Abstract
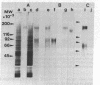
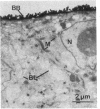
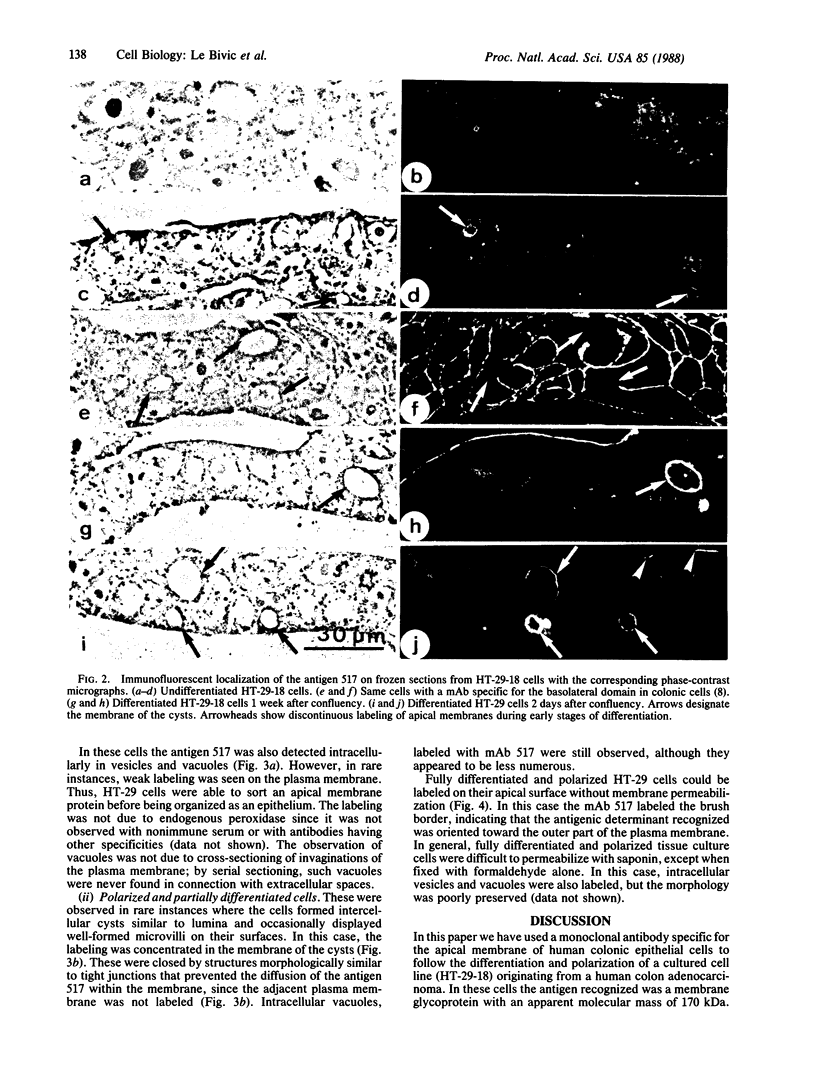
A monoclonal antibody that recognizes a membrane glycoprotein specific for the apical membrane of human colonic epithelial cells has been used to follow the differentiation and polarization of a cell line, HT-29, derived from a human colon adenocarcinoma. When these cells formed a polarized epithelium, the antigen was concentrated at the apical plasma membrane. It was also found intracellularly in vesicles and vacuoles. When HT-29 cells were undifferentiated and unpolarized, the antigen was not expressed significantly at the plasma membrane but was found concentrated in the membranes of intracellular vacuoles. Cells not yet organized into an epithelium may thus synthesize a membrane protein specific for their future apical membranes and store it intracellularly until the polarization process takes place. Intermediary stages of differentiation were occasionally recognized. They are characterized by a small number of cells surrounding an intercellular lumen. These lumina displayed apical membrane features (the presence of the apical antigen, of some microvilli, and of junctional complexes), although the cells were not fully differentiated. The differentiation process in HT-29 cells is apparently similar to that observed during embryonic development of the intestine. Therefore, HT-29 cells represent a useful model system to study epithelial differentiation in vitro.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bretscher A., Weber K. Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell. 1980 Jul;20(3):839–847. doi: 10.1016/0092-8674(80)90330-x. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Colony P. C., Neutra M. R. Epithelial differentiation in the fetal rat colon. I. Plasma membrane phosphatase activities. Dev Biol. 1983 Jun;97(2):349–363. doi: 10.1016/0012-1606(83)90092-1. [DOI] [PubMed] [Google Scholar]
- Coudrier E., Reggio H., Louvard D. Characterization of an integral membrane glycoprotein associated with the microfilaments of pig intestinal microvilli. EMBO J. 1983;2(3):469–475. doi: 10.1002/j.1460-2075.1983.tb01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudouet B., Robine S., Huet C., Sahuquillo-Merino C., Blair L., Coudrier E., Louvard D. Changes in villin synthesis and subcellular distribution during intestinal differentiation of HT29-18 clones. J Cell Biol. 1987 Jul;105(1):359–369. doi: 10.1083/jcb.105.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratecos D., Knibiehler M., Benoit V., Sémériva M. Plasma membranes from rat intestinal epithelial cells at different stages of maturation. I. Preparation and characterization of plasma membrane subfractions originating from crypt cells and from villous cells. Biochim Biophys Acta. 1978 Oct 4;512(3):508–524. doi: 10.1016/0005-2736(78)90161-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Bivic A., Arsanto J. P. Differential expression of alkaline phosphatase and ATPase activities in human colon carcinoma cell line HT-29.18 during differentiation. Biol Cell. 1987;60(1):41–47. doi: 10.1111/j.1768-322x.1987.tb00544.x. [DOI] [PubMed] [Google Scholar]
- Louvard D., Maroux S., Vannier C., Desnuelle P. Topological studies on the hydrolases bound to the intestinal brush border membrane. I. Solubilization by papain and Triton X-100. Biochim Biophys Acta. 1975 Jan 28;375(2):235–248. [PubMed] [Google Scholar]
- Madara J. L., Neutra M. R., Trier J. S. Junctional complexes in fetal rat small intestine during morphogenesis. Dev Biol. 1981 Aug;86(1):170–178. doi: 10.1016/0012-1606(81)90327-4. [DOI] [PubMed] [Google Scholar]
- Mathan M., Moxey P. C., Trier J. S. Morphogenesis of fetal rat duodenal villi. Am J Anat. 1976 May;146(1):73–92. doi: 10.1002/aja.1001460104. [DOI] [PubMed] [Google Scholar]
- Matlin K. S. The sorting of proteins to the plasma membrane in epithelial cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2565–2568. doi: 10.1083/jcb.103.6.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggio H., Webster P., Louvard D. Use of immunocytochemical techniques in studying the biogenesis of cell surfaces in polarized epithelia. Methods Enzymol. 1983;98:379–395. doi: 10.1016/0076-6879(83)98166-1. [DOI] [PubMed] [Google Scholar]
- Remy L., Marvaldi J., Rua S., Secchi J., Lechene de la Porte P. The role of intracellular lumina in the repolarization process of a colonic adenocarcinoma cell line. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;46(4):297–305. doi: 10.1007/BF02890318. [DOI] [PubMed] [Google Scholar]
- Robine S., Huet C., Moll R., Sahuquillo-Merino C., Coudrier E., Zweibaum A., Louvard D. Can villin be used to identify malignant and undifferentiated normal digestive epithelial cells? Proc Natl Acad Sci U S A. 1985 Dec;82(24):8488–8492. doi: 10.1073/pnas.82.24.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Fuller S. D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973 May;57(2):551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trugnan G., Rousset M., Chantret I., Barbat A., Zweibaum A. The posttranslational processing of sucrase-isomaltase in HT-29 cells is a function of their state of enterocytic differentiation. J Cell Biol. 1987 May;104(5):1199–1205. doi: 10.1083/jcb.104.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Salas D. E., Salas P. J., Rodriguez-Boulan E. Modulation of the expression of an apical plasma membrane protein of Madin-Darby canine kidney epithelial cells: cell-cell interactions control the appearance of a novel intracellular storage compartment. J Cell Biol. 1987 May;104(5):1249–1259. doi: 10.1083/jcb.104.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Feramisco J. R., Ash J. F. Fluorescent localization of contractile proteins in tissue culture cells. Methods Enzymol. 1982;85(Pt B):514–562. doi: 10.1016/0076-6879(82)85050-7. [DOI] [PubMed] [Google Scholar]
- Zweibaum A., Pinto M., Chevalier G., Dussaulx E., Triadou N., Lacroix B., Haffen K., Brun J. L., Rousset M. Enterocytic differentiation of a subpopulation of the human colon tumor cell line HT-29 selected for growth in sugar-free medium and its inhibition by glucose. J Cell Physiol. 1985 Jan;122(1):21–29. doi: 10.1002/jcp.1041220105. [DOI] [PubMed] [Google Scholar]
- Zweibaum A., Triadou N., Kedinger M., Augeron C., Robine-Léon S., Pinto M., Rousset M., Haffen K. Sucrase-isomaltase: a marker of foetal and malignant epithelial cells of the human colon. Int J Cancer. 1983 Oct 15;32(4):407–412. doi: 10.1002/ijc.2910320403. [DOI] [PubMed] [Google Scholar]