Abstract
There are eight lysozyme genes in the Anopheles gambiae genome. Transcripts of one of these genes, LYSC-1, increased in Anopheles gambiae cell line 4a3B by 24 h after exposure to heat-killed Micrococcus luteus. Lysozyme activity was also identified in conditioned media from the cell line from which the protein was purified to homogeneity using ion exchange and gel filtration. Mass spectrometric analysis of the purified protein showed 100% identity to lysozyme c-1. Purified lysozyme c-1 was tested against non-mosquito derived as well as culturable bacteria isolated from mosquito midguts. Lysozyme c-1 had negligible effects on the growth of most mosquito-derived bacteria in vitro but did inhibit the growth of M. luteus. Although Lys c-1 did not directly kill most bacteria, knockdown of LYSC-1 resulted in significant mortality in mosquitoes subjected to hemocoelic infections with Escherichia coli but not M. luteus thus suggesting that this protein plays an important role in antibacterial defense against selected bacteria.
Keywords: insect immunity, mosquito, antibacterial protein, muramidase
1. Introduction
Lysozymes (EC 3.2.1.17) hydrolyze the β-1, 4-glycosidic linkage between N-acetylmuramic acid and N-acetylglucosamine of peptidoglycan, a cell wall component of bacteria. They are widespread in nature, occurring in bacteriophage, plants and animals. The role of this enzyme as a defense molecule has been well documented in vertebrates (Jolles and Jolles, 1984; Markart et al., 2004) and insects (Dunn, 1986; Hultmark, 1996; Powning and Davidson, 1973; Jolles et al., 1979; Hultmark et al., 1980; Abraham et al., 1995). In both vertebrates and invertebrates, lysozymes may act through multiple pathways to affect bacterial killing. First, these enzymes directly inhibit or kill a number of bacterial species through both enzymatic and nonenzymatic mechanisms (Nash et al. 2006). Lysozyme generally exhibits greater microbiocidal activity against Gram positive than Gram negative bacteria. However, the degree of activity against Gram negative bacteria varies interspecifically (Mai and Hu 2009; Yu et al. 2002) and is affected by osmolarity, ionic concentration and the presence of synergistic co-factors (Skerritt 2004). Second, lysozymes may play broader roles in regulating the overall response to bacteria. For example, it has been suggested that the generation of small peptidoglycan fragments by lysozyme is a key aspect of the pattern recognition process that functions to initiate antibacterial responses in insects (Boman and Hultmark, 1987; Boman, 1991; Dunn et al., 1994). Indeed, inhibition of lysozyme resulted in reduced activation of the melanization pathway in Tenebrio molitor (Park et al 2007). Conversely, lysozymes may be essential to downregulation of the inflammatory response in some situations. Knockout of a mouse lysozyme resulted in the prolonged upregulation of an inflammatory response to infection by Micrococcus luteus, suggesting that the degradation of peptidoglycan eliminates a key signal of infection (Markart et al., 2004). Many invertebrates have more than one lysozyme and it is possible that these actions are carried out by separate enzymes with specific peptidoglycan degrading activities or temporal and spatial expression profiles.
In dipteran insects, a large expansion of the c-type lysozyme gene family has occurred (Daffre et al., 1994; Li et al., 2005; Ursic-Bedoya et al., 2005). In the mosquito Anopheles gambiae, there are eight c-type lysozymes with different tissue and stage expression profiles, biochemical properties (pI and molecular weight), and with varying conservation of amino acids critical for function (Li et al., 2005). One of these proteins, lysozyme c-1, is a typical basic lysozyme with the required amino acids for muramidase activity. It is constitutively expressed in most tissues of adult mosquitoes but is upregulated during bacterial infections (Li et al., 2005; Dong et al., 2006; Dong et al., 2009). We previously reported detection of this lysozyme in conditioned medium from An. gambiae derived 4a3B cells (Li and Paskewitz, 2006). To begin to clarify the roles of lysozymes in mosquito immunity, we now report on purification of Lys c-1 from this source and tests of its activity against selected bacteria as well as bacteria isolated from midguts of An. gambiae and An. stephensi females. We also conducted lysozyme c-1 knockdown experiments in An. gambiae female mosquitoes and analyzed the survival of mosquitoes after challenge with bacteria. Results of the Lys c-1 purification, its antibacterial activity and whole insect responses after gene silencing are presented.
2. Materials and methods
2.1. Mosquitoes, bacteria, and cell line
The G3 and 4a rr strains of Anopheles gambiae were acquired through the Malaria Research and Reference Reagent Resource Center (MR4, American Type Culture Collection Manassas, VA, USA) and the Center for Disease Control and Prevention and have been in culture for at least 5 years at the Department of Entomology, UW-Madison. A colony of An. stephensi was acquired through Johns Hopkins University (gift of Prof. Marcelo Jacobs Lorena) and has also been in culture for 5 years at UW. Mosquitoes were reared as described previously (Paskewitz et al., 1999). These mosquito colonies were used for the isolation of midgut bacteria. The G3 strain was used for gene silencing experiments.
A list of selected bacteria used in this study and their sources is given in Table 2. Some strains of bacteria used in this study were selected on the basis of previous reports of mosquito midgut bacterioflora (Lindh et al., 2005; Favia et al., 2007; Gusmao et al., 2007; Dillon and Dillon, 2004; Azambuja et al., 2005; Demaio et al., 1996). These included Bacillus subtilis, Enterobacter sp., Asaia sp. (gifts from Dr. Nichole Broderick, Dept. of Plant Pathology, UW Madison). Bacteria were maintained on Luria Broth (LB) plates as well on tryptic soy broth (TSA) plates. Glycerol stocks were maintained at −80 °C.
Table 2.
Muramidase activity of Lys c-1 against various bacteria in lysoplate assays. Mosquito derived bacteria were indentified by BLAST search based on 16s rRNA sequences. Purified Lys c-1 (10 μL of 0.01 mg/mL) was incubated with various bacteria derived from female mosquitoes as well as stock bacteria.
| Bacterial isolates from | GenBank accession no. |
Identification (top matches)++ | Sequence length/query coverage/identity% |
Muramidase activity in lysoplate assays** |
|
|---|---|---|---|---|---|
| An. gambiae G3 | Gram-type | Purified Lys c-1 | |||
| G3-1 | FJ816020 | Elizabethkingia meningoseptica * | 1402/99/99 | Gram− | nd |
| G3-3 | FJ816021 | Asaia krungthepensis | 1361/99/99 | Gram− | nd |
| G3-6 | FJ816022 | Micrococcus luteus | 1387/99/100 | Gram+ | nd |
| An. gambiae 4a rr | |||||
| GS-1 | FJ816023 | Erwinia sp. | 1403/100/100 | Gram− | nd |
| GS-2 | FJ816024 | Serratia sp. | 1350/99/99 | Gram− | nd |
| GS-3 | FJ816025 | Uncultured bacterium | 1385/100/99 | Gram+ | 5.5 |
| GS-4 | FJ816026 | Klebsiella oxytoca | 1400/100/99 | Gram− | tr |
| GS-6 | FJ816027 | Uncultured bacterium | 1411/100/99 | Gram− | nd |
| GS-7 | FJ816028 | Elizabethkingia meningoseptica* | 1374/100/99 | Gram− | nd |
| An. Stephensi | |||||
| ST-2 | FJ816029 | Serratia sp. | 1335/100/99 | Gram− | nd |
| ST-3 | FJ816030 | Bacterium spbL1type1-2 | 1327/100/100 | Gram− | nd |
| Non-mosquito derived bacteria | |||||
| Micrococcus luteus | Gram+ | 6 | |||
| Serratia marcescens | Gram− | nd | |||
| Enterobacter cloacae | Gram− | nd | |||
| Staphylococcus epidermidis | Gram+ | nd | |||
| Bacillus subtilis | Gram+ | 7 | |||
| Bacillus thuringiensis | Gram+ | nd | |||
| Asaia sp. | Gram− | nd | |||
| Pseudomonas aeruginosa | Gram− | nd | |||
| Enterobacter sp. | Gram− | nd | |||
|
Micrococcus lysodeikticus (lyophilized) |
Gram+ | 14 | |||
| Escherichia coli DH5α | Gram− | nd | |||
Top score from NCBI BLAST database nr/nt; Query coverage is over the entire length of sequenced PCR product as indicated
also known as Chryseobacterium meningosepticum; Zone of clearance measured in mm; Measurements were made after O/N incubation of the bacteria with lysozyme sample; nd-absence of detectable zone of clearance; tr-trace zone of clearance (below 5 mm).
Anopheles gambiae cell line 4a3B was provided by Dr. Hans-Michael Mueller (Müller et al., 1999). This cell line has been in culture since 2000. Cells are routinely cultured in Schneider's Drosophila medium (Lonza, Walkersville, MD, USA) supplemented with 5-10% fetal calf serum and an antibiotic/antimycotic mixture containing 10,000 units of penicillin (base), 10,000 μg of streptomycin (base), and 25 μg of amphotericin B/mL utilizing penicillin G (sodium salt), streptomycin sulfate, and amphotericin B as Fungizone® Antimycotic in 0.85% saline (Invitrogen Corporation, Carlsbad, CA, USA) used at a final dilution of 1:100. For the purpose of Lys c-1 purification, cells were cultured in serum-free medium to confluence before conditioned medium was harvested. Conditioned medium was collected after removal of cell debris by centrifugation and then stored at −20 °C.
2.2. Isolation and identification of culturable bacteria from mosquito midguts
For the isolation of culturable bacteria, 2-3 day old female mosquitoes were cold anaesthetized for 5 min on ice and subsequently surface sterilized twice in 70% ethanol for 2 min. Mosquitoes were transferred to sterile saline (0.9% NaCl w/v in water) and were dissected under aseptic conditions. Ten midguts were transferred to 500 μL sterile saline and were homogenized using sterile pestles with a motor driven hand homogenizer (Kimble/Kontes, Vineland, New Jersey, USA).
Homogenates were sonicated in a water bath sonicator which operated at 125 watts for 2 min to facilitate the release of bacteria from the gut tissues. Serial dilutions (from 10−1 to 10−5) from this homogenate were prepared in 0.9% sterile saline and 100 μl from each dilution were plated onto tryptic soy agar (3 g/L tryptic soy broth and 1.5% agar w/v). Plates were incubated at 28 °C for 48 h. Bacteria were analyzed for their morphology, color, texture and size. Bacterial colonies with unique morphologies were picked from different dilutions and streaked on TSA plates to isolate pure cultures. After three serial isolation steps, the bacteria were stored as glycerol stocks at −80 °C. For the identification of bacteria, genomic DNA from each isolated bacteria was prepared using the Master Pure Gram Positive DNA purification kit (Epicenter Biotechnologies, Madison, WI). Approximately 50-100 ng genomic DNA was used as a template for PCR amplification of the 16S rRNA gene using 27F and 1492R primers (gift of Dr. Jo Handlesman, Dept. of Bacteriology, UW Madison, WI). The sequences for these primers are: 27F: 5-AGR GTT TGA TYM TGG CTC AG-3; 1492R: 5-GGY TAC CTT GTT ACG ACT T-3. Amplification conditions were as follows: 94 °C - 4 min; followed by 30 cycles of 94 °C - 45 s; 58 °C - 45 s; 72 °C - 1.5 min; final extension of 72 °C - 10 min. PCR products were analyzed on 1% agarose gels stained with ethidium bromide. A DNA band of approximately 1.4 kb was excised from the gels and purified using a QIAquick gel extraction kit (QIAGEN Sciences, Maryland, USA). The purified PCR product was sequenced with the same primer sets used for PCR amplification (Biotech Center, UW Madison, WI, USA) and analyzed by FinchTV software (Geospiza Inc., Seattle, WA). Each manually corrected sequence over the entire length of the 16s RNA gene (1327-1411bp) was blasted against NCBI nr/nt database. Searches yielding the highest identity scores are reported as the closest matches for each respective bacterial sequence. Sequences from midgut derived bacteria were submitted to GenBank and the accession numbers are given in Table 2.
2.3. One dimensional SDS-PAGE and immunoblotting
Purified Lys c-1 was fractionated by 10% SDS-PAGE gels (NuPage, Invitrogen, USA). The gels were silver stained or used for immunoblotting. Polyclonal antibodies were produced to a peptide specific to Lys c-1 and do not cross react with other lysozymes (Li et al., 2005). In all experiments, the anti-Lys c-1 antibody (9122) was used at a 1:500 and blots were incubated overnight at 4 °C. Bound antibodies were detected by a peroxidase labeled goat anti-rabbit IgG (Kirkegaard and Perry Labs, Maryland, USA) at 1:5000 using a diaminobenzidine reagent (DAB; Sigma-Aldrich) as per supplied instructions.
2.4. Immune challenge to the cell line
The 4a3B cells were cultured in Schneider's Drosophila medium as described above. For immune challenge, M. luteus was grown overnight in tryptic soy broth medium (30 g/L). This culture was then heat inactivated by autoclaving (121 °C for 15 min). The cells were pelleted by centrifugation at 7800 g for 10 min at room temperature and the pellet was resuspended in 0.1 M MOPS, pH-6.5. Five hundred microliters of a bacterial cell suspension (A600 of 1.0) were added to each T25 flask containing 10 mL of 4a3B cells three days after passaging and were further incubated at 28 °C for 24 or 72 h. Conditioned medium was collected from three different flasks and lysozyme activity was monitored from each of three flasks by turbidometric assays.
2.5. Purification and identification of Lys c-1
Frozen conditioned medium was thawed at room temperature. The pH of the medium was raised to 8.0 with 0.1 N NaOH without any loss of lysozyme activity. Changing the pH of the conditioned medium caused the medium to become turbid. The precipitates were removed by filtration through 0.8 μm membrane filters. Clean filtrate was allowed to pass through an ion exchange column (1×10 cm) packed with CM C-25 Sephadex beads pre-equilibrated with 0.1 M Tris-Cl pH-8.0 containing 0.15 M NaCl. The column was washed with 20 mL of 0.1 M Tris-Cl pH 8.0, 0.15 M NaCl. Lysozyme activity was eluted with 0.6 M NaCl in 0.1 M Tris-Cl pH 8.0. The fractions with highest lysozyme activity were pooled and 300 μL of this mixed sample was loaded onto a pre-equilibrated Sephadex G-75 (Sigma) gel filtration column (1 × 20 cm). Lysozyme was eluted with 0.1 M Tris-Cl, pH 8.0 containing 0.15 M NaCl and 0.01 % Triton X-100. Each fraction was assessed for lysozyme activity via turbidity assays and for purity on SDS-PAGE. Protein concentration was measured by Biorad's protein assay reagent (Biorad) and/or EZQ™ Protein Quantitation Kit (Invitrogen). About 200 μL (0.01 mg/mL) of the purified sample was loaded on a 12.5% preparative polyacrylamide gel (NuPage, Invitrogen, USA). The protein band at the molecular mass of 14 kDa was excised from the gel using a sterile scalpel blade and processed for MS analysis. In gel digestion and mass spectrometric analysis was done at the Mass Spectrometry Facility (Biotechnology Center, University of Wisconsin-Madison). The digestion was performed as outlined on the website: http://www.biotech.wisc.edu/ServicesResearch/MassSpec/ingel.htm. The digested peptides were subjected to Matrix-Assisted Laser Desorption/Ionization-Time of Flight-Time of Flight (MALDI TOF-TOF) mass spectrometer (Applied Biosystems Inc., Foster City, CA). The peptide fingerprint was subjected to subsequent tandem MS analysis. Raw data was deconvoluted using GPS Explorer™ software (Applied Biosystems) and submitted for peptide mapping and MS/MS ion search analysis against non-redundant NCBI database with an in-house licensed Mascot search engine (Matrix Science, London, UK).
2.6. dsRNA mediated silencing of Lys c-1 and bacterial infections in the KD mosquitoes
An in vitro transcription template was produced using a one-step PCR protocol. The primers used to prepare the templates for LYSC-1 and GFP contains T7 sequence on both forward and reverse primers at the 5′-end. The primer sequences are: (Fwd 5′-TAATACGACTCACTATAGGGATGAAAGTGTTTTCCACAGTTTTG-3′, Rev 5′-TAATACGACTCACTATAGGGAAAAACAGGAGCTAACATTCGG -3′). These primers were used to amplify a product from mosquito cDNA. The unrelated GFP sequences (Fwd: 5′-TAATACGACTCACTATAGGGCGTGATCAAGCCCGACA-3′, Rev: 5′-TAATACGACTCACTATAGGGCTTCGGCGTGCTC-3′) were used to amplify a product from phMGFP vector (Promega). The PCR product was purified using a QIAquick gel extraction kit (QIAGEN) and 1-2 μg of the product were used as template for transcription. The MEGAscript™ RNAi kit (Ambion, Austin, Texas) was used for transcription and the production of dsRNA following the instruction manual. All dsRNA preparations were quantified by measuring absorbance at 260 nm, checked for integrity on an agarose gel, and stored at −80 °C.
One day old An. gambiae G3 females were injected with 1.4 μg dsLYSC-1 RNA or dsGFP RNA (as control). After 24 h the mosquitoes were injected with 0.1 μL live E. coli (A600=0.004) or M. luteus (A600=0.4). The survival rate of the mosquitoes was monitored for seven days and specificity and efficiency of the knockdown were examined using RT-PCR. Survival of the mosquitoes after infection with bacteria were analyzed using Log-rank and Gehan-Breslow-Wilcoxon tests (GraphPad Software Inc., CA, USA). At least 45 mosquitoes were alive at the start of the monitoring period (beginning 24 h after bacterial challenge) for each experiment. A subset of mosquitoes were also examined for melanotic nodules following M. luteus inoculation. The results of two independent experiments are presented. Expression of LYSC-2, LYSC-8, defensin (DEF1) and cecropin (CEC1) was also monitored in bacteria challenged mosquitoes after LYSC-1 KD using RT-PCR.
2.7. Real time quantitative (QPCR) and semiquantitative RT-PCR
Real time PCR was used to compare relative levels of LYSC-1 transcripts in the cell line before and after treatment with heat killed M. luteus. The protocol used for real time PCR was has been described previously (Paskewitz et al., 2008). Briefly, total RNA was isolated from 1 mL cells (~ 1.5×106 cells/mL) using MasterPure™ Complete DNA and RNA Purification Kit (Epicentre Biotechnologies, Madison, WI, USA) according to the supplied instructions. Total RNA was treated with RQ1 DNAse (Promega) at concentration of 1 unit/μg RNA preparation to remove genomic DNA. RNA concentration was measured with micro-spectrophotometry (Nanodrop NT1000, Thermo Fisher Scientific, Waltham, MA). RNA quality was assessed on an Agilent 2100 bioanalyzer (Agilent Technologies, Inc. Santa Clara, CA, USA) using the Agilent RNA 6000 Nano Kit. Total RNA was also checked for genomic DNA contamination with each set of primers used and across all conditions/replicates. Threshold cycle (Ct) values above 32 in the QPCR amplification were considered free of significant genomic DNA contamination. Five hundred nanograms of total RNA were used to synthesize cDNA using a High-Capacity cDNA Archive Kit (Applied Biosystems). The resulting cDNA was stored at −20 °C.
Primers based on An. gambiae cDNA sequences for internal reference genes (ribosomal protein S7 (Salazar et al., 1993) actin and RPL31) and LYSC-1 were designed using the Beacon designer software (Premier Biosoft International, Palo Alto, CA, USA). The primers were synthesized by IDT Technologies (Coralville, IA, USA). The treatments are compared to the calibrator as control cells (minus M. luteus) at zero hour time point versus 24 and 72 h post challenge with M. luteus.
Semiquantitative RT-PCR was performed to analyze transcript abundance in adult female mosquitoes following RNAi and bacterial challenge. Total RNA was isolated from five mosquitoes from respective treatments as described above. One hundred nanograms of total RNA were used to synthesize cDNA using a High-Capacity cDNA Archive Kit. The resulting cDNA was stored at −20 °C until use. Primers based on An. gambiae cDNA sequences for immune effector genes LYSC-2, LYSC-8, cecropin (CEC1) and defensin (DEF1) were designed using the Beacon designer software; other primers were as described above. Sources and sequences of gene specific primer sets are given in Table 1. Final concentrations of reagents used for RT-PCR were 1X buffer, 200 μM dNTPs, 0.5 μM each primer, 0.2 μL (4 units) Advantage Taq polymerase (Clontech Laboratories Inc., CA, USA). PCR cycle conditions were: an initial denaturation at 95 °C for 3 min; then repeated cycles of 95 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s; and a final extension step at 72 ° C for 10 min. For LYSC-1, LYSC-2, LYSC-8, CEC1 and DEF1, 35 cycles were used while 25 cycles were used for S7.
Table 1.
Sources and sequences of primers used for semiquantitative RT-PCR and real time PCR.
| Gene | GenBank Accession no. |
Sense primer 5′-3′ | Antisense primer 5′-3′ |
|---|---|---|---|
| LYSC-1(RT-PCR) | DQ007317 | ATGAAAGTGTTTTCCACAGTTTTG | AAAAACAGGAGCTAACATTCGG |
| LYSC-1(real time PCR) | DQ007317 | AATGTGAACTGGCAAAGG | AGTCGTTTGAACCGTAGC |
| LYSC-2 | AY659929 | CTACCATCAGAAAGCAAATCAG | TAGCCTCACCAACGGAAC |
| LYSC-8 | DQ004402 | AACGAGAGCCGATACGATAC | CGCACCAGTAGTAGTTGTTG |
| Cecropin (CEC1) | AF200686 | CCAACAACAATGAACTTCTCC | CTGCTGCCTTGAACACTC |
| Defensin (DEF1) | X93562 | AAGAGGGTGTGCCGTTCC | GCCTGTGTTGTAAACTATTATCC |
| RPL31 | XP 320630 | TGGACAGCATTTGGGACGATTC | TCACCTGGCAACGAAGTAAAGC |
| RPS7 | L20837 | CGCTATGGTGTTCGGTTCC | TGCTGCAAACTTCGGCTAT |
2.8. Lysozyme activity assays
2.8.1. Turbidometric assay
For turbidometric assays, 30 μL of the protein (0.01mg/mL) sample was incubated with 970 μL of M. luteus cells (0.3 mg/mL of purchased Micrococcus lysodeikticus cells; Sigma) prepared in 0.1 M MOPS, pH 6.5. Decreases in turbidity were monitored at 475 nm over a period of 60 s using a preset program in a Genesys 5 Spectrophotometer (Spectronic Instruments Inc. Rochester, NY, USA). For simplicity the absorbance values versus time were plotted directly.
2.8.2. Zonal inhibition assay
The zonal inhibition assay was carried out as described previously (Nasr et al., 2003). A solution of 1% agarose was prepared in 0.1 M sodium phosphate buffer pH 6.5 containing 0.05 M NaCl. The melted agarose was mixed with lyophilized powder of M. luteus cells to give a final concentration of 0.5 mg/mL cells and then poured in sterile petri-plates. After solidification, 1.5 mm wells were made and 10 μL (0.01mg/mL) of test samples were added to the wells. Plates were incubated at 37°C overnight. Buffer served as negative control. In addition to lyophilized M. luteus, Lys c-1 activity was tested against growing bacteria isolated from mosquitoes as well as representative non-mosquito derived Gram-positive and Gram-negative bacteria. Each bacterium was grown on TSA (Tryptic Soy Broth 30g/L containing 1.5% agar) plates. Wells (1.5 mm in diameter) were punched in the agar using sterile plastic tip and 10 μL (of 0.01 mg/mL) of the purified lysozyme preparation was applied to wells. Plates were incubated at 30 °C for overnight and the zone of clearance was measured.
3. Results
3.1. Lysozyme activity in the 4a3B cell line following bacterial challenge
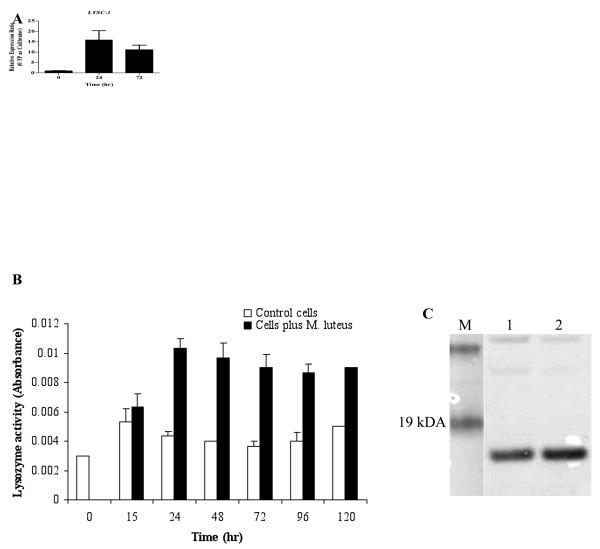
The abundance of LYSC-1 transcripts was measured by real time quantitative PCR before and after treatment of 4a3B cells with heat-killed M. luteus (Fig. 1A). Expression of the LYSC-1 transcripts increased dramatically (15.8 fold; average of three replicates) 24 h after treatment in three replicate experiments and remained significantly elevated at 72 h post treatment (10 fold).
Figure 1.
Lysozyme expression in the Anopheles gambiae 4a3B cell line. A. Quantitative PCR for LYSC-1 expression in 4a3b cell line following exposure to heat killed M. luteus. 0 TP as Calibrator=Zero time point as calibrator. B. Muramidase activity in the conditioned medium of control and bacteria challenged cells. Lysozyme activity was assayed via turbidometric assay from 0 to 120 h in three replicates. Error bars represent standard deviation of the mean of three independent measurements. C. Western blot of conditioned media from 4a3b cell line before (lane 1) and 24 h after (lane 2) M. luteus induction.
To monitor muramidase activity in conditioned medium from the 4a3B cell line, turbidity assays were performed. Results (Fig. 1B) indicated that lysozyme activity increases in conditioned medium for a prolonged period following exposure to heat-killed M. luteus and remains elevated through 120 h post challenge. Correspondence of this activity with elevated levels of Lys c-1 (rather than another of the An. gambiae lysozymes) in the conditioned medium 24 h post challenge was confirmed by Western blotting with anti-Lys-c-1 antibodies (Fig. 1C).
3.2. Purification and identification of lysozyme c-1 from the conditioned medium
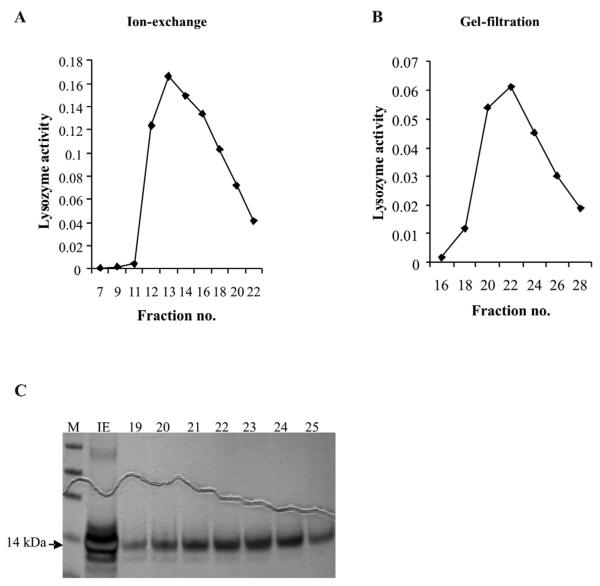
Lysozyme was purified from the cell culture medium of 4a3B cell line using a two step purification protocol. Almost all of the lysozyme activity could be recovered following the first ion exchange step (Fig. 2A). Gel filtration on Sephadex-G75 of fractions exhibiting the highest lysozyme activity (Fig. 2B) from the ion exchange step yielded one major purified band of about 14 kDa and a very faint band (probably in the femtograms range) running below the lysozyme band (Fig. 2C). We confirmed this upper protein as Lys c-1 (GenBank accession no. AAC47326) by ESI-MS and MS/MS (Biotech Center, UW Madison). The lower protein band could not be identified as even a 10X concentrated protein sample was below the detection limit of the MS instrument.
Figure 2.
Purification of lyoszyme c-1 from conditioned medium of 4a3B cells. A. Ion-exchange chromatography of the crude cell culture medium. B. Gel filtration chromatography of peak fractions from A. on Sephadex G-75. C. Silver stained gel from ion-exchange peak and G-75 fractions; Lane IE- ion exchange peak, lanes marked 19-25 are the respective fractions from B. Fraction 22-24 were used for characterization of activity under varied conditions. Lysozyme band is seen at an apparent molecular mass of 14 kDa marked by an arrow.
3.3. Zonal inhibition assays against midgut-derived bacterial isolates and other bacteria
Because several lysozymes have been reported as active against Gram-negative bacteria (Abraham et al., 1995; Ibrahim et al., 2001), we tested the mosquito enzyme for its activity against Gram-negative as well as Gram-positive bacteria. For these assays, we chose bacteria that were isolated from the midguts of laboratory colonies An. gambiae and An. stephensi, as well as other bacteria from various sources. The respective bacteria were grown on tryptic soy broth plates and the purified enzyme was applied to the wells. The results are described in Table 2. We tested antibacterial activity in two samples, one from the peak fraction of the ion exchange step and second in the purified fraction after second step of gel filtration. The antibacterial activity was higher in the partially purified fraction of the first ion exchange step as compared to the pure Lys c-1 indicating that additional antibacterial proteins are secreted in the conditioned medium that exert antibacterial effect in concert. In this partially purified fraction, the growth of most bacteria was inhibited except mosquito-derived Elizabethkingia meningoseptica, Asaia krungthepensis, non-mosquito derived Asaia sp., Serratia marcescens and Pseudomonas aeruginosa (data not shown). By contrast, purified Lys c-1 was strongly active only against M. luteus and Bacillus subtilis. It had trace activity against two mosquito-derived bacteria (GS-3 and GS-4; Table 2). Unexpectedly, Lys c-1 was not active against a mosquito-derived strain of M. luteus from An. gambiae G3.
3.4. Lysozyme c-1 knockdown reduces the survival of mosquitoes upon infection with bacteria
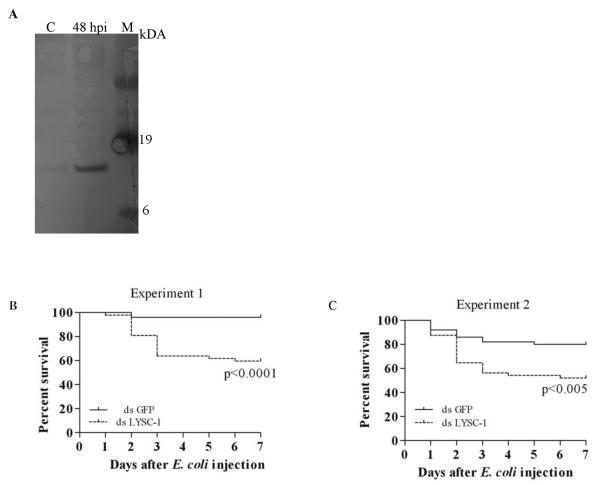
Since Lys c-1 did not directly kill many bacteria but is strongly induced by bacteria in hemolymph from whole insects (Fig 3A) and in 4a3B cells (Fig. 1), we tested whether gene knockdown would affect survival after exposure to mildly pathogenic bacterial strains. RT-PCR results documented efficient KD of LYSC-1 that persisted to at least seven days after injection of E. coli. The survival of mosquitoes injected with dsLYSC-1 was compared to control dsGFP injected mosquitoes in two independent experiments using Log-Rank and Gehan-Breslow-Wilcoxon test of survival curves. Silencing of LYSC-1 caused significant mortality in the mosquitoes infected with E. coli as compared to a control group (p<0.0001 experiment 1 and p<0.005 in experiment 2; Fig. 3B and C). The majority of the mortality was concentrated in the first 3 days after administration of the bacteria.
Figure 3.
Induction and silencing of lysozyme c-1 in adult mosquitoes. A. Western blot demonstrating induction of lysozyme c-1 in mosquito hemolymph following inoculation with M. luteus (C=control; hpi= hour post infection; M=molecular mass marker. B and C. Kaplan Meier survival curves of mosquitoes showing effect of LYSC-1 silencing after E. coli injection in two independent experiments. One day old An. gambiae females were injected with 1.4 μg dsLYSC-1 or an equal concentration of dsGFP as control. After 24 h the mosquitoes were injected with E. coli. The mortality was monitored over seven days.
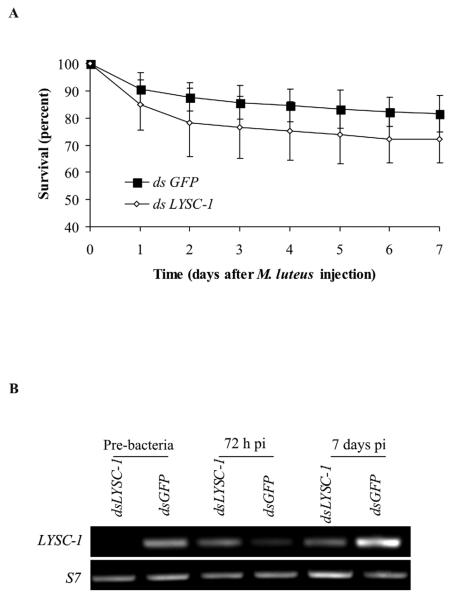
M. luteus infection in the LYSC-1 KD mosquitoes did not have any significant effects on their survival (Fig. 4A). These bacteria are rapidly melanized in A. gambiae and LYSC-1 KD did not alter that response. However, in contrast to the results for E. coli, LYSC-1 transcripts re-appeared 96 h after M. luteus injection of KD mosquitoes (Fig. 4B). This result was repeated in three independent experiments, although the timing of re-appearance of the transcript varied somewhat.
Figure 4.
Effect of LYSC-1 KD on survival of mosquitoes after M. luteus injection. A. Mortality curve following M. luteus infection 24 h after LYSC-1 KD. Results of four independent experiments are shown. Error bars depicts standard deviation. B. Semiquantiative RT-PCR analysis of LYSC-1 transcripts following injection of M. luteus.
3.5. Effects of LYSC-1 knockdown on transcript abundance of defensin, cecropin, LYSC-2 and LYSC-8 in mosquitoes
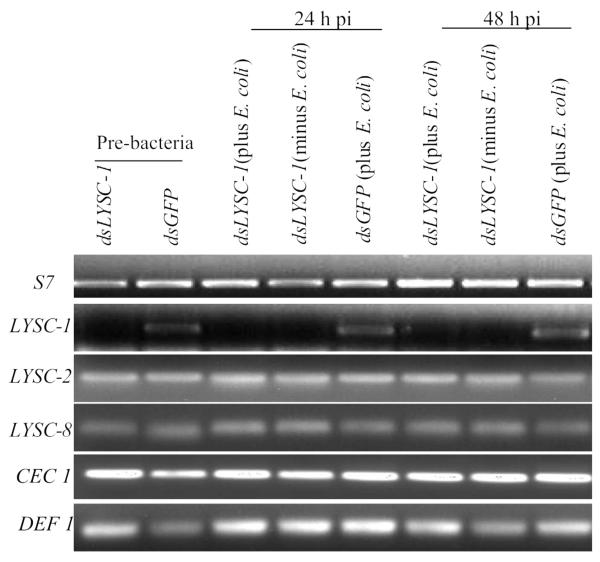
Since Lys c-1 does not kill bacteria directly but does enhance survival of the insects after bacterial exposure, we hypothesized that this protein might produce small peptidoglycan fragments which are needed to upregulate signaling cascades resulting in the production of antimicrobial peptides. We measured transcript abundance for LYSC-2, LYSC-8, DEF1 and CEC1 following LYSC-1 knockdown and after the introduction of E. coli. These four genes were selected because they are all induced by bacterial inoculation. This was compared with dsGFP mosquitoes that were challenged with bacteria and with dsLYSC-1 mosquitoes that were not challenged. We found no evidence that LYSC-1 knockdown affected transcript abundance for the other immune genes after bacterial injection (Fig. 5).
Figure 5.
Semiquantitative RT-PCR analysis of selective immunity gene transcripts in LYSC-1 KD and E. coli injected mosquitoes over 72 h.
4. Discussion
Expression of eight lysozyme genes in An. gambiae has been documented (Li et al., 2005; Kang et al., 1996) but the functional roles of these proteins are unknown. Transcripts of several lysozymes were more abundant in adult An. gambiae mosquitoes following infection with bacteria or malaria parasites (Dong et al., 2006; Li et al., 2005). LYSC-1 and LYSC-2 transcript abundance increased when adult female mosquitoes were challenged with E. coli or M. luteus (Li et al., 2005) which suggested a possible role for these proteins in immunity. Upregulation of LYSC-1 was even more striking in the An. gambiae 4a3B cell line after bacterial challenge and we used this finding to further investigate the activity of this enzyme and its role in mosquito immunity.
Lysozymes are defined by their ability to cleave peptidoglycan in the cell wall of bacteria and this generally limits direct growth inhibition to Gram-positive bacteria (Nakimbugwe et al., 2006) although there are a growing number of exceptions (Clarke and DuPont, 1992; Yu et al., 2002; Gandhe et al., 2007). We investigated whether Lys c-1 can affect the growth of mosquito midgut-derived bacteria since the midgut is likely to be the most common site of entry in natural settings. Most of the bacteria identified in this study have been previously reported from mosquitoes, including some insects that had been field-collected (Straif et al., 1998; Gonzalez-Ceron et al., 2003; Favia et al., 2007; Lindh et al., 2008; Dong et al., 2009). Nine out of eleven mosquito-derived bacteria belonged to the Gram-negative group suggesting that the midgut environment is favorable for this type of bacteria. Gram-negative bacteria are generally considered resistant to lysozyme, due to the outer layer of lipopolysaccharide and protein which shields the peptidoglycan layer (Masschalck and Michiels, 2003). Secretion of lysozyme inhibitors also has been proposed as a resistance mechanism of several bacteria (Masschalck and Michiels, 2003). In keeping with these strong defense mechanisms, purified Lys c-1 did not show growth inhibitory attributes against most bacteria when cultured under neutral conditions in vitro. Perhaps most surprisingly, no activity was detected against a mosquito-derived M. luteus strain (Table 2) suggesting that this Gram positive strain may exhibit unique adaptations for life in the mosquito gut. Lys c-1 is expressed in the midgut and in the salivary glands so resident gut bacteria are likely to be exposed to this protein. Purified Lys c-1 did exhibit activity against non-mosquito derived strains of M. luteus and Bacillus subtilis, against a commercially available preparation of lyophilized M. luteus, and against two mosquito-derived bacteria, Klebsiella oxytoca (GS-4) and GS-3 (most closely related to an uncultured bacterium from an ant lion (Dunn and Stabb, 2005).
Although purified Lys c-1 from the 4a3B cell line did not show significant growth inhibitory activity against most mosquito-derived bacteria, the protein increased in conditioned media after exposure to heat-killed bacteria and in hemolymph from adult insects after injection of live M. luteus. An increase in c-type lysozyme gene expression following immune challenge has been reported for several Lepidoptera (Fujimoto et al., 2001; Lee & Brey, 1995; Sun et al., 1991; Mulnix and Dunn, 1994) and for adult mosquitoes (Li et al., 2005) and cultured cells from Aedes aegypti or A. albopictus (Gao & Fallon, 2000; Hernandez et al., 2003). The strong increase in Lys c-1 activity after immune challenge with Gram-negative or Gram-positive bacteria is intriguing since Lys c-1 can directly kill only a small number of Gram-positive bacterial species in vitro. To verify that Lys c-1 was important for mosquito survival after bacterial challenge, we silenced the gene through RNA interference. The impact of Lys c-1 depletion varied by bacterial species demonstrating specificity in the response to the two bacteria. Silencing of LYSC-1 in adult mosquitoes reduced their survival upon subsequent infection with E. coli. In the absence of LYSC-1, the mosquitoes died at a rapid rate during the first three days after infection but thereafter the mortality curve plateaued. By contrast, no significant mortality was observed after M. luteus infection in the KD mosquitoes although this bacterium is susceptible to Lys c-1 activity in vitro. Redundancy in antibacterial mechanisms could account for this observation. M. luteus is melanized within the hemocoel within a few minutes or hours after introduction. This response appears to be quite effective since M. luteus is rapidly cleared from the insect, unlike E. coli which persists for many days after infection (Gorman and Paskewitz, 2000). In the beetle, Tenebrio molitor, melanization is regulated by lysozyme which is necessary for the generation of peptidoglycan fragments that are bound by a peptidoglycan recognition protein (Park et al. 2007). However, knockdown of A. gambiae LYSC-1 did not affect rapid melanization of M. luteus.
We also considered the possibility that these effects (plateau and failure to affect survival after inoculation with M luteus) resulted from lack of persistence of the silencing phenotype. When we examined mosquitoes that had been silenced and then injected with E. coli, no transcripts were detected at any time out to 7 days after the bacterial inoculation. However, LYSC-1 transcripts reappeared 48-96 h after M. luteus infection. This result indicates that silencing is not systemic and that LYSC-1 may be expressed in a unique subset of cells only after M. luteus introduction. Since extensive melanization of this bacteria occurs along the dorsal vessel (unpub. obs.) and in hemocytes (Baton et al. 2009), we hypothesize that the generation of replacements for the hemocytes involved in this reaction occurs and that these new cells now express Lys c-1. Finally, we note that studies that rely on gene silencing to examine functional roles of proteins over extended timeframes will need to incorporate controls to verify the persistence of silencing during the entire observational period.
Since Lys c-1 did not directly kill E. coli in in vitro assays but did affect survival after exposure to this organism, we considered three other possible roles for the enzyme. First, Lys c-1 might produce peptidoglycan fragments that could activate transcription pathways that result in the production of antimicrobial peptides, in a manner similar to that identified for Gram positive bacteria and the TOLL pathway by Park and colleagues (2007). Second, Lys c-1 might assist in the removal of peptidoglycan, thereby downregulating an antimicrobial response that is likely to be costly to the insect. Finally, this lysozyme might act synergistically with other antimicrobial mechanisms to destroy bacteria.
We tested the hypothesis that the loss of Lys c-1 might affect the expression of other antimicrobial proteins which are responsible for clearing bacteria. Bacterially-induced upregulation of AMP (antimicrobial peptide) gene expression might not occur if a failure to produce small peptidoglycan fragments resulted in inefficient activation of the antibacterial peptide signaling cascades. Conversely, AMP gene expression might be hyper-elevated if most peptidoglycan fragments were not rendered non-immunogenic. In Drosophila, this latter function is attributed to catalytic peptidoglycan recognition proteins (PGRPs) that hydrolyze amide bonds between N-acetylmuramic acid and L-alanine moieties of PGN (peptidoglycan), thereby “scavenging” PGN and downregulating AMP transcription pathways (Mellroth et al., 2003; Zaidman et al., 2006). We examined transcript levels of CEC1, DEF1 or LYSC-2/8 in LYSC-1 KD and E. coli challenged adult female mosquitoes. However, no significant differences in transcript levels were noted in comparison with control insects following introduction of E coli.
It remains possible that Lys c-1 acts synergistically with other antimicrobial mechanisms, and/or affects key immune functions. Chalk et al. (1995) reported synergy between a chicken lysozyme and insect cecropin (but not defensin) at physiological concentrations against E. coli. Human peptidoglycan recognition proteins (i.e. PGLYRP) have been documented to act synergistically against both Gram-positive and Gram-negative bacteria in conjunction with lysozyme and lysostaphin (Lu et al., 2006; Cho et al., 2005; Wang et al., 2007). This synergism is likely to occur in vivo because both PGLYRPs and lysozyme are found within granules in the most abundant phagocyte, the polymorphonuclear leukocyte (Lu et al., 2006; Wang et al., 2007). Phagocytosis by granulocytic hemocytes is important for clearance of bacteria in insects (Strand, 2008; Hillyer et al., 2003; 2004) and some insect PGRPs as well as Lys c-1 (Castillo et al. 2006) are produced by hemocytes. Therefore, it is possible that Lys c-1 depletion may cause impairment in removal of bacterial loads through synergy with other antibacterial peptides in the hemolymph plasma or following phagocytosis by granulocytes.
In conclusion we have isolated and purified a lysozyme corresponding to Lys c-1 from a mosquito-derived cell line 4a3B. Lys c-1 had limited direct effects on the growth of culturable bacteria isolated from the community in mosquito midguts. However its depletion through RNAi led to increased mortality in adult mosquitoes following challenge with Gram-negative bacteria. We found this system a useful tool to produce, isolate and characterize an important but neglected immune protein in vitro given the challenge in isolating it from whole mosquitoes in sufficient amounts for biochemical studies. Future studies will investigate the kinetics of Lys c-1 against specific targets under various conditions. Combining the cell culture system and selective gene inhibition by RNA interference will allow further investigation of the role of lysozyme c-1 in antibacterial immune responses.
Acknowledgements
We thank Dr. Nichole Broderick and Jo Handelsman for sharing protocol for bacteria isolation experiments from mosquitoes and for providing bacterial strains; Dr. John Lindquist for supplying bacteria; Erica Yashiro for helping with sequence analysis; Heather Sage and Ellie Walker for immune challenge to cell lines; Patrick Irwin for statistical analyses and Beth Schadd and Eric J. Shelley for rearing mosquitoes used in these experiments. This work was supported by NIH grant RO1 AI 037083 to SMP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham EG, Nagaraju J, Salunke D, Gupta HM, Dalta RK. Purification and partial characterization of an induced antibacterial protein in the silkworm, Bombyx mori. J. Invertebr. Pathol. 1995;65:17–24. doi: 10.1006/jipa.1995.1003. [DOI] [PubMed] [Google Scholar]
- Azambuja P, Garcia ES, Ratcliffe NA. Gut microbiota and parasite transmission by insect vectors. Trends Parasitol. 2005;21:568–572. doi: 10.1016/j.pt.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Boman HG, Hultmark D. Cell-free immunity in insects. Ann. Rev. Microbiol. 1987;41:103–126. doi: 10.1146/annurev.mi.41.100187.000535. [DOI] [PubMed] [Google Scholar]
- Boman HG. Antibacterial peptides: key components needed in immunity. Cell. 1991;65:205–207. doi: 10.1016/0092-8674(91)90154-q. [DOI] [PubMed] [Google Scholar]
- Castillo JC, Robertson AE, Strand MR. Characterization of hemocytes from the mosquitoes Aedes aegypti and Anopheles gambiae. Insect Biochem. Mol. Biol. 2006;36:891–903. doi: 10.1016/j.ibmb.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalk R, Albuquerque CMR, Ham PJ, Townson H. Full sequence and characterization of two insect defensins: immune peptides from the mosquito Aedes aegypti. Proc. R. Soc. Lond. B. 1995;261:217–221. doi: 10.1098/rspb.1995.0139. [DOI] [PubMed] [Google Scholar]
- Cho JH, Fraser IP, Fukase K, Kusumoto Yukari., Fujimoto Shoichi., Stahl GL, Ezekowitz RAB. Human peptidoglycan recognition protein S is an effector of neutrophil-mediated innate immunity. Blood. 2005;106:2551–2558. doi: 10.1182/blood-2005-02-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A, Dupont C. O-acetylated peptidoglycan: its occurrence, pathological significance and biosynthesis. Can. J. Microbiol. 1992;38:85–91. doi: 10.1139/m92-014. [DOI] [PubMed] [Google Scholar]
- Daffre S, Kylsten P, Samakovlis C, Hultmark D. The lysozyme locus in Drosophila melanogaster: an expanded gene family adapted for expression in the digestive tract. Mol. Gen. Genet. 1994;242:152–162. doi: 10.1007/BF00391008. [DOI] [PubMed] [Google Scholar]
- Demaio J, Pumpuni CB, Kent M, Beier JC. The midgut bacterial flora of wild Aedes triseriatus, Culex pipiens and Psorophora columbiae mosquitoes. Am. J. Trop. Med. Hyg. 1996;54:219–223. doi: 10.4269/ajtmh.1996.54.219. [DOI] [PubMed] [Google Scholar]
- Dziarski R. Peptidoglycan recognition proteins (PGRPs) Mol. Immunol. 2004;40:877–886. doi: 10.1016/j.molimm.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Dillon RJ, Dillon VM. The gut bacteria of insects: nonpathogenic interactions. Annu. Rev. Entomol. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- Dimopoulos G, Christophides GK, Meister S, Schultz J, White KP, Barillas-Mury C, Kafatos FC. Genome expression analysis of Anopheles gambiae: responses to injury, bacterial challenge, and malaria infection. Proc. Natl. Acad. Sci. USA. 2002;99:8814–8819. doi: 10.1073/pnas.092274999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Aguilar R, Xi Z, Warr E, Mongin E, Dimopoulos G. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2006;2:e52. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Manfredini F, Dimopoulos G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009;5:e1000423. doi: 10.1371/journal.ppat.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn PE. Biochemical aspects of insect immunology. Ann. Rev. Entomol. 1986;31:321–339. [Google Scholar]
- Dunn PE, Bohnert TJ, Russell V. Regulation of antibacterial protein synthesis following infection and during metamorphosis of Manduca sexta. Ann. N.Y. Acad. Sci. 1994;15:117–130. doi: 10.1111/j.1749-6632.1994.tb33567.x. [DOI] [PubMed] [Google Scholar]
- Dunn AK, Stabb EV. Culture-independent characterization of the microbiota of the ant lion Myrmeleon mobilis (Neuroptera: Myrmeleontidae) Appl Environ Microbiol. 2005;71:8784–8794. doi: 10.1128/AEM.71.12.8784-8794.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favia G, Ricci I, Damiani C, Raddadi N, Crotti E, Marzorati M, Rizzi A, Urso R, Brusetti L, Borin S, Mora D, Scuppa P, Pasqualini L, Clementi E, Genchi M, Corona S, Negri I, Grandi G, Alma A, Kramer L, Esposito F, Bandi C, Sacchi L, Daffonchio D. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc. Natl. Acad. Sci. USA. 2007;104:9047–9051. doi: 10.1073/pnas.0610451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S, Tsuda I, Kishimoto K, Yamano Y, Morishima I. Protein purification, cDNA cloning and gene expression of lysozyme from eri-silkworm, Samia cynthia ricini. Comp. Biochem. Physiol. B. 2001;128:709–718. doi: 10.1016/s1096-4959(00)00368-7. [DOI] [PubMed] [Google Scholar]
- Gandhe AS, Janardhan G, Nagaraju J. Immune upregulation of novel antibacterial proteins from silkmoths (Lepidoptera) that resemble lysozymes but lack muramidase activity. Insect Biochem. Mol. Biol. 2007;37:655–666. doi: 10.1016/j.ibmb.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Gao Y, Fallon AM. Immune activation upregulates lysozyme gene expression in Aedes aegypti mosquito cell culture. Insect Biochem. Mol. Biol. 2000;9:553–558. doi: 10.1046/j.1365-2583.2000.00216.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Ceron L, Santillan F, Rodriguez MH, Mendez D, Hernandez-Avila JE. Bacteria in midguts of field-collected Anopheles albimanus block Plasmodium vivax sporogonic development. J. Med. Entomol. 2003;40:371–374. doi: 10.1603/0022-2585-40.3.371. [DOI] [PubMed] [Google Scholar]
- Guan R, Roychowdhury A, Ember B, Kumar S, Boons GJ, Mariuzza RA. Structural basis for peptidoglycan binding by peptidoglycan recognition proteins. Proc. Natl. Acad. Sci. USA. 2004;101:7168–17173. doi: 10.1073/pnas.0407856101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusmão DS, Santos AV, Marini DC, Russo-Ede S, Peixoto AM, Bacci-Júnior M, Berbert-Molina MA, Lemos FJ. First isolation of microorganisms from the gut diverticulum of Aedes aegypti (Diptera: Culicidae): new perspectives for an insect-bacteria association. Mem. Inst. Oswaldo. Cruz. 2007;102:919–924. doi: 10.1590/s0074-02762007000800005. [DOI] [PubMed] [Google Scholar]
- Hernandez VP, Higgins L, Fallon AM. Characterization and cDNA cloning of an immune-induced lysozyme from cultured Aedes albopictus mosquito cells. Dev. Comp. Immunol. 2003;27:11–20. doi: 10.1016/s0145-305x(02)00065-4. [DOI] [PubMed] [Google Scholar]
- Hillyer JF, Schmidt SL, Christensen BM. Hemocyte-mediated phagocytosis and melanization in the mosquito Armigeres subalbatus following immune challenge by bacteria. Cell Tissue Res. 2003;313:117–127. doi: 10.1007/s00441-003-0744-y. [DOI] [PubMed] [Google Scholar]
- Hillyer JF, Schmidt SL, Christensen BM. The antibacterial innate immune response by the mosquito Aedes aegypti is mediated by hemocytes and independent of Gram type and pathogenicity. Microbes Infect. 2004;6:448–459. doi: 10.1016/j.micinf.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA. The immune response of Drosophila. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- Hultmark D. Insect lysozymes EXS. 1996;75:87–102. doi: 10.1007/978-3-0348-9225-4_6. [DOI] [PubMed] [Google Scholar]
- Hultmark D, Steiner H, Rasmuson T, Boman HG. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 1980;106:7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- Ibrahim HR, Thomas U, Pellegrini A. A helix-loop-helix peptide at the upper lip of the active site cleft of lysozyme confers potent antimicrobial activity with membrane permeabilization action. J. Biol. Chem. 2001;276:43767–43774. doi: 10.1074/jbc.M106317200. [DOI] [PubMed] [Google Scholar]
- Jollès J, Schoentgen F, Croizier G, Croizier L, Jollès P. Insect lysozymes from three species of Lepidoptera: their structural relatedness to the C (chicken) type lysozyme. J. Mol. Evol. 1979;14:267–721. doi: 10.1007/BF01732494. [DOI] [PubMed] [Google Scholar]
- Jolles P, Jolles J. What is new in lysozyme research. Always a model system, today as yesterday. Mol. Cell. Biochem. 1984;63:165–189. doi: 10.1007/BF00285225. [DOI] [PubMed] [Google Scholar]
- Kang D, Romans P, Lee JY. Analysis of a lysozyme gene from the malaria vector mosquito, Anopheles gambiae. Gene. 1996;174:239–244. doi: 10.1016/0378-1119(96)00088-1. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Brey PT. Isolation and characterization of the lysozyme-encoding gene from the silkworm Bombyx mori. Gene. 1995;161:199–203. doi: 10.1016/0378-1119(95)00199-g. [DOI] [PubMed] [Google Scholar]
- Lindh JM, Terenius O, Faye I. 16S rRNA gene-based identification of midgut bacteria from field-caught Anopheles gambiae sensu lato and A. funestus mosquitoes reveals new species related to known insect symbionts. Appl. Environ. Microbiol. 2005;71:7217–7223. doi: 10.1128/AEM.71.11.7217-7223.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Calvo E, Marinotti O, James AA, Paskewitz SM. Characterization of the c-type lysozyme gene family in Anopheles gambiae. Gene. 2005;360:131–139. doi: 10.1016/j.gene.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Li B, Paskewitz SM. A role for lysozyme in melanization of Sephadex beads in Anopheles gambiae. J. Insect. Physiol. 2006;52:936–942. doi: 10.1016/j.jinsphys.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu X, Wang M, Qi J, Wang H, Li X, Gupta D, Dziarski R. Peptidoglycan recognition proteins are a new class of human bactericidal proteins. J. Biol. Chem. 2006;281:5895–5907. doi: 10.1074/jbc.M511631200. [DOI] [PubMed] [Google Scholar]
- Mai W, Hu C. cDNA cloning, expression and antibacterial activity of lysozyme C in the blue shrimp (Litopanaeus stylirostris) Prog. Natural Sci. 2009;19:837–844. [Google Scholar]
- Markart P, Korfhagen TR, Weaver TE, Akinbi HT. Mouse lysozyme M is important in pulmonary host defense against Klebsiella pneumoniae infection. Am. J. Respir. Crit. Care Med. 2004;169:454–458. doi: 10.1164/rccm.200305-669OC. [DOI] [PubMed] [Google Scholar]
- Masschalck B, Michiels CW. Antimicrobial properties of lysozyme in relation to foodborne vegetative bacteria. Crit. Rev. Microbiol. 2003;29:191–214. doi: 10.1080/713610448. [DOI] [PubMed] [Google Scholar]
- Mellroth P, Karlsson J, Steiner H. A scavenger functions for a Drosophila peptidoglycan recognition protein. J. Biol. Chem. 2003;278:7059–7064. doi: 10.1074/jbc.M208900200. [DOI] [PubMed] [Google Scholar]
- Müller HM, Dimopoulos G, Blass C, Kafatos FC. A hemocyte-like cell line established from the malaria Vector Anopheles gambiae expresses six prophenoloxidase genes. J. Biol. Chem. 1999;274:11727–11735. doi: 10.1074/jbc.274.17.11727. [DOI] [PubMed] [Google Scholar]
- Mulnix AB, Dunn PE. Structure and induction of a lysozyme gene from the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 1994;24:271–281. doi: 10.1016/0965-1748(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Nakimbugave D, Masschalck B, Atanassova M, Zewdie-Bosüher A, Michiels CW. Comparison of bactericidal activity of six lysozymes at atmospheric pressure and under high hydrostatic pressure. Int. J. Food. Microbiol. 2006;108:355–363. doi: 10.1016/j.ijfoodmicro.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Nasr NM, Fallon AM. Detection of lysozyme-like enzymatic activity secreted by an immune-responsive mosquito cell line. J. Invertebr. Pathol. 2003;82:162–166. doi: 10.1016/s0022-2011(03)00030-2. [DOI] [PubMed] [Google Scholar]
- Park JW, Kim CH, Kim JH, Je BR, Roh KB, Kim SJ, Lee HH, Ryu JH, Lim JH, Oh BH, Lee WJ, Ha NC, Lee BL. Clustering of peptidoglycan recognition protein-SA is required for sensing lysine-type peptidoglycan in insects. Proc. Natl. Acad. Sci. USA. 2007;104:6602–6607. doi: 10.1073/pnas.0610924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-Excel-based tool using pairwise correlations. Biotechnol. Lett. 2004;26:509–515. doi: 10.1023/b:bile.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- Powning RF, Davison WJ. Studies on insect bacteriolytic enzymes. I. Lysozyme in haemolymph of Galleria mellonella and Bombyx mori. Comp. Biochem. Physiol. B. 1973;45:669–686. [PubMed] [Google Scholar]
- Paskewitz SM, Reese-Stardy S, Gorman MJ. An easter-like serine protease from Anopheles gambiae exhibits changes in transcript abundance following immune challenge. Insect Mol. Biol. 1999;8:329–337. doi: 10.1046/j.1365-2583.1999.83124.x. [DOI] [PubMed] [Google Scholar]
- Paskewitz SM, Li B, Kajla MK. Cloning and molecular characterization of two invertebrate-type lysozymes from Anopheles gambiae. Insect Mol Biol. 2008;17:217–225. doi: 10.1111/j.1365-2583.2008.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet J, Dziarski R. Peptidoglycan recognition proteins: pleiotropic sensors and effectors of antimicrobial defences. Nat. Rev. Microbiol. 2007;5:264–277. doi: 10.1038/nrmicro1620. [DOI] [PubMed] [Google Scholar]
- Salazar CE, Mills-Hamm D, Kumar V, Collins FH. Sequence of a cDNA from the mosquito Anopheles gambiae encoding a homologue of human ribosomal protein S7. Nucleic Acids Res. 1993;21:4147. doi: 10.1093/nar/21.17.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerrett SJ. Lysozyme in pulmonary host defense. Am. J. Resp. Crit. Care Med. 2004;169:435–436. doi: 10.1164/rccm.2312018. [DOI] [PubMed] [Google Scholar]
- Sun SC, Asling B, Faye I. Organization and expression of the immunoresponsive lysozyme gene in the giant silk moth, Hyalophora cecropia. J. Biol. Chem. 1991;266:6644–6649. [PubMed] [Google Scholar]
- Straif SC, Mbogo CNM, Toure AM, Walker ED, Kaufman M, Toure YT, Beier JC. Midgut Bacteria in Anopheles gambiae and An. funestus (Diptera: Culicidae) from Kenya and Mali. J. Med. Entomol. 1998;35:222–226. doi: 10.1093/jmedent/35.3.222. [DOI] [PubMed] [Google Scholar]
- Strand MR. Insect hemocytes and their role in immunity. In: Beckage NE, editor. Insect Immunity. Academic Press; San Diego, CA: 2008. pp. 25–48. [Google Scholar]
- Ursic-Bedoya RJ, Mitzey AM, Obraztsova M, Lowenberger C. Molecular cloning and transcriptional activation of lysozyme-encoding cDNAs in the mosquito Aedes aegypti. Insect Mol. Biol. 2005;14:89–94. doi: 10.1111/j.1365-2583.2004.00534.x. [DOI] [PubMed] [Google Scholar]
- Wang M, Liu LH, Wang S, Li X, Lu X, Gupta D, Dziarski R. Human peptidoglycan recognition proteins require zinc to kill both Gram-positive and Gram-negative bacteria and are synergistic with antibacterial peptides. J. Immunol. 2007;178:3116–3125. doi: 10.4049/jimmunol.178.5.3116. [DOI] [PubMed] [Google Scholar]
- Yu KH, Kim KN, Lee JH, Lee HS, Kim SH, ChoKY-Nam MH, Lee HI. Comparative study on characteristics of lysozymes from the hemolymph of three lepidopteran larvae, Galleria mellonella, Bombyx mori, Agrius convolvuli. Dev. Comp. Immunol. 2002;26:707–713. doi: 10.1016/s0145-305x(02)00027-7. [DOI] [PubMed] [Google Scholar]
- Zaidman-Rémy A, Hervé M, Poidevin M, Pili-Floury S, Kim MS, Blanot D, Oh BH, Ueda R, Mengin-Lecreulx D, Lemaitre B. The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity. 2006;24:463–473. doi: 10.1016/j.immuni.2006.02.012. [DOI] [PubMed] [Google Scholar]