Abstract
Benzyl isothiocyanate (BITC), a constituent of edible cruciferous vegetables, inhibits growth of human breast cancer cells in culture. The present study provides in vivo evidence for efficacy of BITC for prevention of mammary cancer in MMTV-neu mice. Administration of BITC at 1 and 3 mmol/kg diet for 25 weeks markedly suppressed the incidence and/or burden of mammary hyperplasia and carcinoma in female MMTV-neu mice without causing weight loss or affecting neu protein level. For example, cumulative incidence of hyperplasia/carcinoma was significantly lower in mice fed BITC-supplemented diets compared with control mice (P= 0.01 by Fisher’s test). The BITC-mediated prevention of mammary carcinogenesis correlated with suppression of cell proliferation and increased apoptosis. The average number of Ki-67 positive cells in the carcinoma lesions of 3 mmol BITC group was lower by about 21% (P<0.05) compared with tumors from control mice. Apoptotic bodies in the mammary tumor were higher by about 2 to 2.5-fold in the 1 and 3 mmol BITC treatment groups (P<0.05) compared with control group. The BITC administration also resulted in overexpression of E-cadherin and infiltration of CD3+ T-cells in the tumor. Even though BITC treatment increased cytotoxicity of natural killer (NK) cells in vitro, dietary feeding of BITC failed to augment NK cell lytic activity in an ex vivo assay. The present study demonstrating efficacy of BITC against mammary cancer in an animal model provides impetus to determine its activity in a clinical setting.
Keywords: Benzyl isothiocyanate, Breast Cancer, Chemoprevention
Introduction
Breast cancer remains a leading cause of cancer related deaths in women despite significant advances in screening techniques, allowing for early detection of the disease, as well as continuous evolution of molecularly targeted therapies (1-4). The known risk factors for breast cancer include family history, Li-Fraumeni syndrome, atypical hyperplasia of the breast, late age at first full-term pregnancy, early menarche, and late menopause (5-7). Because some of these risk factors are not modifiable, novel strategies for prevention of breast cancer are highly desirable. Some success in prevention of mammary carcinogenesis has been achieved using selective estrogen-receptor modulators such as tamoxifen, but this strategy is ineffective against estrogen receptor negative breast cancers (8,9). In addition, selective estrogen-receptor modulators suffer from severe side effects including increased risk of uterine cancer, thromboembolism, cataracts, and perimenopausal symptoms (8,9). Accordingly, novel agents that are relatively safe but which could prevent onset and/or progression of breast cancer irrespective of the hormone receptor status are attractive. Natural products have received increased attention in recent years for the discovery of novel cancer chemopreventive and/or cancer therapeutic agents (10).
Benefit of a diet rich in cruciferous vegetables as a modifier of the breast cancer risk is recognized by population-based, observational studies. For example, a case-control study involving >300 breast cancer cases and controls (matched by age, menopausal status, etc.) documented an inverse correlation between urinary levels of isothiocyanates (ITCs) as a biological measure of cruciferous vegetable intake and the risk of breast cancer (11). Consumption of cruciferous vegetables was inversely associated with the risk of mammary cancer in premenopausal women in another population based case-control study (12).
Anti-carcinogenic effect of cruciferous vegetables is attributed to ITCs, which are generated upon hydrolysis of corresponding glucosinolates through catalytic mediation of myrosinase (13,14). Benzyl isothiocyanate (BITC) is one such compound that has attracted increasing attention because of its ability to inhibit chemically-induced cancer in animal models (13,14). For example, BITC is a potent inhibitor of mouse lung carcinogenesis induced by polycyclic hydrocarbons and hepatocarcinogenesis in rats induced by diethylnitrosamine (15,16). Dietary administration of N-acetylcysteine conjugate of BITC inhibited benzo[a]pyrene-induced lung tumorigenesis in A/J mice (17).
Recent studies from our laboratory have shown that BITC inhibits viability of cultured human breast cancer cells by causing apoptosis (18). On the other hand, a spontaneously immortalized non-tumorigenic human mammary epithelial cell line (MCF-10A) is markedly more resistant to BITC-mediated growth inhibition and apoptosis induction compared with breast cancer cells (18). We also showed that the proapoptotic effect of BITC against human breast cancer cells is triggered by generation of reactive oxygen species due to inhibition of mitochondrial respiratory chain (18,19).
Despite compelling epidemiological and cellular evidence suggesting anti-cancer effect of BITC (11,12,18,19), studies on its in vivo efficacy against breast cancer are lacking. Here, we show that dietary administration of BITC inhibits incidence and/or burden of mammary hyperplasia and carcinoma in female MMTV-neu mice without causing any side effects.
Materials and Methods
Reagents
BITC (purity ~98%) was purchased from LKT laboratories. Cell culture media, antibiotic mixture, and fetal bovine serum were purchased from Invitrogen. Antibody against neu was purchased from ThermoFisher Scientific; antibody against Ki-67 was from DakoCytomation; antibody against CD31 was purchased from Santa Cruz Biotechnology; anti-CD3 antibody was from Biocare Medical; and anti-E-cadherin antibody was purchased from BD Transduction Laboratories. ApopTag Plus Peroxidase In situ Apoptosis detection kit for terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay was procured from Chemicon International-Millipore. Control and BITC supplemented AIN-76A diets were prepared by Harlan-Teklad and stored at 4°C in air-tight containers purged with nitrogen gas. Stability of BITC in the diet was checked by high-performance liquid chromatography essentially as described by Jiao et al. (20).
Chemoprevention protocol
Six-week old female transgenic MMTV-neu mice were purchased from Jackson Laboratories and acclimated for 2 weeks prior to start of the experiment. Spontaneous development of breast cancer in MMTV-neu mice is driven by mammary epithelial expression of the rat erbB2 oncogene under control of the MMTV promoter (21). The mice were randomized into 3 groups with 32 mice in each group. The control mice were placed on basal AIN-76A diet, whereas the experimental groups of mice were fed AIN-76A diet supplemented with either 1 mmol BITC/kg (149.2 mg BITC/kg diet) or 3 mmol BITC/kg (447.6 mg BITC/kg diet). The diet was replenished every 3-4 days at the time of cage change. Diet consumption and body weights were recorded once weekly. Each mouse in every group was also monitored on alternate days for signs of distress including impaired movement or posture, indigestion, and areas of redness or swelling. Mice were sacrificed at 33 weeks of age (25 weeks on control or BITC-supplemented diets). The mammary tissues and vital organs (heart, lung, liver, kidney) were collected at necropsy.
Histopathological analyses of mammary neoplastic lesions
Concentration-effect relationship for dietary BITC administration on incidence and burden (area of the mammary gland occupied by the neoplastic lesion) of mammary hyperplasia and carcinoma were assessed by histopathological analyses of H&E stained sections. Mammary glands from control and BITC-fed mice were fixed in 10% neutral-buffered formalin, dehydrated, embedded in paraffin, and sectioned at 4-5 μm thickness. Three step sections encompassing the periphery and center of each mammary gland were included in the analysis. Hyperplasia was characterized by thickened and multi-layered mammary ducts and cells with a large nucleus. Carcinoma was characterized by the loss of ductal structure, uncontrolled expansion of enlarged cells, and invasion of healthy breast tissue. A Leica microscope equipped with a grid was used for measurement of the area of the hyperplasia/carcinoma lesions at ×100 magnification.
Immunohistochemistry
Mammary tissues were sectioned at 4-5 μm thickness, deparaffinized, and rehydrated. The sections were quenched with 3% hydrogen peroxide and blocked with normal serum. The sections were then incubated with anti-neu (erbB2), anti-Ki-67, anti-E-cadherin, anti-CD31, or anti-CD3 antibody and washed with Tris-buffered saline followed by incubation with appropriate biotinylated secondary antibody. A characteristic brown color was developed by incubation with 3,3-diaminobenzidine. For CD3 staining to determine infiltration of T cells, a characteristic pink color was developed by incubation with Vulcan red. The sections were examined under a Leica microscope. At least five non-overlapping representative images were captured from each section using a camera mounted onto the microscope. The images were analyzed using Image ProPlus 5.0 software from Media Cybernetics (Bethesda, MD) for quantitation.
Detection of apoptotic bodies by TUNEL assay
The paraffin-embedded tissue sections (4-5 μm thickness) were deparaffinized, rehydrated, and used to visualize apoptotic cells by TUNEL staining according to the manufacturer’s protocol as described by us previously (22).
Measurement of lytic Activity of natural killer (NK) and/or dendritic cells (DC)
Basal media for NK cells and DC was RPMI 1640 containing 10% fetal bovine serum, antibiotic mixture, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, non-essential amino acids, 50 μmol/L HEPES, and 50 μmol/L 2-mercaptoethanol. The NK cells were activated by culture for 5 days in medium supplemented with 1000 units/mL interleukin-2. The DC were cultured for 5 days in basal media supplemented with 0.5 mmol/L NG-methyl-L-arginine monoacetate, 500 ng/mL interleukin-4, 500 ng/mL granulocyte macrophage-colony stimulating factor, and 25 ng/mL Flt-3. The BRI-JM04 cells, established from spontaneously developing tumor of a MMTV-neu mouse (23; a generous gift from Dr. Anne Lenferink, Biotechnology Research Institute, Montreal, Canada), were used as target cells to measure lytic activity of NK cells and/or DC. The BRI-JM04 cells were maintained in DMEM supplemented with 10% fetal bovine serum, 2 mmol/L L-glutamine, and antibiotics. For in vitro cytotoxicity assay, the NK cells and DC were isolated from the spleen and bone marrow, respectively, of 10-week old wild-type (untreated) female FVB mice (24,25). After isolation, the NK cells and DC were maintained in above described media in the absence or presence of 0.1, 1 or 3 μmol/L BITC. After 5 days, the BITC-containing media was removed, and the NK cells (2×105 cells/ml) were plated in 96-well plates alone or with DC at a 10:8 (NK cells:DC ratio). After 24 h, BRI-JM04 target cells labeled with 51Cr were added to the wells at specified ratios. The plates were spun down after 4 h and 50 μL of the supernatant was used for analysis of 51Cr release using a scintillation counter. The NK cells and DC respectively isolated from the spleen and bone marrow of control and BITC-fed MMTV-neu mice were used to determine ex vivo lytic activity against BRI-JM04 target cells essentially as described above.
Statistical analyses
Statistical significance of difference in hyperplasia/carcinoma incidence between control and BITC treatment groups was determined by Fisher’s test. Statistical significance of difference in burden (area of the neoplastic lesion) of mammary neoplastic lesions between groups was determined by a one-sided Wilcoxon test. A two-tailed t-test was used to determine the statistical significance of difference in cell proliferation (Ki-67 expression), vessel area (CD31 staining), and apoptotic bodies (TUNEL) between control and BITC treatment groups.
Results
Dietary BITC administration inhibited mammary cancer in MMTV-neu mice
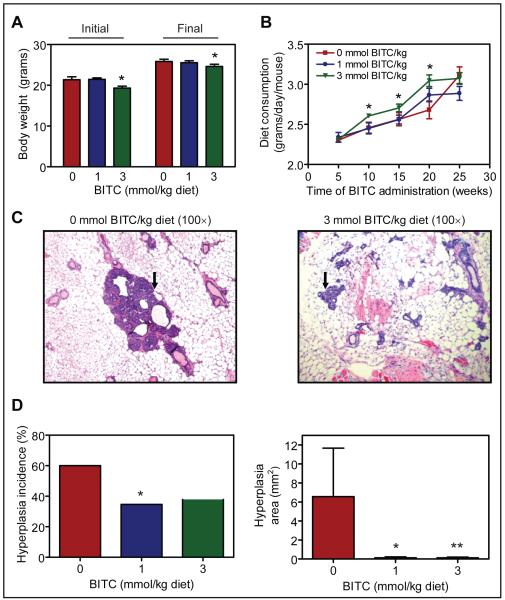
Dose-dependent inhibition of polycyclic aromatic hydrocarbon (benzo[a]pyrene, 5-methychrysene, and dibenz[a,h]anthracene)-induced pulmonary tumor incidence and/or multiplicity in mice by gavage of BITC has been demonstrated previously (26). For example, the benzo[a]pyrene-induced pulmonary tumor multiplicity in female A/J mice was reduced by about 63% and 81% by gavage of 6.7 and 13.4 μmol BITC, respectively, administered 15 min prior to the carcinogen challenge (26). In the present study, we used a well-characterized mouse model (21) to determine mammary cancer chemopreventive efficacy of dietary BITC administration. Doses of the BITC for the present study were selected from previous chemoprevention studies involving chemically-induced tumor models (26-28). The BITC-supplemented diet was well-tolerated by the mice and did not result in weight loss compared with age-matched controls (Fig. 1A). Histology of the vital organs of mice placed on BITC-supplemented diets was also normal (results not shown). However, there were 7 deaths in the control group and 6 and 3 mice each respectively died before the termination of the study in the 1 and 3 mmol BITC/kg diet groups. Necropsies on the deceased mice by a veterinary technician were inconclusive to pinpoint the cause of death. The average body weight of the mice in the 3 mmol BITC/kg diet group was smaller compared with control mice at the onset of the study and this differential was maintained at the termination of the experiment (Fig. 1A). Average food consumption (grams/day/mouse) was modestly but statistically significantly higher in 3 mmol BITC/kg diet group compared with the control group (Fig. 1B). The H&E stained sections depicting hyperplastic structures (identified by an arrow) in representative mammary gland of a control mouse and a mouse from the 3 mmol BITC/kg diet group are shown in Figure 1C. The incidence of mammary hyperplasia in control mice was about 60%, which was reduced by about 42% in the 1 mmol BITC/kg diet group (P=0.06 compared with control by Fisher’s test) and by about 37% in the 3 mmol BITC/kg diet group (P=0.09 compared with control by Fisher’s test) (Fig. 1D). Statistical comparison between the control group and combined 1 and 3 mmol BITC/kg diet groups revealed significant reduction in mammary hyperplasia incidence upon dietary BITC administration (P=0.05 by Fisher’s test; control versus combined 1 and 3 mmol BITC/kg diet groups).
Fig. 1.
A, initial and final body weights of MMTV-neu mice fed basal diet or diet supplemented with 1 or 3 mmol BITC/kg diet. B, average diet consumption over time in mice of control and BITC groups. For data in panels A and B, Columns or points, mean (n= 25 for the control group, n= 26 for the 1 mmol BITC/kg diet group, and n= 29 for the 3 mmol BITC/kg diet group); bars, SE; *, P<0.05, significantly different compared with control. C, H&E stained sections depicting mammary hyperplasia (identified by an arrow) in a representative mouse of control group and 3 mmol BITC/kg diet group. D, hyperplasia incidence (*P=0.06 compared with control) and hyperplasia area (*P=0.06 and **P=0.08 compared with control) in the breast of control mice and mice fed BITC-supplemented diets (n= 25 for the control group, n= 26 for the 1 mmol BITC/kg diet group, and n= 29 for the 3 mmol BITC/kg diet group).
Mean area of the mammary hyperplasia in the control group (6.6 ± 5.1 mm2) was higher compared with that in mice fed 1 mmol BITC/kg diet (0.12 ± 0.09 mm2; P= 0.06 compared with control) or 3 mmol BITC/kg diet (0.13 ± 0.08 mm2; P=0.08 compared with control) (Fig. 1D). Average area of the hyperplasia from the combined 1 and 3 mmol BITC/kg diet groups was statistically significantly lower compared with that of control mice (P=0.04 by Wilcoxon test compared with control). These results indicated that dietary BITC administration inhibited mammary hyperplasia incidence and burden in MMTV-neu mice without causing side effects.
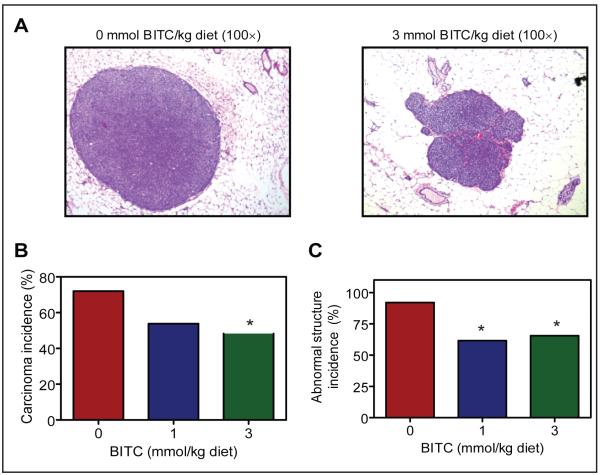
As expected, several mice in each group developed palpable tumors by the end of the study. The H&E stained sections depicting carcinoma in mammary gland of a representative control mouse and a mouse from the 3 mmol BITC/kg diet group are shown in Figure 2A.
Fig. 2.
A, H&E stained sections depicting mammary carcinoma in a representative mouse of the control group and a mouse of the 3 mmol BITC/kg diet group. B, incidence of carcinoma in the breast tissue of mice of the control group, 1 mmol BITC/kg diet group, and 3 mmol BITC/kg diet group. *,P=0.07 compared with control by Fisher’s test). C, cumulative incidence of abnormal structures in the breast tissue of mice of the control group, 1 mmol BITC/kg diet group and 3 mmol BITC/kg diet group (n= 25 for the control group, n= 26 for the 1 mmol BITC/kg diet group, and n= 29 for the 3 mmol BITC/kg diet group). *,P= 0.01 compared with control by Fisher’s test.
Approximately 72% of the control mice developed carcinoma (Fig. 2B). The incidence of carcinoma was reduced by about 25% in mice fed 1 mmol BITC/kg diet compared with control, although the difference did not reach statistical significance (P=0.15 by Fisher’s test). The incidence of mammary carcinoma was lower b ~33% (P=0.07 by Fisher’s test) in mice fed 3 mmol BITC/kg diet compared with control mice (Fig. 2B). When the results were computed to include hyperplasia, carcinoma in situ, and adenocarcinoma (collectively termed as abnormal structure), the incidence of the abnormal structure was significantly lower in mice fed BITC-supplemented diets compared with control mice (Fig. 2C; P=0.01 by Fisher’s test).
Effect of dietary BITC administration on neu protein expression
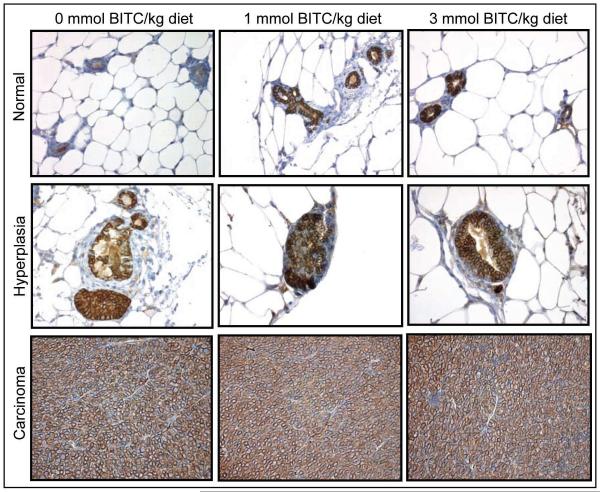
Next, we considered the possibility that BITC-mediated prevention of mammary carcinogenesis in MMTV-neu mice was due to suppression of the transgene expression. As expected, high level of neu protein was detectable in the mammary hyperplasia and carcinoma of the control mice (Fig. 3). The expression of the neu protein was not altered by dietary feeding of BITC (Fig. 3). These observations led us to conclude that the BITC-mediated prevention of mammary carcinogenesis in MMTV-neu mice was not due to the suppression of the transgene expression.
Fig. 3.
Immunohistochemical analysis of neu protein expression in normal breast, hyperplasia, and carcinoma of representative mice of the control group and the BITC-fed groups. Representative images at ×400 magnification are shown.
BITC administration inhibited cell proliferation and increased apoptosis in the tumor
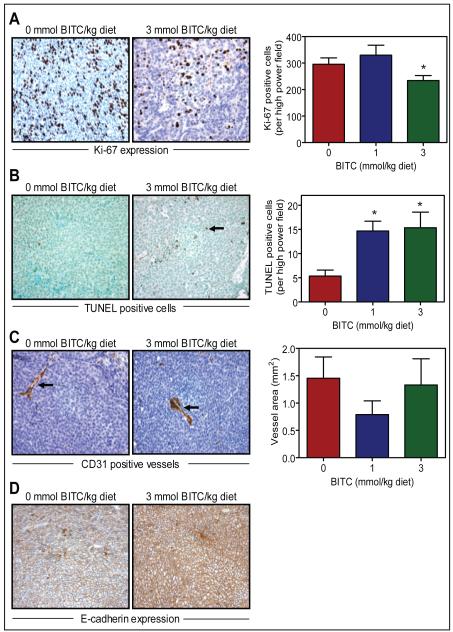
Figure 4A depicts immunohistochemical analysis of Ki-67 expression in mammary tumor from a representative mouse of both the control group and the 3 mmol BITC/kg diet group. The Ki-67 is a large nuclear protein expressed during all active phases of the cell cycle and is a well-accepted marker of cellular proliferation (29). As can be seen in Figure 4A (right panel), expression of the Ki-67 was significantly lower in the mammary tumor of the mice fed 3 mmol BITC/kg diet compared with mice placed on basal AIN-76A diet. For example, average number of the Ki-67 positive cells/high power field in the tumor of 3 mmol BITC/kg diet was lower by about 21% (P<0.05 compared with control by two-tailed Student’s t-test) compared with tumors from control mice (Fig. 4A). The BITC administration resulted in a statistically significant increase in number of apoptotic bodies in the tumor as visualized by TUNEL assay (Fig. 4B). The TUNEL-positive apoptotic bodies in the mammary tumor were higher by about 2-fold in the 1 mmol BITC/kg diet group and by about 2.5-fold in the 3 mmol BITC/kg diet group (P<0.05 by two-tailed Student’s t-test) compared with control (Fig. 4B). These results indicated that dietary BITC administration resulted in reduced cell proliferation and increased apoptosis in the mammary tumor especially at the 3 mmol/kg diet dose.
Fig. 4.
Immunohistochemical analyses for (A) Ki-67 expression, (B) TUNEL-positive apoptotic bodies, (C) CD31-positive blood vessels, and (D) E-cadherin expression in tumor from mice of control and BITC groups. Representative images at ×400 magnification are shown. Columns, mean (n= 7); bars, SE. Statistical significance of difference was determined by two-tailed Student’s t-test. *,P<0.05 compared with control.
Effect of BITC administration on tumor angiogenesis
Because neoangiogenesis (formation of new blood vessels) is critical for tumor growth (30), mammary carcinoma from the control and 3 mmol BITC/kg diet group were stained for angiogenesis marker CD31 (also known as platelet cell endothelial adhesion molecule). Immunohistochemical staining for CD31 in the mammary tumor of a representative mouse from both the control group and the 3 mmol BITC/kg diet group are shown in Figure 4C (vessels are identified by arrows). Even though the vessel area was smaller in the tumors from BITC-fed mice compared with control, the difference did not reach statistical significance (Fig. 4C; right panel).
BITC administration resulted in overexpression of tumor E-cadherin expression
E-cadherin is considered a suppressor of invasion and growth of many epithelial cancers (31). We therefore compared expression of E-cadherin in mammary tumor from the control and BITC-fed mice (Fig. 4D). Expression of E-cadherin was markedly higher in the tumor of 3 mmol BITC-fed mice compared with control mice. Collectively, these results indicated that the BITC-mediated inhibition of mammary carcinogenesis in MMTV-neu mice was associated with overexpression of E-cadherin.
Effect of BITC on activity of NK cells
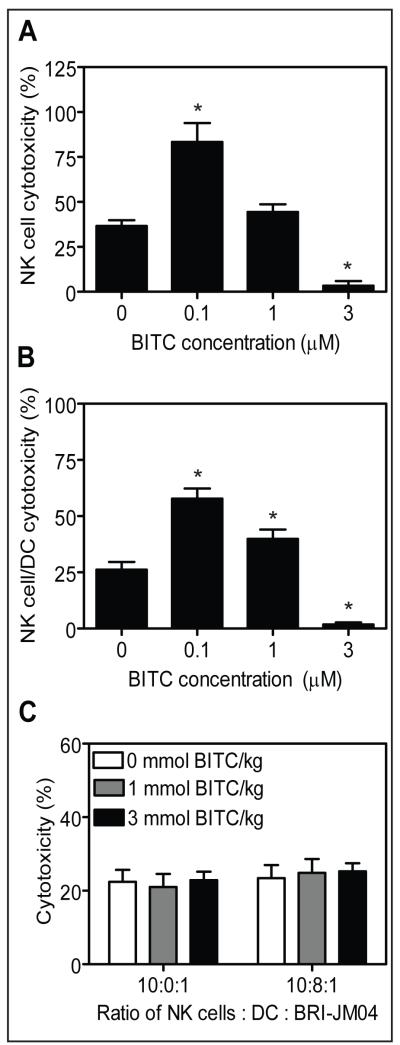
NK cells play an important role in immune surveillance by eliminating tumor cells through granule-mediated exocytosis and induction of apoptosis (32). Initially, we determined lytic activity of NK cells cultured in the presence of varying concentrations of BITC for 5 days against BRI-JM04 target cells. As can be seen in Figure 5A, the NK cytotoxicity was increased t ~83% in the presence of 0.1 μmol/L BITC compared t ~37% specific cytotoxicity observed in absence of BITC. Interestingly, this effect was abolished at higher concentrations of BITC, possibly due to a cytotoxic effect of BITC on NK cells especially at the 3 μmol/L concentration. Because interaction of NK cells with DC results in co-activation of both cell types (33), we also determined in vitro cytotoxicity following co-culture of NK cells and DC for 5 days with or without BITC. Similar to NK cells alone, presence of 0.1 μmol/L BITC, but not the 1 or 3 μmol/L dose, in culture media augmented cytotoxicity of NK cells and DC co-cultures (Fig. 5B). These experiments, which were repeated three times with similar results, suggested that low concentrations of BITC could augment lytic activity of NK cells and NK cells-DC co-cultures. Next, we tested ex vivo cytotoxicity of NK cells and DC isolated from the spleen and bone marrow, respectively, of control and BITC-fed mice using BRI-JM04 as a target cell line. The results presented in Figure 5C do not show any significant improvement in cytotoxicity of NK cells alone or NK cells-DC co-cultures from BITC-fed mice compared with control mice.
Fig. 5.
A, NK cells isolated from the spleen of wild-type FVB mice were cultured for 5 days in the absence or presence of 0.1, 1 or 3 μmol/L BITC and then tested for cytotoxicity against BRI-JM04 target cells. B, cytotoxicity of NK cells (isolated from the spleen of FVB mice) and dendritic cells (DC; isolated from the bone marrow of FVB mice) co-cultured for 5 days in the absence or presence of the indicated concentrations of BITC. The BRI-JM04 cells were used as target cells. In panels A and B, the experiment was repeated 3 times in triplicate and the results were consistent. Representative data from a single experiment are shown. Columns, mean (n= 3); bars, SE. *,P<0.05, compared with control by two-tailed Student’s t-test. C, ex vivo cytotoxicity of NK cells and DC isolated from spleen and bone marrow, respectively, of MMTV-neu mice of the control group, 1 mmol BITC/kg diet group or 3 mmol BITC/kg diet group against BRI-JM04 target cells. Columns, mean (n= 25 for the control group, n= 26 for the 1 mmol BITC/kg diet group, and n= 29 for the 3mmol BITC/kg diet group); bars, SE.
BITC administration increased tumor infiltration of T cells
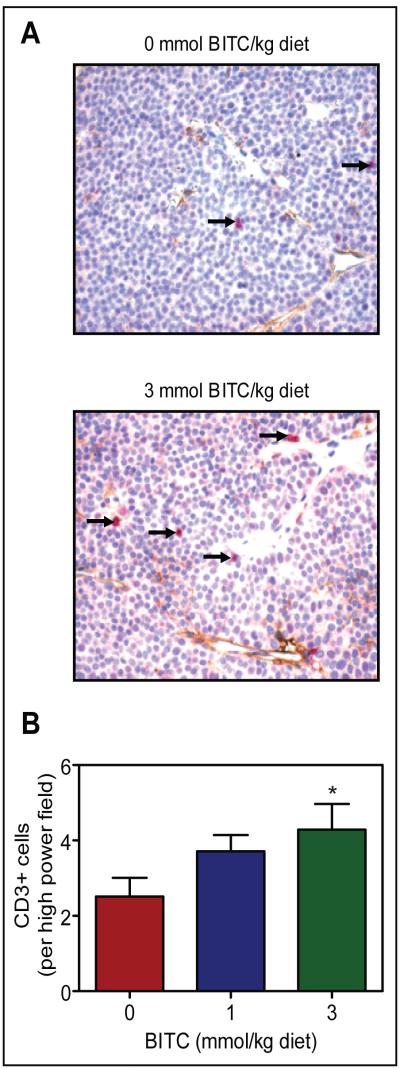
In addition to NK cells, T cells are also implicated in surveillance of tumors (34,35). Presence of infiltrating T cells in the tumor was examined by immunohistochemistry of CD3, a pan T cell marker. Infiltrating T cells (identified by arrows) were visible in the tumors of control mice (Fig. 6A) as well as in the tumors of 1 and 3 mmol BITC/kg diet groups (Fig. 6B). Fraction of infiltrating CD3+ T cells in the tumor of mice fed 3 mmol BITC/kg diet was ~70% higher in comparison with control mice (P=0.06 by two-tailed Student’s t-test). These results suggested that the anti-cancer effect of dietary BITC administration was accompanied by an increase in T cell surveillance.
Fig. 6.
A, immunohistochemical analysis of CD3+ T cells (pink) in the tumor of a representative mouse of the control group and a mouse of the 3 mmol BITC/kg diet group. B, quantitation of CD3+ T cells in the tumors of the control group, 1 mmol BITC/kg diet group, and 3 mmol BITC/kg diet group. Columns, mean (n= 7); bars, SE. *,P=0.06 compared with control by two-tailed Student’s t-test.
Discussion
Overexpression of neu due to the amplification of neu gene is linked with breast cancer (36). The neu positive tumors account for approximately 20% of all breast cancers and these tumors tend to be more aggressive with poor prognosis (37). In the present study, we used a mouse model with mammary specific expression of the neu oncogene to test in vivo chemopreventive efficacy of dietary BITC administration. We found that BITC administration in the diet suppresses incidence and/or burden of mammary hyperplasia and carcinoma in MMTV-neu mice without causing weight loss or any other adverse effects. However, we were puzzled by deaths of some mice in each group before termination of the study. Because more mice died in the control group and the necropsy was inconclusive, we conclude that the deaths were not related to BITC administration. The BITC-mediated prevention of mammary carcinogenesis was evident at concentrations that can be generated through dietary intake of cruciferous vegetables (13,14).
We have shown previously that BITC treatment suppresses growth of human breast cancer cells (MCF-7 and MDA-MB-231) in association with apoptosis induction regardless of estrogen responsiveness or p53 status (18). Cultured cancer cells are invaluable not only for rapid screening of potential cancer chemopreventive agents but also for elucidation of the mechanism of their action. However, in vivo validation of the cellular findings is a critical step in clinical development of promising anti-cancer agents. Consistent with cellular data (18,19), dietary BITC administration elicits growth inhibitory and proapoptotic response in vivo in the MMTV-neu model. Expression of the proliferation marker Ki-67 in the mammary tumor of MMTV-neu mice is reduced significantly upon administration of 3 mmol BITC/kg diet. Likewise, the spontaneous tumors from BITC-fed mice exhibit significantly higher count of TUNEL-positive apoptotic bodies in comparison with control tumors. Based on these observations, we conclude that cell proliferation and apoptosis are valid biomarkers to assess BITC response in future clinical trials.
E-cadherin, a single-span transmembrane glycoprotein of five repeats and cytoplasmic domain, plays an important role in normal physiologic processes including development, cell polarity, and tissue morphology (38). E-cadherin is considered a tumor suppressor because of its role in inhibition of epithelial-mesenchymal transition (31,39,40). E-cadherin is frequently down-regulated during cancer progression and correlates with poor prognosis (41). Expression of E-cadherin has been shown to reduce progression and invasiveness of breast tumors (42). The present study reveals that dietary BITC administration causes a marked increase in expression of E-cadherin protein in the tumor of the MMTV-neu mice. Our preliminary unpublished studies indicate that BITC treatment indeed inhibits migration of MDA-MB-231 human breast cancer cells at pharmacologically relevant concentrations (Singh SV, unpublished results). Thus it is reasonable to conclude that E-cadherin overexpression probably contributes to mammary cancer prevention by dietary BITC administration. However, further studies are needed to elucidate the mechanism of BITC-mediated induction of E-cadherin protein expression.
The NK cells are the earliest responders of the innate immune system and have been broadly implicated in tumor immune surveillance (32,43). We assessed the immunomodulatory potential of BITC in vitro and found that it can augment NK cell cytotoxicity at low concentrations. However, augmentation of NK cell activity was not evident in an ex vivo assay. The T cells are involved in cell-mediated immunity, and have been implicated in the control of tumor development (44,45). We observed an increase in T cell infiltration in the tumors from BITC-fed MMTV-neu mice compared with controls. These results suggest that the BITC-mediated prevention of mammary carcinogenesis may be mediated by infiltration of cytotoxic T cells.
In conclusion, the present study indicates that dietary BITC administration inhibits mammary carcinogenesis in MMTV-neu mice without causing weight loss or any other side effects. We show further that the BITC-mediated prevention of mammary cancer correlates with reduced cell proliferation, increased apoptosis, CD3+ T cell infiltration, and overexpression of E-cadherin. These preclinical observations merit clinical investigation of BITC for its efficacy against human breast cancer.
Acknowledgments
Grant support: This investigation was supported in part by the USPHS grant CA129347-02 awarded by the National Cancer Institute and a grant from the Ayers Foundation.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Jemal A, Ward E, Thun MJ. Temporal trends in breast cancer mortality by state and race. Cancer Causes Control. 2008;19:537–45. doi: 10.1007/s10552-008-9113-1. [DOI] [PubMed] [Google Scholar]
- 3.van de Ven SM, Elias SG, van den Bosch MA, Luijten P, Mali WP. Optical imaging of the breast. Cancer Imaging. 2008;8:206–15. doi: 10.1102/1470-7330.2008.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz M, Estevez LG, Alvarez I, et al. Evaluation of international treatment guidelines and prognostic tests for the treatment of early breast cancer. Cancer Treat Rev. 2008;34:701–9. doi: 10.1016/j.ctrv.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 6.Hulka BS, Stark AT. Breast cancer: cause and prevention. Lancet. 1995;346:883–7. doi: 10.1016/s0140-6736(95)92713-1. [DOI] [PubMed] [Google Scholar]
- 7.Kelsey JL, Bernstein L. Epidemiology and prevention of breast cancer. Annu Rev Public Health. 1996;17:47–67. doi: 10.1146/annurev.pu.17.050196.000403. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 9.Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–24. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 10.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–37. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 11.Fowke JH, Chung FL, Jin F, et al. Urinary isothiocyanate levels, brassica, and human breast cancer. Cancer Res. 2003;63:3980–6. [PubMed] [Google Scholar]
- 12.Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134:1134–8. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- 13.Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev. 2000;32:395–411. doi: 10.1081/dmr-100102342. [DOI] [PubMed] [Google Scholar]
- 14.Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab. 2002;3:233–55. doi: 10.2174/1389200023337496. [DOI] [PubMed] [Google Scholar]
- 15.Wattenberg LW. Inhibitory effects of benzyl isothiocyanate administered shortly before diethylnitrosamine or benzo[a]pyrene on pulmonary and forestomach neoplasia in A/J mice. Carcinogenesis. 1987;8:1971–3. doi: 10.1093/carcin/8.12.1971. [DOI] [PubMed] [Google Scholar]
- 16.Sugie S, Okumura A, Tanaka T, Mori H. Inhibitory effects of benzyl isothiocyanate and benzyl thiocyanate on diethylnitrosamine-induced hepatocarcinogenesis in rats. Jpn. J. Cancer Res. 1993;84:865–70. doi: 10.1111/j.1349-7006.1993.tb02059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang YM, Conaway CC, Chiao JW, et al. Inhibition of benzo(a)pyrene-induced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugates of benzyl and phenethyl isothiocyanates during the postinitiation phase is associated with activation of mitogen-activated protein kinases and p53 activity and induction of apoptosis. Cancer Res. 2002;62:2–7. [PubMed] [Google Scholar]
- 18.Xiao D, Vogel V, Singh SV. Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Mol Cancer Ther. 2006;5:2931–45. doi: 10.1158/1535-7163.MCT-06-0396. [DOI] [PubMed] [Google Scholar]
- 19.Xiao D, Powolny AA, Singh SV. Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger ROS-dependent apoptosis in human breast cancer cells. J Biol Chem. 2008;283:30151–63. doi: 10.1074/jbc.M802529200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiao D, Eklind KI, Choi CI, Desai DH, Amin SG, Chung FL. Structure-activity relationships of isothiocyanates as mechanism-based inhibitors of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Res. 1994;54:4327–33. [PubMed] [Google Scholar]
- 21.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–82. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh SV, Warin R, Xiao D, et al. Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res. 2009;69:2117–25. doi: 10.1158/0008-5472.CAN-08-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenferink AE, Magoon J, Cantin C, O’Connor-McCourt MD. Investigation of three new mouse mammary tumor cell lines as models for transforming growth factor (TGF)-beta and Neu pathway signaling studies: identification of a novel model for TGF-beta-induced epithelial-to-mesenchymal transition. Breast Cancer Res. 2004;6:R514–30. doi: 10.1186/bcr907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giezeman-Smits KM, Okada H, Brissette-Storkus CS, et al. Cytokine gene therapy of gliomas: induction of reactive CD4+ T cells by interleukin-4-transfected 9L gliosarcoma is essential for protective immunity. Cancer Res. 2000;60:2449–57. [PubMed] [Google Scholar]
- 25.Brissette-Storkus CS, Kettel JC, Whitham TF, et al. Flt-3 ligand (FL) drives differentiation of rat bone marrow-derived dendritic cells expressing OX62 and/or CD161 (NKR-P1) J Leukoc Biol. 2002;71:941–9. [PubMed] [Google Scholar]
- 26.Hecht SS, Kenney PMJ, Wang M, Upadhyaya P. Benzyl isothiocyanate: an effective inhibitor of polycyclic aromatic hydrocarbon tumorigenesis in A/J mouse lung. Cancer Lett. 2002;187:87–94. doi: 10.1016/s0304-3835(02)00410-x. [DOI] [PubMed] [Google Scholar]
- 27.Hecht SS, Kenney PM, Wang M, Trushin N, Upadhyaya P. Effects of phenethyl isothiocyanate and benzyl isothiocyanate, individually and in combination, on lung tumorigenesis induced in A/J mice by benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Lett. 2000;150:49–56. doi: 10.1016/s0304-3835(99)00373-0. [DOI] [PubMed] [Google Scholar]
- 28.Boysen G, Kenney PM, Upadhyaya P, Wang M, Hecht SS. Effects of benzyl isothiocyanate and 2-phenethyl isothiocyanate on benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone metabolism in F-344 rats. Carcinogenesis. 2003;24:517–25. doi: 10.1093/carcin/24.3.517. [DOI] [PubMed] [Google Scholar]
- 29.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 31.Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol. 2003;19:207–35. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 32.Terabe M, Berzofsky JA. NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol. 2007;28:491–6. doi: 10.1016/j.it.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez NC, Lozier A, Flament C, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–11. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 34.Georgiannos SN, Renaut A, Goode AW, Sheaff M. The immunophenotype and activation status of the lymphocytic infiltrate in human breast cancers, the role of the major histocompatibility complex in cell-mediated immune mechanisms, and their association with prognostic indicators. Surgery. 2003;134:827–34. doi: 10.1016/s0039-6060(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 35.Reome JB, Hylind JC, Dutton RW, Dobrzanski MJ. Type 1 and type 2 tumor infiltrating effector cell subpopulations in progressive breast cancer. Clin Immunol. 2004;111:69–81. doi: 10.1016/j.clim.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Carter WB, Hoying JB, Boswell C, Williams SK. HER2/neu overexpression induces endothelial cell retraction. Int J Cancer. 2001;91:295–9. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1061>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 37.Gusterson BA, Gelber RD, Goldhirsch A, et al. International (Ludwig) Breast Cancer Study Group Prognostic importance of c-erbB-2 expression in breast cancer. J Clin Oncol. 1992;10:1049–56. doi: 10.1200/JCO.1992.10.7.1049. [DOI] [PubMed] [Google Scholar]
- 38.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–32. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 39.Agiostratidou G, Hulit J, Phillips GR, Hazan RB. Differential cadherin expression: potential markers for epithelial to mesenchymal transformation during tumor progression. J Mammary Gland Biol Neoplasia. 2007;12:127–33. doi: 10.1007/s10911-007-9044-6. [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–29. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Mohammadizadeh F, Ghasemibasir H, Rajabi P, Naimi A, Eftekhari A, Mesbah A. Correlation of E-cadherin expression and routine immunohistochemistry panel in breast invasive ductal carcinoma. Cancer Biomarkers. 2009;5:1–8. doi: 10.3233/CBM-2009-0551. [DOI] [PubMed] [Google Scholar]
- 42.Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5:R217–22. doi: 10.1186/bcr651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–43. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 44.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–68. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Girardi M, Oppenheim DE, Steele CR. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–9. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]