Abstract
Cortisol is essential for regulating all cell types in the body, including those in the brain. Most information concerning cortisol’s cerebral effects comes from work in nonhumans. This is a first effort to use functional magnetic resonance imaging (fMRI) to study the time course and locus of cortisol’s effects on selected brain structures in resting humans. We repeatedly scanned 21 healthy young adults over 45 min to examine changes in the brain’s activity 5 min before, and for 40 min after, an IV injection of 10 mg of hydrocortisone (N = 11) or saline placebo (N = 10). At 15–18 min postinjection we observed in the hydrocortisone group reduced activity in the hippocampus and amygdala that reached a peak response minimum at 25–30 min postinjection (−1 Standard Deviation) relative to placebo. No such effect was seen in the thalamus. Functional MRI appears to be a safe, noninvasive method to study the time course and anatomical effects of glucocorticoids in the human brain.
Keywords: cortisol, hydrocortisone, human, brain, magnetic resonance imaging, amygdala, hippocampus, thalamus
Acute effects of hydrocortisone on the human limbic system: An fMRI study
This is an initial report of the use of functional magnetic resonance imaging (fMRI) to study the acute effects of cortisol in the human brain. Diurnal glucocorticoid secretion regulates all cells in the body, including those of the brain (De Kloet and Reul, 1987). High-level cortisol release is a central component of the response to acute and chronic stress (Van Cauter, et al., 1996; Gunnar and Vazquez, 2001; Edwards, et al., 2003). Chronically high levels of cortisol resulting from stress, depression, and Cushing’s disease reduce hippocampal volume (Sapolsky, et al., 1990; Bremner, 2006) and alter cognition and emotional reactivity (Starkman et al., 1981; 1992; 2003).
Acute stress causes a rapid rise in circulating cortisol that readily passes the blood-brain barrier, allowing it to reach receptors located in the cortex, limbic system, hippocampus, thalamus, and hypothalamus. These “fast” effects of cortisol on the brain have been viewed as a critical regulator of behavior (Dallman, 2005); they appear to mediate cortisol’s short-term effects on cognition, emotion, and motivation (Diorio, et al., 1993; Buchanan, et al., 2001; Mizoguchi, et al., 2004), as well as working memory and long-term memory (al’Absi, et al., 2002; Roozendaal, 2002; Grillon, et al., 2004; Hoffman and al’Absi, 2004). The importance of these behavioral effects stands in contrast to our lack of precise knowledge of the real-time effects of stress levels of cortisol in the human brain. Our information about the sites of glucocorticoid actions in the central nervous system comes primarily from studies in vitro and in animal models (McEwen, et al., 1968; Alexis, et al., 1983; Coirini, et al., 1983; De Kloet, et al., 1984), with a few studies in primates (Sanchez, et al., 2000), and fewer still in the human (de Leon, et al., 1997; de Quervain, et al., 2003; Croissant and Olbrich, 2004). We currently have no clear information on the exact time course by which endogenous cortisol acts on the brain after entering into the bloodstream or which structures are the first to participate in these immediate responses. Since fMRI provides a rapid and noninvasive method to image the brain, it has the potential to serve as a useful tool for documenting the time-course and spatial effects of cortisol in human subjects. We observed the acute effects of hydrocortisone on specified limbic and paralimbic structures in human subjects undergoing repeated fMRI acquisitions. The choice of structures was based on cortisol’s known functional effects on the hippocampus (Buchanan and Lovallo, 2001; Buchanan, et al., 2006) and amygdala (Shepard, et al., 2000; Dedovic, et al., 2009). Similarly, cortisol has been reported to act on thalamic receptors in rat and hamster (Sutanto, et al., 1988). We report here on the time course and direction of response to hydrocortisone of these selected brain regions using the blood oxygenation level dependent (BOLD) signal obtained using fMRI in resting humans.
Materials and Methods
Participants
We tested twenty-one healthy volunteers (11 females) who met the following inclusion criteria: ages 18 to 55 years; good physical health by self report and medical history interview without a history of allergies or current treatment with steroids; normal mental status and absence of psychiatric diagnosis (DSM-IV) (American Psychiatric Association, 1994) as assessed by interview; absence of prescription medications; no cardiac pacemakers or any nonremovable metal objects that could make it unsafe to enter the magnet; ability to understand the study requirements and follow directions. All volunteers signed a consent form approved by the Institutional Review Board at the University of Texas Health Sciences Center, San Antonio, TX and were paid for their participation.
Cortisol administration
Participants were randomly assigned to receive an intravenous injection of either hydrocortisone (N = 11) or saline (N = 10) in a double-blind procedure. These groups were similar in age (M ± SD = 29.6 ± 9.65 and 26.9 ± 5.93, respectively; t = 0.77, p > 0.05) and gender composition (6 and 5 females respectively; χ2 = 0.043, p > 0.05). The protocol was designed to mimic the effects of a rapid release of cortisol into the bloodstream as might accompany an acute stress response. The use of a bolus injection also allowed a precise documentation of the onset and time course of cortisol’s effects from the ongoing scans. Hydrocortisone (Hawkins Chemical Co., Minneapolis, MN) or saline was packaged by Innovative Pharmacy Solutions, Edmond, OK in identical plastic bottles containing single doses of either saline alone or 10 mg of hydrocortisone in saline solution. A 20-gauge intravenous catheter was inserted into a peripheral vein in the right arm and connected to a saline drip. The hydrocortisone or saline was administered as a bolus injection via the intravenous catheter. Prior work has shown that 20 mg of oral hydrocortisone produced blood levels of cortisol similar to those seen during mental stress protocols in the laboratory (Buchanan, et al., 2001). The smaller dose was chosen here because of the more rapid onset of the effects of the bolus administration.
Brain scanning
Following screening and catheter insertion, volunteers were placed in the scanner and instructed to remain still with their eyes gently closed during the duration of the procedure. Scanning was carried out on a Siemens 3T Trio MRI scanner. Functional scans were acquired continuously at 5-sec intervals over 45 min, with CORT or PLACEBO injection occurring at the 5-min mark. Functional imaging utilized a gradient echo, echoplanar sequence sensitive to the BOLD signal, acquiring 24 sagital slices (TR = 5000 ms, TE = 40 ms, flip angle = 20 degrees, voxel size = 4 mm3 and a 1 mm gap, FOV = 256 cm). After functional image acquisition, a higher resolution coplanar T1-weighted series was obtained for each subject and used for anatomical reference (TR = 500 ms, TE = 20 ms, flip angle = 90 degrees, voxel size of 2 × 2 × 4 mm and a 1mm gap, FOV = 256 cm). Finally, a high-quality 3D anatomical image was acquired with an 0.8 mm3 voxel size and a 256 × 256 × 256 data matrix (TR = 33 ms, TE = 12 ms, and flip angle = 60 degrees). Total time of data acquisition was approximately 2 hr. Due to the length of the scan time, data preprocessing included motion correction.
Structural imaging was conducted to provide anatomically accurate delineations of limbic and paralimbic regions of interest (ROIs) that were selected based on previous research on structures affected by cortisol, including the thalamus, insular cortex, amygdala, hippocampus, and parahippocampal gyrus. Bilateral anatomically driven ROIs for each structure were defined using the Harvard-Oxford Structural Probability Atlas (thresholded at 70% probability) distributed with FSL neuroimaging analysis software (http://www.fmrib.ox.ac.uk/fsl/data/atlas-descriptions.html). The ROIs were then registered into each subject’s space, and a time series was extracted. In order to minimize momentary signal fluctuations, the data were averaged in 5-min increments.
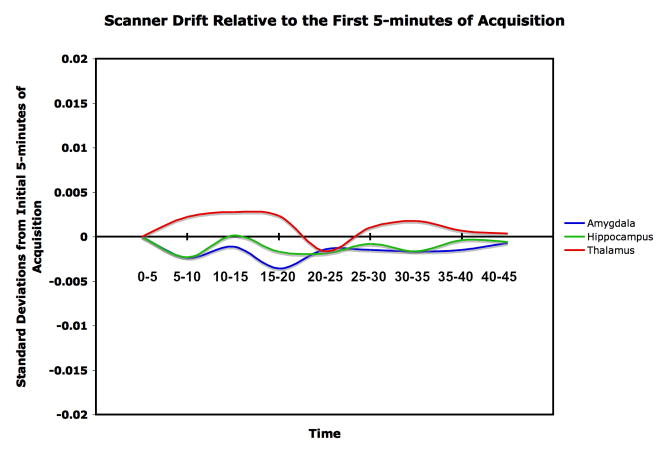
The data were acquired over a 45-min session with repeated fMRI acquisitions. With long acquisitions, scanner drift can affect the data. We dealt with this in two ways: First, the scanner used in this study has daily quality assurance procedures in place specifically to minimize such drift. Second, to ensure that the present data were free of scanner drift, we examined the activity of the hippocampus, amygdala, and thalamus in the placebo group, because signals should be constant over time if scanner drift is absent. Using minutes 0 to 5 as a baseline, change in signal strength was registered in SD units for each succeeding 5-min segment. As shown in Figure 1, BOLD signal strength remained at < ± 0.005 SD units over the next 40 min. An alternative strategy would be to filter the BOLD signal series using a low time constant filter in the range of .008 Hz. However, this was considered undesirable in the case of the present data because the filtration would also remove true time series signal caused by the response to cortisol, which is also on the order of minutes. Instead, we chose to standardize the data using Z scores and to examine the response to cortisol as a difference from placebo in successive 5-min segments.
Figure 1.
BOLD-signal consistency over 45 min for the placebo group. The figure shows that for the three structures in question, there was < ± 0.005 SD change in signal strength in any time period relative to the baseline period at 0–5 min.
All of the BOLD measurements from the placebo group were therefore put into a common distribution with the mean set to zero and the SD set to 1.0. Each measurement from the cortisol group was then assigned a Z value based on its SD units, and each subject’s standardized score was extracted at each time point. Because the BOLD signal does not provide a calibrated metric, we elected to compare the placebo vs. hydrocortisone conditions in SD units that are equivalent to Cohen’s d′, which is also expressed as the difference in SD units between two conditions or two groups. This provides a statistical comparison between the hydrocortisone and placebo groups that is interpretable as an effect size in which an effect size of 0.2 to 0.3 is considered a “small” effect, one around 0.5 a “medium” effect, and 0.8 and greater, as a “large” effect.
Results
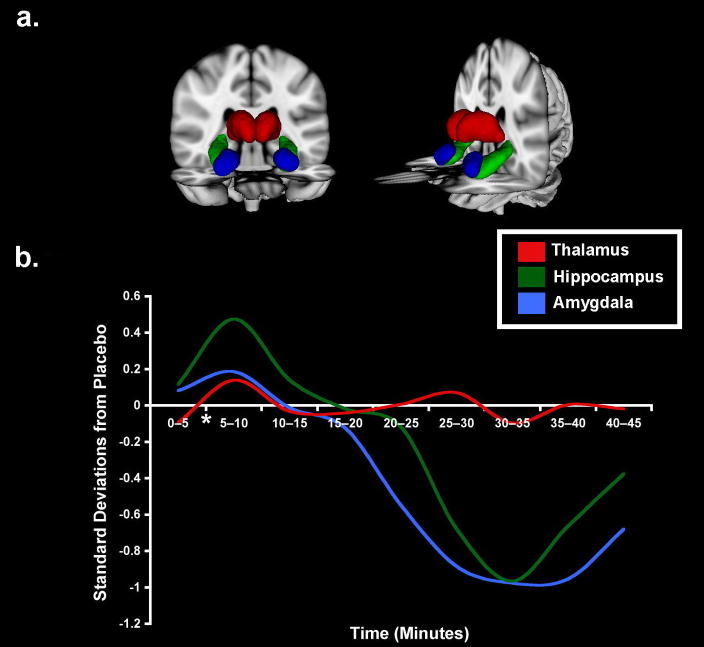
Results are summarized in Figure 2. Relative to the saline group we observed reduced BOLD signals bilaterally in the amygdalae and parahippocampal gyri, with effect sizes approaching 1.0 at 20 to 25 min postinjection and persisting for as long as 40 min postinjection, the end of the scanning period. In comparison the thalamus had variations remaining at < 0.2 SD throughout the period of observation. Similarly, the insula and posterior parahippocampal gyri did not respond to hydrocortisone (not shown). Table 1 shows the mean ± standard error difference from placebo to hydrocortisone for each 5-min time period during the scanning session.
Figure 2.
Normalized BOLD-signal changes in response to hydrocortisone administration. (A) 3D rendering of regions of interest from which time courses were extracted. (B) Hydrocortisone group differences from placebo in Z score units averaged over 5-min segments. Cortisol or placebo was administered at the end of the first 5 min.
Table 1.
Difference between placebo group and hydrocortisone group in BOLD signal strength obtained at the hippocampus, amygdala, and thalamus over each 5-min time segment during the scanning session. Entries show mean ± standard error.
| Mean Difference from Placebo | ||||||
|---|---|---|---|---|---|---|
| Time Period (minutes) | Hippocampus | Amygdala | Thalamus | |||
| Mean | SE | Mean | SE | Mean | SE | |
| 0–5 | 0,17 | 0,21 | 0,20 | 0,29 | −0,32 | 0,20 |
| 5–10 | 0,99 | 0,19 | 0,44 | 0,27 | 0,55 | 0,20 |
| 10–15 | −0,11 | 0,23 | −0,55 | 0,31 | −0,12 | 0,26 |
| 15–20 | −0,64 | 0,14 | −1,11 | 0,27 | −0,01 | 0,21 |
| 20–25 | −0,93 | 0,39 | −3,81 | 0,42 | 0,12 | 0,25 |
| 25–30 | −3,72 | 0,23 | −5,88 | 0,34 | 0,41 | 0,32 |
| 30–35 | −5,25 | 0,29 | −7,39 | 0,31 | −0,52 | 0,27 |
| 35–40 | −5,12 | 0,24 | −8,07 | 0,27 | −0,03 | 0,29 |
| 40–45 | −3,58 | 0,23 | −7,06 | 0,34 | 0,01 | 0,25 |
Discussion
These results demonstrate the use of the fMRI BOLD signal to detect the timing and locus of glucocorticoid effects on selected structures of the human brain. The present observations were made under a restricted set of conditions with the subjects at rest. The time course of the data we present indicates that reduced activation in the amygdala and parahippocampal gyrus is the initial response of these structures to a stress-level of glucocorticoid exposure. Although these results must be interpreted with caution in terms of the underlying cellular physiology, there is some reason to consider the effects to reflect a hyperpolarization of the neurons in these structures.
The threshold of 20 min for hydrocortisone’s effects to become observable in the present study, and the direction of response in the parahippocampus and amygdala, are consistent with the mechanism and timing of “fast” glucocorticoid actions in the brain. In animal models and tissue studies, these fast effects are mediated by cortisol’s effect on membrane receptors, and the onset of neuronal response to systemically administered glucocorticoids is on the order of 15 to 20 min (Makara and Haller, 2001; Dallman, 2005). In contrast, cortisol’s longer lasting genomic effects are exerted by actions on intracellular glucocorticoid and mineralocorticoid receptors and subsequent entry into the cell nucleus, leading to alterations of gene expression, a chain of events that requires longer times to develop (Makara and Haller, 2001; Dallman, 2005). The primary mechanism of these fast glucocorticoid effects on membrane is hyperpolarization (Chen, et al., 1991; Makara and Haller, 2001; Logothetis and Wandell, 2004), an effect that is consistent with the reduction of metabolic activity in the structures that responded in the present study.
Although the structures featured in this report are all known to possess receptors for glucocorticoids, the thalamus, insula, and parahippocampal gyrus showed no change in metabolism, as indexed by the lack of a BOLD signal response, while the activity of the hippocampus and amygdala declined substantially by 30–35 min postinjection. The difference in responses across structures may be due to receptor distribution and density or to the dose of hydrocortisone. The response differences are therefore functional effects occurring under these conditions of testing and do not reflect the presence or absence of glucocorticoid receptors.
Other studies have reported on cortisol’s effects using positron emission tomography (PET). Oral hydrocortisone administered to healthy elderly persons led to reduced hippocampal metabolism seen as lowered uptake of fluorine deoxyglucose (de Leon, et al., 1997). In a PET study of memory retrieval, oral hydrocortisone impaired retrieval of material learned 24 hrs earlier, and this memory deficit was associated with reduced cerebral blood flow in the parahippocampal gyrus measured using O15 water (de Quervain, et al., 2003). These findings of reduced hippocampal blood flow and glucose metabolism are consistent with our present results. In considering which method to use in tracking hydrocortisone effects in the present study, we decided to fMRI in preference to O15 water because of fMRI’s fast data acquisition time and high temporal resolution. In these imaging studies, cortisol was administered orally, so the exact onset of cortisol’s effects was not observed. In the present study, we used an IV administration allowing hydrocortisone’s entry to the bloodstream to be precisely known. We scanned in 5-sec segments and ultimately averaged these into 5-min blocks to provide sufficient smoothing of the data while retaining enough temporal resolution to visualize the time course of hydrocortisone’s effects. A similar acquisition with PET using O15 water would have had a minimal temporal resolution of 10 min. An additional consideration is that PET entails the use of radiation vs. none for fMRI. A similar fMRI approach was used in this facility to track the effects of glucose infusion at the hypothalamus (Mahankali, et al., 2000).
This initial set of observations provides a basis for more extensive in vivo study of cortisol’s real-time effects on the human brain. The present observations were made in resting persons exposed to a single dose, and we examined a restricted set of brain structures chosen for their known responsiveness to cortisol. Future studies should explore such effects at different doses and under differing behavioral conditions. The dose chosen for use here was within the physiological range and was intended to mimic the effect of a mild behavioral stressor, such as mental arithmetic or a public speaking simulation (al’Absi, et al., 1997).
The direction of hydrocortisone’s effects may vary as a function of the behavioral state of the subject, as the glucocorticoid response of the amygdala and hippocampus differs depending on the resting vs. stressed state of the animal (Makara and Haller, 2001; Roozendaal, 2002), and similar directional effects may occur in the human. In addition, cortisol administered prior to a fear conditioning procedure resulted in altered responses in the anterior cingulate gyrus, the lateral orbitofrontal cortex, and medial prefrontal cortex (Stark, et al., 2006). This suggests that cortisol’s actions may be seen in a wider range of structures during stress as opposed to the resting state. Future work should map the response to a range of doses that might approximate the levels of cortisol seen during mild and severe psychological stress and occurring during physiological stressors, such as a marathon run, which produces blood levels far higher than the current dose (Dessypris, et al., 1980). Whether the direction of effect or the range of structures affected would remain the same is therefore a question for further study (van Stegeren, 2009). Finally, the longer-term response to glucocorticoid via its genomic actions is a potential candidate for the use of this technique, and would require extending the period of observation beyond the 40 min explored here.
The present results demonstrate the feasibility of studying cortisol’s real-time effects in the human brain, and they raise questions for future study. The IV administration used here provides new information about the timing of the brain’s response to cortisol following its entry into the blood stream.
Acknowledgments
Role of the funding source: This work was supported by the Medical Research Service of the Department of Veterans Affairs (W.R.L.), the National Institutes of Health, NIAAA (AA12207) (W.R.L.), NARSAD (Essel Young Investigator, D.C.G.), and the UTHSCSA GCRC (M01-RR-01346). We thank Sibel Cakr, M.D. for help with the intravenous injections.
Footnotes
AUTHOR CONTRIBUTIONS
W.R.L. and D.C.G. designed the study and details of the protocol. J.L.R. analyzed the fMRI and anatomical data and conducted the statistical analyses. P.T.F. contributed to the study design, the imaging protocol, and the interpretation of the data. All authors contributed to the writing of the paper and approve of its content.
Conflict of interest The authors have no financial interest in any product associated with this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al’Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34:266–275. doi: 10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hugdahl K, Lovallo WR. Adrenocortical stress responses and altered working memory performance. Psychophysiology. 2002;39:95–99. doi: 10.1017/S0048577202001543. [DOI] [PubMed] [Google Scholar]
- Alexis MN, Stylianopoulou F, Kitraki E, Sekeris CE. The distribution and properties of the glucocorticoid receptor from rat brain and pituitary. J Biol Chem. 1983;258:4710–4714. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8:445–461. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Brechtel A, Sollers JJ, Lovallo WR. Exogenous cortisol exerts effects on the startle reflex independent of emotional modulation. Pharmacol Biochem Behav. 2001;68:203–210. doi: 10.1016/s0091-3057(00)00450-0. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Impaired memory retrieval correlates with individual differences in cortisol response but not autonomic response. Learn Mem. 2006;13:382–387. doi: 10.1101/lm.206306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YZ, Hua SY, Wang CA, Wu LG, Gu Q, Xing BR. An electrophysiological study on the membrane receptor-mediated action of glucocorticoids in mammalian neurons. Neuroendocrinology. 1991;53(Suppl 1):25–30. doi: 10.1159/000125791. [DOI] [PubMed] [Google Scholar]
- Coirini H, Marusic ET, De Nicola AF, Rainbow TC, McEwen BS. Identification of mineralocorticoid binding sites in rat brain by competition studies and density gradient centrifugation. Neuroendocrinology. 1983;37:354–360. doi: 10.1159/000123575. [DOI] [PubMed] [Google Scholar]
- Croissant B, Olbrich R. Stress response dampening indexed by cortisol in subjects at risk for alcoholism. J Stud Alcohol. 2004;65:701–707. doi: 10.15288/jsa.2004.65.701. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Fast glucocorticoid actions on brain: back to the future. Front Neuroendocrinol. 2005;26:103–108. doi: 10.1016/j.yfrne.2005.08.001. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Reul JM. Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology. 1987;12:83–105. doi: 10.1016/0306-4530(87)90040-0. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Veldhuis HD, Wagenaars JL, Bergink EW. Relative binding affinity of steroids for the corticosterone receptor system in rat hippocampus. J Steroid Biochem. 1984;21:173–178. doi: 10.1016/0022-4731(84)90380-7. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, McRae T, Rusinek H, Convit A, De Santi S, Tarshish C, Golomb J, Volkow N, Daisley K, Orentreich N, McEwen B. Cortisol reduces hippocampal glucose metabolism in normal elderly, but not in Alzheimer’s disease. J Clin Endocrinol Metab. 1997;82:3251–3259. doi: 10.1210/jcem.82.10.4305. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Henke K, Aerni A, Treyer V, McGaugh JL, Berthold T, Nitsch RM, Buck A, Roozendaal B, Hock C. Glucocorticoid-induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. Eur J Neurosci. 2003;17:1296–1302. doi: 10.1046/j.1460-9568.2003.02542.x. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Dessypris A, Wagar G, Fyhrquist F, Makinen T, Welin MG, Lamberg BA. Marathon run: effects on blood cortisol -- ACTH, iodothyronines -- TSH and vasopressin. Acta Endocrinol (Copenh) 1980;95:151–157. doi: 10.1530/acta.0.0950151. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Hucklebridge F, Clow A, Evans P. Components of the diurnal cortisol cycle in relation to upper respiratory symptoms and perceived stress. Psychosom Med. 2003;65:320–327. doi: 10.1097/01.psy.0000033123.70631.8e. [DOI] [PubMed] [Google Scholar]
- Grillon C, Smith K, Haynos A, Nieman LK. Deficits in hippocampus-mediated Pavlovian conditioning in endogenous hypercortisolism. Biol Psychiatry. 2004;56:837–843. doi: 10.1016/j.biopsych.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hoffman R, al’Absi M. The effect of acute stress on subsequent neuropsychological test performance. Arch Clin Neuropsychol. 2004;19:497–506. doi: 10.1016/j.acn.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Mahankali S, Liu Y, Pu Y, Wang J, Chen CW, Fox PT, Gao JH. In vivo fMRI demonstration of hypothalamic function following intraperitoneal glucose administration in a rat model. Magn Reson Med. 2000;43:155–159. doi: 10.1002/(sici)1522-2594(200001)43:1<155::aid-mrm20>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Makara GB, Haller J. Non-genomic effects of glucocorticoids in the neural system. Evidence, mechanisms and implications. Prog Neurobiol. 2001;65:367–390. doi: 10.1016/s0301-0082(01)00012-0. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weiss JM, Schwartz LS. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Takeda S, Aburada M, Tabira T. Endogenous glucocorticoids are essential for maintaining prefrontal cortical cognitive function. J Neurosci. 2004;24:5492–5499. doi: 10.1523/JNEUROSCI.0086-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000;861:288–295. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- Stark R, Wolf OT, Tabbert K, Kagerer S, Zimmermann M, Kirsch P, Schienle A, Vaitl D. Influence of the stress hormone cortisol on fear conditioning in humans: evidence for sex differences in the response of the prefrontal cortex. Neuroimage. 2006;32:1290–1298. doi: 10.1016/j.neuroimage.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biol Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Giordani B, Gebarski SS, Schteingart DE. Improvement in learning associated with increase in hippocampal formation volume. Biol Psychiatry. 2003;53:233–238. doi: 10.1016/s0006-3223(02)01750-x. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Schteingart DE, Schork MA. Depressed mood and other psychiatric manifestations of Cushing’s syndrome: relationship to hormone levels. Psychosom Med. 1981;43:3–18. doi: 10.1097/00006842-198102000-00002. [DOI] [PubMed] [Google Scholar]
- Sutanto W, van Eekelen JA, Reul JM, de Kloet ER. Species-specific topography of corticosteroid receptor types in rat and hamster brain. Neuroendocrinology. 1988;47:398–404. doi: 10.1159/000124954. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996;81:2468–2473. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH. Imaging stress effects on memory: a review of neuroimaging studies. Can J Psychiatry. 2009;54:16–27. doi: 10.1177/070674370905400105. [DOI] [PubMed] [Google Scholar]