(3):439–443. doi: 10.1016/0361-9230(86)90252-2. [DOI] [PubMed] [Google Scholar]