Abstract
The lateral cilia of the gill of Crassostrea virginica are controlled by a dopaminergic–serotonergic innervation. Dopamine is the neurotransmitter causing cilio-inhibition. High levels of manganese are neurotoxic to people, causing Manganism, a Parkinson-like disease. Clinical interventions for Manganism have not been very successful. Recently, p-Aminosalicylic acid (PAS) was reported as an effective treatment of severe Manganism in humans; however, its mechanism of action is unknown. Previously, we reported that manganese treatments caused disruption of the dopaminergic innervation of gill of C. virginica. Here we compared the effects of manganese on gill innervation in the presence of PAS, EDTA or Acetylsalicylic acid (ASA), and examined whether co-treating animals with PAS could block the deleterious effects of manganese on the oyster's dopaminergic innervation of the gill. Beating rates of the lateral cilia of the gill were measured by stroboscopic microscopy. Pre-treating gill preparations with PAS or EDTA blocked the neurotoxic effects of manganese, while ASA did not. In other experiments, animals exposed to three day treatments with manganese produced a dose dependent impairment of the dopaminergic, cilio-inhibitory system, which was decreased by co-treatment with PAS. The study shows that PAS protects the animal against neurotoxic effects of manganese and the mechanism of action of PAS in alleviating Manganism is more likely related to its chelating abilities than its anti-inflammatory actions.
Keywords: Ciliary activity, Crassostrea virginica, Dopamine, Gill, Manganism, p-Aminosalicylic acid
1. Introduction
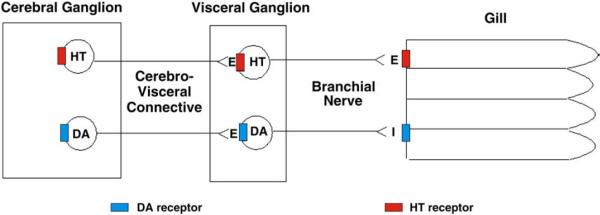
The Eastern oyster, Crassostrea virginica, has a reciprocal dopaminergic and serotonergic innervation of the lateral ciliated cells of gill epithelia, similar to that of Mytilus edulis (Carroll and Catapane, 2007). The innervation originates in the cerebral and visceral ganglia, and controls the gill via the branchial nerve. Excitation of the dopaminergic circuit results in a terminal release of dopamine at the level of the gill epithelium that slows down and stops the beating rates of the lateral cilia. Excitation of the serotonergic circuit results in a terminal release of serotonin that speeds up the beating rates of the lateral cilia (Fig. 1). Since the animal's CNS activity is directly related to a measurable peripheral response, the ganglia and gill preparations of C. virginica can serve as a useful model with which to study dopaminergic and serotonergic systems and the chemicals that affect the release and activity of these biogenic amines.
Fig. 1.
Schematic representation of the innervation of the lateral ciliated cells of the gill of Crassostrea virginica. Serotonin (HT), Dopamine (DA), E=excitatory neurotransmitter, I=inhibitory neurotransmitter.
C. virginica readily accumulates manganese into its ganglia and tissues (Murray et al., 2007). Manganese accumulations are associated with reduced levels of dopamine in the oyster's cerebral ganglia, visceral ganglia and gill, while having no effect on levels of other biogenic amines, including serotonin, epinephrine and octopamine (King et al., 2008). Treating animals with manganese in the presence of p-Aminosalicylic acid (PAS) blocked the manganese-induced loss of ganglionic and gill dopamine levels (King et al., 2008). Recently, using ganglia and gill preparations, it was shown that oysters pretreated with manganese had an impaired cilio-inhibitory response to exogenous application of dopamine to either the cerebral ganglia, visceral ganglia or directly to the gill (Martin et al., 2008). Increased manganese exposure caused greater disruption of the cilio-inhibitory mechanism, while in all cases, caused no impairment to the animal's serotonergic cilio-excitatory system demonstrating the specificity of manganese toxicity on the animal's dopaminergic system.
Manganese is present in animal tissues and is required as an enzyme cofactor or activator for numerous reactions of metabolism (Cotzias, 1958). While essential in trace amounts, excessive exposure can result in manganese toxicity. The primary cause of toxicity is believed to be the inhalation of manganese from the atmosphere (Andersen et al., 1999) and the risk of high levels of airborne manganese is of concern for workers involved in mining and manganese ore processing. Toxic manganese exposure is also possible in a number of other occupational settings including welding, steel or dry battery manufacturing, and agricultural use of manganese ethylene (bis)dithiocarbamate (MANEB) or other organomanganese fungicides (Olanow, 2004; NAS, 1973; Meco et al., 1994; Reidy et al., 1992; Iregren, 1999). More recently, because of greater industrial use of manganese containing chemicals and commercial use of compounds like methylcyclopentadienyl manganese tricarbonyl (MMT) as an antiknock gasoline additive, there is a growing concern about the potential health consequences in the general population due to chronic low-level ambient exposure to manganese (Hudnell et al., 1999; Kaiser, 2003).
Toxic manganese exposure results in extrapyramidal symptoms similar to Idiopathic Parkinson's Disease (Barbeau, 1984; Calne et al., 1994; Dobson et al., 2004), a dopaminergic cell disorder of the substantia nigra pars compacta. This manganese-induced Parkinsonism was first described in 1837 in two manganese ore-crushing mill workers (Couper, 1837) and has since been referred to as Manganism (Mena et al., 1967; Barbeau, 1984; Donaldson, 1987; Gorell et al., 1999). Symptoms common to both disorders include gait imbalance, rigidity, tremors and bradykinesia (Mena et al., 1967; Rosenstock et al., 1971; Mena et al., 1970; Huang et al., 1989), suggesting an overlapping etiology of neuronal damage in the substantia nigra and resulting deficiency of the neurotransmitter dopamine for the striatum. However there are a number of clinical features of Manganism that differentiate it from Parkinson's Disease including earlier behavioral and cognitive dysfunction, symmetry of effects, an intention rather than resting tremor, difficulty turning, more prominent dystonia, poor response to Levodopa, and a characteristic “cock walk” (Calne et al., 1994; Olanow, 2004; Barbeau et al., 1976; Huang et al., 1993; Lu et al., 1994; Koller et al., 2004; Jankovic, 2005; Cersosimo and Koller, 2006), suggesting different or more extensive damage in the basal ganglia or to the dopaminergic system.
Although manganese-induced Parkinsonism has been recognized for some time, the primary mechanism underlying manganese neurotoxicity remains elusive. Human and animal studies have shown that toxic manganese exposure results in metal accumulations in various areas of the basal ganglia and dysfunction of cells of both the striatum and the globus pallidus (Calne et al., 1994; Eriksson et al., 1987; Erikson et al., 2004a, 2004b; Brenneman et al., 1999; Nagatomo et al., 1999; Newland, 1999; Pal et al., 1999; Baek et al., 2003). Considering the clinical similarities between Manganism and Parkinson's Disease, and the fact that manganese accumulates in brain regions rich in dopaminergic neurons, it has long been suggested that manganese neurotoxicity involves a disruption in dopaminergic neurotransmission (Neff et al., 1969; Hornykiewicz, 1972; Graham, 1984). Some studies have shown that manganese selectively targets dopaminergic neurons in the human basal ganglia (Olanow, 2004; Pal et al., 1999) and decreased dopamine levels in the striatum (Rosenstock et al., 1971; Eriksson et al., 1987; Parenti et al., 1986; Vescovi et al., 1991; Sistrunk et al., 2007). However, the mechanism by which manganese produces dopaminergic dysfunction in humans is not fully resolved and reports postulate that the mechanism underlying manganese toxicity may be more related to downstream neuronal pathways rather than deficits in nigrostriatal function (Calne et al., 1994; Pal et al., 1999; Huang et al., 1998, Olanow, 2004).
Lack of an effective treatment for manganese toxicity has been a major obstacle in the clinical management of manganese-induced Parkinsonism, which often progresses even after exposure ends (Huang et al. 1993, 1998). Chelation therapy has been widely used to treat pathologies associated with metal toxicity, and a number of studies have shown that EDTA treatments lowered the body burden of manganese in patients with Manganism (Calne et al., 1994; Discalzi et al., 2000; Ono et al., 2002). However, reports are conflicting as to whether EDTA chelation therapy caused any symptomatic improvements, especially in patients with severe Manganism (Crossgrove and Zheng, 2004; Hernandez et al., 2006). In 1992, Ky et al. were the first to report that the drug p-Aminosalicylic acid (PAS) was clinically successful in the treatment of two cases of chronic serious manganese poisoning. Often administered with Isoniazid, PAS had been used for many years as a tuberculostatic agent, but the mechanism by which PAS was able to alleviate the symptoms of Manganism is unknown. Clinically it is unclear as to whether PAS is acting as an anti-inflammatory agent or as an effective manganese chelator in the treatment of manganese neurotoxicity and further studies are needed to determine the drug's exact mechanism of action (Jiang et al., 2006).
The present study sought to examine whether PAS could block the neurotoxic effects of manganese on the dopaminergic innervation of lateral ciliated cells of the gill and to gain better insight into whether the neuroprotective effect of PAS was related to its chelating or anti-inflammatory properties.
2. Materials and methods
2.1. Animals and gill observation
Oysters were kept and incubated in Instant Ocean® artificial seawater (ASW) obtained from Aquarium Systems Inc. (Mentor, OH, USA). Dopamine, serotonin, PAS (4-Aminosalicyclic acid) and acetylsalicylic acid (ASA) were obtained from Sigma-Aldrich (St. Louis, MO, USA). All other reagents including manganese chloride (MnCl2.4H2O, ASC grade) and calcium disodium EDTA (EDTA) were obtained from Fisher Scientific (Pittsburgh, PA, USA). Just prior to use, serotonin was freshly dissolved in ASW, and dopamine was dissolved in ASW containing 10 mg% ascorbic acid buffered with sodium bicarbonate, pH 7.2, to retard dopamine oxidation as described by Malanga (1975).
Adult C. virginica of approximately 80 mm shell length were obtained from Frank M. Flower and Sons Oyster Farm in Oyster Bay, NY, USA. They were maintained in the laboratory for up to two weeks in temperature-regulated aquaria in ASW at 16–18 °C, specific gravity of 1.024±0.001, salinity of 31.9 ppt, and pH of 7.2±0.2. Each animal was tested for health prior to experimentation by the resistance it offered to being opened. Animals that fully closed in response to tactile stimulation and required at least moderate hand pressure to being opened were used for the experiments. In order to observe the lateral ciliated cells of the gill epithelium, gills were positioned in observation chambers so the cilia of the medial gill lamina were viewed at 100−200× magnification through an Olympus CK inverted microscope with transmitted stroboscopic light from a Grass Instruments PS 22 Photo Stimulator. The beating rates of the lateral cilia were measured by the method of Catapane et al. (1978) by synchronizing the flashing rate of the stroboscope with the beating rate of the cilia. Because the lateral cilia beat in a metachronal wave pattern (Aiello and Sleigh, 1972) when synchronization is achieved the lateral cilial waves appear motionless in a characteristic horseshoe like configuration. At all multiple synchronizing rates above the one corresponding to the true beating frequency, the wavelength of the beating cilia will appear to be a fraction of the true wavelength. The beating rates of the lateral cilia are expressed as beats/s±sem.
2.2. Acute treatments of isolated gill preparations
To determine if acute addition of manganese chloride, PAS, ASA or EDTA had any effect on the basal beating rates of the lateral cilia, isolated gill preparations were prepared by excising gills from animals, sectioning them into half inch portions and positioning them in observation chambers containing ASW for microscopic observation. Increasing concentrations of manganese chloride (1 μM–1 mM) were applied and beating rates measured. Similar experiments were conducted using PAS, ASA, and EDTA.
2.3. Visceral ganglion preparations
Visceral ganglion preparations (VG preparations) were prepared by dissecting the animals so that the gill with the ipsilateral branchial nerve, visceral ganglion, and attached cerebrovisceral connective (CVC) was positioned in an observation chamber containing ASW. The observation chambers had a plexiglass barrier separating the visceral ganglion from the gill so that superfusion of serotonin or dopamine could be specifically applied to either the ganglion or gill without leakage of the chemical to the other chambers (Catapane et al., 1978).
2.4. Electrical stimulations of the branchial nerve and CVC
The branchial nerve of isolated gill preparation and the CVC of VG preparations were stimulated with suction electrodes made from IV tubing and a 20 gauge stainless steel wire. The tips were heated and pulled to match the diameter of the branchial nerve or CVC. Bipolar repeating pulses of 2 ms duration at 10V and either 5 Hz (low frequency) or 20 Hz (high frequency) were applied to the nerves with a Grass S48 Stimulator for 10 min while cilia of the lateral ciliated epithelial cells were observed. Ciliary beating rates after low and high frequency electrical stimulations of the CVC were also determined after manganese chloride (100 μM and 1.0 mM) was added to the gill chamber of VG preparations in the presence or absence of PAS, EDTA or ASA.
2.5. Short term treatments of oysters with manganese or manganese and PAS
To determine if PAS could block the impaired cilio-inhibitory response to dopamine seen in animals receiving short-term manganese treatments, oysters were incubated for 3 days in temperature-controlled aerated containers of ASW containing added manganese with or without PAS. To ensure that each oyster would receive equal drug exposure during the treatment period and not just close up, healthy specimens were shucked by removing their right shell before being placed in individual containers of ASW containing manganese chloride or manganese chloride plus PAS. Control animals were similarly prepared without exposure to added manganese or PAS. Control and experimentally treated animals tolerated the 3-day treatment well. Survival was excellent and all animals used in the subsequent experiments had visible signs of heart pumping. After 3 days, control and treated animals were dissected and VG preparations were prepared to determine beating rates of the lateral cilia after superfusion of dopamine or serotonin to the VG or directly to the gill of VG preparations.
2.6. Statistical analysis
Statistical analysis for experiments was determined by two-way ANOVA with Tukey post-test, Student t-test or Pearson correlation.
3. Results
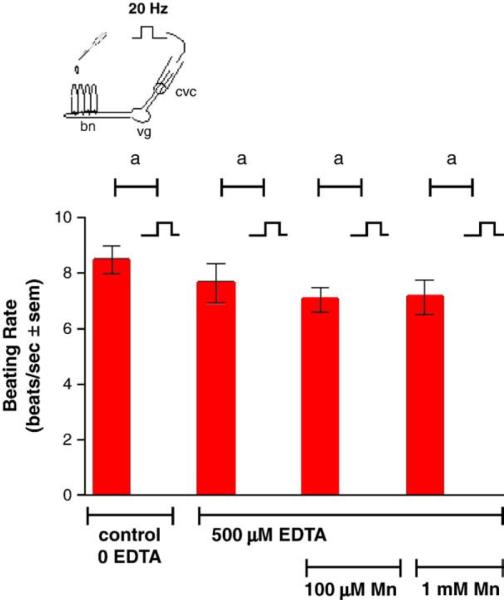
Adding manganese chloride directly to isolated gill preparations as high as 1 mM, did not significantly alter basal beating rates of the lateral cilia (Fig. 2). Stimulating the branchial nerve with suction electrodes produces a change in the beating rate of the lateral cilia that is dependent on the frequency of stimulation. Low frequency bipolar pulses (5 Hz, 10V, and 2 ms duration) for 10min accelerate the beating rate, while high frequency bipolar pulses (20 Hz, 10 V, and 2 ms duration) for 10 min decreased the beating rate (Fig. 3). There is generally a latency time of 5–20 s between application of the stimulation and changes in beating rates. The changes in beating rates are long lasting, going on for 10min or more. Adding 100 μM of manganese to the gill for 10 min prior to stimulations did not alter the effects of 5 Hz stimulation, but did prevent 20 Hz stimulations from decreasing beating rates (Fig. 4). Adding 100 μM of PAS to the gill prior to 100 μM of manganese prevented manganese from blocking the effects of 20 Hz stimulations (Fig. 5).
Fig. 2.
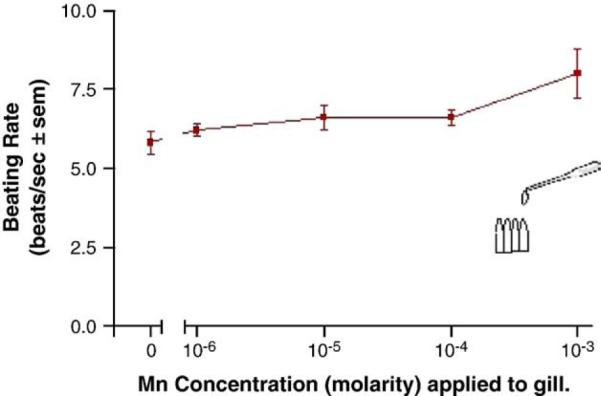
Effects of applying manganese to isolated gill.N=5 for each concentration. Pearson correlation r was 0.9350. Statistical analysis was determined by a two tailed t-test of the basal ciliary activity (0 manganese) to that of each manganese concentration and showed no significant differences between the manganese concentrations and basal activity.
Fig. 3.
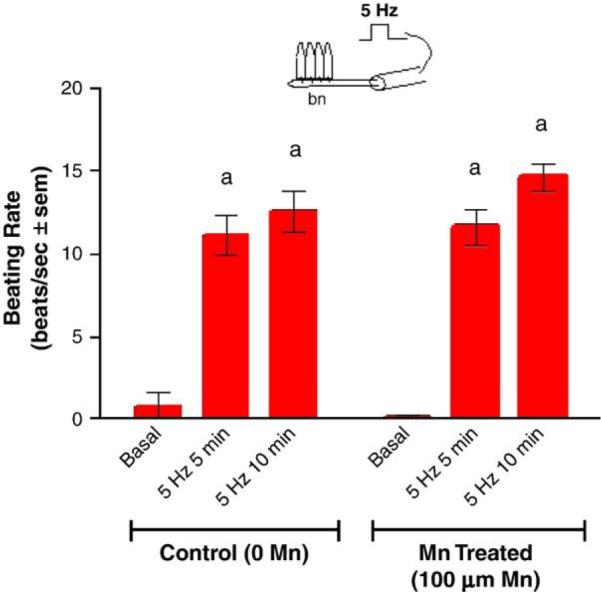
The effects of 100 μM manganese on beating rates of lateral cilia in response to low frequency electrical stimulations (5 Hz, 10V, and 2 ms duration for 10 min) of the branchial nerve. Statistical analysis was determined by a two-way ANOVA with Tukey post-test, ap<0.001 compared to basal, n=5.
Fig. 4.
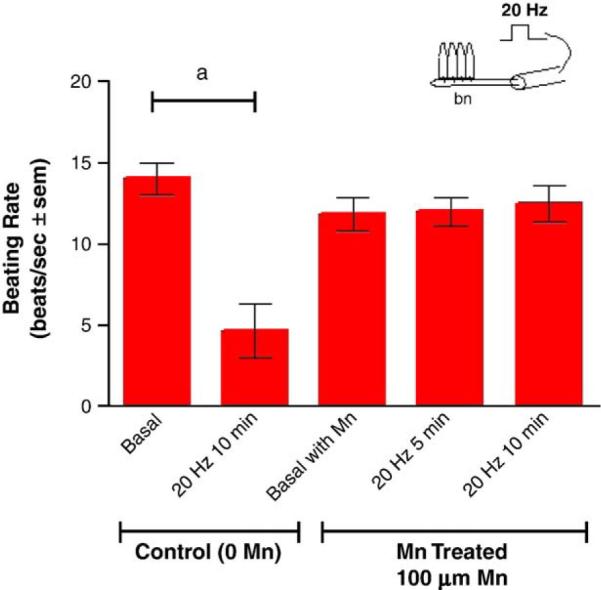
The effects of 100 μM manganese on changes in beating rates of lateral cilia in response to high frequency stimulations (20 Hz, 10V, and 2 ms duration for 10 min) of the branchial nerve. The cilia were activated with 10−5 M HT prior to applying stimulations. Statistical analysis was determined by a two-way ANOVA with Tukey post-test. N=5 for each treatment, ap<0.0l for comparison of 20 Hz to basal. There were no significant differences between the beating rates before and after 20 Hz stimulations of the manganese treated gills.
Fig. 5.
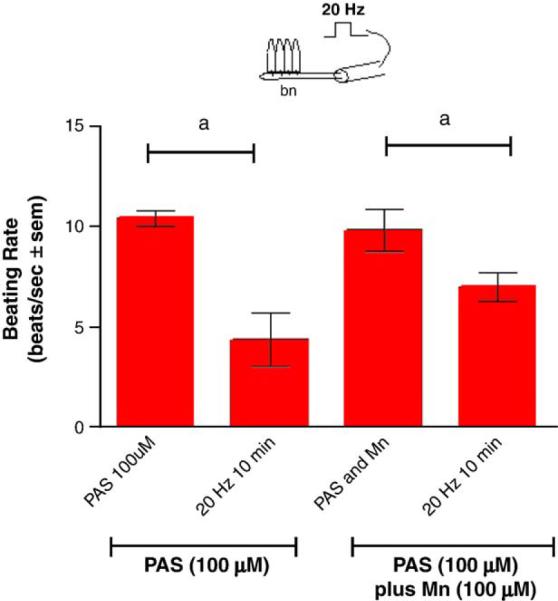
The effects of 100 μM PAS, with and without 100 μM PAS, with and without 100 μM Mn, on the actions of manganese on beating rates of lateral cilia in response to high frequency stimulations (20 Hz, 10V, and 2 ms duration for 10 min) to the branchial nerve. The cilia were activated with 10−5 M HT prior to applying stimulations. N=5 for each treatment. Statistical analysis was determined by a two-way ANOVA with Tukey post-test, ap<0.0l for comparison of before and after stimulations.
In other experiments, the CVC of VG preparations were stimulated for 10 min at either low frequency or high frequency and the lateral cilia of the gill similarly showed a differential response.
Stimulating the CVC at 5 Hz increased the beating rates of the lateral cilia, while stimulating at 20 Hz decreased the beating rates (Fig. 6). The presence of added manganese chloride (100 μM and 1.0 mM) to the gill chamber had no effect on the cilio-excitatory effects of 5 Hz stimulations to the CVC but prevented the cilio-inhibitory effects of the 20 Hz stimulations to the CVC (Fig. 7). This neurotoxic effect of manganese could be blocked by adding PAS (500 μM) to the gill chamber before manganese additions, while the presence of PAS alone in the gill chamber had no direct effects on the cilio-inhibitory actions of 20 Hz electrical stimulations of the CVC (Fig. 8). Similar results were obtained when EDTA (100 μM and 1.0 mM), a known metal chelator, was present in the gill chamber before manganese additions (Fig. 9). While both PAS and EDTA were able to block the neurotoxic effects of manganese, the anti-inflammatory agent ASA (100 μM and 1.0 mM) showed no blocking activity against manganese when substituted for PAS in the gill chamber (Fig. 10).
Fig. 6.
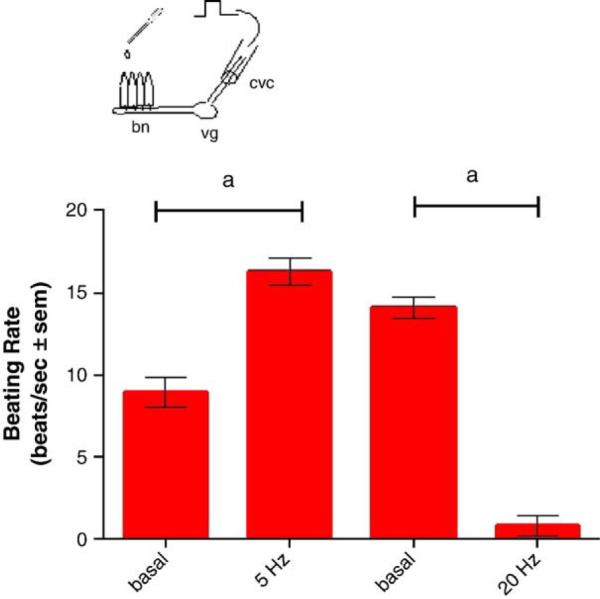
Effects of low and high frequency stimulations (5 Hz or 20 Hz, 10 V, and 2 ms duration) of the CVC on beating rates of lateral cilia. The CVC was stimulated for 5 min at 5 Hz and beating rates recorded 5 min later. After a 10 minute rest, the CVC was stimulated at 20 Hz for 5 min and the beating rates recorded 5min later. Statistical significance was determined by a two-way ANOVA with Tukey post-test. ap<0.001 for comparison before and after stimulations, n=9 for each test.
Fig. 7.
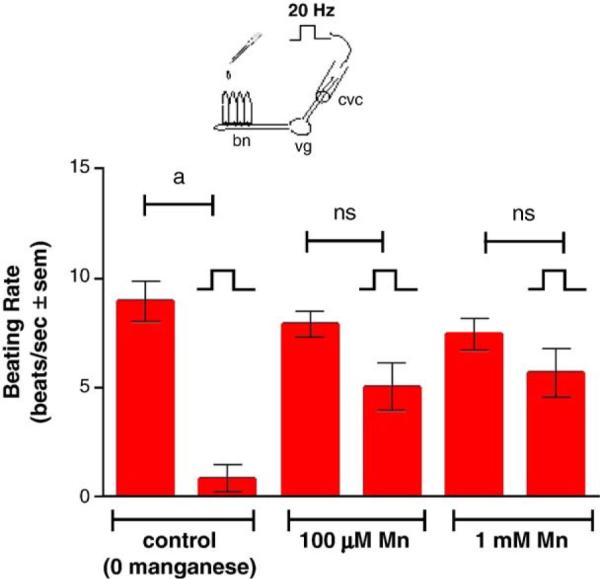
Effects of different concentrations of manganese applied to the gills on high frequency stimulations (20 Hz, 10V, and 2 ms duration for 10 min) of the CVC on beating rates of lateral cilia. The cilia were activated with 10−5 M HT prior to applying stimulations. Statistical significance was determined by a two-way ANOVA with Tukey post-test. ap<0.001, ns not significant for comparison of before and after stimulations. N=9 for each treatment.
Fig. 8.
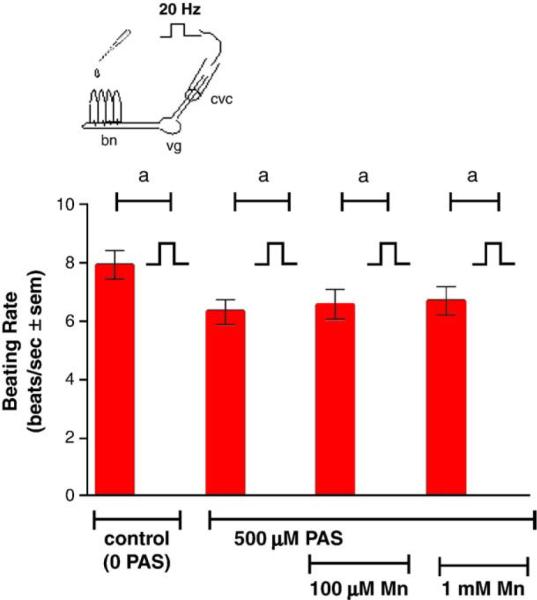
Effects of 500 μM PAS, with and without Mn, on high frequency stimulations (20 Hz, 10 V, 2 ms duration for 10 min) of the CVC on beating rates of lateral cilia. The cilia were activated with 10−5 M HT prior to applying stimulations. Statistical significance was determined by a two-way ANOVA with Tukey post-test. ap<0.001 for comparison of before and after stimulations. N=6 for each treatment. Note that the beating rate after stimulation for each treatment was 0 beats/s.
Fig. 9.
Effects of 500 μM EDTA, with and without Mn, on high frequency stimulations (20 Hz, 10 V, 2 ms duration for 10 min) of the CVC on beating rates of lateral cilia. The cilia were activated with 10−5 M HT prior to applying stimulation and statistical significance was determined by a two-way ANOVA with Tukey post-test. ap<0.001 for comparisons of before and after stimulations. N=6 for each treatment Note that the beating rate after stimulation for each treatment was 0 beats/s.
Fig. 10.
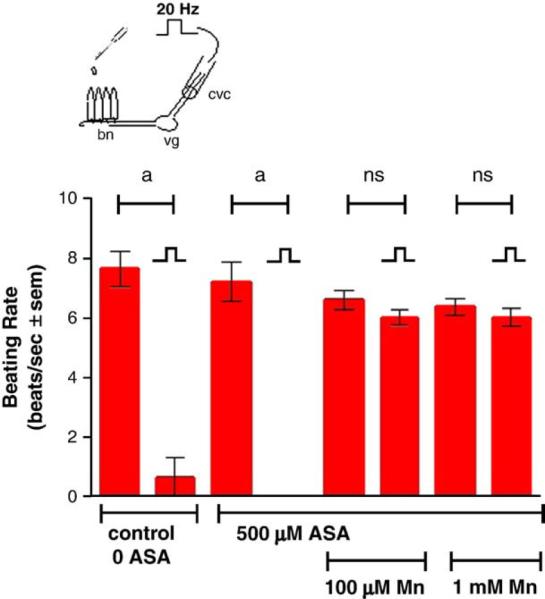
Effects of 500 μM ASA with and without Mn, on high frequency (20 Hz, 10 V, and 2 ms duration for 10 min) of the CVC on beating rates of lateral cilia. The cilia were activated with 10−5 M HT prior to applying stimulations. Statistical significance was determined by a two-way ANOVA with Tukey post-test. ap<0.001, ns=not significant for comparisons of before and after stimulations. N=9 for each treatment. Note that the beating rate after stimulation for the 500 μM ASA treatment was 0 beats/s.
When whole animals were treated for 3 days in temperature-regulated aerated containers of ASW in the presence of manganese, there was a decreased response of the lateral cilia upon superfusion of dopamine to the visceral ganglia of VG preparations. Compared to controls, the animals incubated with 50 μM manganese demonstrated statistically significant impairment of their cilio-inhibitory response to superfusion of dopamine to their visceral ganglia, and higher manganese treatments (500 μM) resulted in greater disruption of the animal's dopaminergic system (Fig. 11). To determine the effects of PAS on whole animals and to determine whether co-treating animals with PAS and manganese could block the neurotoxic effects of manganese on the animal's dopaminergic system, oysters were treated for 3 days with PAS alone, or co-treated with PAS and manganese. Oysters treated with PAS, as high as 2 mM, had excellent survival and the treatment did not alter basal beating rates or the cilio-inhibitory effect of dopamine when applied to the visceral ganglia of VG preparations (Fig. 12). In animals co-treated with PAS and manganese, the presence of PAS reduced the neurotoxic effect of manganese on the animal's dopaminergic system. A 50 μM dose of PAS decreased the ability of a 500 μM dose of manganese from reducing the response of the lateral cilia to superfusion of dopamine to visceral ganglia (Fig. 13).
Fig. 11.
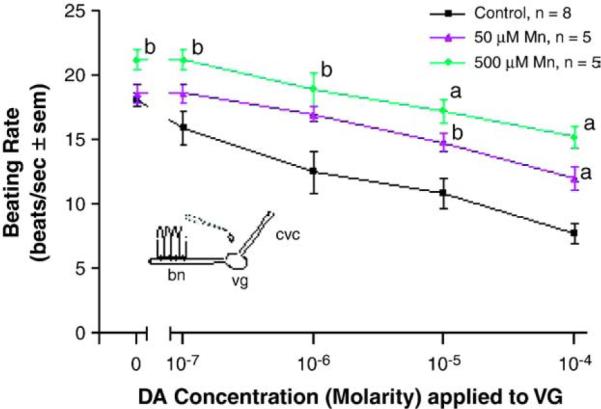
The changes in beating rates (beats/s±sem) of lateral cilia in response to superfusion of dopamine to the visceral ganglia of VG preparations of controls and animals treated for 3 days with 50 or 500 μM Mn. The cilia were activated with 10−5 M and Turkey post-test. ap<0.01, bp<0.05 for comparison to control at the same dopamine doses.
Fig. 12.
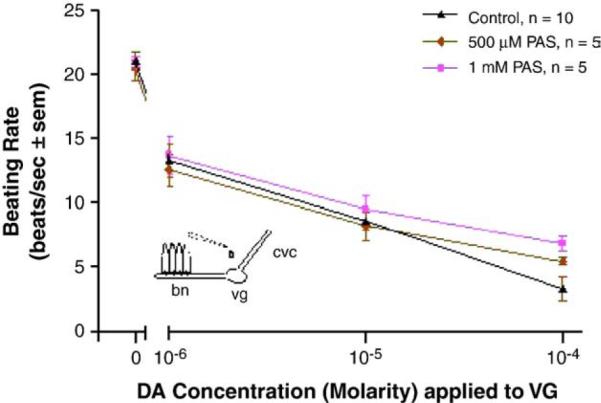
The changes in beating rates (beats/s±sem) of lateral cilia in response to application of dopamine to the visceral ganglia of VG preparations of controls and animals treated for 3 days with PAS. The cilia were activated with 10−5 M serotonin. Statistical analysis was determined by a two-way ANOVA with Tukey post-test. ap<0.05 for comparison to control and the 10−4 M dopamine concentration. There were no significant differences among treatments for other dopamine concentrations.
Fig. 13.
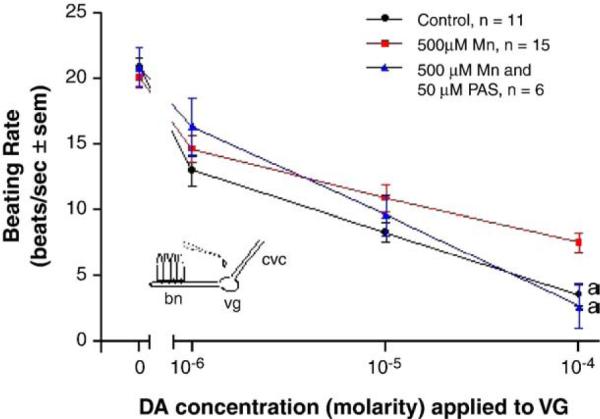
The effects of PAS treatments on the neurotoxic actions of manganese on changes in beating rates (beats/s±sem) of lateral cilia in response to superfusion of dopamine to the visceral ganglia of VG preparations. Animals were treated with 500 μM manganese with and without 50 μM PAS for 3 days. The cilia were activated with 10−5 M serotonin. Statistical analysis was determined by a two-way ANOVA with Tukey post-test. ap<0.01 for comparison of control and PAS-treated to Mn-treated.
4. Discussion
The study shows that the nervous system of C. virginica regulates the beating of the lateral cilia of the gill. In a manner similar to that of the related bivalve, M. edulis (Catapane et al., 1979), low frequency stimulations to the branchial nerve or CVC cause a terminal release of serotonin that accelerates beating rates, while high frequency stimulations cause a terminal release of dopamine that decreases beating rates. Acutely adding increased amounts of manganese directly to gill does not alter the beating rates of the cilia within the concentrations tested. However, 100 μM of manganese applied to the gill blocked the cilio-inhibitory response to high frequency electrical stimulations to the branchial nerve and CVC, while not impairing the effects of low frequency stimulations. The neurotoxic effects of manganese were blocked by adding PAS and EDTA to the gill, but not by adding ASA. The results of the short-term experiments similarly show that treating animals for 3 days with manganese impairs the dopaminergic innervation to the gill and that co-treatments with PAS block the impairments. Previous studies showed that although short-term manganese treatments decreased endogenous dopamine levels in the ganglia and gill, the neurotoxic mechanism of action of manganese is at the post-synaptic dopamine receptor because applying dopamine directly to the gill of manganese treated animals was ineffective in slowing down beating rates (Martin et al., 2008). The present study strengthens this by demonstrating that the acute and short-term neurotoxic effects of manganese are blocked by adding PAS or EDTA directly to the gill. The study also showed that the mechanism of action of PAS in blocking the effects of manganese mimicked that of the metal chelator EDTA. In similar experiments the drug ASA, an anti-inflammatory agent related to PAS, was not able to block the neurotoxic effects of manganese.
The mechanism by which manganese produces dopaminergic dysfunction in humans is not fully resolved and reports postulate that the mechanism underlying manganese toxicity may be more related to downstream neuronal pathways rather than deficits in nigrostriatal function or dopamine content (Calne et al., 1994; Pal et al., 1999; Huang et al., 1998, Olanow, 2004). Evidence from nonhuman primate data suggests a manganese-induced post-synaptic decrease of D2-like dopamine receptor levels (Eriksson et al., 1987). Lack of an effective treatment for manganese intoxication has been a major obstacle in the clinical management of Manganism, which often progresses even after exposure ends (Huang et al., 1993, 1998). Unlike Parkinson's Disease, patients with Manganism have a poor response to L-DOPA treatments (Huang et al., 1993). Chelation therapy has been widely used to treat pathologies associated with metal toxicity. Early studies comparing the manganese-chelating abilities of various compounds found a greater binding capacity of manganese with chelators containing N and O electron donating centers, than thiol centered chelators (Khandelwal et al., 1980) and a number of studies have shown that EDTA treatments did indeed lower the body burden of manganese in patients with Manganism (Calne et al., 1994; Discalzi et al., 2000; Ono et al., 2002). However, reports are conflicting as to whether EDTA chelation therapy caused any symptomatic improvements, especially in patients with severe manganese-induced Parkinsonism (Crossgrove and Zheng, 2004; Hernandez et al., 2006). Considering its amino, carboxyl and hydroxyl functional groups, the drug PAS is a potential therapeutic manganese-chelating agent. Early studies had showed that PAS could mobilize manganese from the livers and testis of manganese-intoxicated rats (Tandon et al., 1975), and enhance the fecal excretion of manganese in manganese-intoxicated rabbits (Tandon, 1978). Clinical studies in China demonstrated that PAS treatments were successful in alleviating symptoms in two patients with chronic manganese poisoning (Ky et al, 1992). In addition, Jiang et al. (2006) reported that PAS treatments reversed many of the clinical symptoms in a woman with severe chronic Manganism, and after a 17 year follow-up, the drug was considered to have a beneficial prognostic effect. Questions remained as to whether the therapeutic actions of PAS in the successful treatment in patients with manganese neurotoxicity were due to the drug's anti-inflammatory moiety or its chelation ability. Recently Zheng et al. (2009) found that high and prolonged PAS treatments could reduce body fluid and tissue levels of manganese-exposed Sprague–Dawley rats and that PAS was likely acting as a chelating agent to mobilize and remove tissue manganese. The present study focused on the ability of PAS to block manganese-induced neurotoxicity of the dopaminergic system in C. virginica and found that the therapeutic actions of PAS were more in line with that of the known metal chelator EDTA than with the related anti-inflammatory agent ASA. These results lend further support to the hypothesis that the mechanism of action of PAS in the treatment of manganese-induced Parkinsonism is chelation. The work also shows that the gill–ganglion preparation of C. virginica is a useful preparation to study the physiology and pharmacology of biogenic amines and drugs affecting them.
Acknowledgments
This work was supported in part by grants 2R25GM0600305 of the Bridge Program of NIGMS, 0516041071 of the CSTEP Program of the New York State Department of Education and 0622197 of the DUE Program of NSF.
References
- Aiello E, Sleigh MA. The metachronal wave of lateral cilia of Mytilus edulis. J. Cell Biol. 1972;54:493–506. doi: 10.1083/jcb.54.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ME, Gearhart JM, Clewell HJ., III Pharmacokinetic data needs to support risk assessments for inhaled and ingested manganese. Neurotoxicology. 1999;20:161–171. [PubMed] [Google Scholar]
- Baek SY, Lee MJ, Jung HS, Kim HJ, Lee CR, Yoo C, Lee JH, Lee H, Yoon CS, Kim YH, Park J, Kim JW, Jeon BS, Kim Y. Effect of manganese exposure on MPTP neurotoxicities. Neurotoxicology. 2003;24:657–665. doi: 10.1016/S0161-813X(03)00033-0. [DOI] [PubMed] [Google Scholar]
- Barbeau A. Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C Cotzias) Neurotoxicology. 1984;5:13–35. [PubMed] [Google Scholar]
- Barbeau A, Inoué N, Cloutier T. In: Role of manganese in dystonia. : Advances in Neurology. Eldridge R, Fahn S, editors. vol. 14. Raven Press; New York: 1976. pp. 339–352. [PubMed] [Google Scholar]
- Brenneman KA, Cattley RC, Ali SF, Dorman DC. Manganese induced developmental neurotoxicity in the CD rat: is oxidative damage a mechanism of action? Neurotoxicology. 1999;20:477–488. [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: similarities and difference. Neurology. 1994;44:1583–1586. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- Carroll MA, Catapane EJ. The nervous system control of lateral ciliary activity of the gill of the bivalve mollusc, Crassostrea virginica. Comp. Biochem. Physiol. A. 2007;148:445–450. doi: 10.1016/j.cbpa.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catapane EJ, Stefano GB, Aiello E. Pharmacological study of the reciprocal dual innervation of the lateral ciliated gill epithelium by the CNS of Mytilus edulis (Bivalvia) J. Exp. Biol. 1978;74:101–113. [Google Scholar]
- Catapane EJ, Stefano GB, Aiello E. Neurophysiological correlates of the dopamine cilio-inhibitory mechanism of Mytilus edulis. J. Exp. Biol. 1979;83:315–323. doi: 10.1242/jeb.83.1.315. [DOI] [PubMed] [Google Scholar]
- Cersosimo MG, Koller WC. The diagnosis of manganese-induced parkinsonism. NeuroToxicology. 2006;27:340–346. doi: 10.1016/j.neuro.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Cotzias GC. Manganese in health and disease. Physiol. Rev. 1958;38:503–532. doi: 10.1152/physrev.1958.38.3.503. [DOI] [PubMed] [Google Scholar]
- Couper J. On the effects of black oxide of manganese when inhaled into the lungs. Br. Ann. Med. Pharmacol. 1837;1:41–42. [Google Scholar]
- Crossgrove J, Zheng W. Manganese toxicity upon overexposure. Biomedicine. 2004;17(8):544–553. doi: 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discalzi G, Pira E, Hernandez EH, Valentini C, Turbiglio M, Meliga F. Occupational Mn parkinsonism: magnetic resonance imaging and clinical patterns following CaNa2-EDTA chelation. Neurotoxicology. 2000;21:863–866. [PubMed] [Google Scholar]
- Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann. N.Y. Acad. Sci. 2004;1012:115–128. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- Donaldson J. The physiopathologic significance of manganese in brain: its relation to schizophrenia and neurodegenerative disorders. Neurotoxicology. 1987;8:451–462. [PubMed] [Google Scholar]
- Eriksson H, Magiste K, Plantin LO, Fonnum F, Hedstrtm K, Theodorsson G, Norheim E, Kristensson K, Stalberg E, Heilbronn E. Effects of manganese oxide on monkeys as revealed by a combined neurochemical, histological and neurophysiological evaluation. Arch. Toxicol. 1987;61:46–52. doi: 10.1007/BF00324547. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Dobson AW, Dorman DC, Aschner M. Manganese exposure and induced oxidative stress in the rat brain. Sci. Total Environ. 2004a:334–335. 409–416. doi: 10.1016/j.scitotenv.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson KM, Syversen T, Steinnes E, Aschner M. Globus pallidus: a target brain region for divalent metal accumulation associated with dietary iron deficiency. J. nutr. Biochem. 2004b;15:335–341. doi: 10.1016/j.jnutbio.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Rybicki BA, Cole Johnson C, Peterson EL. Occupational metal exposures and the risk of Parkinson's disease. Neuroepidemiology. 1999;18:303–308. doi: 10.1159/000026225. [DOI] [PubMed] [Google Scholar]
- Graham DG. Catecholamine toxicity: a proposal for the molecular pathogenesis of manganese neurotoxicity and Parkinson's disease. Neurotoxicology. 1984;5:83–95. [PubMed] [Google Scholar]
- Hernandez EH, Discalzi G, Valentini C, Venturi F, Chiò A, Carmellino C, Rossi L, Sacchetti A, Pira E. Follow-up of patients affected by manganese-induced Parkinsonism after treatment with CaNa2EDTA. Neurotoxicology. 2006;27:333–339. doi: 10.1016/j.neuro.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine and extrapyramidal motor function and dysfunction. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1972;50:390–415. [PubMed] [Google Scholar]
- Huang CC, Chu NS, Lu CS, Chen RS, Calne DB. Long-term progression in chronic Manganism – ten years follow-up. Neurology. 1998;50:698–700. doi: 10.1212/wnl.50.3.698. [DOI] [PubMed] [Google Scholar]
- Huang CC, Chu NS, Lu CS, Wang JD, Tsai JL, Tzeng JL. Chronic manganese intoxication. Arch. Neurol. 1989;46:1104–1106. doi: 10.1001/archneur.1989.00520460090018. [DOI] [PubMed] [Google Scholar]
- Huang CC, Lu CS, Chu NS, Hochberg F, Lilienfeld D, Olanow W. Progression after chronic manganese exposure. Neurology. 1993;43:1479–1483. doi: 10.1212/wnl.43.8.1479. [DOI] [PubMed] [Google Scholar]
- Hudnell HK, Mergler D, Wittberg RA. Possible effects of chronic exposure to environmental airborne manganese on neurological function in children. Abstract: Int. Conference on Heavy Metals; Ann Arbor, Michigan. 1999. [Google Scholar]
- Iregren A. Manganese neurotoxicity in industrial exposures: proof of effects, critical exposure level, and sensitive tests. Neurotoxicology. 1999;20:315–324. [PubMed] [Google Scholar]
- Jankovic J. Searching for a relationship between manganese and welding and Parkinson's disease. Neurology. 2005;64:2021–2028. doi: 10.1212/01.WNL.0000166916.40902.63. [DOI] [PubMed] [Google Scholar]
- Jiang W, Mo X, Du F, Fu X, Zhu X, Gao H, Xie J, Liao F, Pira E, Zheng W. Effective treatment of manganese-Induced occupational Parkinsonism with p-aminosalicylic acid: a case of 17-year follow-up study. J. Occup. Environ. Med. 2006;48:644–649. doi: 10.1097/01.jom.0000204114.01893.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J. Manganese: a high octane dispute. Science. 2003;300:926–928. doi: 10.1126/science.300.5621.926. [DOI] [PubMed] [Google Scholar]
- Khandelwal S, Kachru DN, Tandon SK. Chelation in metal intoxication IX. Influence of amino and thiol chelators on excretion of manganese in poisoned rabbits. Toxicol. Letts. 1980;6:131–135. doi: 10.1016/0378-4274(80)90180-0. [DOI] [PubMed] [Google Scholar]
- King C, Myrthil M, Carroll MA, Catapane EJ. Effects of p-aminosalicylic acid on the neurotoxicity of manganese and levels of dopamine and serotonin in the nervous system and innervated organs of Crassostrea virginica. In Vivo. 2008;29:29–34. [PMC free article] [PubMed] [Google Scholar]
- Koller WC, Lyons KE, Truly W. Effect of levodopa treatment for Parkinsonism in welders. A double-blind study. Neurology. 2004;62:730–733. doi: 10.1212/01.wnl.0000113726.34734.15. [DOI] [PubMed] [Google Scholar]
- Ky SQ, Deng HS, Xie PY, Hu W. A report of two cases of chronic serious manganese poisoning treated with sodium para-aminosalicylic acid. Br. J. Ind. Med. 1992;49:66–69. doi: 10.1136/oem.49.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CS, Huang CC, Chum NS, Calne DB. Levodopa failure in chronic manganism. Neurology. 1994;44:1600–1602. doi: 10.1212/wnl.44.9.1600. [DOI] [PubMed] [Google Scholar]
- Malanga CJ. Dopaminergic stimulation of frontal ciliary activity in the gill of Mytilus edulis. Comp. Biochem. Physiol. C. 1975;51:25–34. doi: 10.1016/0306-4492(75)90033-7. [DOI] [PubMed] [Google Scholar]
- Martin K, Huggins T, King C, Carroll MA, Catapane EJ. The neurotoxic effects of manganese on the dopaminergic innervation of the gill of the bivalve mollusc, Crassostrea virginica. Comp. Biochem. Physiol. C. 2008;148:152–159. doi: 10.1016/j.cbpc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meco G, Bonfanti V, Vanacore N, Fabrizio E. Parkinsonism after chronic exposure to the fungicide maneb (manganese ethylene-bis-dithiocarbamate. Scand. J. Work Environ. Health. 1994;20:301–305. doi: 10.5271/sjweh.1394. [DOI] [PubMed] [Google Scholar]
- Mena I, Court J, Fuenzalida S, Papavasiliou PS, Cotzias GS. Modification of chronic manganese poisoning. N. Engl. J. Med. 1970;282:5–10. doi: 10.1056/NEJM197001012820102. [DOI] [PubMed] [Google Scholar]
- Mena I, Marin O, Fuenzalida S, Cotzias GC. Chronic manganese poisoning. Clinical picture and manganese turnover. Neurology. 1967;17:128–136. doi: 10.1212/wnl.17.2.128. [DOI] [PubMed] [Google Scholar]
- Murray S, Lovell A, Carroll MA, Catapane EJ. Distribution of manganese in the oyster Crassostrea virginica raised in Jamaica Bay, NY and its accumulations in oysters acutely exposed to high levels. Annals of the 2007 SICB Conference.2007. [Google Scholar]
- Nagatomo S, Umehara F, Hanada K, Nobuhara Y, Takenaga S, Arimura K, Osame M. Manganese intoxication during total parenteral nutrition: report of two cases and review of the literature. J. Neurol. Sci. 1999;162:102–105. doi: 10.1016/s0022-510x(98)00289-5. [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences . Medical and biological effects of environmental pollutants: manganese. National Academy Press; Wash. DC: 1973. pp. 1–101. [Google Scholar]
- Neff NH, Barrett RE, Costa E. Selective depletion of caudate nucleus dopamine and serotonin during chronic manganese dioxide administration to squirrel monkeys. Experientia. 1969;25:1140–1141. doi: 10.1007/BF01900234. [DOI] [PubMed] [Google Scholar]
- Newland MC. Animal models of manganese's neurotoxicity. Neurotoxicology. 1999;20:415–432. [PubMed] [Google Scholar]
- Olanow CW. Manganese-induced parkinsonism and Parkinson's disease. Ann. N.Y. Acad. Sci. 2004;1012:209–223. doi: 10.1196/annals.1306.018. [DOI] [PubMed] [Google Scholar]
- Ono K, Komai K, Yamada M. Myoclonic involuntary movement associated with chronic manganese poisoning. J. Neurol. Sci. 2002;199:93–96. doi: 10.1016/s0022-510x(02)00111-9. [DOI] [PubMed] [Google Scholar]
- Pal PK, Samii A, Calne DB. Manganese neurotoxicity: a review of clinical features. Neurotoxicology. 1999;20:227–238. [PubMed] [Google Scholar]
- Parenti M, Flauto C, Parati E, Vescovi A, Groppetti A. Manganese neurotoxicity: effects of L-DOPA and pargyline treatments. Brain Res. 1986;367:8–13. doi: 10.1016/0006-8993(86)91571-4. [DOI] [PubMed] [Google Scholar]
- Reidy TJ, Bowler RM, Rauch SS, Pedroza GI. Pesticide exposure and neuropsychological impairment in migrant farm workers. Arch. Clin. Neuropsychol. 1992;l7:85–95. [PubMed] [Google Scholar]
- Rosenstock HA, Simons DG, Meyer JS. Chronic Manganism. Neurologic and laboratory studies during treatment with levodopa. JAMA. 1971;217:1354–1358. doi: 10.1001/jama.217.10.1354. [DOI] [PubMed] [Google Scholar]
- Sistrunk SC, Ross MK, Filipov NM. Direct effects of manganese compounds on dopamine and its metabolite DOPAC: an in vivo study. Environ. Toxicol. Pharmacol. 2007;23:286–296. doi: 10.1016/j.etap.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon SK. Chelation in metal intoxication. VI. Influence of PAS and CDTA on the excretion of manganese in rabbits given MnO2. Toxicology. 1978;9:379–385. doi: 10.1016/0300-483x(78)90021-5. [DOI] [PubMed] [Google Scholar]
- Tandon SK, Chandra SV, Singh J, Husain R, Seth PK. Chelation in metal intoxication: I. In vivo effect of chelating agents on liver and testis of manganese administered rats. Environ. Res. 1975;9:18–25. doi: 10.1016/0013-9351(75)90045-6. [DOI] [PubMed] [Google Scholar]
- Vescovi A, Facheris L, Zaffaroni A, Malanca G, Parati EA. Dopamine metabolism alterations in a manganese-treated pheochromocytoma cell line (PC12) Toxicology. 1991;67:129–142. doi: 10.1016/0300-483x(91)90137-p. [DOI] [PubMed] [Google Scholar]
- Zheng W, Jiang Y, Zhang Y, Jiang W, Wang X, Cowan DM. Chelation therapy of manganese intoxication with para-aminosalicylic acid (PAS) in Sprague–Dawley rats. Neurotoxicology. 2009;30:240–248. doi: 10.1016/j.neuro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]