Abstract
This paper describes the construction of a mixed monolayer of ferrocenylalkanethiol and encapsulated horseradish peroxidase (HRP) at a gold electrode for amperometric detection of H2O2 at trace levels. By tuning the alkanethiol chain lengths that tether the HRP enzyme and the ferrocenylalkanethiol (FcC11SH) mediator, facile electron transfer between FcC11SH and HRP can be achieved. Unlike most HRP-based electrochemical sensors, which rely on HRP-facilitated H2O2 reduction (to H2O), the electrocatalytic current is resulted from an HRP-catalyzed oxidation reaction of H2O2 (to O2). Upon optimizing other experimental conditions (surface coverage ratio, pH, and flow rate), the electrocatalytic reaction proceeding at the electrode was used to attain a low amperometric detection level (0.64 nM) and a dynamic range spanning over three orders of magnitude. Not only does the thin hydrophilic porous HRP capsule allow facile electron transfer, it also enables H2O2 to permeate. More significantly, the enzymatic activity of the encapsulated HRP is retained for a considerably longer period (> three weeks) than naked HRP molecules attached to an electrode or those wired to a redox polymer thin film. By comparing to electrodes modified with denatured HRP that are subsequently encapsulated or embedded in a poly-L-lysine matrix, it is concluded that the encapsulation has significantly preserved the native structure of HRP and therefore its enzymatic activity. The electrode covered with FcC11SH and encapsulated HRP is shown to be capable of rapidly and reproducibly detecting H2O2 present in complex sample media.
Introduction
One of the forefronts of analytical chemistry is the development of heterogeneous enzyme biosensors wherein enzymes are immobilized onto solid surfaces and used to generate signals corresponding to specific analytes in solution.1–4 Since efficient catalytic activity at surface largely governs the ultimate performance of an enzyme biosensor, retention of the enzymatic activity and prevention of enzyme leaching are two important criteria for constructing successful sensors.5, 6 In general, to avoid serious enzyme leaching from the surface, covalent linkage of enzyme molecules onto surface is more advantageous than physical adsorption. As for retaining the enzymatic activity, numerous strategies have been explored. These strategies include, but are not limited to, entrapment of enzyme molecules in sol-gel,7, 8 hydrogel,9 conducting or redox polymer matrices10, 11 and nanoporous materials,12, 13 attachment of enzymes onto highly hydrophilic surfaces (e.g., polylysine,14 polyethylene glycol,15, 16 chitosan,17, 18 and dextran,19, 20), and separation of enzyme layers from sample solutions using polymeric membranes21, 22, and ion-exchange polyion membranes.23, 24
Several recent papers have described the synthesis of “single-enzyme nanoparticles (SENs)” in bulk and applications of them to study enzymatic reactions in homogeneous solutions. The common procedure resorts to coupling monomers onto certain residues of an enzyme molecule, followed by initiation of polymerization of monomers (e.g., acrylamide) and cross-linking of the resultant polymer into a network. As a result, individual enzyme molecules are encapsulated into a polymeric and hydrophilic exterior. Kim and Grate were the first to encapsulate trypsin and chymotrypsin with a porous vinyl polymer/siloxane network and demonstrated that small molecules can readily move across the shell to reach the enzyme molecules.25 A particularly attractive feature is that the encapsulated enzymes sustain their activities over an extended period of time. Using HRP as a model system, Liu and coworkers enclosed single horseradish peroxidase (HRP) molecules in a nanogel shell and showed that the shell thickness can be augmented via further polymerization of acrylamide monomers added in solution.26 Lin, Wang, and coworkers immobilized HRP nanoparticles comprising of over 1000 cross-linked HRP molecules onto an electrode and achieved a mediator- and promoter-free electrochemical detection of H2O2 at micromolar concentrations.27 Interestingly, encapsulated single HRP molecules have not been immobilized at a solid substrate and utilized as part of a heterogeneous biosensor for durable detection at low H2O2 concentrations.
We wish to report the modification of gold electrodes with a mixed monolayer of ferrocenylalkanethiol/encapsulated HRP for sensitive amperometric H2O2 detection. Instead of forming the HRP capsules in a homogeneous solution, enclosure of the HRP molecules with a porous, hydrophilic shell is carried out “in-situ” at the electrode interface. The ferrocenylalkanethiol serves as a mediator for generating a large electrocatalytic current. We found that the ferrocene group located at the end of the alkyl chain, with an oxidation potential higher than ferrocene or its derivative in solution, participates in an HRP-catalyzed H2O2 oxidation reaction to O2. This is in contrast to many previously studied mediators in solution, which tend to partake in HRP-catalyzed H2O2 reduction reaction to produce H2O.28–33 In addition to preserving the HRP enzymatic activity and enabling the analyte permeability across the capsule, we also show that, through optimizing lengths of the molecules tethering the HRP and mediator molecules and the enzyme/mediator ratio in the mixed monolayer, facile electron transfer between the HRP and ferrocenylalkanethiol molecules can be achieved. As a result, a remarkable sensitivity for electrochemical H2O2 detection is obtained and the robustness of the electrode is demonstrated to be suitable for H2O2 measurement in complex sample media.
Experimental Section
Materials
11-Ferrocenyl-1-undecanethiol (Fc-C11SH) was purchased from Dojindo Laboratories (Kumamoto, Japan). N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), 3-mercaptopropionic acid (MPA), horseradish peroxidase VI (HRP), poly-L-lysine, and hydrogen peroxide were acquired from Sigma-Aldrich (St. Louis, MO). N-acryloxysuccinimide, 4-dimethylaminoantipyrine (DMAP), ammonium persulfate, N,N,N',N'-tetramethylethylenediamine, acrylamide, N,N’-methylene bisacrylamide, 4-dimethylaminoantipyrine, and boric acid were obtained from Thermo-Fisher Scientific (Pittsburgh, PA). 2,2’-Bipyridineruthenous dichloride (Ru(bpy)3Cl2) was obtained from Simth G. Frederick Chemical Company (Powell, OH). Aqueous solutions were prepared using Millipore water (18 MΩ•cm, Simplicity Model, Billerica, MA). The wired HRP/polymer solution for preparing the electrode was purchased from Bioanalytical System Inc. (BAS, West Lafayette, IN).
Instrumentation
Electrochemical experiments in quiescent solutions or flowing solution streams were carried out using a CHI-832 electrochemical workstation (CH Instruments, Austin, TX). Polycrystalline, 3 mm diameter gold disk working electrodes (BAS), a Ag/AgCl reference electrode (BAS), and a platinum coil auxiliary electrode were used in preliminary studies on the electrode reaction mechanism. A 3 mm gold disk embedded in a flat PEEK block (BAS) served as the working electrode for the amperometric detection of H2O2. The design of the homemade thin-layer flow cell is analogous to that of the commercially available flow cell (CH Instruments), except that a stainless steel tubing (1/16” OD, 0.02” ID and 4” long) was connected to the outlet of the cell as the auxiliary electrode.
Procedures
Electrode Modification
Gold disk electrodes were cleaned electrochemically in 0.1 M sulfuric acid by cycling potential between 0.0 and 2.0 V vs. Ag/AgCl for 10 min, rinsing with water and drying under nitrogen. This was followed by immersing the electrodes in 60 µL of dimethyl sulfoxide (DMSO) containing 0.83 mM FcC11SH and 4.2 mM MPA for 1 h. Then, the electrodes were submerged in a 2.5 mM MPA solution for 1 h to obtain an optimal FcC11SH coverage. To attach HRP, the electrodes were first kept in 60 µL of phosphate buffered saline (PBS, pH 5.5) containing 75 mM EDC and 15 mM NHS for 0.5 h. Subsequently they were soaked in 2 mg/mL HRP solution (pH 5.5) overnight. To encapsulate HRP, the electrodes covered with FcC11SH/HRP were first submerged in 3.8 mL of borate buffer (100 mM, pH = 9.3) that also contained 0.5 mg 4-dimethylaminoantipyrine.34 Next, 0.2 mL DMSO comprising 4.0 mg N-acryloxysuccinimide was gradually added and the reaction was allowed to proceed for 2 h at room temperature. After acryloylation, the electrodes were transferred to a 3.5 mL borate buffer that had been purged with nitrogen, and the radical polymerization at the surface of the acryloylated HRP was initiated by adding 30 µL aqueous solution containing 3 mg ammonium persulfate and 3 µL of N,N,N',N'-tetramethylethylenediamine for 1 h. To the resultant solution, acrylamide monomers and N,N’-methylene bisacrylamide (molar ratio = 10:1), dissolved in 0.5 mL water, were incrementally added within 1 h. The polymerization reaction was allowed to undergo for another 1 h under nitrogen. The as-prepared electrodes were stored in PBS (pH = 7.4) at 4 °C.
The wired HRP/polymer electrode was prepared following the user manual (BAS).35 Briefly, 0.5 µL of a surfactant solution was cast onto the electrode surface with a microsyringe. Upon drying, the electrode was coated with 0.5 µL of the HRP/polymer solution and allowed to dry overnight. The electrode was stored in a refrigerator at 4 °C when not used.
Detection of H2O2 at different electrodes
For amperometric detection of H2O2 at the wired HRP/polymer electrode, 0.1 V vs. Ag/AgCl was applied while the sample, injected via a six-port rotary valve (Valco, Houston, TX) into a 50 mM KClO4 solution, was delivered by a syringe pump (Kd Scientific, Holliston, MA) at 0.1 mL/min. At 0.1 V, the mediator, osmium bis(2,2'-bipyridine) chloride (Os(bpy)2Cl3+), attached to the polystyrene polymer, is reduced via an electrocatalytic cycle that converts H2O2 to H2O.11 For H2O2 detection at electrodes modified with FcC11SH/encapsulated HRP and its counterpart without HRP encapsulation, the experiments were conducted by holding the electrode potential at 0.5 V.
To evaluate the stability of the electrodes modified with FcC11SH/encapsulated HRP, after amperometric measurements of at least five injections of 1.0 nM H2O2, the electrodes were stored in PBS (pH 7.4) at 4 °C before the tests in the following days.
Real sample preparation and analysis
The neuroblastoma B104 cells, kindly donated by Dr. V. Pikov at Huntington Medical Research Institute (Pasadena, CA), can also be obtained from American Type Culture Collection (Manassas, VA). Cells were seeded onto plates or dishes in Dulbecco’s modified Eagle’s medium (DMEM, Mediatech, Manassas, VA) supplemented with 100 U penicillin and 100 µg/mL streptomycin at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air. Cells were plated at the density of 5 × 103 cells and adhered onto the 96-well plates. H2O2 (0.5 µM) was added to the plates and after 24 h of incubation an aliquot of the cell culture was injected into the flow cell for determining the remaining H2O2. H2O2 in a cell-free medium was also measured to assess the matrix effect on the electrochemical H2O2 detection. Concurrent with the cell incubation in the presence of H2O2, cell viability (cytotoxicity) was evaluated through the standard MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Mediatech, Manassas, VA) assay.36 Briefly, 100 µL DMEM were used to wash the residual H2O2. Then 180 µL media and 20 µL of MTT (dissolved in PBS at a concentration of 5 mg/mL) were added to each well and incubated for 4 h at 37 °C in dark. When taken up by living cells, MTT is converted to a water-insoluble blue product (formazan). The formazan product was dissolved by adding 150 µL DMSO to each well. The absorption value at 595 nm was determined with a Tecan Genios plate reader (Tecan USA, Durham, NC). All measurements were performed in triplicates, and the cell viability was presented as the percentage of survival relative to that in control culture.
Results and Discussion
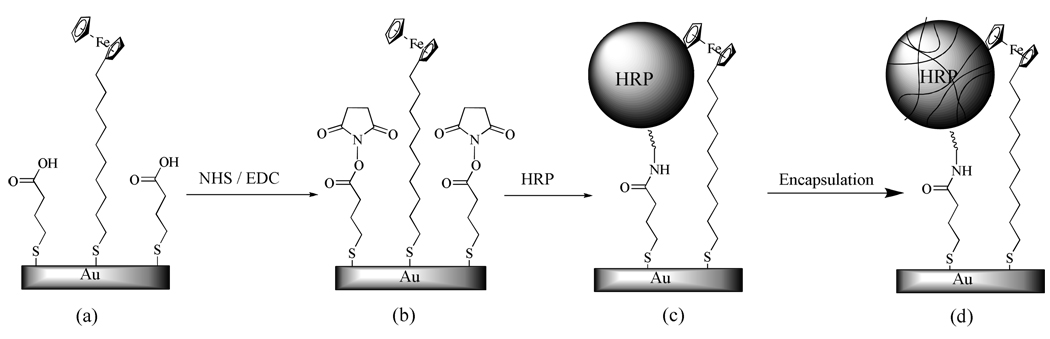
Scheme 1 depicts the general steps for modifying Au electrodes with mixed monolayers of FcC11SH and encapsulated HRP. First, a mixed monolayer of FcC11SH and mercaptopropionic acid (MPA) with a desired FcC11SH surface coverage is self-assembled onto a gold electrode (step a). Subsequently, HRP molecules are attached to the MPA moieties via amine coupling with the NHS/EDC chemistry (steps b and c). Encapsulation of the HPR molecules is accomplished by first acryloylating the amine groups remaining on the HRP molecule, followed by radical polymerization of the acrylamide monomers (step d) around HRP.
Scheme 1.
Steps for constructing the mixed monolayer of FcC11SH and encapsulated HRP at a Au electrode: (a) formation of a mixed monolayer of MPA/FcC11SH, (b) activation of and NHS ester attachment onto MPA, (c) cross-linking of HRP, and (d) encapsulation of HRP through acryloylation. For illustration, the molecules are not drawn to scale.
Key to the successful construction of the monolayer of FcC11SH/encapsulated HRP for electrochemical detection of H2O2 is that the chain length of the ferrocenylalkanethiol must be longer than that of the carboxyl-terminated alkanethiol. This allows the Fc groups to be in close proximity of HRP. In addition, the ratio of FcC11SH/HRP (or that of FcC11SH/MPA in the first step) should be optimized. Consequently HRP molecules are surrounded by FcC11SH and the electron transfer between HRP and H2O2 can be effectively mediated by FcC11SH. We found that mixed monolayers containing 7–15% of FcC11SH (estimated from charges under the ferrocene oxidation peaks) produce high electrocatalytic currents. FcC11SH surface coverage in this range can be regulated either by controlling the molar ratio between FcC11SH and MPA solutions used for the mixed monolayer formation or using MPA to partially replace a preformed mixed monolayer of FcC11SH/MPA (cf. details in Experimental Section). Rubin et al. found that 7% of 16-ferrocenylhexadecanethiol in a mixed monolayer that also contains glucose oxidase yields the highest sensitivity towards glucose detection.37 The coverage of FcC11SH in the mixed monolayer is relatively low for the optimal signal, because the enzyme molecules are much bulkier than FcC11SH and only the Fc groups positioned next to the enzyme molecules are capable of mediating the electron transfer. We should mention that Liu and coworkers have shown that HRP encapsulated in a homogeneous solution has a typical shell thickness between 2 and 5 nm.26 Such a thickness is sufficiently small and the electron transfer between FcC11SH and HRP is therefore not impeded. The electron transfer between the electrode and the Fc moieties is known to be facile when the alkanethiol linker is not particularly long.38, 39 The mediated electron transfer is analogous to that occurs to a monolayer of redox active proteins immobilized onto an alkanethiol layer (i.e., monolayer protein voltammetry40, 41).
Traditionally, mediated electrochemical detection of H2O2 utilizing an HRP-modified electrode and a mediator (M) in solution, can be generalized using notations similar to those in a recent review by Heller and Feldman:42
| (1) |
| (2) |
| (3) |
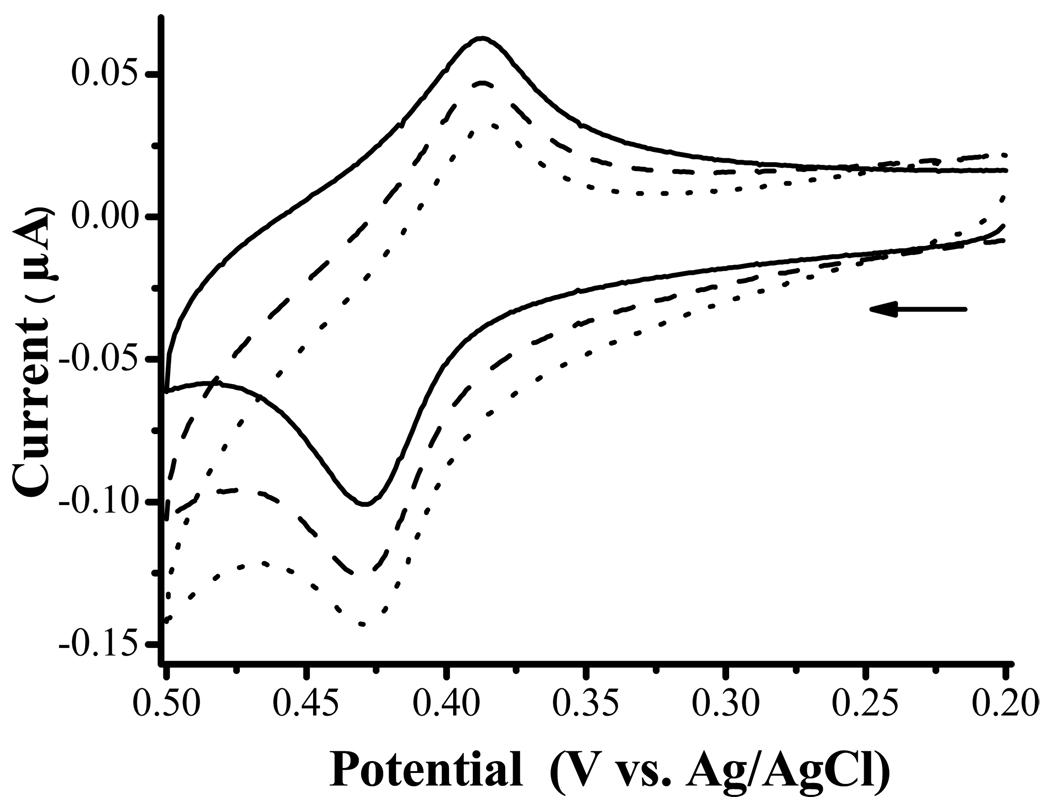
where HRP+ represents the oxidized form of HRP and M+ denotes the oxidized form of the mediator. When M is Fc or a Fc derivative in solution, by setting the electrode potential at a value (typically < 0.3 V vs. Ag/AgCl33) to reduce the enzymatically generated Fc+ back to Fc, the catalytic cycle continuously converts H2O2 to H2O. The commercially successful wired HRP/polymer electrode utilizing Os(bpy)32+ as the mediator follows the same electrode reaction.11 However, we did not observe any electrocatalytic current when the as-prepared electrode was held at such a potential. Rather, considerably high oxidation current was observed when a potential greater than 0.5 V was applied. At 0.5 V or beyond, the Fc group at the end of the alkyl chain is oxidized (cf. solid line curve in Figure 1). The oxidation potential of FcC11SH, taken as the average of the anodic (0.43 V) and cathodic (0.39 V) peak potentials, is 0.41 V. When HRP was added into a PBS solution housing an FcC11SH/MPA-modified electrode, we observed an increase in the anodic peak (cf. dashed line curve in Figure 1). Addition of H2O2 further increased the anodic peak and reduced the cathodic peak (dotted line curve). These behaviors are also characteristic of an electrocatalytic reaction (EC’ mechanism43).
Figure 1.
CVs acquired at an Au electrode modified with a mixed monolayer of FcC11SH and MPA in a 0.1 M PBS solution (solid line curve), in a PBS solution containing 0.2 mM HRP (dashed) and in a PBS solution containing 0.2 mM HRP and 0.2 mM H2O2 (dotted). The scan rate was 5 mV/s and the arrow indicates the initial scan direction.
A number of papers have shown that, in the presence of an oxidant, HRP can be first oxidized to a form that contains a four-valent Fe center (i.e., oxyperoxidase44–46) and is capable of oxidizing H2O2 to O2. This form, denoted herein as Fe(IV)-HRP or HRP2+, may be responsible for the observed electrocatalytic oxidation of H2O2:
| (5) |
| (6) |
| (7) |
where Red is the reduced form of the oxidant that is capable of oxidizing HRP to the Fe(IV)-HRP. From the dashed line curve in Figure 1, it is evident that the oxidized FcC11SH at the electrode, viz., Fc+C11SH, can oxidize HRP. Therefore, we suggest that Fc+C11SH may behave as an oxidant and has converted the surface-confined HRP to its oxidized form HRP2+. The regeneration of FcC11SH explains the increase of anodic current from the solid to the dashed line curves. In the presence of H2O2, HRP2+ oxidizes H2O2 to O2 and itself is converted back to the native HRP. Thus, the anodic current is further amplified (cf. the dotted line curve in Figure 1).
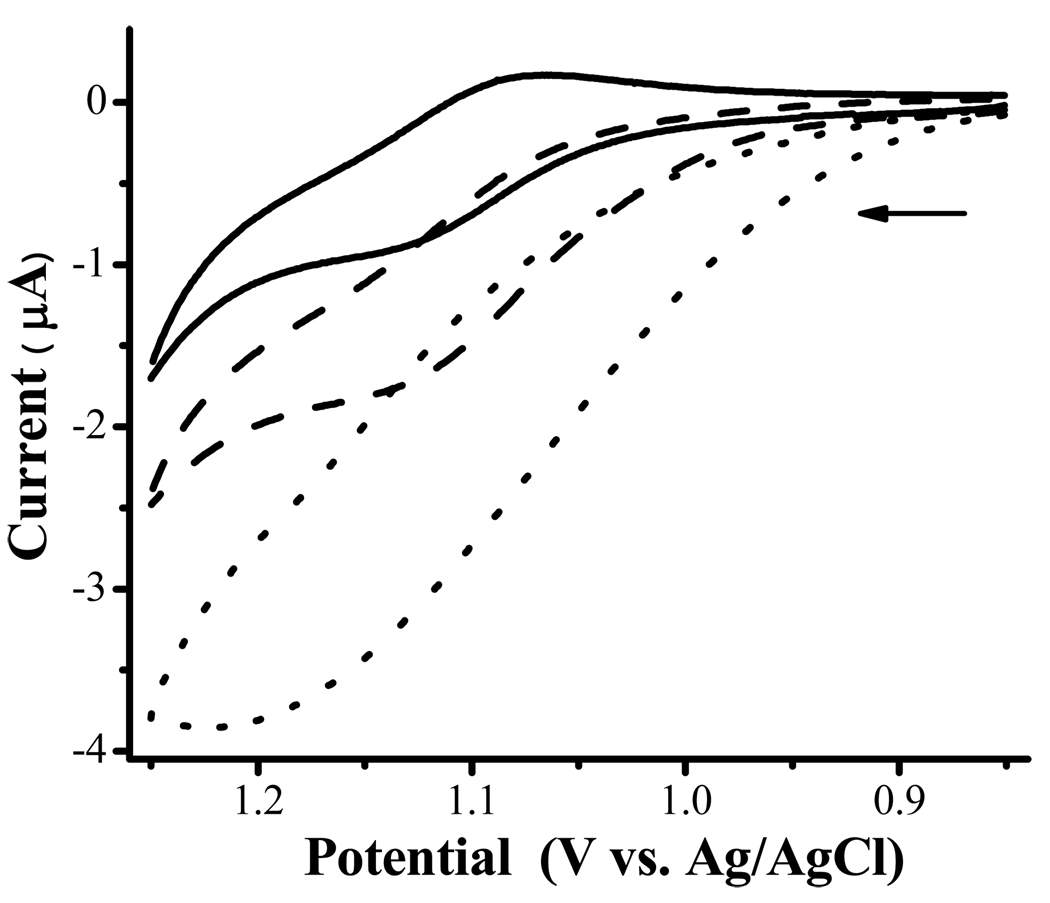
The above mechanism is reasonable considering that the potential of oxyperoxidase or HRP2+ is approximately between 0.5 and 0.7 V,47 which is quite close to the oxidation potential of Fc+C11SH. This is also close to the diffusion-limited oxidation of H2O2 to O2 at Pt electrodes.11, 48 The close proximity between FcC11SH and HRP molecules at the electrode may have lowered the energy barrier for the electron transfer. Thus, achieving H2O2 oxidation at a less positive potential at an electrode less catalytic than Pt is an attractive aspect of the as-prepared electrode. To provide further support to the above mechanism, we conducted another experiment by employing Ru(bpy)32+ whose oxidation potential is rather high (1.11 V vs. Ag/AgCl43). When HRP is present in the solution, a sigmoidal CV of Ru(bpy)32+ was observed (See the dashed voltammogram in Figure 2). In the presence of H2O2, the increase in the steady-state anodic current is even more pronounced (dotted line curve). In this case, the electrogenerated Ru(bpy)33+ acts as an oxidant in the same fashion as Fc+C11SH to facilitate the HRP-catalyzed electrooxdiation of H2O2 to O2. Overall, the lack of a H2O2 reduction reaction is not entirely surprising, given that the potentials of redox proteins in thin films depend on a variety of factors such as the substrate electrode, the interaction of the proteins with the film materials, and the identity of the redox proteins.49, 50
Figure 2.
CVs acquired at a bare Au electrode in a PBS solution containing 0.2 mM Ru(bpy)32+ (solid line curve), 0.2 mM Ru(bpy)32+ and 0.2 mM HRP (dashed), and 0.2 mM Ru(bpy)32+, 0.2 mM HRP, and 0.2 mM H2O2 (dotted). The scan rate was 5 mV/s and the arrow indicates the initial scan direction.
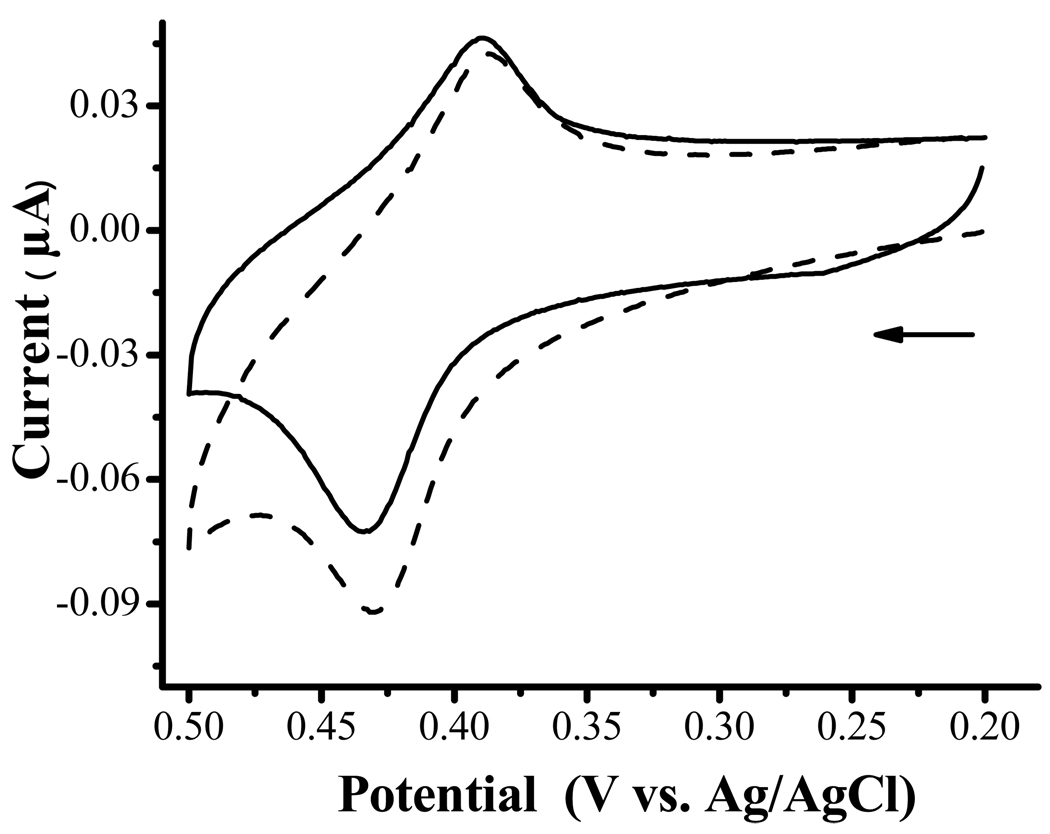
When HRP is immobilized and encapsulated, the oxidation current corresponding to the co-immobilized FcC11SH increases with H2O2 (Figure 3). Such a behavior is in remarkable resemblance to that obtained with HRP in solution (cf. Figure 1 and Figure 2). Thus, we conclude that the FcC11SH/encapsulated HRP electrode can efficiently catalyze the oxidation of H2O2 and encapsulating HRP does not appear to hinder (1) the diffusion of H2O2 from the solution to the HRP molecules and (2) the facile electron transfer reaction between Fc+C11SH and HRP. We should note that H2O2 detection is often accomplished by using electrodes modified with various oxidases.50, 51 These electrodes generally require O2 for sustained enzymatic function. Thus, the generation of O2 from the electrocatalytic oxidation of H2O2 could potentially help prevent oxygen depletion in various detection schemes.
Figure 3.
CVs at a mixed monolayer of FcC11SH/encapsulated HRP acquired in 0.1 M PBS (solid line curve) and PBS containing 20 µM H2O2 (dashed). Scan rate = 5 mV/s and the arrow indicates the initial scan direction.
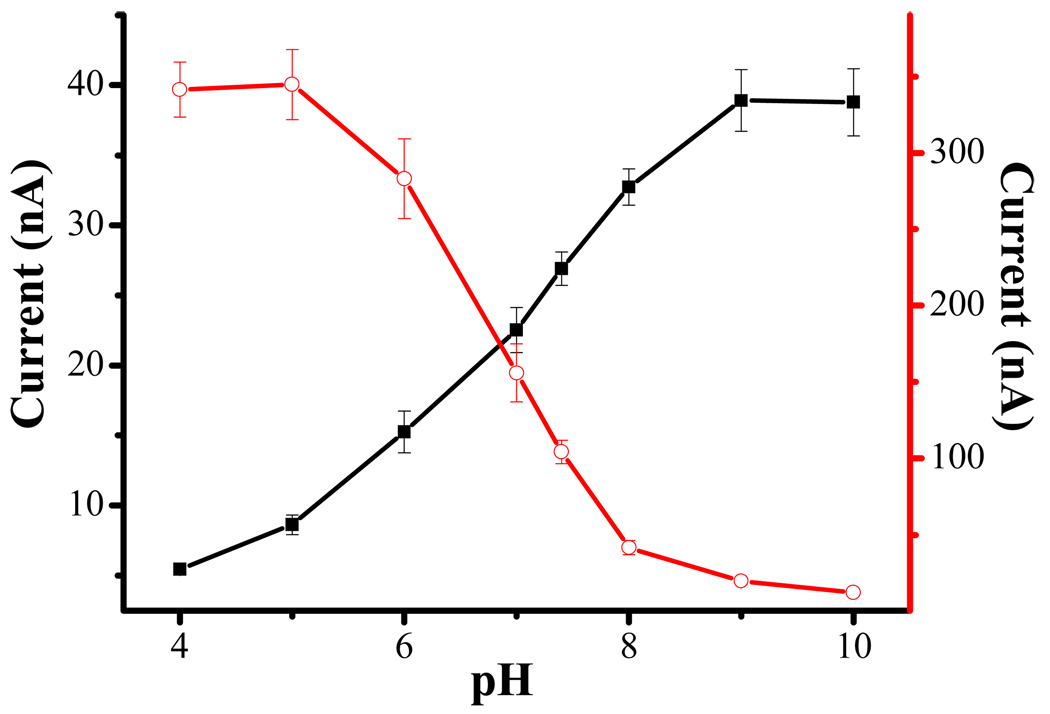
The amperometric detection of H2O2 in a thin-layer flow cell housing an FcC11SH/encapaulsted HRP electrode was optimized. We found that, in the range of flow rates studied (0.015–0.14 mL/min), 0.080 mL/min produced the highest current (data not shown). This is understandable, as a higher flow rate increases the mass transfer rate of H2O2. But as the flow rate becomes too high, the reaction time becomes rather short and the effect outweighs the contribution by the increased mass transfer at a high flow rate. We also studied the dependence of the electrocatalytic current on solution pH (filled squares in Figure 4) and compared the behavior observed at a wired HRP/polymer electrode (open circles). Two points are worth noting: (1) both types of HRP electrodes possess enzymatic activity across a wide pH range (5.0–10.0 for the FcC11SH/encapaulsted HRP electrode and 4.0–8.0 for the wired HRP/polymer electrode), and (2) the pH dependence of the wired HRP/polymer electrode is opposite to that of the FcC11SH/encapaulsted HRP electrode. The pH dependences of these two types of electrodes can be rationalized by the difference between the aforementioned mechanisms. For the HRP-catalyzed H2O2 reduction (cf. eq. 1), the equation describes the pH dependence of redox potential of the H2O2/H2O couple. As such, an increase in pH would decrease the potential or the oxidizing power of H2O2. The net effect is a decrease in the mediator (Os(bpy)32+) turn-over rate or catalytic current. On the contrary, for HRP-catalyzed H2O2 oxidation (cf. eq. 7), H2O2 is oxidized and the potential of the redox couple O2/H2O2 is also pH dependent . In this case, an increase in pH would also decrease the potential, but the reducing power of H2O2 is increased. As the consequence, the driving force of redox reaction 7 is increased and the Fc+C11SH mediator turn-over rate is higher. Notice that the current begins to level off at pH < 5.0 and > 9.0 at the wired HRP/polymer and the FcC11SH/encapsulated HRP electrodes, respectively. In both cases, the electrocatalytic currents are governed by the mass transfer of H2O2 from the bulk solution to the electrode surface. The contrast in the pH dependences again strongly suggests that H2O2 is oxidized at the electrode prepared in this work.
Figure 4.
Dependence of electrocatalytic currents on solution pH: currents recorded at a wired HRP/polymer electrode (open circles) and the FcC11SH/encapsulated HRP electrode (filled squares). For both electrodes, 1 µM H2O2 was used. RSD values ranging from 1.86 to 10.2% are plotted as the error bars (n = 3).
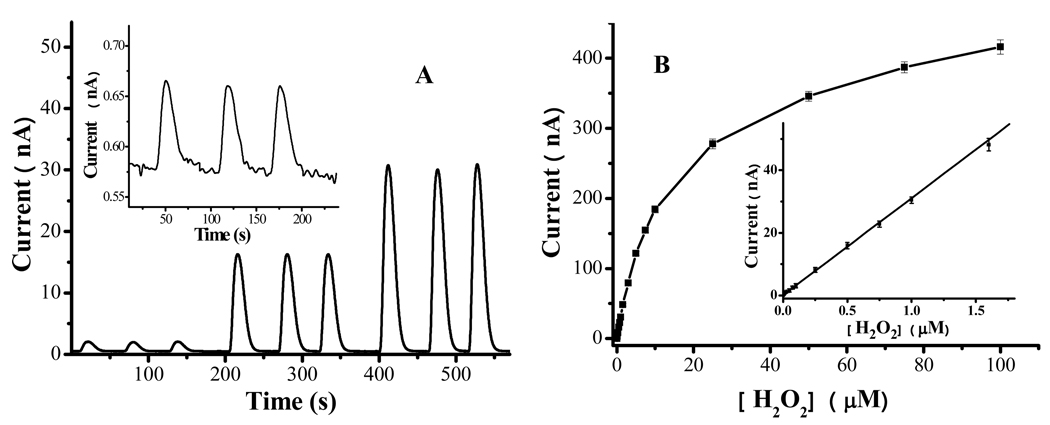
Having optimized the experimental condition, we performed amperometric detection of H2O2 across a wide range of concentrations. Figure 5A depicts a representative chronoamperomogram showing three consecutive injections of three different H2O2 concentrations. It is clear that reproducible peaks can be obtained and the peak height is proportional to the three concentrations. From the variations of the baseline signals, we estimated the detection limit (3σ) for H2O2 at this electrode to be 0.64 nM. This is a reasonable detection limit since injections of 1.0 nM H2O2 produced well-resolved peaks with a good signal-to-noise ratio (cf. inset of Figure 5A). Figure 5B displays the calibration plot for [H2O2] between 1.0 nM and 100 µM and the inset shows the linear portion of the plot (from 1.0 nM to 1.6 µM). The dynamic range is comparable to that obtained at the wired HRP/polymer electrode (from 0.50 nM to 1.0 µM). The excellent linearity (R2 = 0.999) suggests that the dynamic range spans over three orders of magnitude and the RSD values range within 1.3–4.1%, which are considered to be excellent.52 We also assessed the reproducibility among three different electrodes. The RSD value (3.4%) is also rather small and the difference can be attributed to the slight variation in the FcC11SH coverage among different electrodes. At the wired HRP/polymer electrode, the detection limit was found to be lower (0.033 nM). The lower detection limit is expected, because the 3-D polymer network can incorporate more mediator and enzyme molecules51 than the mediator and enzyme molecules dispersed in a 2-D mixed monolayer. However, we should point out that the calibration curve for the wired HRP/polymer electrode deviates from linearity at H2O2 concentration below 0.5 nM. Thus the detection limit is not much lower than that of the FcC11SH/encapsulated HRP electrode.
Figure 5.
(A) Amperometric response at an FcC11SH/encapsulated HRP electrode upon consecutive injections of three different H2O2 concentrations (0.05, 0.50 and 1.0 µM). The electrode was held at 0.5 V vs. Ag/AgCl, while the injected H2O2 samples were delivered at 0.08 mL/min. Inset: Amperometric response to three consecutive injections of 1.0 nM H2O2. (B) Dependence of the electrocatalytic current on [H2O2] in the range between 1 nM and 100 µM, i (nA) = 0.029 [H2O2] (nM) + 0.56 (nA). The inset shows the linear portion of the plot (r2 = 0.999 between 1.0 nM and 1.6 µM). Each concentration was repeated three times and the error bars represent the RSD values.
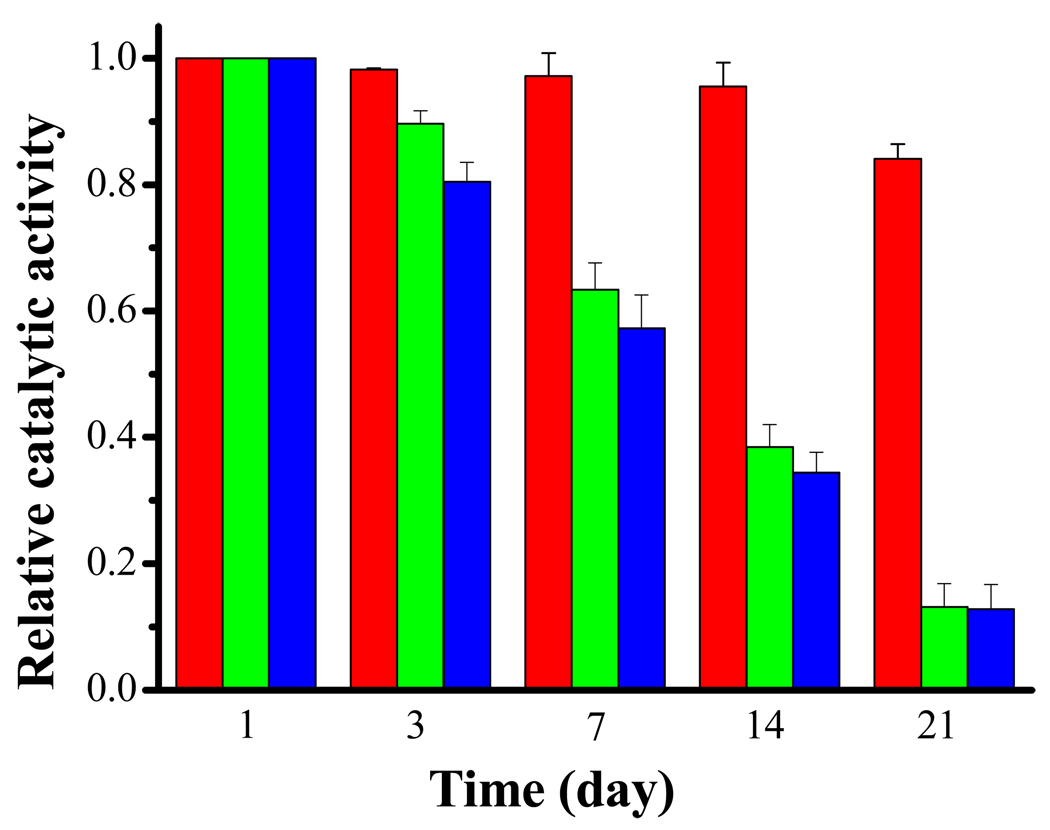
Figure 6 is a bar graph comparing the durability among the three types of electrodes, viz., the FcC11SH/encapsulated HRP electrode (red), the FcC11SH/HRP electrode (green), and the wired HRP/polymer electrode (blue). It is apparent that both the wired HRP/polymer and the FcC11SH/HRP (i.e., HRP is not encapsulated) begin to degrade after about three days. The stability of the wired HRP/polymer electrode is consistent with the one-week shelf life noted by the vendor.35 In contrast, the FcC11SH/encapsulated HRP electrode remains stable for almost three weeks and only loses 16% of its catalytic efficiency after 21 days.
Figure 6.
Comparison of stability among the FcC11SH/encapsulated HRP electrode (red), the FcC11SH/HRP electrode (green), and the wired HRP/polymer electrode (blue). Error bars represent the RSD values (n = 3), which range between 0.14 and 8.1%.
To determine whether the retention of catalytic activity of HRP is due to the preservation of the native structure or due to the isolation of the HRP from the sample solution by the hydrogel, we also modified electrodes with native and denatured HRP molecules and subsequently covered them with a poly-L-lysine (PLL) film. Specifically, following the attachment of HRP onto the MPA/FcC11SH mixed monolayer (cf. Scheme 1) PLL was cross-linked using the NHS/EDC chemistry. In a separate experiment, an electrode covered with FcC11SH/HRP was immersed in a 15% methanol solution at 60 °C for 20 min to denature HRP, which was followed by attachment of the PLL matrix. While the former electrode showed excellent amperometric response to injected H2O2, essentially no signals were detected at the latter electrode. In addition, we found that denatured HRP that had been encapsulated also did not produce electrocatalytic currents. Since Rusling and coworkers have demonstrated that enzymes such as HRP can retain near native structures for extended periods when cross-linked to PLL films,14 the above observations suggest that encapsulation of HRP renders an environment that retains the native structure of HRP and prolongs its enzymatic activity.
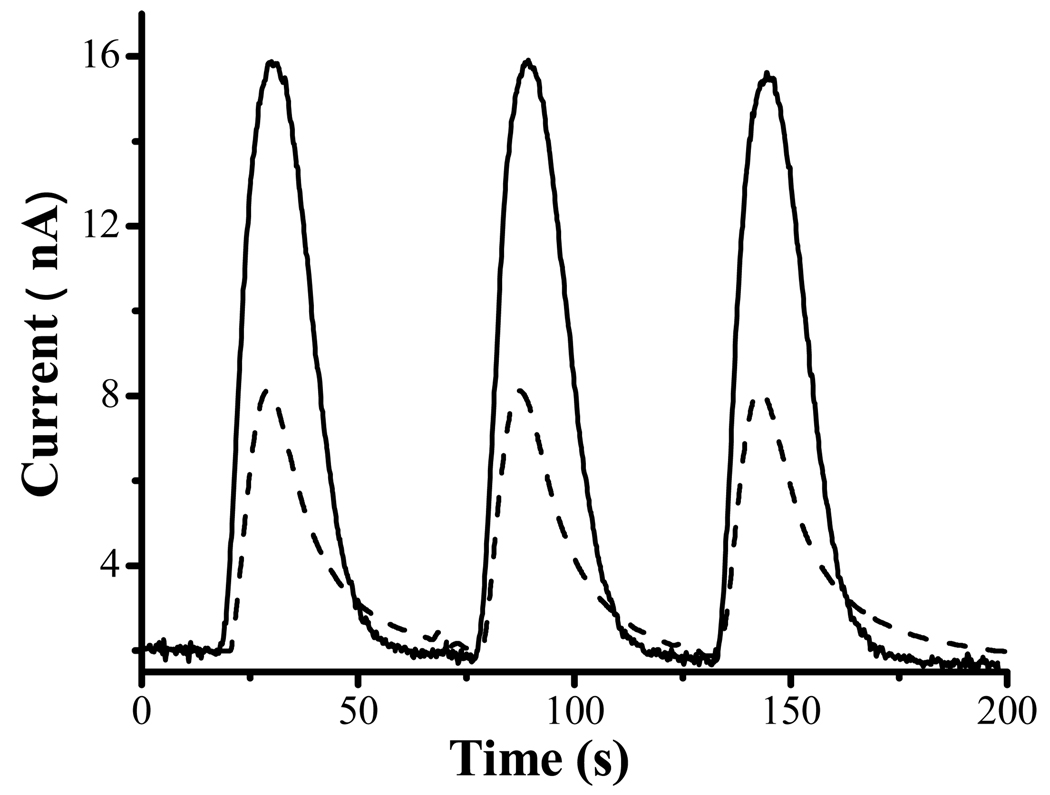
Finally, we applied the FcC11SH/encapsulated HRP electrode to the detection of H2O2 in a real sample. In neuroscience research, reactive oxygen species such as H2O2 are widely believed to cause oxidative stress/damage to cells.53 H2O2 or species that produce H2O2 are generally introduced to neuronal cell cultures and cell viability is monitored over a period of time.36, 54, 55 For example, we56, 57 and others58, 59 have shown that redox metals (e.g., Cu2+ and Fe3+), upon complexation with amyloid peptides, can generate H2O2 with cellular redox species. Knowledge about the percent of H2O2 along the course of incubation affords accurate information about the dosage of H2O2 that inflicts cell death. To demonstrate the viability of using the FcC11SH/encapsulated HRP electrode for H2O2 detection in a complex sample matrix, we first spiked a cell-free medium with 500.0 nM H2O2 and determined the amount of H2O2 to be 494.4 ± 5.7 nM (RSD = 1.14%). The excellent accuracy also demonstrates that the cell culture matrix does not cause appreciable surface contamination and degradation of the HRP activity and the various species in the culture medium do not react with H2O2. In a separate experiment, we added 500.0 nM H2O2 into a B104 neuroblastoma cell culture sample, and, through a MTT assay36 (see also details in Experimental Section), found that only 45% of the cells survived after 20 h. The injection peaks presented by the dashed line curve in Figure 7 clearly show that H2O2 remaining in the cell culture can be detected rapidly and reproducibly (RSD = 0.82% for the three injection peaks). The H2O2 concentration was determined to be 188.1 ± 1.1 nM, which is in excellent agreement with that obtained at the wired HRP/polymer electrode (197.8 ± 3.3 nM). The remaining H2O2 is about 38% of the original dosage, suggesting that a large fraction of the original dosage has reacted and caused the cell death. From this experiment, we conclude that encapsulation of the HRP molecule affords reliable analyses for H2O2 in biological samples.
Figure 7.
Amperometric responses corresponding to three consecutive injections of a cell-free medium containing 500 nM H2O2 (solid line curve) and the medium containing B-104 neuroblastoma that had been incubated with 500 nM H2O2 for 20 h (dashed). The flow rate used was 0.08 mL/min and the electrode potential was held at 0.5 V vs. Ag/AgCl.
Conclusion
Gold electrodes modified with mixed monolayers of FcC11SH/encapsulated HRP have been successfully applied to the amplified amperometric detection of H2O2 at trace levels. By electrogenerating ferrocenium at the end of an alkanethiol tether, a catalytic cycle is created, which results in an increased oxidation current or signal amplification. The electrode process was elucidated to be an HRP-catalyzed oxidation reaction of H2O2 to O2. Construction of the FcC11SH/encapsulated HRP electrode is straightforward and the electrode performance was optimized by studying the influences of FcC11SH surface coverage, solution pH, and flow rate on the amplified signals. The dynamic range and sensitivity are highly comparable to those achievable with the commercially available wired HRP/polymer electrode. The as-prepared electrode yields a detection limit of 0.64 nM, which is quite remarkable considering that both the enzyme and mediator molecules are spread in a monolayer (instead of across a thick film). Compared to electrodes at which HRP molecules are exposed, the encapsulation of the preimmobilized HPR is demonstrated to enhance the durability of the electrode and to render the amenability to detection of H2O2 in complex sample matrix. The significantly improved stability can be ascribed to the porous acrylonated exterior formed at the surface of HRP molecules, which allows H2O2 to reach the HRP core while retaining the native structure of the HRP molecule.
Acknowledgment
Partial support of this work by an NIH-NINDS grant (No: 1SC1NS070155-01 to FZ), the RIMI Program at California State University, Los Angeles (P20-MD001824-01 to FZ), a NSF-RUI grant (No. 0555224 to FZ), and the Natural Science Foundation of China (Nos. 20676153 and 20876179 to YL) is gratefully acknowledged. YP also thanks the China Scholarship Council for financial support.
Reference
- 1.Zhang X, Ju H, Wang J, editors. Electrochemical Sensors, Biosensors and their Biomedical Applications. New York: Academic Press; 2007. [Google Scholar]
- 2.Cooper J, Cass T, editors. Biosensors. 2nd ed. Oxford: Oxford University Press; 2004. [Google Scholar]
- 3.Ramsay G, editor. Commercial Biosensors: Applications to Clinical, Bioprocess, and Environmental Samples. New York: Wiley-Interscience; 1998. [Google Scholar]
- 4.Borisov SM, Wolfbeis OS. Chem. Rev. 2008;108:423–461. doi: 10.1021/cr068105t. [DOI] [PubMed] [Google Scholar]
- 5.Wang J. Chem. Rev. 2008;108:814–825. doi: 10.1021/cr068123a. [DOI] [PubMed] [Google Scholar]
- 6.Privett BJ, Shin JH, Schoenfisch MH. Anal. Chem. 2008;80:4499–4517. doi: 10.1021/ac8007219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang G, Xu JJ, Chen HY, Lu ZH. Biosens. Bioelectron. 2003;18:335–343. doi: 10.1016/s0956-5663(02)00152-5. [DOI] [PubMed] [Google Scholar]
- 8.Kang X, Wang J, Tang Z, Wu H, Lin Y. Talanta. 2009;78:120–125. doi: 10.1016/j.talanta.2008.10.063. [DOI] [PubMed] [Google Scholar]
- 9.Holtz JH, Asher SA. Nature. 1997;389:829–832. doi: 10.1038/39834. [DOI] [PubMed] [Google Scholar]
- 10.Yu X, Sotzing GA, Papadimitrakopoulos F, Rusling JF. Anal. Chem. 2003;75:4565–4571. doi: 10.1021/ac034188r. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Janle E, Huang TH, Gitzen J, Kissinger PT, Vreeke M, Heller A. Anal. Chem. 1995;67:1326–1331. [Google Scholar]
- 12.Ma D, Li M, Patil AJ, Mann S. Adv. Mater. 2004;16:1838–1841. [Google Scholar]
- 13.Kumar R, Maitra AN, Patanjali PK, Sharma P. Biomaterials. 2005;26:6743–6753. doi: 10.1016/j.biomaterials.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 14.Guto PM, Kumar CV, Rusling JF. J. Phys. Chem. B. 2007;111:9125–9131. doi: 10.1021/jp071525h. [DOI] [PubMed] [Google Scholar]
- 15.Russell RJ, Pishko MV, Simonian AL, Wild JR. Anal. Chem. 1999;71:4909–4912. doi: 10.1021/ac990901u. [DOI] [PubMed] [Google Scholar]
- 16.Lahiri J, Isaacs L, Tien J, Whitesides GM. Anal. Chem. 1999;71:777–790. doi: 10.1021/ac980959t. [DOI] [PubMed] [Google Scholar]
- 17.Wei X, Cruz J, Gorski W. Anal. Chem. 2002;74:5039–5046. doi: 10.1021/ac020216e. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Wang MK, Zhao F, Xu ZA, Dong SJ. Biosens. Bioelectron. 2005;21:984–988. doi: 10.1016/j.bios.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Stevens MM, Allen S, Davies MC, Roberts CJ, Schacht E, Tendler SJB, VanSteenkiste S, Williams PM. Langmuir. 2002;18:6659–6665. [Google Scholar]
- 20.Sigal GB, Bamdad C, Barberis A, Strominger J, Whitesides GM. Anal. Chem. 1996;68:490–497. doi: 10.1021/ac9504023. [DOI] [PubMed] [Google Scholar]
- 21.Schoenfisch MH, Rothrock AR, Shin JH, Polizzi MA, Brinkley MF, Dobmeier KP. Biosens. Bioelectron. 2006;22:306–312. doi: 10.1016/j.bios.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Yu BZ, Long N, Moussy Y, Moussy F. Biosens. Bioelectron. 2006;21:2275–2282. doi: 10.1016/j.bios.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Moattisirat D, Poitout V, Thome V, Gangnerau MN, Zhang Y, Hu Y, Wilson GS, Lemonnier F, Klein JC, Reach G. Diabetologia. 1994;37:610–616. doi: 10.1007/BF00403381. [DOI] [PubMed] [Google Scholar]
- 24.Heitner-Wirguin C. J. Membr. Sci. 1996;120:1–33. [Google Scholar]
- 25.Kim J, Grate JW. Nano Lett. 2003;3:1219–1222. [Google Scholar]
- 26.Yan M, Ge J, Liu Z, Ouyang PK. J. Am. Chem. Soc. 2006;128:11008–11009. doi: 10.1021/ja064126t. [DOI] [PubMed] [Google Scholar]
- 27.Liu GD, Lin YH, Ostatna V, Wang J. Chem. Commun. 2005:3481–3483. doi: 10.1039/b504943a. [DOI] [PubMed] [Google Scholar]
- 28.Kenausis G, Chen Q, Heller A. Anal. Chem. 1997;69:1054–1060. doi: 10.1021/ac961083y. [DOI] [PubMed] [Google Scholar]
- 29.Mulchandani A, Pan ST. Anal. Biochem. 1999;267:141–147. doi: 10.1006/abio.1998.2983. [DOI] [PubMed] [Google Scholar]
- 30.Patolsky F, Zayats M, Katz E, Willner I. Anal. Chem. 1999;71:3171–3180. doi: 10.1021/ac9901541. [DOI] [PubMed] [Google Scholar]
- 31.Su XD, O'Shea SJ. Anal. Biochem. 2001;229:241–246. doi: 10.1006/abio.2001.5429. [DOI] [PubMed] [Google Scholar]
- 32.Castillo J, Gaspar S, Sakharov I, Csoregi E. Biosens. Bioelectron. 2003:705–714. doi: 10.1016/s0956-5663(03)00011-3. [DOI] [PubMed] [Google Scholar]
- 33.Tatsuma T, Okawa Y, Watanabe T. Anal. Chem. 1989;61:2352–2355. [Google Scholar]
- 34.Schmidt A, Schumacher JT, Reichelt J, Hecht HJ, Bilitewski U. Anal. Chem. 2002;74:3037–3045. doi: 10.1021/ac0108111. [DOI] [PubMed] [Google Scholar]
- 35. http://www.basinc.com/mans/PE-man.pdf.
- 36.Lin YC, Huang YC, Chen SC, Liaw CC, Kuo SC, Huang LJ, Gean PW. Neurochem. Res. 2009;34:923–930. doi: 10.1007/s11064-008-9860-0. [DOI] [PubMed] [Google Scholar]
- 37.Rubin S, Chow JT, Ferraris JP, Zawodzinski TA. Langmuir. 1996;12:363–370. [Google Scholar]
- 38.Creager SE, Weber K. Langmuir. 1993;9:844–850. [Google Scholar]
- 39.Smalley JF, Feldberg SW, Chidsey CED, Linford MR, Newton MD, Liu Y-P. J. Phys. Chem. 1995;99:13141–13149. [Google Scholar]
- 40.Clark RA, Bowden EF. Langmuir. 1997;13:559–565. [Google Scholar]
- 41.El Kasmi A, Wallace JM, Bowden EF, Binet SM, Linderman RJ. J. Am. Chem. Soc. 1998;120:225–226. [Google Scholar]
- 42.Heller A, Feldman B. Chem. Rev. 2008;108:2482–2505. doi: 10.1021/cr068069y. [DOI] [PubMed] [Google Scholar]
- 43.Bard AJ, Faulkner LR, editors. Electrochemical Methods: Fundamentals and Applications. New York: John Wiley & Sons; 2001. [Google Scholar]
- 44.Jenzer H, Jones W, Kohler H. J. Biol. Chem. 1986;261:5550–5556. [PubMed] [Google Scholar]
- 45.Zhou YL, Hu NF, Zeng YH, Rusling JF. Langmuir. 2002;18:211–219. [Google Scholar]
- 46.Huang R, Hu NF. Biophys. Chem. 2003;104:199–208. doi: 10.1016/s0301-4622(02)00367-8. [DOI] [PubMed] [Google Scholar]
- 47.Everse J, Grisham MB, Everse KE, editors. Peroxidases in Chemistry and Biology. Boca Raton, FL: CRC Press; 1990. [Google Scholar]
- 48.Hu Y, Zhang Y, Wilson G. Anal. Chim. Acta. 1993;281:503–511. [Google Scholar]
- 49.Rusling JF. Acc. Chem. Res. 1998;31:363–369. [Google Scholar]
- 50.Rusling JF, Zhang Z. In: Biomolecular Films. Rusling JF, editor. New York: Marcel Dekker; 2003. pp. 1–64. [Google Scholar]
- 51.Heller A. Acc. Chem. Res. 1990;23:128–134. [Google Scholar]
- 52.Skoog DA, Holler FJ, Crouch SR, editors. Principles of Instrumental Analysis. 6th ed. Belmont, CA: Thomson Brooks/Cole; 2007. [Google Scholar]
- 53.Gaggelli E, Kozlowski H, Valensin D, Valensin G. Chem. Rev. 2006;106:1995–2044. doi: 10.1021/cr040410w. [DOI] [PubMed] [Google Scholar]
- 54.Heo SR, Han AM, Kwon YK, Joung I. Neurosci. Lett. 2009;450:45–50. doi: 10.1016/j.neulet.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 55.Baruch-Suchodolsky R, Fischer B. Biochemistry. 2009;48:4354–4370. doi: 10.1021/bi802361k. [DOI] [PubMed] [Google Scholar]
- 56.Jiang DL, Men LJ, Wang JX, Zhang Y, Chickenyen S, Wang YS, Zhou FM. Biochemistry. 2007;46:9270–9282. doi: 10.1021/bi700508n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang D, Li X, Williams R, Patel S, Men L, Wang Y, Zhou F. Biochemistry. 2009;48:7939–7947. doi: 10.1021/bi900907a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang XD, Cuajungco MP, Atwood CS, Hartshorn MA, Tyndall JDA, Hanson GR, Stokes KC, Leopold M, Multhaup G, Goldstein LE, Scarpa RC, Saunders AJ, Lim J, Moir RD, Glabe C, Bowden EF, Masters CL, Fairlie DP, Tanzi RE, Bush AI. J. Biol. Chem. 1999;274:37111–37116. doi: 10.1074/jbc.274.52.37111. [DOI] [PubMed] [Google Scholar]
- 59.Streltsov VA, Varghese JN. Chem. Commun. 2008:3169–3171. doi: 10.1039/b803911a. [DOI] [PubMed] [Google Scholar]