Abstract
Purpose
CD44 is one of the most common markers used for identification of highly tumorigenic sub-populations of human carcinoma cells, but little is known about the function of CD44 or its major ligand, hyaluronan, in these cells. The purpose of this study is to investigate the involvement of hyaluronan and its interaction with CD44 in the properties of a tumorigenic sub-population of primary ovarian carcinoma cells.
Experimental Design
A tumorigenic sub-population was identified in ascites fluids from ovarian carcinoma patients by expression of high CD133 levels. Treatment with small hyaluronan oligosaccharides, which dissociate constitutive hyaluronan polymer-CD44 interactions, was used to test the importance of hyaluronan-CD44 interaction in assembly of multidrug and monocarboxylate transporters and receptor tyrosine kinases in the plasma membrane of cells with high CD133 levels, and in the tumorigenic capacity of the CD133-high sub-population.
Results
Although total CD44 levels were similar in cells with high or low CD133 expression, CD44 was present in close association with transporters, receptor tyrosine kinases and emmprin (CD147) in the plasma membrane of cells with high CD133 levels. Treatment with small hyaluronan oligosaccharides reduced association of the transporters and receptor tyrosine kinases with CD44 in the plasma membrane, diminished drug transporter activity, and inhibited intra-peritoneal tumorigenesis in these cells.
Conclusions
We conclude that hyaluronan-CD44 interaction plays an important role in the properties of highly tumorigenic cells by stabilizing oncogenic complexes in their plasma membrane, and that treatment with hyaluronan-CD44 antagonists provides a logical therapeutic approach for abrogating the properties of these cells.
Introduction
Ovarian carcinoma is the most lethal malignancy of the female reproductive system. Most ovarian cancers arise from the surface epithelium of the ovary but the detailed mechanisms whereby ovarian carcinomas develop and progress are unclear. Most patients are diagnosed with advanced disease that involves accumulation of peritoneal ascites fluid containing tumor and inflammatory cells, implantation and invasion of various adjacent organs by tumor cells, and metastases. Standard therapy of surgery combined with chemotherapy results in complete remission in most patients, but relapse and onset of chemoresistant disease are very common (1, 2). Thus improved therapeutic strategies, including adjuvant treatments that sensitize recurring tumors to chemotherapeutic agents, are crucial for reducing mortality. Resistance of cancers to chemotherapeutic agents can arise in various ways but in recent years many studies have focused on the potential role of highly tumorigenic, therapy-resistant sub-populations of cancer cells that are present within many types of tumors and are often termed cancer stem cells or tumor-initiating cells (3, 4). Although the origins and characteristics of these cells are still controversial and may simply reflect tumor heterogeneity, it seems clear that highly malignant, multidrug-resistant sub-populations are present in many tumors (4-6).
One of the most common markers used for identification of highly tumorigenic sub-populations in carcinomas is CD44 (4), and recently it was shown that CD44 is a useful marker for isolating these sub-populations from patient-derived ovarian carcinomas (7). Hyaluronan, the major ligand for CD44, is a very large polysaccharide that is assembled into pericellular and extracellular matrices in many tissues and serves both structural and signaling functions (8). Hyaluronan is enriched in the tumor stroma and pericellular matrix around cancer cells in a variety of malignant cancer types, including ovarian carcinomas (9). In previous work, we observed that hyaluronan accumulates rapidly in ascites and at sites of initial attachment of tumor cells to the mesentery and peritoneal wall following inoculation of murine ovarian or mammary tumor cells into the peritoneum of syngeneic mice (10). Other investigators have demonstrated functional involvement of hyaluronan and CD44 in ovarian cancer cell adhesion and migration in culture systems (11, 12). In addition, we and others have shown that hyaluronan-CD44 interactions co-regulate several oncogenic signaling pathways in various cancer cells (8, 13-15). Of particular interest is the apparent role of hyaluronan-CD44 interactions in assembly or stabilization of signaling complexes containing receptor tyrosine kinases or transporters in the plasma membrane and the functional importance of these interactions in malignant and drug-resistant properties of cancer cells (16-21).
CD133 is a stem cell marker that is commonly used for identifying highly malignant, tumor-initiating sub-populations (4) although, as with all such markers, its usefulness varies widely with respect to tumor type or stage and details of technical application (22). Nevertheless, recent studies have shown that CD133-expressing cells are present within ovarian carcinomas and that these cells exhibit properties associated with tumor-initiating cells (23, 24). Consequently, we employed CD133 to separate tumorigenic and non-tumorigenic sub-populations from the ascites of patients with ovarian carcinoma, and used these cells to investigate the role of hyaluronan-CD44 interactions. We found that cells with high levels of CD133 (“CD133-hi” cells) express elevated levels of hyaluronan, emmprin (CD147), receptor tyrosine kinases (RTKs), monocarboxylate (lactate) transporters (MCTs), and ATP-binding cassette (ABC) family multidrug transporters, as compared to cells with low levels of CD133 (“CD133-lo” cells). Surprisingly, we found that CD133-hi and CD133-lo cells express similar levels of CD44. However, in the CD133-hi cells, CD44 is concentrated in the plasma membrane and is associated therein with RTKs, MCTs and ABC transporters, whereas this was not the case in CD133-lo cells. Moreover, treatment with an antagonist of hyaluronan-CD44 interactions, i.e. small hyaluronan oligosaccharides (8), reduced association of CD44 with the RTKs, MCTs and ABC transporters, induced their internalization, reduced drug transporter activity, and inhibited tumorigenic capacity in the CD133-hi cells.
Materials and Methods
Reagents
Fetal bovine serum (FBS) was purchased from Atlas Biologicals (Fort Collins, CO). RPMI1640 was purchased from Sigma (St. Louis, MO). The following antibodies were obtained for these studies: breast cancer resistance protein/ABCG2 (BCRP) clone BXP-21 (Kamiya Biomedical Company; Seattle, WA), CD44/HCAM (DF1485) and MCT4 (Santa Cruz Biotechnology; Santa Cruz, CA), Alexa555-tagged CD44 (Cedarlane Laboratories, Burlington, NC), P-glycoprotein/ABCB1 (Pgp) (Calbiochem; La Jolla, CA), β-actin (Sigma), CD133/1(AC-133)-PE antibody (Mitenyi Biotec GmbH, Germany), CD133 and EGFR (Cell Signaling Technology, Beverly, MA), emmprin/CD147 (BD Biosciences; Franklin Lakes, NJ), goat anti-mouse HRP and goat anti-rabbit HRP (Chemicon; Temecula, CA), and Alexa Fluor® 488, 555, 647 (Invitrogen; Carlsbad, CA). DRAQ5 nuclear stain was obtained from Biostatus Limited (Leicestershire, UK). Western blotting detection reagent (ECL) was purchased from Pierce (Rockford, IL). Hyaluronan oligosaccharides used in this study were a mixed fraction of average molecular weight ~2.5×103, composed of 2-10 disaccharide units, that were fractionated from testicular hyaluronidase (Sigma, type 1-S) digests of hyaluronan polymer (Sigma, sodium salt, cat # H5388); fractionation was by trichloroacetic acid precipitation followed by serial dialysis, using membranes of Amicon Ultra Ultracel 5,000 MWCO (Millipore; Billerica, MA) and Spectra/Por Membrane 1,000 MWCO (Spectrum Laboratories; Rancho Dominguez, CA). All other chemicals were of reagent grade or higher.
Primary ovarian carcinoma cell preparation and culture
Use of patient ascites fluids was approved by Medical University of South Carolina Institutional Review Board. Cells were collected from ascites of patients with stage III/IV ovarian carcinoma by centrifugation at 500 g for 10 minutes at room temperature. The cells were washed twice with red blood cell lysis buffer (150 mM ammonium chloride, 10 mM potassium bicarbonate, 0.1 mM EDTA, pH 7.4) and once with Hank's Balanced Salt Solution, before being plated in RPMI1640 with 2.38 g/l Hepes, 2 g/l sodium bicarbonate, Penicillin/ Streptomycin, and 10% FBS, pH 7.4, at 37°C in a humidified 5% CO2-95% air incubator. After 1 hour, non-adherent cells were removed (rapidly adherent cells are mainly macrophages and fibroblasts) and replated, and the subsequent adherent cells maintained in monolayer culture. For preparation of lysates, cells were seeded in 10 cm dishes 48 h prior to treatment. For preparation of confocal microscopy slides, cells were seeded in 4-well multi-chamber culture slides (BD Biosciences) 48 h prior to treatment.
Magnetic sorting of CD133-hi and CD133-lo cells
The primary ovarian carcinoma cells from patient ascites were separated by magnetic cell sorting using the EasySep PE selection kit (StemCell Technologies, BC, Canada) according to the manufacturer's protocols. Briefly, cells were digested by 0.25% Trypsin-EDTA (Invitrogen, NY, USA), resuspended in recommended medium (PBS with 2% FBS and 1mM EDTA), containing species-specific FcR blocking antibody. After gently agitating the mixture for 15 minutes, mouse anti-human CD133/1(AC-133)-PE antibody was added and cells were incubated at room temperature for 15 minutes. The cells were washed once with recommended medium before EasySep PE Selection Cocktail was added and incubated at room temperature for 15 minutes. Subsequently, EasySep Magnetic Nanoparticles were added and the cells separated according to manufacturer's recommendations using the EasySep Magnet. Both the CD133-hi and CD133-lo fractions were subsequently cultured in RPMI feed medium, as described above, for 48 hours prior to in vitro experiments. Alternatively the cells were counted, mixed with 1:10 PBS-diluted, growth factor-reduced Matrigel, and injected directly into mice.
Immunoblot analysis
Whole cell lysates were prepared for immunoblotting using a RIPA buffer modified to contain 1 mM PMSF, 10 μg/ml aprotinin and leupeptin, 2 mM sodium orthovanadate, and 10 mM sodium fluoride. Protein content was determined by BCA assay (Pierce; Rockford, IL), and aliquots containing 50 μg of protein were solubilized in SDS sample buffer, resolved on Pierce 4-20% reducing polyacrylamide gels, transferred to nitrocellulose (Osmonics, Westborough MA) with a Pierce apparatus, and subject to immunoblot analysis as previously described (19).
Microscopy
Cells were plated, fixed, permeabilized, blocked for nonspecific binding, incubated with the indicated antibodies, and washed as previously described (21). Cells were then incubated with AlexaFluor® secondary antibodies and Draq5 nuclear stain for 2 h. Slides were processed as described (21) and viewed on a Leica Total Confocal System, Spectral Prism 2, Acoustic Optical Beam Splitter (TCS SP2 AOBS) in the Josh Spruill Molecular Morphology & Imaging Center in the Department of Cell Biology & Anatomy. Alternatively, slides were viewed by epifluorescence using an Olympus BX-60 microscope. Images were acquired using a 12.5-megapixel cooled digital camera (BP70; Olympus) and DP Controller software. Image processing and compilation were done using Adobe Photoshop Software (Adobe Systems, Inc.).
Hyaluronan Production
Hyaluronan levels were assayed in conditioned medium by an ELISA-like assay (25).
FURA 2-AM Assay
FURA 2-AM efflux was measured as described (26). Ovarian carcinoma cells were seeded in 96 well plates and incubated for 48h prior to the experiment, to a final density of ~70% confluence. Cells were treated −/+ 100 μg/ml hyaluronan oligomers in feed medium containing 2.5 μM FURA 2-AM. Following a 1 hour incubation, plates were read (excitation: 340 nm, emission: 500 nm) in an FLx800 Microplate Fluorescence Reader (Biotek Instruments, Inc.; Winooski, VT) from the bottom to determine FURA 2-AM levels in the cell layer.
Tumorigenicity in vivo
Approximately 5-week-old SCID mice provided by the Hollings Cancer Center, MUSC, Xenograft Core Facility were used. Employing a 27-gauge syringe, CD133-hi cells or CD133-lo cells suspended in 400 μl of 1:10 PBS-diluted Matrigel were injected into the intraperitoneal cavity of the SCID mice. The animals were weighed weekly (as a measure of ascites accumulation) and sacrificed after 7 weeks. Alternatively, animals inoculated with CD133-hi cells were treated by intra-peritoneal injection of 500 μg hyaluronan oligomers dissolved in 200 μl phosphate-buffered saline (PBS), or PBS alone, once per week for 6 weeks, following one week without treatment. Immediately after euthanasia, ascites fluid was removed by syringe, volume measured, and cells processed for further analysis. All surgical procedures were carried out in an animal surgery unit within the facilities for laboratory animals provided by the Division of Laboratory Animal Resources and these studies were approved by Medical University of South Carolina's Institutional Animal Care and Use Committee. It should be noted that extensive experience has shown that the hyaluronan oligomers have no significant adverse effects in mice (20, 27-29).
Results
Identification of a CD133-hi tumorigenic sub-population of ovarian carcinoma cells in patient ascites
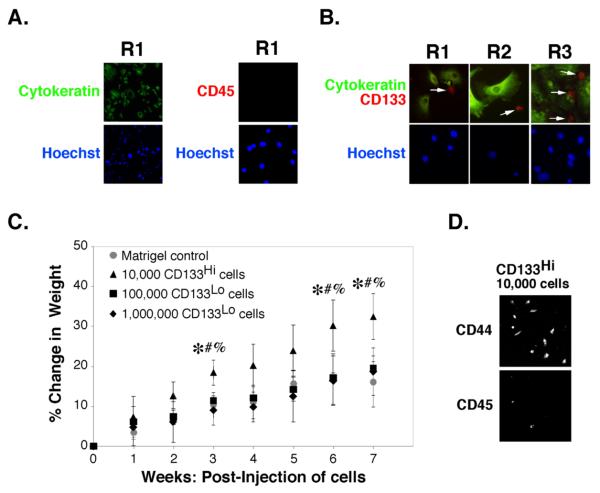
Carcinoma cells were isolated from the ascites of three patients (R1, R2, and R3) with Stage III/IV ovarian carcinoma by routine procedures and monitored for the presence of cytokeratin to identify carcinoma cells and CD45 for cells derived from the circulation, such as macrophages. One hundred percent of the cells in these carcinoma preparations stained positively for cytokeratin and negatively for CD45 (Fig. 1A).
FIGURE 1. Identification of a CD133-hi tumorigenic sub-population of carcinoma cells in patient ascites.
A,B. Unsorted ovarian carcinoma cells from patients R1, R2 or R3 were plated on chamber slides for 24h and processed for immuno-staining as indicated. Cells were visualized by epifluorescence. Arrows in B indicate CD133-hi cells. C, D. Carcinoma cells from patient R1, magnetically sorted for CD133, were injected into the peritoneum of SCID mice as indicated. C. Tumor growth after injection of 104 CD133-hi cells in Matrigel was compared to tumor growth after injection of 105 or 106 CD133-lo cells in Matrigel (or Matrigel alone, as control), as assessed by percent change in weight due to ascites accumulation [(weight at week n/weight at day 0)-1]*100. Significant differences were found between CD133-hi @ 104 cells and: CD133-lo @ 105 cells (*p<.05), CD133-lo @ 106 cells (#p<.05), or Matrigel control (%p<.05), as determined by one-way ANOVA with Bonferroni comparison of significant differences between points. D. After 7 weeks, ascites was drawn from the peritoneum of animals receiving CD133-hi cells and cells plated for 24h before staining for CD44 and CD45. Ascites was absent from animals that received CD133-lo cells or Matrigel alone and significant numbers of cells could not be retrieved from these animals.
Immunostaining of the carcinoma cells revealed a minor sub-population of cells that stained strongly for CD133 (Fig. 1B). These cells were smaller and less flattened than the CD133-lo carcinoma cells. Although cytokeratin-positive, the CD133-hi cells expressed lower levels of cytokeratin than the CD133-lo cells, consistent with previous observations that less differentiated epithelial cells exhibit low keratin levels. In addition, the CD133-hi cells did not stain with antibody against CD45, demonstrating that they are not leukocytic in origin. Magnetic sorting of the carcinoma cells from patients R1, R2 and R3 for the CD133 antigen, as described in Methods, consistently resulted in a CD133-hi fraction that was less than 10% of the total starting pool.
To confirm the relative tumorigenicity of CD133-hi and CD133-lo sub-populations found in previous studies (23, 24), we seeded them into the intra-peritoneal cavity of SCID mice. Injection of various numbers of CD133-hi and CD133-lo cells from patient R1 revealed that 104 CD133-hi cells was sufficient to initiate tumor growth and ascites accumulation (as measured by increased animal weight relative to controls), whereas injection of 10- and 100-times this number of CD133-lo cells, i.e. 105 and 106 CD133-lo cells, did not give rise to tumors (Fig 1C). Indeed, attempts to withdraw cells from the peritoneum seven weeks after injection of CD133-lo cells failed to yield significant numbers of viable cells, whereas large numbers of cells were obtained from the ascites of animals that had been injected with CD133-hi cells (e.g. see Fig. 6B). We analyzed the cells obtained from ascites of animals receiving CD133-hi cells by immunocytochemistry and found that most cells were positive for CD44 but few cells stained for CD45, indicating that most cells were not macrophages or other cells from the circulation (Fig. 1D). These results confirmed the efficacy of CD133 as a marker for identifying cells with high tumorigenic capacity, as previously reported (23, 24).
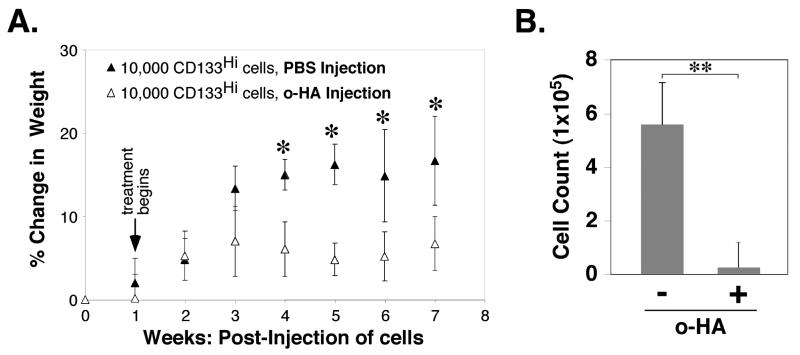
FIGURE 6. Hyaluronan oligomer treatment inhibits growth of CD133-hi ovarian carcinomas in vivo.
104 CD133-hi cells, obtained by magnetic sorting for CD133, were injected in Matrigel into the peritoneum of SCID mice at day 0. Beginning one week after injection of cells, PBS (200μl) or hyaluronan oligomers (500μg; 25mg/kg) in PBS were injected intraperitoneally once per week for 6 weeks. A. Tumor growth, as assessed by percent change in weight due to ascites accumulation, was calculated as in Fig. 1C. B. Numbers of cells that accumulated intraperitoneally after 7 weeks. Error bars express standard deviation in the mean of results from 4 or more animals. Significant differences were observed between treated and untreated animals (A: *p<.05; B: **p<.01) as determined by Student's t-test.
Elevated expression of drug transporters, lactate transporters, RTKs, emmprin and hyaluronan in CD133-hi primary ovarian carcinoma cells
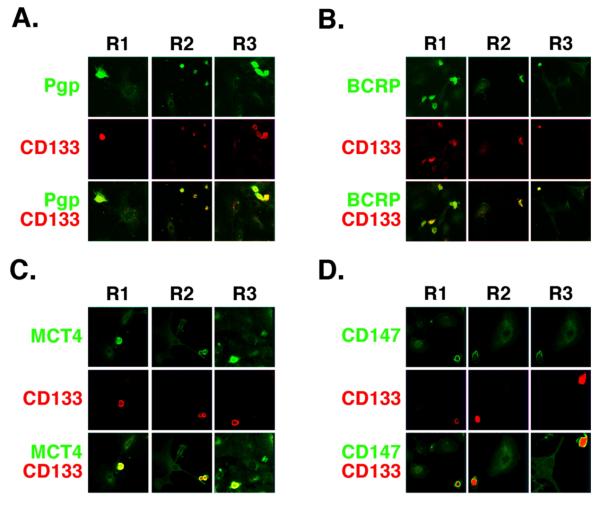
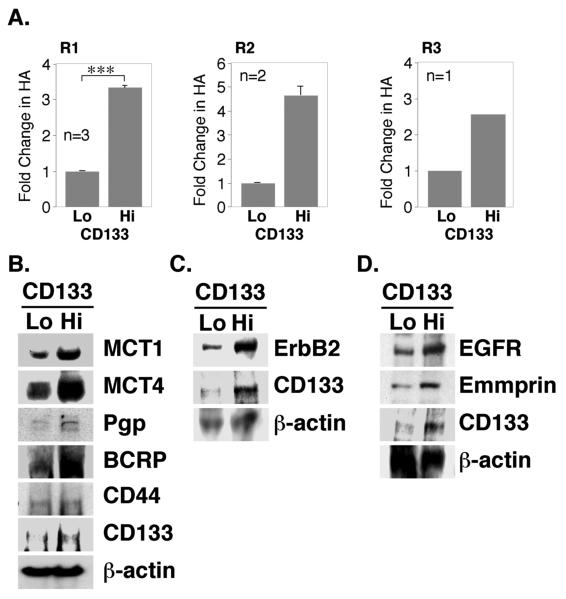
In past studies we and others have found that the RTKs, ErbB2 and EGFR (16, 19, 30), the ABC drug transporters, P-glycoprotein/ABCB1 (Pgp) and breast cancer resistance protein/ABCG2 (BCRP) (20, 31, 32), MCT1 and MCT4 (21), and the matrix metalloproteinase inducer, emmprin (21), are present in close association with CD44 in the plasma membrane of various types of malignant tumor cell lines. Consequently, we examined the distribution of Pgp, BCRP, MCT4, EGFR, emmprin, and CD44 by immunostaining of the ovarian carcinoma cells. We found that each type of protein is enriched in the CD133-hi cells as compared with CD133-lo cells within the same cultures of cells obtained from patients R1, R2 and R3 (Fig. 2; Fig. 5A). Closer examination showed that in each case, these proteins were concentrated in the plasma membrane of the CD133-hi cells (see below). Similar results were obtained with ErbB2 and MCT1 (not shown). Previous studies showed that organization of these various proteins in the plasma membrane of tumor cell lines is dependent on hyaluronan-CD44 interaction (16, 19-21, 30) and that, in some cases, they are stimulated by increased hyaluronan production (18, 19). Thus, we also measured the amounts of hyaluronan secreted by CD133-hi and CD133-lo cells, after separating the cells by magnetic sorting. We found 2.5-5-fold more hyaluronan in media from cultures of the CD133-hi sub-fractions from patients R1, R2, and R3, when compared to the corresponding CD133-lo sub-fractions (Fig. 3A).
FIGURE 2. Co-distribution of signaling and transporter proteins in CD133-hi primary ovarian carcinoma cells.
A-D. Low power confocal images of unsorted ovarian carcinoma cells from patients R1, R2 and R3 that were plated on chamber slides for 24h and stained for CD133 (red) and either Pgp (A), BCRP (B), MCT4 (C), or emmprin/CD147 (D) (green). Most cells lacked significant staining for CD133 (CD133-lo cells); these cells showed relatively low levels of Pgp, BCRP, MCT4 and emmprin. A few cells stained brightly for CD133 (CD133-hi cells); these cells also stained strongly for Pgp, BCRP, MCT4 and emmprin. Similar results were obtained with EGFR and CD44 (see Fig. 5A), as well as with ErbB2 and MCT1 (not shown).
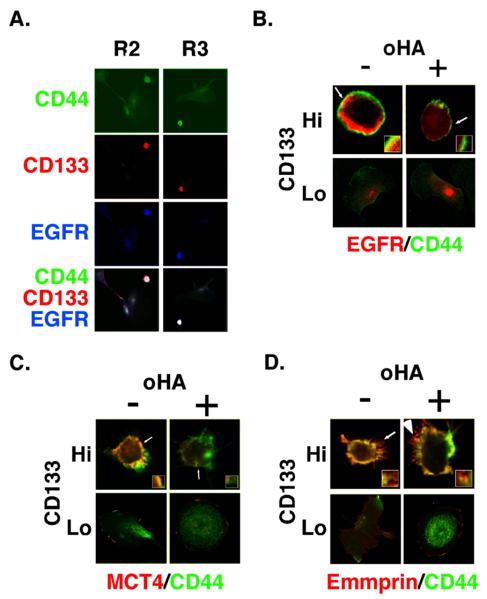
FIGURE 5. Co-localization of EGFR and MCT4 with CD44 in CD133-hi cells and disruption by hyaluronan oligomer treatment.
A. Low power confocal images of unsorted ovarian carcinoma cells from patients R2 and R3 that were plated on chamber slides for 24h and stained for CD44, CD133, and EGFR. CD133-lo cells, which comprised the majority of cells, showed dispersed staining for CD44 and low levels of EGFR. CD133-hi cells stained strongly for CD44 and EGFR. B-D. Higher power images of unsorted ovarian carcinoma cells from patient R1, that were plated on chamber slides for 24h, treated with and without 100 μg/ml hyaluronan oligomers for 1 hour, and then processed for immuno-staining. Cells were stained for CD44 (green), for EGFR (B), MCT4 (C) or emmprin (D) (red), and for CD133 in order to distinguish CD133-hi and CD133-lo cells (not shown). CD133-hi and CD133-lo cells were visualized by confocal microscopy at a z-plane corresponding to the approximate center of the cell. Note co-localization (yellow) of CD44 with EGFR, MCT4 and emmprin at the plasma membrane of untreated CD133-hi cells and internalization of EGFR (B) and MCT4 (C), but not emmprin (D), in hyaluronan oligomer-treated CD133-hi cells. Similar results were obtained for MCT1 and ErbB2 (not shown). Note that internalization of MCT4 is difficult to visualize here due to dispersion throughout the cytoplasm, but is clearly visible on increasing gain (e.g. see (21)). Increased gain did not reveal intracellular emmprin. Arrows indicate areas of the plasma membrane and adjacent cytoplasm shown at higher magnification in the insets.
FIGURE 3. Elevated expression of hyaluronan, signaling proteins and transporter proteins in CD133-hi primary ovarian carcinoma cells.
A. Ovarian carcinoma cells from patients R1, R2 and R3, magnetically sorted for CD133, were plated in 6-well dishes for 24 hours. Conditioned media were collected and hyaluronan quantified by an ELISA-like assay. Results were normalized to cell number. For R1 cells, error bars express standard deviation in the mean of three separately sorted batches; significant differences (***p<.001) between CD133-lo and CD133-hi cells were observed. For R2 cells, error bars represent range of two measurements. Due to limitations in amounts of primary material, the statistical significance of differences could not be determined in R2 and R3 cells since n<3; however, the differences obtained were large. B-D. Ovarian carcinoma cells from patient R1, magnetically sorted for CD133, were plated in 6-well dishes for 24h. Western blot analysis of whole cell lysates (50μg/lane) demonstrated elevated expression of CD133, MCT1, MCT4, Pgp, BCRP, ErbB2, EGFR, and emmprin in CD133-hi cells. Expression of CD44 (B) was approximately equivalent in CD133-hi and CD133-lo cell lysates. Panel B shows a blot obtained with lysates from one batch of sorted cells, whereas panels C and D show two separate blots obtained with lysates from a second sort.
To confirm the results obtained by immunostaining, we also used Western blotting to measure the relative amounts of the various proteins, after separating patient R1 cells by magnetic sorting. As expected, CD133 was highly enriched in the CD133-hi sub-fraction compared to the CD133-lo sub-fraction (Fig. 3B-D). In addition, we found that expression of lactate transporters (MCT1 and MCT4), ABC-family drug transporters (Pgp and BCRP), RTKs (ErbB2 and EGFR) and emmprin is elevated in the CD133-hi fraction when compared to the CD133-lo fraction (Fig. 3B-D). Unexpectedly, we did not find significant differences in levels of CD44 between the two fractions (Fig. 3B).
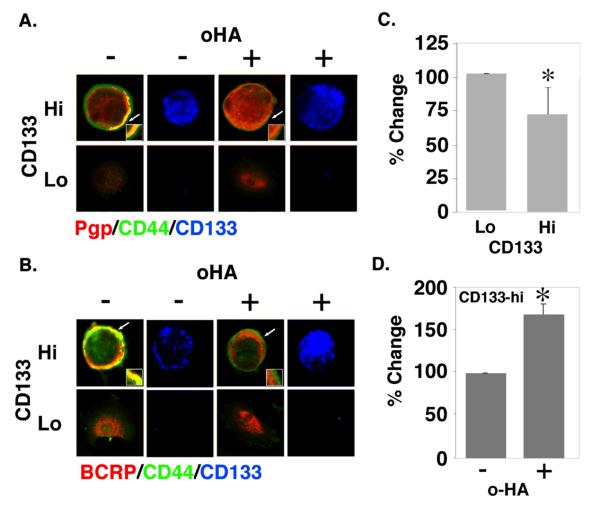
Co-localization of transporters and RTKs with CD44 in CD133-hi primary ovarian carcinoma cells and disruption by treatment with hyaluronan oligomers
Examination of cells from patients R1, R2 and R3 by immunostaining indicated enrichment of multi-drug transporters and MCTs in the plasma membrane of CD133-hi cells when compared to their CD133-lo counterparts (Fig. 2A-C). Confocal analysis of these unsorted, immunostained, carcinoma cells showed clearly that membrane localization of Pgp (red; Fig. 4A) and BCRP (red; Fig. 4B) is elevated in CD133-hi versus CD133-lo cells. Furthermore, CD44 (green) was found to co-localize with Pgp and BCRP in the membrane of the CD133-hi cells to a much greater extent than in the CD133-lo cells (Fig. 4A, B), even though the total amounts of CD44 were similar in both cell types (Fig. 3B). Similar results were obtained when the cells were stained for CD44 and MCT4 (Fig. 5C) or MCT1 (data not shown). In addition to these transporters, we found that the RTK, EGFR, is co-distributed with CD44 in CD133-hi cells (Fig. 5A) and is co-localized with CD44 in the plasma membrane of these cells (Fig. 5B). Similar results were obtained with ErbB2 (not shown).
FIGURE 4. Co-localization of drug transporters with CD44 in CD133-hi cells and disruption by hyaluronan oligomer treatment.
A, B. Unsorted ovarian carcinoma cells from patient R1 were plated on chamber slides for 24h, treated with and without 100 μg/ml hyaluronan oligomers for 1 hour, and then processed for immuno-staining. Cells were stained for CD44 (green), for Pgp (A) or BCRP (B) (red), and for CD133 in order to distinguish CD133-hi and CD133-lo cells (blue; shown separately). CD133-hi and CD133-lo cells were visualized by confocal microscopy at a z-plane corresponding to the approximate center of the cell. Note co-localization (yellow) of CD44 and transporters at the plasma membrane of untreated CD133-hi cells, and internalization of Pgp (A) and BCRP (B) in hyaluronan oligomer-treated CD133-hi cells. Arrows indicate areas of the plasma membrane and adjacent cytoplasm shown at higher magnification in the insets. C, D. Ovarian carcinoma cells from patient R1, magnetically sorted for CD133, were treated with and without 100 μg/ml hyaluronan oligomers for 1 hour in feed medium containing 2.5 μM FURA 2-AM, and analyzed for fluorescence as described in Methods. CD133-hi cells showed higher efflux activity than CD133-lo cells (C); the hyaluronan oligomers inhibited efflux in the CD133-hi cells (D). Error bars express standard deviation in the mean of triplicate wells. Significant differences were observed (*p<.05) as determined by Student's t-test. The results are representative of three or more independent experiments.
We have previously found that treatment of malignant cell lines with an antagonist of hyaluronan-CD44 interaction, i.e. hyaluronan oligomers, results in dissociation of CD44-drug transporter and CD44-MCT complexes from the plasma membrane, internalization of the respective transporters into the cytoplasm, and loss of their function (20, 21). In analogous fashion, treatment of the ovarian carcinoma cells for 1 hour with hyaluronan oligomers resulted in dissociation of Pgp (Fig. 4A), BCRP (Fig. 4B) and MCT4 (Fig. 5C) from CD44 at the plasma membrane of CD133-hi cells and internalization into the cytoplasm. We obtained similar results with MCT1 (not shown) and the RTKs, EGFR (Fig. 5B), and ErbB2 (not shown). Emmprin, which is associated with drug resistance in cancer cells (15) and is highly expressed in malignant ovarian carcinomas (33), is also enriched in CD133-hi cells and co-localizes with CD44 at the plasma membrane (Figs. 2D and 5D). However, treatment with hyaluronan oligomers had little or no effect on membrane localization of emmprin (Fig. 5D), in similar fashion to our previous findings in a breast carcinoma cell line (21).
Since the CD133-hi cells express higher levels of Pgp than the CD133-lo cells, we compared the ability of magnetically sorted CD133-hi and CD133-lo cells to efflux FURA 2-AM, a fluorescent substrate for Pgp. In cells expressing Pgp, intact FURA 2-AM is rapidly transported out of the cell, whereas in the absence of Pgp it is cleaved to FURA-2 and accumulates in the cytosol (26, 34). Thus transporter activity is inversely proportional to accumulation of cytosolic FURA 2 fluorescence. We found that CD133-hi cells efflux higher levels of FURA-2 than their CD133-lo counterparts, indicating that transporter activity is indeed greater in the CD133-hi cells (Fig. 4C). Since treatment with hyaluronan oligomers caused internalization of Pgp, we also measured the effect of the oligomers and found they caused accumulation of FURA-2 in the cytoplasm (Fig. 4D), indicating that they inhibit transporter activity, as found previously in malignant cell lines (20).
Inhibition of CD133-hi ovarian carcinoma growth by hyaluronan oligomer treatment in vivo
As described above, we found that CD133-hi cells gave rise to tumors after injection of relatively low cell numbers but CD133-lo cells failed to give tumors, even with inoculates of high cell numbers (Fig. 1). We tested the effect of hyaluronan oligomers on growth of tumors initiated by injection of CD133-hi cells. Animals were injected intra-peritoneally with 104 CD133-hi cells. Treatment with PBS or hyaluronan oligomers in PBS, by intra-peritoneal injection, was initiated after allowing these cells to grow in vivo for a week. Treatment continued subsequently once per week for six weeks. Analyses of ascites accumulation (Fig. 6A) and numbers of cells obtained from the ascites after 7 weeks (Fig. 6B) indicated that the hyaluronan oligomers abrogate tumor growth.
Discussion
The origins and defining characteristics of solid tumor cancer stem/tumor-initiating cells are controversial, and these cells may simply represent a highly malignant, drug-resistant tumor cell sub-population within a hierarchy of cells with various levels and combinations of oncogenic characteristics (4-6). Nevertheless, increased understanding of the properties of this sub-population of cells may provide important insights into tumor malignancy and resistance to therapy. Several groups have isolated cells with the apparent properties of tumor-initiating cells from ovarian carcinoma cell lines or primary ovarian tumors (7, 23, 24, 35, 36). CD133 has been shown to be an effective marker for identifying these cells from ovarian carcinomas (23, 24), as has the combination of CD44 and CD117 (c-kit) (7), albeit no single marker or combination of markers is likely to be completely selective (5). Although CD44 and CD133 are commonly used to identify tumor-initiating cells from a variety of tumor types (4), little is known about their functions in these cells. In this study we have examined the potential role of interactions of CD44 with its major ligand, hyaluronan, in the drug-resistant and tumorigenic properties of a sub-population of ovarian carcinoma cells that were isolated using CD133 as a marker. We have found that cells with high levels of CD133 express elevated levels of hyaluronan, emmprin, RTKs (ErbB2 and EGFR), MCT-1 and -4, and ABC-family multidrug transporters (Pgp and BCRP), compared to cells with low levels of CD133. Although CD44 is present in similar amounts in CD133-hi and CD133-lo cells, CD44 is enriched in the plasma membrane of CD133-hi cells, and is co-localized with the RTKs, MCTs and ABC transporters in the membrane of CD133-hi cells but not CD133-lo cells.
Past studies using co-immunoprecipitation and confocal microscopy have provided strong evidence for association of CD44 sub-fractions with RTKs, MCTs or ABC transporters, and most of these studies have demonstrated dependence of these interactions on binding of hyaluronan to CD44 (16, 19-21, 30-32). In this study, we probed the role of hyaluronan-CD44 interactions in CD133-hi cells by treating the cells for a short period of time (1 hour) with small hyaluronan oligomers. Past studies have shown that these oligomers, which interact monovalently with CD44, compete for multivalent, polymeric hyaluronan-receptor interactions (37-40) and mimic the effects of other antagonists of hyaluronan-CD44 interaction, such as soluble hyaluronan-binding proteins and antibodies or siRNA against CD44 (19, 27). In addition, the oligomers inhibit hyaluronan synthesis but this effect requires a much longer time period (20). We found that treatment of the CD133-hi cells with hyaluronan oligomers inhibits association of CD44 with the RTKs, MCTs and ABC transporters, induces their internalization, reduces drug transporter activity, and inhibits the capacity for tumor growth in the CD133-hi cells. We showed previously that treatment with hyaluronan oligomers also inhibits tumor growth by a highly malignant, drug-resistant sub-population of C6 rat glioma cells prepared by the side-population method (29). These results strongly support an important role for hyaluronan-CD44 interactions in the characteristic properties of highly tumorigenic sub-populations such as tumor-initiating cells. However, this study has also highlighted another important point, i.e. that hyaluronan-dependent organization of CD44 into membrane complexes is more likely to be significant for tumor-initiating cell properties than levels of CD44 expression.
The usefulness of CD44 as a marker of malignant properties has been a dilemma for many years. For example, in some studies evidence was obtained for CD44 as an essential participant in metastasis (41, 42), whereas in other studies opposing relationships have been documented (43). Likewise, although CD44 has been useful as a marker for tumor-initiating cells in many human carcinomas, it has not been useful for several other human cancers (4). One obvious reason for this discrepancy is that CD44 is widely expressed in numerous cell types, and that in tumors such as gliomas most of the cancer cells express CD44. Despite this, certain sub-fractions of CD44-expressing human tumor cells reproducibly possess malignant or tumor-initiating cell characteristics, especially in human carcinomas and carcinoma cell lines (44-47). We find that, although CD113-hi and CD133-lo sub-fractions of ovarian carcinoma cells express similar levels of CD44, only in CD133-hi cells is CD44 localized to plasma membrane complexes containing RTKs and transporters. Moreover, constitutive multivalent interactions of CD44 with hyaluronan appear to be crucial since the small, monovalent hyaluronan oligomers destabilize these complexes and inhibit tumor progression in vivo. Also, even though CD44 has not been a useful marker for identifying mouse carcinoma tumor-initiating cells (4), over-expression of an antagonist of hyaluronan-CD44 interactions, in this case soluble CD44, inhibited mouse mammary carcinoma growth (48) and lung colonization (49) in a highly malignant syngeneic mouse model; a mutant form of soluble CD44 defective in hyaluronan binding did not have these effects, indicating that hyaluronan interactions were involved. These results suggest that the organizational status rather than level of expression of CD44 is critical to malignant and tumor-initiating cell phenotypes and that hyaluronan-CD44 interaction is required to activate this status.
The question remains how hyaluronan-CD44 interaction leads to organization of signaling complexes. In past studies it has been shown that cell-autonomous hyaluronan-CD44 interactions regulate several oncogenic pathways that promote malignant and drug-resistant properties of cancer cells (8, 15). Several studies suggest that hyaluronan-CD44 interactions influence these pathways by stabilizing RTK and transporter-containing signaling complexes in the plasma membrane of malignant cells (16, 17, 19-21, 30). Moreover, emmprin, which is highly expressed in ovarian carcinomas (33) and is enriched in the CD133-hi cells, cooperates in assembly or stabilization of these complexes (19, 21). At this point, it is not clear whether these widespread effects of hyaluronan-CD44-emmprin interactions on complex formation are due to unique involvement of specific sub-fractions of CD44 splice variants with each complex, general interactions with membrane sub-compartments, or global effects of a hyaluronan-based pericellular matrix. However, considerable evidence points towards involvement of CD44 variants (16, 21) and CD44-mediated complex assembly within lipid micro-domains (17, 19, 50). Despite these caveats, the present study suggests that these interactions drive the properties of tumor-initiating cells by stabilizing oncogenic complexes, and may explain why cell surface CD44 is so commonly associated with these cells.
In summary, our results indicate that hyaluronan-CD44 interactions have functional importance in the properties of highly tumorigenic sub-populations of ovarian carcinoma cells and that utilization of antagonists of these interactions, such as small hyaluronan oligomers, may provide an improved therapeutic approach.
Translational Relevance.
CD44 is a common marker for identification of human carcinoma tumor-initiating cells but its function in these cells is unknown. We treated a highly tumorigenic sub-population of primary ovarian carcinoma cells isolated from patient ascites with small hyaluronan oligosaccharides, an established antagonist of interactions between CD44 and its major ligand, hyaluronan. The oligosaccharides reduced association of receptor tyrosine kinases, multidrug transporters and lactate transporters with CD44 in the plasma membrane, diminished drug transporter activity, and inhibited intra-peritoneal tumorigenic capacity in these cells. These and previous results suggest that multivalent hyaluronan-CD44 interactions play an important role in the malignant and chemoresistant properties characteristic of tumor-initiating cells by stabilizing oncogenic complexes in their plasma membrane. Treatment with hyaluronan-CD44 antagonists such as small hyaluronan oligosaccharides may provide a logical therapeutic approach for targeting these cells, especially as an adjuvant in prevention of tumor recurrence as commonly occurs in ovarian cancer.
Acknowledgments
We thank Drs. William Creasman and Matthew Kohler for supplying ascites fluids and Dr. Bernard Maria for helpful discussions. This work was supported by grant OC050368 from the Department of Defense and grants R01 CA073839 and R01 CA082867 from the National Institutes of Health (to Bryan P. Toole)
Non-standard abbreviations
- ABC
ATP-binding cassette
- BCRP
breast cancer resistance protein/ABCG2
- MCT
monocarboxylate (lactate) transporter
- Pgp
P-glycoprotein/ABCB1
- RTK
receptor tyrosine kinase
References
- 1.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 2.Landen CN, Jr., Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26:995–1005. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- 3.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–84. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 4.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 5.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–3. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shipitsin M, Polyak K. The cancer stem cell hypothesis: in search of definitions, markers, and relevance. Lab Invest. 2008;88:459–63. doi: 10.1038/labinvest.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S, Balch C, Chan MW, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–20. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–39. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 9.Tammi RH, Kultti A, Kosma VM, et al. Hyaluronan in human tumors: Pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Semin Cancer Biol. 2008;18:288–95. doi: 10.1016/j.semcancer.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Yeo TK, Nagy JA, Yeo KT, Dvorak HF, Toole BP. Increased hyaluronan at sites of attachment to mesentery by CD44- positive mouse ovarian and breast tumor cells. Am J Pathol. 1996;148:1733–40. [PMC free article] [PubMed] [Google Scholar]
- 11.Carpenter PM, Dao AV. The role of hyaluronan in mesothelium-induced motility of ovarian carcinoma cells. Anticancer Res. 2003;23:3985–90. [PubMed] [Google Scholar]
- 12.Bourguignon LY, Gilad E, Peyrollier K. Heregulin-mediated ErbB2-ERK signaling activates hyaluronan synthases leading to CD44-dependent ovarian tumor cell growth and migration. J Biol Chem. 2007;282:19426–41. doi: 10.1074/jbc.M610054200. [DOI] [PubMed] [Google Scholar]
- 13.Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–92. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 14.Bourguignon LY. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol. 2008;18:251–9. doi: 10.1016/j.semcancer.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toole BP, Slomiany MG. Hyaluronan, CD44 and Emmprin: partners in cancer cell chemoresistance. Drug Resist Updat. 2008;11:110–21. doi: 10.1016/j.drup.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bourguignon LY, Zhu H, Zhou B, et al. Hyaluronan promotes CD44v3-Vav2 interaction with Grb2-p185(HER2) and induces Rac1 and Ras signaling during ovarian tumor cell migration and growth. J Biol Chem. 2001;276:48679–92. doi: 10.1074/jbc.M106759200. [DOI] [PubMed] [Google Scholar]
- 17.Bourguignon LY, Singleton PA, Diedrich F, Stern R, Gilad E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem. 2004;279:26991–7007. doi: 10.1074/jbc.M311838200. [DOI] [PubMed] [Google Scholar]
- 18.Misra S, Ghatak S, Toole BP. Regulation of MDR1 expression and drug resistance by a positive feedback loop involving hyaluronan, phosphoinositide 3-kinase, and ErbB2. J Biol Chem. 2005;280:20310–5. doi: 10.1074/jbc.M500737200. [DOI] [PubMed] [Google Scholar]
- 19.Ghatak S, Misra S, Toole BP. Hyaluronan regulates constitutive ErbB2 phosphorylation and signal complex formation in carcinoma cells. J Biol Chem. 2005;280:8875–83. doi: 10.1074/jbc.M410882200. [DOI] [PubMed] [Google Scholar]
- 20.Slomiany MG, Dai L, Bomar PA, et al. Abrogating drug resistance in malignant peripheral nerve sheath tumors by disrupting hyaluronan-CD44 interactions with small hyaluronan oligosaccharides. Cancer Res. 2009;69:4992–8. doi: 10.1158/0008-5472.CAN-09-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slomiany MG, Grass GD, Robertson AD, et al. Hyaluronan, CD44 and emmprin regulate lactate efflux and membrane localization of monocarboxylate transporters in human breast carcinoma cells. Cancer Res. 2009;69:1293–301. doi: 10.1158/0008-5472.CAN-08-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaBarge MA, Bissell MJ. Is CD133 a marker of metastatic colon cancer stem cells? J Clin Invest. 2008;118:2021–4. doi: 10.1172/JCI36046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrandina G, Bonanno G, Pierelli L, et al. Expression of CD133-1 and CD133-2 in ovarian cancer. Int J Gynecol Cancer. 2008;18:506–14. doi: 10.1111/j.1525-1438.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 24.Baba T, Convery PA, Matsumura N, et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28:209–18. doi: 10.1038/onc.2008.374. [DOI] [PubMed] [Google Scholar]
- 25.Gordon LB, Harten IA, Calabro A, et al. Hyaluronan is not elevated in urine or serum in Hutchinson-Gilford Progeria Syndrome. Hum Genet. 2003;113:178–87. doi: 10.1007/s00439-003-0958-9. [DOI] [PubMed] [Google Scholar]
- 26.Fu LW, Zhang YM, Liang YJ, Yang XP, Pan QC. The multidrug resistance of tumour cells was reversed by tetrandrine in vitro and in xenografts derived from human breast adenocarcinoma MCF- 7/adr cells. Eur J Cancer. 2002;38:418–26. doi: 10.1016/s0959-8049(01)00356-2. [DOI] [PubMed] [Google Scholar]
- 27.Ghatak S, Misra S, Toole BP. Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumor cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway. J Biol Chem. 2002;277:38013–20. doi: 10.1074/jbc.M202404200. [DOI] [PubMed] [Google Scholar]
- 28.Zeng C, Toole BP, Kinney SD, Kuo JW, Stamenkovic I. Inhibition of tumor growth in vivo by hyaluronan oligomers. Int J Cancer. 1998;77:396–401. doi: 10.1002/(sici)1097-0215(19980729)77:3<396::aid-ijc15>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Gilg AG, Tye SL, Tolliver LB, et al. Targeting hyaluronan interactions in malignant gliomas and their drug-resistant multipotent progenitors. Clin Cancer Res. 2008;14:1804–13. doi: 10.1158/1078-0432.CCR-07-1228. [DOI] [PubMed] [Google Scholar]
- 30.Bourguignon LY, Gilad E, Brightman A, Diedrich F, Singleton P. Hyaluronan-CD44 interaction with leukemia-associated RhoGEF and epidermal growth factor receptor promotes Rho/Ras co-activation, phospholipase C epsilon-Ca2+ signaling, and cytoskeleton modification in head and neck squamous cell carcinoma cells. J Biol Chem. 2006;281:14026–40. doi: 10.1074/jbc.M507734200. [DOI] [PubMed] [Google Scholar]
- 31.Miletti-Gonzalez KE, Chen S, Muthukumaran N, et al. The CD44 receptor interacts with P-glycoprotein to promote cell migration and invasion in cancer. Cancer Res. 2005;65:6660–7. doi: 10.1158/0008-5472.CAN-04-3478. [DOI] [PubMed] [Google Scholar]
- 32.Colone M, Calcabrini A, Toccacieli L, et al. The multidrug transporter P-glycoprotein: a mediator of melanoma invasion? J Invest Dermatol. 2008;128:957–71. doi: 10.1038/sj.jid.5701082. [DOI] [PubMed] [Google Scholar]
- 33.Davidson B, Goldberg I, Berner A, Kristensen GB, Reich R. EMMPRIN (extracellular matrix metalloproteinase inducer) is a novel marker of poor outcome in serous ovarian carcinoma. Clin Exp Metastasis. 2003;20:161–9. doi: 10.1023/a:1022696012668. [DOI] [PubMed] [Google Scholar]
- 34.Homolya L, Hollo Z, Germann UA, et al. Fluorescent cellular indicators are extruded by the multidrug resistance protein. J Biol Chem. 1993;268:21493–6. [PubMed] [Google Scholar]
- 35.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–9. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 36.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci U S A. 2006;103:11154–9. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Underhill CB, Toole BP. Binding of hyaluronate to the surface of cultured cells. J Cell Biol. 1979;82:475–84. doi: 10.1083/jcb.82.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Underhill CB, Toole BP. Physical characteristics of hyaluronate binding to the surface of simian virus 40-transformed 3T3 cells. J Biol Chem. 1980;255:4544–9. [PubMed] [Google Scholar]
- 39.Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000;275:26967–75. doi: 10.1074/jbc.M002527200. [DOI] [PubMed] [Google Scholar]
- 40.Hosono K, Nishida Y, Knudson W, et al. Hyaluronan oligosaccharides inhibit tumorigenicity of osteosarcoma cell lines MG-63 and LM-8 in vitro and in vivo via perturbation of hyaluronan-rich pericellular matrix of the cells. Am J Pathol. 2007;171:274–86. doi: 10.2353/ajpath.2007.060828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gunthert U, Hofmann M, Rudy W, et al. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- 42.Weber GF, Bronson RT, Ilagan J, et al. Absence of the CD44 gene prevents sarcoma metastasis. Cancer Res. 2002;62:2281–6. [PubMed] [Google Scholar]
- 43.Lopez JI, Camenisch TD, Stevens MV, et al. CD44 attenuates metastatic invasion during breast cancer progression. Cancer Res. 2005;65:6755–63. doi: 10.1158/0008-5472.CAN-05-0863. [DOI] [PubMed] [Google Scholar]
- 44.Shipitsin M, Campbell LL, Argani P, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–73. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 45.Liu R, Wang X, Chen GY, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356:217–26. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 46.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10:R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peterson RM, Yu Q, Stamenkovic I, Toole BP. Perturbation of hyaluronan interactions by soluble CD44 inhibits growth of murine mammary carcinoma cells in ascites. Am J Pathol. 2000;156:2159–67. doi: 10.1016/S0002-9440(10)65086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu Q, Toole BP, Stamenkovic I. Induction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 function. J Exp Med. 1997;186:1985–96. doi: 10.1084/jem.186.12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bacso Z, Nagy H, Goda K, et al. Raft and cytoskeleton associations of an ABC transporter: P-glycoprotein. Cytometry A. 2004;61:105–16. doi: 10.1002/cyto.a.20081. [DOI] [PubMed] [Google Scholar]