Abstract
Objectives
To examine whether the quantity of cartilage or semiquantitative scores actually differ in knees with mild radiographic osteoarthritis (OA) compared with knees without OA.
Methods
Framingham OA Study participants had knee tibiofemoral MRI-based measurements of cartilage. Using 3D FLASH-water excitation sequences, cartilage volume (VC), thickness (ThCtAB) and subregional ThCtAB were measured and cartilage scored semiquantitatively (using WORMS). Using weight bearing radiographs, we defined mild OA as K/L=2 and nonOA as K/L=0. Differences between OA and nonOA knees in median cartilage measurements were tested using the Wilcoxon rank sum test.
Results
Among 948 participants (one knee each), neither VC nor regional thickness (ThCtAB) were different in mild versus nonOA knees. In mild OA, cartilage erosions in focal areas were missed when cartilage was quantified over large regions like the medial tibia. For some but not all subregions of cartilage, especially among men, ThCtAB was lower (p<.05) in mild OA than nonOA knees. Because semiquantitative scores captured focal erosions, median WORMS scores were higher in mild OA than nonOA (all p<.05). In moderate/severe OA (K/L grades 3 or 4), OA knees had much lower ThCtAB and higher WORMS scores than knees with nonOA.
Conclusions
In mild OA, the focal loss of cartilage is missed by quantitative measures of cartilage volume or thickness over broad areas. Regional cartilage volume and thickness (e.g. medial tibia) are not different in mild OA versus nonOA. Subregional thickness may be decreased in mild OA. Semiquantitative scoring which assesses focal cartilage damage differentiates mild OA from nonOA.
Keywords: knee osteoarthritis, MRI, cartilage
Introduction
Evaluation of knee osteoarthritis (OA) has been based primarily on plain radiographs, which depict osseous changes thought to occur late in the disease process.[1] Loss of cartilage is not directly visible on radiographs and can only be inferred indirectly by joint space narrowing.
Cartilage thickness and volume as assessed by magnetic resonance imaging (MRI) have emerged in recent years as important quantitative measurements of a joint's osteoarthritic status. The proliferation of studies using these measurements has been spurred by careful validation studies showing that thickness and volume of cartilage can be estimated accurately by careful quantitative measurement of images from high resolution MRIs.[2-6] Since one central element of the pathology of OA consists of wearing away of articular cartilage, cartilage thickness and volume should diminish as OA develops.[7-9] Also, quantitative methods for measuring cartilage damage are sought to aid in the diagnosis of early OA.[10, 11]
Another approach to characterize cartilage from MRI is the use of semiquantitative measures like the whole-organ scoring method (WORMS),[12] which evaluate cartilage surface morphology by the extent and depth of cartilage lesions, using an ordinal scale to quantify these changes. Satisfactory specificity and sensitivity for detecting focal chondral lesions by MRI have been demonstrated in vivo and in cadaveric studies.[13-15]
We used cartilage and plain radiographic data from a large community-based study of knees with and without radiographic knee OA to examine whether cartilage thickness and volume, denuded bone area, and a semiquantitative measurement of cartilage morphology were decreased in knees with OA compared to knees without OA, and to describe the relationship between quantitative and semiquantitative measurements.
Methods
Subjects
For this cross-sectional study, participants were members of the Framingham Osteoarthritis Study Cohort, which consists of 2 separate groups: members of the Framingham Heart Study Offspring cohort,[16] and a newly recruited cohort from the town of Framingham, Massachusetts, the community sample. Details of recruitment have been reported elsewhere[17] (see supplemental file 1). Both groups were selected from the community without respect to knee pain or OA status.
Approval for the study was obtained from the Boston University Medical Center Institutional Review Board.
Assessment of MRI
In the Framingham Offspring subgroup, only participants who reported knee pain underwent MRI of the knee(s). All subjects in the community sample underwent bilateral knee MRI, regardless of whether they had symptoms. Participants in whom MRI was contraindicated did not undergo scanning, and in one subject with a total knee replacement only the native knee was scanned (see supplemental file 2).
Quantitative Analysis
Using the coronal 3D FLASH-water excitation sequences, the cartilage plates of the medial tibia (MT), lateral tibia (LT), central medial femur (cMF) and central lateral femur (cLF) were quantified with 3D digital post-processing using proprietary software (Chondrometrics Works, Chondrometrics, Ainring, Germany)[18] (see figure 1). In brief, manual segmentation of the total subchondral bone area (tAB), (including denuded bone areas, but excluding osteophytes) [8] and of the cartilage surface (AC) were performed in MT and LT and in the weight-bearing central subregion of the femoral condyles (cMF and cLF), as described previously.[19] Segmentation was performed by eight technicians who had received formal training in cartilage segmentation prior to the study, and all segmentations underwent quality control review by an expert (F.E.). If necessary, segmentations were corrected by the original readers. Based on these segmentations, the following measures were computed: a) total cartilage volume (VC), b) total subchondral bone area (tAB), c) total mean cartilage thickness (ThCtAB) including denuded cartilage areas as 0 mm cartilage thickness, but not including osteophyte cartilage, d) percent denuded area (dAB), defined as percentage of total cartilage bone surface are denuded of cartilage, and e) total mean cartilage thickness excluding denuded cartilage area (ThCcAB).[5]
Figure 1.
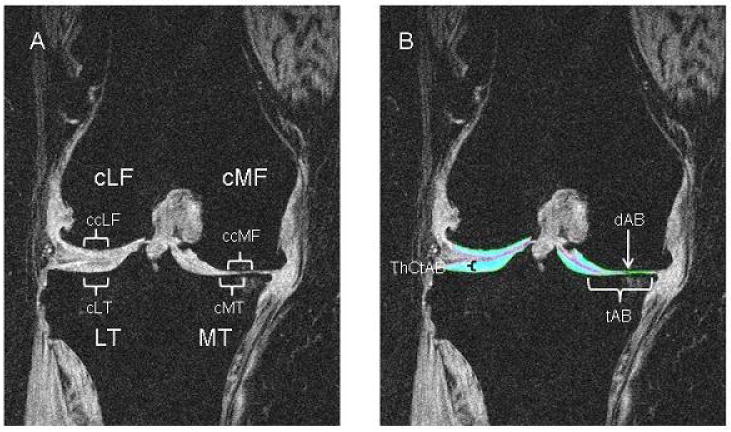
Coronal MRI of the knee of a patient with medial OA (K/L=4). A: without segmentation of the cartilage, showing the different regions (medial and lateral tibia, MT and LT; medial and lateral Femur, cLF and CMF) and subregions (central parts of the regions, cMT, cLT, ccLF and ccMF) as used in the paper. B: same plane with segmentation of the femorotibial cartilage plates (cartilage=turquoise). Cartilage thickness (ThCtAB) is preserved in the lateral tibial. There is severe damage on the medial side with small denuded area of subchondral bone (dAB) (this is just to the lateral side of the turquoise cartilage). The total area of subchondral bone (tAB) does not include osteophytes (e.g. there is a small medial osteophyte which is excluded).
To identify subregions of cartilage loss in mild/advanced OA and using recently published technology, [20] the MT and LT plates were subdivided into 5 subregions (each covering approx. 20% of the tAB) and the cMF and cLF plates in 3 subregions (each covering 33% of the tAB), respectively. Based on recent work suggesting early loss in central areas,[21, 22] we focused specifically on the central medial and lateral tibia (cMT and cLT) and on the central medial and lateral femora (ccMF, ccLF). In a previous study test–retest, coefficients of variations (with joint repositioning) ranged from 2.0% to 3.6%.[4] Regional measurement displayed precision errors between 1.5% and 4.7%.[20]
Semiquantitative Analysis
Using the proton density-weighted fat suppressed sequences, MRIs were analyzed with the semi-quantitative whole-organ scoring method (WORMS) by two experienced musculoskeletal radiologists (AG, FR), which in its full scope incorporates various features of different diarthoidal tissues.[12] For the purposes of this paper, we focused only on articular cartilage integrity so as to create a parallel with other measures of cartilage loss. Scores for cartilage using WORMS range from 0 (normal) to 6 (no cartilage remaining in a region) (see supplemental file 3 for details of scoring). Cartilage was evaluated in 10 subregions of the tibiofemoral joint: anterior, central, and posterior segments of the medial and lateral femur and tibia. Because our focus was only on cartilage in the tibiofemoral joint, we did not include patellofemoral regions in our analysis. To adapt the WORMS scores to the plate areas described above, we assigned the following WORMS subregions to the plates: the central medial tibia subregion corresponds to the cMT plate and the central medial femur to the cMF plate. We averaged the three subregions read for WORMS in the medial tibia (anterior, central and posterior) to depict the MT plate. In a random sample of 170 knees, inter-rater agreement for cartilage semiquantitative scoring showed a weighted k value = 0.73 (95% CI: 0.71, 0.75).
Assessment of radiographs
In all subjects, bilateral fixed flexion weight bearing posteroanterior (PA) [23] views of the knees were obtained. All radiographs were read for the Kellgren/Lawrence (K/L) grade (0-4) [24] (weighted kappa [intrarater reliability] = 0.83 (95% CI: 0.74–0.91)). by an academically based musculoskeletal radiologist (PA) Kellgren and Lawrence used different descriptions of their grades.[25], with Grade 2 usually described as having definite osteophytes and possible narrowing, but with nonweight bearing films used in their atlas. To standardize this approach, because OA is usually defined as showing cartilage loss and because in our highly standardized weight bearing films, we rarely encountered a knee with definite osteophytes that did not show probable narrowing, we agreed in advance that Kellgren and Lawrence grade 2 would be defined as definite osteophytes and probable mild narrowing.
Analysis plan
The overall goal of the analysis was to determine whether knees with OA (Kellgren and Lawrence grades 1 to 4) had quantitative and semiquantitative measurements that differed from knees with no OA (K/L=0), with a special emphasis whether this was true for mild OA (K/L=2). We pursued two different analytic approaches:
We performed plate-specific analysis to examine differences between different cartilage measurements (quantitative: VC, tAB, ThCtAB, dAB and ThCcAB; semiquantitative: WORMS score) and the K/L grades (0 to 4); this was done separately for men and women, as gender is an important confounder,[26-29] and for medial and lateral compartments. Because some knees would be expected to have OA in the lateral compartment and in that case, the medial compartment cartilage would be unaffected, we diagnosed medial tibiofemoral OA as follows: joint space narrowing present only in the medial compartment or medially more than laterally on radiographs. If we could not decide upon the location of the disease based on this rule, we made the distinction based on the location of the largest osteophytes.[30] Lateral disease was diagnosed similarly, and these definitions were applied for each K/L grade ≥ 1. For each cartilage measure, we calculated medians with interquartile ranges. Differences between K/L scores in median cartilage-measurements were tested using the Wilcoxon rank sum test. As WORMS are ordinal data, only non-parametric tests can be used in their analysis. Given this, we chose to use non-parametric tests for continuous outcomes as well so as to permit comparison between the results of different analysis.
One might argue that in knees with K/L=0 many knees would already have some kind of cartilage damage. We therefore performed a sensitivity analysis, defining a subgroup of knees with K/L=0 that included only knees without any indication of cartilage damage (maximum WORMS-score in all of the 10 tibiofemoral subregions for cartilage=0).
For all analyses, p-values less than or equal to 0.05 (2-tailed tests) were considered significant. All analyses were conducted using SAS software, version 9.1 (SAS Institute Inc, Cary, North Carolina).
Results
A total of 948 participants in the offspring and community cohorts provided information in all three imaging modalities: proton density-weighted fat-suppressed images for WORMS-readings, 3D FLASH-water excitation sequences for quantitative measurements, and radiographic information on K/L grades. These subjects had a mean age of 63.6 years and a mean body mass index of 28.6 kg/m2. Twenty-five percent reported knee pain on most days in the studied knee. Fifty-seven percent were women. More than three-quarters did not show evidence of OA in the knee joint studies (76% had a K/L=0). Of knees with OA, we classified 189 (83%) as having medial OA. In medial OA K/L=1, 15% had a radiographic joint space narrowing (JSN) grade=1, and 89% in K/L=2. All K/L=3 had a JSN grade 2, and all K/L=4 had a JSN grade 3.
Tables 1 (women) and 2 (men) present the data for VC, tAB, ThCtAB and WORMS by K/L grade separately for the medial compartment. We found similar findings for the lateral compartment. However, the numbers for each K/L-stratum for lateral disease were sparse, ranging from 2 to 7 subjects per stratum and are therefore not presented.
Table 1.
Median [interquartile range] for semiquantitative and quantitative MRI measurements of the medial tibiofemoral joint in women. P-values from Wilcoxon test are printed in italics, and all of these p values are compared with K/L=0. All data are presented with the focus on K/L 2 vs. 0 depicted in bold font.
| K/L = 0 (n=398) |
Subset of K/L = 0 With NO cartilage lesions on MRI (n=235) |
K/L = 1 (n=24) |
K/L = 2 (n=49) |
K/L = 3 (n=34) |
K/L = 4 (n=10) |
|
|---|---|---|---|---|---|---|
| Medial tibia (MT) | ||||||
| Volume (VC) [mm3] | 1684 [1498, 1895] | 1660 [1507, 1853] | 1737 [1499, 1892] | 1870 [1675, 2111] | 1772 [1381, 2062] | 1263 [930, 1540] |
| p-value | 0.84 | 0.0002 | 0.60 | <.0001 | ||
| Subchondral bone area (tAB) [cm2] | 10.59 [9.86, 11.35] | 10.50 [9.86, 11.28] | 10.43 [10.00, 11.10] | 11.46 [10.87, 11.97] | 11.43 [10.78, 12.29] | 11.75 [10.76, 12.89] |
| p-value | 0.71 | <.0001 | <.0001 | <.0001 | ||
| Thickness (ThCtAB) [mm] | 1.60 [1.49, 1.71] | 1.60 [1.49, 1.71] | 1.62 [1.48, 1.76] | 1.65 [1.52, 1.85] | 1.50 [1.27, 1.63] | 0.99 [0.67, 1.19] |
| p-value | 0.65 | 0.08 | 0.005 | <.0001 | ||
| WORMS-score | 0.0 [0.0, 0.0] | 0* | 0.0 [0.0, 1.0] | 1.0 [0.0, 2.0] | 2.7 [2.0, 3.7] | 4.0 [3.7, 5.0] |
| p-value | 0.0002 | <.0001 | <.0001 | <.0001 | ||
| Central medial tibia (cMT) ‡ | ||||||
| Thickness (ThCtAB) [mm] | 2.13 [1.93, 2.33] | 2.12 [1.93, 2.31] | 2.08 [1.89, 2.44] | 2.22 [1.83, 2.44] | 1.73 [1.49, 2.19] | 0.96 [0.29, 1.52] |
| p-value | 0.85 | 0.38 | <.0001 | <.0001 | ||
| WORMS-score | 0.0 [0.0, 0.0] | 0* | 0.0 [0.0, 3.0] | 3.0 [0.0, 3.0] | 5.0 [3.0, 5.0] | 5.0 [5.0, 6.0] |
| p-value | 0.001 | <.0001 | <.0001 | <.0001 | ||
| Central medial femur (cMF) | ||||||
| Volume (VC) [mm3] | 832 [713, 978] | 827 [700, 945] | 888 [674, 1076] | 863 [710, 1072] | 706 [509, 947] | 249 [206, 531] |
| p-value | 0.64 | 0.20 | 0.002 | <.0001 | ||
| Subchondral bone area (tAB) [cm2] | 4.81 [4.27, 5.42] | 4.74 [4.20, 5.32] | 5.03 [4.33, 5.60] | 5.12 [4.54, 6.05] | 5.26 [4.21, 5.91] | 5.28 [4.48, 5.74] |
| p-value | 0.20 | 0.007 | 0.15 | 0.31 | ||
| Thickness (ThCtAB) [mm] | 1.65 [1.53, 1.82] | 1.64 [1.53, 1.78] | 1.58 [1.48, 1.81] | 1.63 [1.41, 1.83] | 1.30 [1.01, 1.55] | 0.47 [0.35, 1.13] |
| p-value | 0.46 | 0.30 | <.0001 | <.0001 | ||
| WORMS-score | 0.0 [0.0, 2.0] | 0* | 2.5 [0.0, 3.0] | 3.0 [2.5, 4.0] | 5.0 [4.0, 5.0] | 6.0 [5.0, 6.0] |
| p-value | 0.001 | <.0001 | <.0001 | <.0001 | ||
| Central part of central medial femur (ccMF) †, ‡ | ||||||
| Thickness (ThCtAB) [mm] | 2.00 [1.80, 2.22] | 2.00 [1.78, 2.18] | 1.83 [1.63, 2.03] | 1.83 [1.49, 2.14] | 1.29 [0.98, 1.66] | 0.18 [0.06, 1.13] |
| p-value | 0.03 | 0.006 | <.0001 | <.0001 | ||
by definition this group has cartilage WORMS=0;
no corresponding WORMS score;
no corresponding volume-measurement
Table 2.
Median [interquartile range] for semiquantitative and quantitative MRI measurements of the medial tibiofemoral joint in men. P-values from Wilcoxon test are printed in italics, and all of these p values are compared with K/L=0. All data are presented with the focus on K/L 2 vs. 0 depicted in bold font.
| K/L = 0 (n=322) |
Subset of K/L = 0 With NO cartilage lesions on MRI (n=169) |
K/L = 1 (n=9) |
K/L = 2 (n=32) |
K/L = 3 (n=21) |
K/L = 4 (n=12) |
|
|---|---|---|---|---|---|---|
| Medial tibia (MT) | ||||||
| Volume (VC) [mm3] | 2527 [2216, 2815] | 2520 [2240, 2787] | 2859 [2342, 2900] | 2519 [2415, 2945] | 2278 [1917, 2571] | 1394 [1190, 1677] |
| p-value | 0.19 | 0.29 | 0.04 | <.0001 | ||
| Subchondral bone area (tAB) [cm2] | 13.66 [12.88, 14.65] | 13.63 [12.93, 14.56] | 14.59 [13.24, 15.51] | 14.50 [13.63, 15.06] | 14.29 [13.58, 15.47] | 13.86 [12.52, 15.89] |
| p-value | 0.09 | 0.004 | 0.05 | 0.74 | ||
| Thickness (ThCtAB) [mm] | 1.87 [1.72, 1.99] | 1.87 [1.72, 1.98] | 1.85 [1.79, 1.96] | 1.82 [1.65, 1.97] | 1.59 [1.32, 1.83] | 0.99 [0.84, 1.19] |
| p-value | 0.96 | 0.27 | 0.003 | <.0001 | ||
| WORMS-score | 0.0 [0.0, 0.0] | 0* | 1.0 [1.0, 1.7] | 0.8 [0.3, 1.5] | 3.3 [1.0, 3.7] | 3.2 [1.0, 5.3] |
| p-value | 0.001 | <.0001 | <.0001 | <.0001 | ||
| Central medial tibia (cMT) ‡ | ||||||
| Thickness (ThCtAB) [mm] | 2.53 [2.28, 2.73] | 2.54 [2.26, 2.73] | 2.51 [2.31, 2.67] | 2.32 [2.04, 2.60] | 2.07 [1.70, 2.41] | 0.85 [0.54, 1.17] |
| p-value | 0.65 | 0.007 | <.0001 | <.0001 | ||
| WORMS-score | 0.0 [0.0, 0.0] | 0* | 2.5 [0.0, 4.0] | 3.0 [3.0, 4.0] | 5.0 [3.5, 6.0] | 6.0 [5.0, 6.0] |
| p-value | 0.001 | <.0001 | <.0001 | <.0001 | ||
| Central medial femur (cMF) | ||||||
| Volume (VC) [mm3] | 1316 [1154, 1523] | 1321 [1166, 1502] | 1378 [1191, 1639] | 1141 [1018, 1417] | 823 [641, 1252] | 361 [336, 538] |
| p-value | 0.72 | 0.06 | <.0001 | <.0001 | ||
| Subchondral bone area (tAB) [cm2] | 6.09 [5.41, 6.81] | 6.09 [5.41, 6.62] | 6.81 [5.19, 7.04] | 5.97 [5.28, 6.99] | 6.49 [5.83, 6.92] | 5.89 [5.68, 7.61] |
| p-value | 0.40 | 0.83 | 0.18 | 0.56 | ||
| Thickness (ThCtAB) [mm] | 2.05 [1.86, 2.24] | 2.05 [1.88, 2.22] | 2.06 [1.83, 2.19] | 1.90 [1.62, 2.10] | 1.40 [0.90, 1.57] | 0.63 [0.42, 1.01] |
| p-value | 0.72 | 0.06 | <.0001 | <.0001 | ||
| WORMS-score | 0.0 [0.0, 2.0] | 0* | 2.0 [0.0, 3.0] | 3.0 [3.0, 4.0] | 5.0 [5.0, 6.0] | 6.0 [6.0, 6.0] |
| p-value | 0.005 | <.0001 | <.0001 | <.0001 | ||
| Central part of central medial femur (ccMF) †, ‡ | ||||||
| Thickness (ThCtAB) [mm] | 2.54 [2.20, 2.82] | 2.54 [2.25, 2.79] | 2.44 [2.11, 2.57] | 2.13 [1.94, 2.56] | 1.45 [0.90, 1.73] | 0.34 [0.10, 0.85] |
| p-value | 0.26 | 0.001 | <.0001 | <.0001 | ||
by definition this group has cartilage WORMS=0;
no corresponding WORMS score;
no corresponding volume-measurement
In general, no significant difference was seen comparing VC in knees with K/L=2 vs. knees with K/L=0, and in some analyses VC was actually higher in knees with K/L=2, e.g. in the medial tibial plate in females (1684 mm3 (interquartile range 1498 to 1895) in K/L=0 compared to 1870 mm3 (1675 to 2111) in K/L=2 (p=0.0002)). For some but not all cartilage regions, VC was lower in K/L=3 knees than in those with K/L=0 (see tables 1 and 2). A consistent decrement in cartilage volume was seen in knees with K/L=4 compared with K/L=0.
In women, cartilage thickness (ThCtAB) was generally not different for K/L=2 vs. K/L=0, with the exception of the central region of cMF (ccMF) where thickness was lower in knees with K/L=2 (see table 1). In men, ThCtAB was diminished in K/L=2 knees vs. those with K/L=0 both in general and in cMF region and both central subregions of the medial femoral and tibial plates (cMT and ccMF), but it was not true for the larger medial tibia region (MT).
When we examined K/L=3 knees, we found significantly lower values for ThCtAB than for K/L=0 knees in both men and women. Again ThCtAB of the subregions (cMT and ccMF) differentiated better between K/L=3 and K/L=0 than ThCtAB of total plates (MT, cMF). For VC, we found conflicting results. In men, VC was diminished in K/L=3 knees vs. those with K/L=0 whereas for women it was not.
In K/L=0, only few MRIs showed any exposed regions of bone dAB (range from 2.5% of knees in men in MT to 0.3% in women in cMF). In K/L=2, less than 20% of all knees had dAB, and <60% among knees with K/L=3 had dAB. However, in K/L=4, all knees had measurable areas of exposed bone (dAB>0). Thus, although often present in severe OA, dAB scores did not discriminate between K/L=2 and K/L=0.
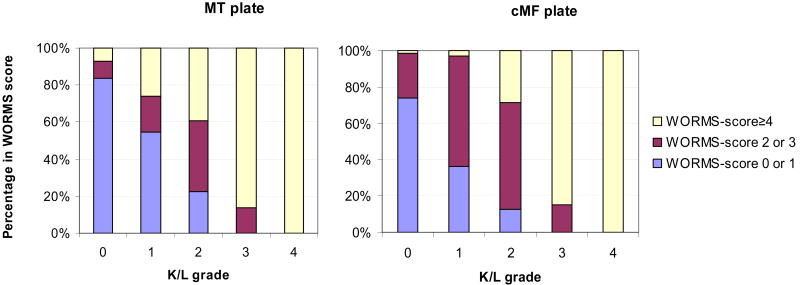
The semiquantitative WORMS score consistently showed significant differences between K/L=2 and K/L=0 knees. To better understand how a semiquantitative assessment could be sensitive to mild changes of OA, we looked at the distribution of WORMS cartilage scores by K/L grade (Figure 2). WORMS scores of 0 and 1 reflect preserved thickness and no morphological lesions. Since WORMS scores 2 and 3 reflect focal erosions with preserved cartilage thickness, we combined these scores. WORMS scores ≥ 4 indicate diffuse cartilage thinning. In K/L=0 the predominant score (about 80% of knees) was WORMS=0 for MT and cMF, whereas in K/L=1 and K/L=2, WORMS scores 2 and 3 predominated, representing focal erosions of cartilage and not diffuse loss or thinning. In K/L=3 and 4, WORMS scores above 4 predominated.
Figure 2.
Percentages of different WORMS-score categories for the MT and cMF plate are plotted against K/L grade (males and females). WORMS-score categories 0 and 1 represents unimpaired cartilage, score 2, 2.5 or 3 signify focal thinning/erosions in small areas, and WORMS scores equal or greater than 4 indicate diffuse cartilage thinning.
Because K/L=0 could include knees with mild OA on MRI, we reperformed analyses in which we restricted nonOA knees to those which not only had K/L=0, but which also had WORMS scores of 0 (we found almost no knees with scores of 1). These results, displayed in tables 1 and 2 in the second column, were similar compared to results for all knees with K/L=0. We did not find different results when analysing for the two cohorts separately. Also, analyses of those with knee pain and mild OA showed similar findings to those of all knees with mild OA irrespective of knee pain.
Discussion
Quantitative measurement of cartilage volume (VC) did not distinguish mild OA (K/L=2) from nonOA. We also found that cartilage thickness over entire cartilage plates, such as the medial tibia, failed to distinguish knees with mild OA from those without OA. For women, even some subregional measurements showed no significant differences between mild OA and nonOA knees, but we did find more consistent differences between mild OA and nonOA when we focused on subregional cartilage plates, especially in men. Denuded area had a value of 0 in most knees with mild OA, suggesting that its value in differenting OA from nonOA is limited. A semiquantitative assessment did distinguish mild from nonOA knees, and most of the mild OA knees showed scores of 2 or 3, evidence of focal erosive cartilage lesions that might not be detected using quantitative measurement which summarizes morphology over broad areas.
We are not aware of prior attempts to evaluate different cartilage measurement techniques in a large cross-sectional study. Most studies have focused on only one quantitative measurement, e.g. volume [9, 11] An additional strength of this study is its large sample size and the independent assessment of quantitative and semiquantitative measurements by MRI radiologists who were blinded to K/L status.
How can we interpret our findings that cartilage volume was actually increased in all plates in women with mild OA (K/L=2) compared to non-OA (K/L=0)? VC is a function of cartilage thickness and the size of the area of the total subchondral bone area (tAB). In our cohort, as described previously, [31] tAB values (bone area) increased in almost all K/L grades 1 to 4 compared to K/L=0, thus providing an explanation why VC was higher e.g. in K/L=2. It was shown in the past that volume should be adjusted for bone size, and our results confirm these findings. [8]
For cartilage thickness measurements, which adjust for bone size, the only sites where there was a significant reduction in cartilage thickness among knees with mild OA (K/L=2) were the central subregions such as the central medial femur (cMF) and central medial tibia (cMT, for males only) and in the central part of the medial femur (ccMF) for both genders. Since cartilage loss occurs most frequently at the load-bearing sites, [15] we are likely sampling from the regions where cartilage loss is occurring. ThCtAB includes denuded areas of subchondral bone (dAB), and the question remains, whether cartilage thickness measured for remaining cartilage only (ThCcAB) would perform better in differentiating early OA from non OA. However, only about 20% of knees with K/L=2 had any denuded area, and the figures for ThCcAB and ThCtAB therefore differ not at all for K/L=0, 1, and 2 (results not shown).
Why are quantitative measures not very sensitive to the development of radiographic OA even at a stage when the x-ray shows evidence of disease? One might argue that in K/L=2 joint space narrowing is not impaired, as defined by some authors.[32] However, in our sample, almost all radiographs with K/L 2 had joint space narrowing grade = 1 (86% in women, and 94% in men). The most comprehensive explanation might provide insights from the semiquantitative measures and regional measures. As shown in Figure 2, in K/L=2 the predominant WORMS scores are 2 and 3, representing small areas of focal thinning which are may not be picked up by quantitative measures that describe the entire cartilage plates. Additionally, swelling of cartilage surrounding cartilage defects could negate the effect of focal losses on quantitative measurements that sum cartilage morphometry over a large region. Cartilage swelling has been shown in animal models to be a feature of early stages of experimental osteoarthritis.[33]
This study has several limitations. Despite the large number of participants, this was a community sample and most persons did not have OA. As a consequence there were small numbers of subjects with OA of a given compartment, especially those with advanced stages and with lateral disease. However, in the compartment of most interest, the medial tibiofemoral compartment with mild OA (K/L=2), we had 80 subjects contributing to the measurements. Furthermore, we cannot make any conclusions pertaining to longitudinal findings. Reproducibility of cartilage volume and surface has been shown to be excellent [20, 34, 35] and it is likely that these measurements are a very sensitive tool for detecting changes over time.[36] While radiographic OA is not the optimal gold standard measure of OA, it is still the standard used to define OA. Framingham Study radiograph readings have provided referenced estimates of population prevalence of OA. Since we do not yet know how to best define OA on MRI, OA is necessarily the standard for the current work.
In conclusion, cartilage loss in mild OA tends to be focal and such loss is best detected by experienced readers applying semiquantitative methods. For cross sectional studies whose goal is to identify those with radiographic OA, these methods may be optimal. Cartilage volume (VC) does a poor job of distinguishing OA from nonOA knees. Decreases in cartilage thickness (ThCtAB), which takes into account bone size, could probably only be used if there is a focus on subregions within the larger cartilage plates.
Supplementary Material
Acknowledgments
Funding and Role of Sponsor: Dr Reichenbach is the recipient of an educational grant by the Swiss Society of Rheumatology.
Supported by National Institutes of Health (NIH) AR47785, NIH AG18393 and by the National Heart, Lung, and Blood Institute (NHLBI)'s Framingham Heart Study N01-HC-25195.
Footnotes
Conflict of interest: Felix Eckstein is CEO of Chondrometrics GmbH, a company providing MR image analysis services. He provides consulting services to Pfizer, MerckSerono, AstraZeneca, and Wyeth. Martin Hudelmaier has a part time appointment with Chondrometrics GmbH.
Ali Guermazi is president of Boston Imaging Core Lab, LLC (BICL), a company providing radiological image assessment services. He is shareholder of Synarc, Inc.
Frank Roemer is shareholder of BICL.
Other authors declare no conflict of interest.
Publisher's Disclaimer: Online First contains unedited articles in manuscript form that have been peer reviewed and accepted for publication but have not yet appeared in the paper journal (edited, typeset versions may be posted when available prior to final publication). Online First articles are citable and establish publication priority; they are indexed by PubMed from initial publication. Citations to Online First articles must include the digital object identifier (DOIs) and date of initial publication.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in ARD and any other BMJPGL products and sublicences such use and exploit all subsidiary rights, as set out in our licence (http://ARD.bmjjournals.com/ifora/licence.pdf).
References
- 1.Altman RD, Fries JF, Bloch DA, Carstens J, Cooke TD, Genant H, et al. Radiographic assessment of progression in osteoarthritis. Arthritis Rheum. 1987;30:1214–25. doi: 10.1002/art.1780301103. [DOI] [PubMed] [Google Scholar]
- 2.Burgkart R, Glaser C, Hyhlik-Durr A, Englmeier KH, Reiser M, Eckstein F. Magnetic resonance imaging-based assessment of cartilage loss in severe osteoarthritis: accuracy, precision, and diagnostic value. Arthritis Rheum. 2001;44:2072–7. doi: 10.1002/1529-0131(200109)44:9<2072::AID-ART357>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Graichen H, von Eisenhart-Rothe R, Vogl T, Englmeier KH, Eckstein F. Quantitative assessment of cartilage status in osteoarthritis by quantitative magnetic resonance imaging: technical validation for use in analysis of cartilage volume and further morphologic parameters. Arthritis Rheum. 2004;50:811–6. doi: 10.1002/art.20191. [DOI] [PubMed] [Google Scholar]
- 4.Eckstein F, Charles HC, Buck RJ, Kraus VB, Remmers AE, Hudelmaier M, et al. Accuracy and precision of quantitative assessment of cartilage morphology by magnetic resonance imaging at 3.0T. Arthritis Rheum. 2005;52:3132–6. doi: 10.1002/art.21348. [DOI] [PubMed] [Google Scholar]
- 5.Eckstein F, Burstein D, Link TM. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed. 2006;19:822–54. doi: 10.1002/nbm.1063. [DOI] [PubMed] [Google Scholar]
- 6.Cohen ZA, McCarthy DM, Kwak SD, Legrand P, Fogarasi F, Ciaccio EJ, et al. Knee cartilage topography, thickness, and contact areas from MRI: in-vitro calibration and in-vivo measurements. Osteoarthritis Cartilage. 1999;7:95–109. doi: 10.1053/joca.1998.0165. [DOI] [PubMed] [Google Scholar]
- 7.von Eisenhart-Rothe R, Graichen H, Hudelmaier M, Vogl T, Sharma L, Eckstein F. Femorotibial and patellar cartilage loss in patients prior to total knee arthroplasty, heterogeneity, and correlation with alignment of the knee. Ann Rheum Dis. 2006;65:69–73. doi: 10.1136/ard.2005.038869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgkart R, Glaser C, Hinterwimmer S, Hudelmaier M, Englmeier KH, Reiser M, et al. Feasibility of T and Z scores from magnetic resonance imaging data for quantification of cartilage loss in osteoarthritis. Arthritis Rheum. 2003;48:2829–35. doi: 10.1002/art.11259. [DOI] [PubMed] [Google Scholar]
- 9.Cicuttini FM, Wluka AE, Forbes A, Wolfe R. Comparison of tibial cartilage volume and radiologic grade of the tibiofemoral joint. Arthritis Rheum. 2003;48:682–8. doi: 10.1002/art.10840. [DOI] [PubMed] [Google Scholar]
- 10.Blumenkrantz G, Majumdar S. Quantitative magnetic resonance imaging of articular cartilage in osteoarthritis. Eur Cell Mater. 2007;13:76–86. doi: 10.22203/ecm.v013a08. [DOI] [PubMed] [Google Scholar]
- 11.Jones G, Ding C, Scott F, Glisson M, Cicuttini F. Early radiographic osteoarthritis is associated with substantial changes in cartilage volume and tibial bone surface area in both males and females. Osteoarthritis Cartilage. 2004;12:169–74. doi: 10.1016/j.joca.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Bredella MA, Tirman PF, Peterfy CG, Zarlingo M, Feller JF, Bost FW, et al. Accuracy of T2-weighted fast spin-echo MR imaging with fat saturation in detecting cartilage defects in the knee: comparison with arthroscopy in 130 patients. AJR Am J Roentgenol. 1999;172:1073–80. doi: 10.2214/ajr.172.4.10587150. [DOI] [PubMed] [Google Scholar]
- 14.Masi JN, Sell CA, Phan C, Han E, Newitt D, Steinbach L, et al. Cartilage MR imaging at 3.0 versus that at 1.5 T: preliminary results in a porcine model. Radiology. 2005;236:140–50. doi: 10.1148/radiol.2361040747. [DOI] [PubMed] [Google Scholar]
- 15.Amin S, LaValley MP, Guermazi A, Grigoryan M, Hunter DJ, Clancy M, et al. The relationship between cartilage loss on magnetic resonance imaging and radiographic progression in men and women with knee osteoarthritis. Arthritis Rheum. 2005;52:3152–9. doi: 10.1002/art.21296. [DOI] [PubMed] [Google Scholar]
- 16.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–25. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 17.Felson DT, Niu J, McClennan C, Sack B, Aliabadi P, Hunter DJ, et al. Knee buckling: prevalence, risk factors, and associated limitations in function. Ann Intern Med. 2007;147:534–40. doi: 10.7326/0003-4819-147-8-200710160-00005. [DOI] [PubMed] [Google Scholar]
- 18.Eckstein F, Hudelmaier M, Wirth W, Kiefer B, Jackson R, Yu J, et al. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis. 2006;65:433–41. doi: 10.1136/ard.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glaser C, Burgkart R, Kutschera A, Englmeier KH, Reiser M, Eckstein F. Femoro-tibial cartilage metrics from coronal MR image data: Technique, test-retest reproducibility, and findings in osteoarthritis. Magn Reson Med. 2003;50:1229–36. doi: 10.1002/mrm.10648. [DOI] [PubMed] [Google Scholar]
- 20.Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27:737–44. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 21.Pelletier JP, Raynauld JP, Berthiaume MJ, Abram F, Choquette D, Haraoui B, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9:R74. doi: 10.1186/ar2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter DJ, Niu J, Zhang Y, Lavalley M, McClennan CE, Hudelmaier M, et al. Premorbid knee OA is not characterized by diffuse thinness: The Framingham Study. Ann Rheum Dis. 2008;67:1545–9. doi: 10.1136/ard.2007.076810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32:128–32. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 24.Kellgren JH, L J. Atlas of standard radiographs. In: Kellgren JH, editor. The epidemiology of chronic rheumatism; a symposium organized by the Council for International Organizations of Medical Sciences. Oxford: Blackwell; 1963. p. 2. [Google Scholar]
- 25.Spector TD, Cooper C. Radiographic assessment of osteoarthritis in population studies: whither Kellgren and Lawrence? Osteoarthritis Cartilage. 1993;1:203–6. doi: 10.1016/s1063-4584(05)80325-5. [DOI] [PubMed] [Google Scholar]
- 26.Hudelmaier M, Glaser C, Hohe J, Englmeier KH, Reiser M, Putz R, et al. Age-related changes in the morphology and deformational behavior of knee joint cartilage. Arthritis Rheum. 2001;44:2556–61. doi: 10.1002/1529-0131(200111)44:11<2556::aid-art436>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 27.Otterness IG, Eckstein F. Women have thinner cartilage and smaller joint surfaces than men after adjustment for body height and weight. Osteoarthritis Cartilage. 2007;15:666–72. doi: 10.1016/j.joca.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Eckstein F, Siedek V, Glaser C, Al-Ali D, Englmeier KH, Reiser M, et al. Correlation and sex differences between ankle and knee cartilage morphology determined by quantitative magnetic resonance imaging. Ann Rheum Dis. 2004;63:1490–5. doi: 10.1136/ard.2003.018226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faber SC, Eckstein F, Lukasz S, Muhlbauer R, Hohe J, Englmeier KH, et al. Gender differences in knee joint cartilage thickness, volume and articular surface areas: assessment with quantitative three-dimensional MR imaging. Skeletal Radiol. 2001;30:144–50. doi: 10.1007/s002560000320. [DOI] [PubMed] [Google Scholar]
- 30.Felson DT, Gale DR, Elon Gale M, Niu J, Hunter DJ, Goggins J, et al. Osteophytes and progression of knee osteoarthritis. Rheumatology (Oxford) 2005;44:100–4. doi: 10.1093/rheumatology/keh411. [DOI] [PubMed] [Google Scholar]
- 31.Wluka AE, Wang Y, Davis SR, Cicuttini FM. Tibial plateau size is related to grade of joint space narrowing and osteophytes in healthy women and in women with osteoarthritis. Ann Rheum Dis. 2005;64:1033–7. doi: 10.1136/ard.2004.029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiphof D, Boers M, Bierma-Zeinstra SM. Differences in descriptions of Kellgren and Lawrence grades of knee osteoarthritis. Ann Rheum Dis. 2008;67:1034–6. doi: 10.1136/ard.2007.079020. [DOI] [PubMed] [Google Scholar]
- 33.Calvo E, Palacios I, Delgado E, Ruiz-Cabello J, Hernandez P, Sanchez-Pernaute O, et al. High-resolution MRI detects cartilage swelling at the early stages of experimental osteoarthritis. Osteoarthritis Cartilage. 2001;9:463–72. doi: 10.1053/joca.2001.0413. [DOI] [PubMed] [Google Scholar]
- 34.Brem MH, Pauser J, Yoshioka H, Brenning A, Stratmann J, Hennig FF, et al. Longitudinal in vivo reproducibility of cartilage volume and surface in osteoarthritis of the knee. Skeletal Radiol. 2007;36:315–20. doi: 10.1007/s00256-006-0208-z. [DOI] [PubMed] [Google Scholar]
- 35.Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27:737–44. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 36.Sharma L, Eckstein F, Song J, Guermazi A, Prasad P, Kapoor D, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58:1716–26. doi: 10.1002/art.23462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.