Abstract
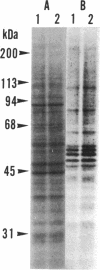
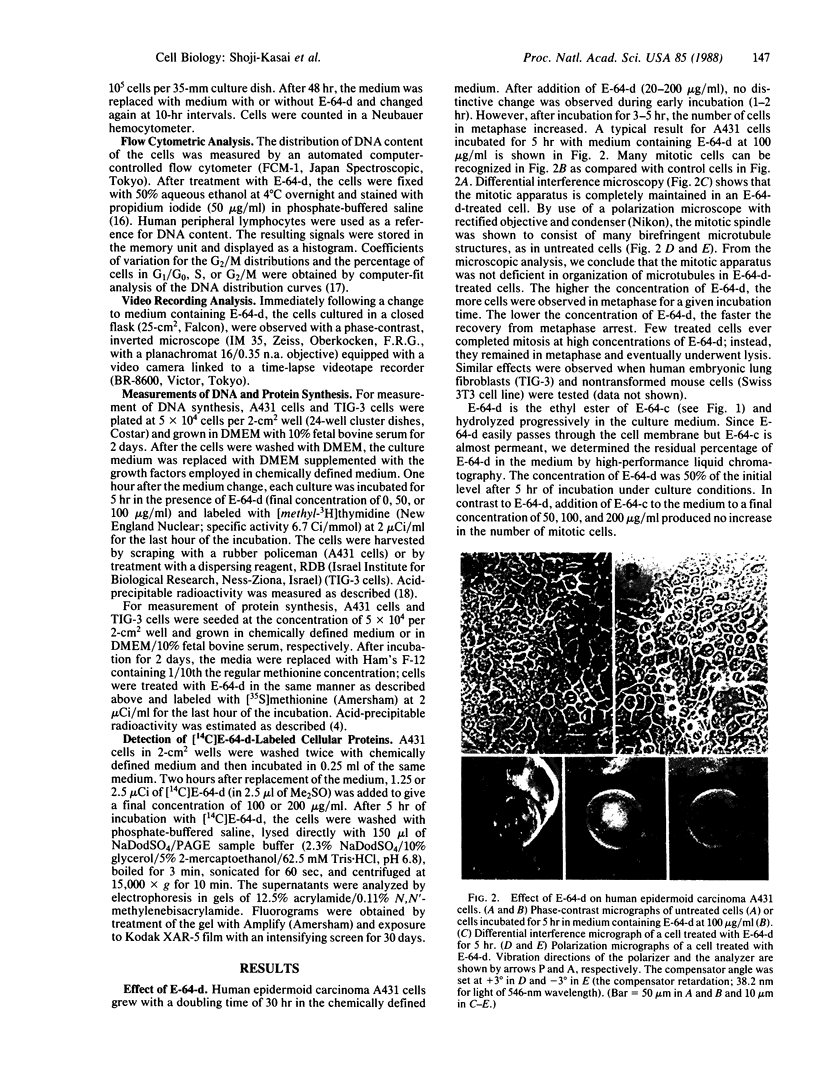
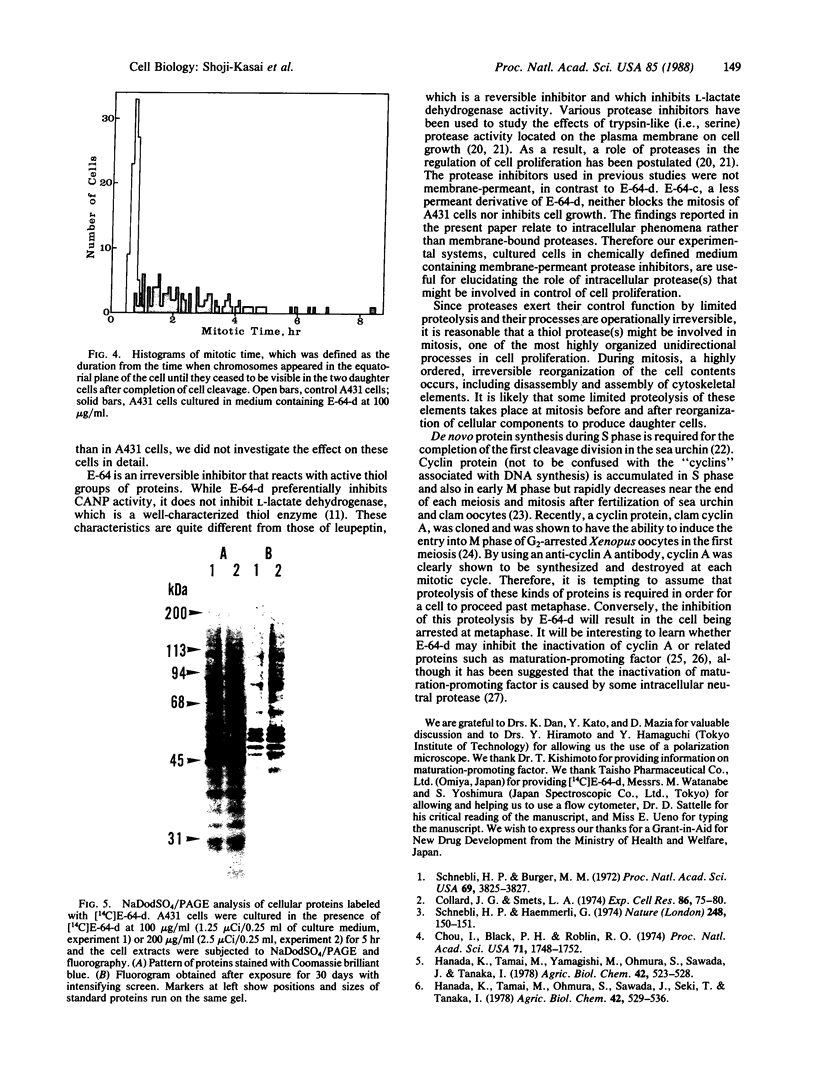
E-64-d (ethyl (2S, 3S)-3-[(S)-3-methyl-1-(3-methylbutylcarbamoyl) butylcarbamoyl]oxirane-2-carboxylate), a membrane-permeant derivative of the thiol protease-specific inhibitor E-64, was found to arrest human epidermoid carcinoma A431 cells at mitotic metaphase. This effect was dose-dependent with a threshold of 20 micrograms/ml in chemically defined culture medium. Cell cycle analysis by flow cytometry showed that the relative proportion of the G2/M population increased 2.5-fold after treatment of the cells with E-64 (100 micrograms/ml) for 5 hr. In addition, time-lapse video analysis showed that E-64-treated cells remained at metaphase for an extended period after rounding-up, whereas untreated cells completed mitosis within 42.0 +/- 5.7 min. Some treated cells were able to complete mitosis, while others did not do so within the limits of our observation. As an approach to the molecular basis of this phenomenon, we have shown that several cellular proteins can be labeled by incubation of cells with radioactive E-64-d.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D. W. Epidermal growth factor inhibits growth of A431 human epidermoid carcinoma in serum-free cell culture. J Cell Biol. 1982 Apr;93(1):1–4. doi: 10.1083/jcb.93.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou I. N., Black P. H., Roblin R. O. Non-selective inhibition of transformed cell growth by a protease inhibitor. Proc Natl Acad Sci U S A. 1974 May;71(5):1748–1752. doi: 10.1073/pnas.71.5.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard J. G., Smets L. A. Effect of proteolytic inhibitors on growth and surface architecture of normal and transformed cells. Exp Cell Res. 1974 May;86(1):75–80. doi: 10.1016/0014-4827(74)90650-8. [DOI] [PubMed] [Google Scholar]
- Dorée M., Peaucellier G., Picard A. Activity of the maturation-promoting factor and the extent of protein phosphorylation oscillate simultaneously during meiotic maturation of starfish oocytes. Dev Biol. 1983 Oct;99(2):489–501. doi: 10.1016/0012-1606(83)90298-1. [DOI] [PubMed] [Google Scholar]
- Evans T., Rosenthal E. T., Youngblom J., Distel D., Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983 Jun;33(2):389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Fabricant R. N., De Larco J. E., Todaro G. J. Nerve growth factor receptors on human melanoma cells in culture. Proc Natl Acad Sci U S A. 1977 Feb;74(2):565–569. doi: 10.1073/pnas.74.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiura S., Nonaka I., Sugita H. Suppression of calcium-induced removal of the Z-line by a thiol-protease inhibitor, E-64-c. J Biochem. 1981 Jul;90(1):283–285. [PubMed] [Google Scholar]
- Kasai Y., Inomata M., Hayashi M., Imahori K., Kawashima S. Isolation and characterization of monoclonal antibodies against calcium-activated neutral protease with low calcium sensitivity. J Biochem. 1986 Jul;100(1):183–190. doi: 10.1093/oxfordjournals.jbchem.a121691. [DOI] [PubMed] [Google Scholar]
- Kosugi Y., Ikebe J., Sekine M., Musha T., Shitara N., Kohno T., Takakura K. Computer aided analysis of cell-cycle phase from cytophotometric histogram. IEEE Trans Biomed Eng. 1978 Sep;25(5):429–434. doi: 10.1109/TBME.1978.326340. [DOI] [PubMed] [Google Scholar]
- Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975 Jul;66(1):188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y., Markert C. L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971 Jun;177(2):129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Matsuo M., Kaji K., Utakoji T., Hosoda K. Ploidy of human embryonic fibroblasts during in vitro aging. J Gerontol. 1982 Jan;37(1):33–37. doi: 10.1093/geronj/37.1.33. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Asami O., Tanaka K., Ichihara A. Increased survival of rat hepatocytes in serum-free medium by inhibition of a trypsin-like protease associated with their plasma membranes. Exp Cell Res. 1984 Nov;155(1):81–91. doi: 10.1016/0014-4827(84)90769-9. [DOI] [PubMed] [Google Scholar]
- Picard A., Peaucellier G., le Bouffant F., Le Peuch C., Dorée M. Role of protein synthesis and proteases in production and inactivation of maturation-promoting activity during meiotic maturation of starfish oocytes. Dev Biol. 1985 Jun;109(2):311–320. doi: 10.1016/0012-1606(85)90458-0. [DOI] [PubMed] [Google Scholar]
- Rosenstreich D. L., Mizel S. B. The participation of macrophages and macrophage cell lines in the activation of T lymphocytes by mitogens. Immunol Rev. 1978;40:102–135. doi: 10.1111/j.1600-065x.1978.tb00403.x. [DOI] [PubMed] [Google Scholar]
- Schnebli H. P., Burger M. M. Selective inhibition of growth of transformed cells by protease inhibitors. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3825–3827. doi: 10.1073/pnas.69.12.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnebli H. P., Haemmerli G. Protease inhibitors do not block transformed cells in the G1 phase of the cell cycle. Nature. 1974 Mar 8;248(5444):150–151. doi: 10.1038/248150a0. [DOI] [PubMed] [Google Scholar]
- Swenson K. I., Farrell K. M., Ruderman J. V. The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell. 1986 Dec 26;47(6):861–870. doi: 10.1016/0092-8674(86)90801-9. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai M., Hanada K., Adachi T., Oguma K., Kashiwagi K., Omura S., Ohzeki M. Papain inhibitions by optically active E-64 analogs. J Biochem. 1981 Jul;90(1):255–257. doi: 10.1093/oxfordjournals.jbchem.a133458. [DOI] [PubMed] [Google Scholar]
- Tamai M., Matsumoto K., Omura S., Koyama I., Ozawa Y., Hanada K. In vitro and in vivo inhibition of cysteine proteinases by EST, a new analog of E-64. J Pharmacobiodyn. 1986 Aug;9(8):672–677. doi: 10.1248/bpb1978.9.672. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Nakamura T., Ichihara A. A unique trypsin-like protease associated with plasma membranes of rat liver. J Biol Chem. 1986 Feb 25;261(6):2610–2615. [PubMed] [Google Scholar]