Abstract
Morphine-3-glucoronide (M3G) is a major morphine metabolite detected in cerebrospinal fluid of humans receiving systemic morphine. M3G has little-to-no affinity for opioid receptors and induces pain by unknown mechanisms. The pain-enhancing effects of M3G have been proposed to significantly and progressively oppose morphine analgesia as metabolism ensues. We have recently documented that morphine activates toll-like receptor-4 (TLR4), beyond its classical actions on μ-opioid receptors. This suggests that M3G may similarly activate TLR4. This activation could provide a novel mechanism for M3G-mediated pain enhancement, as (a) TLR4 is predominantly expressed by microglia in spinal cord and (b) TLR4 activation releases pain-enhancing substances, including interleukin-1 (IL1). We present in vitro evidence that M3G activates TLR4, an effect blocked by TLR4 inhibitors, and that M3G activates microglia to produce IL1. In vivo, intrathecal M3G (0.75 μg) induced potent allodynia and hyperalgesia, blocked or reversed by interleukin-1 receptor antagonist, minocycline (microglial inhibitor), and (+)- and (−)-naloxone. This latter study extends our prior demonstrations that TLR4 signaling is inhibited by naloxone nonstereoselectively. These results with (+)- and (−)-naloxone also demonstrate that the effects cannot be accounted for by actions at classical, stereoselective opioid receptors. Hyperalgesia (allodynia was not tested) and in vitro M3G-induced TLR4 signaling were both blocked by 17-DMAG, an inhibitor of heat shock protein 90 (HSP90) that can contribute to TLR4 signaling. Providing further evidence of proinflammatory activation, M3G upregulated TLR4 and CD11b (microglial/macrophage activation marker) mRNAs in dorsal spinal cord as well as IL1 protein in the lumbosacral cerebrospinal fluid. Finally, in silico and in vivo data support that the glucuronic acid moiety is capable of inducing TLR4/MD-2 activation and enhanced pain. These data provide the first evidence for a TLR4 and IL1 mediated component to M3G-induced effects, likely of at least microglial origin.
Keywords: minocycline, 17-DMAG, (+)-naloxone, nonstereoselective, allodynia, hyperalgesia
The neuroactive metabolites of morphine can contribute to its effects. Morphine is principally metabolized in the liver, but is also metabolized in the central nervous system (CNS) (King et al., 1999). In humans, ~44–55% of morphine is metabolized to morphine-3-glucuronide (M3G), 9–15% to morphine-6-glucuronide (M6G), 8–10% excreted as morphine and the remainder converted into numerous minor metabolites (Christrup, 1997; Andersen et al., 2003). Clinical studies have consistently found M3G and M6G in the CNS following systemic morphine, including in lumbosacral cerebrospinal fluid (CSF) (Sjogren et al., 1998; Dale et al., 2007).
One of the major metabolites of morphine, M6G, is a μ opioid receptor agonist and a more potent analgesic than morphine (Abbot and Palmour, 1988). In contrast to M6G, M3G has been reported to enhance pain, although the mechanisms are unknown. Unlike M6G, M3G has little-to-no affinity for μ, κ, or δ opioid receptors (Labella et al., 1979). To date, all studies of M3G-induced allodynia and hyperalgesia have sought exclusively neuronal mechanisms to explain its effects. Behaviorally, inhibited NMDA receptor activity or increased GABA activity can reduce the effects of M3G (Bartlett et al., 1994b). However, M3G has no affinity for NMDA or GABA receptors (Bartlett et al., 1994a) and in vitro its effects on NMDA are not direct (Hemstapat et al., 2003).
We propose an alternative hypothesis for M3G-induced pain enhancement, through M3G-induced proinflammatory responses by immunocompetent cells including microglia. The proinflammatory molecules released by activated microglia and other immunocompetent cells increase neuronal excitability, which has been shown to increase pain sensitivity in various pain models (for review, Milligan and Watkins, 2009). Studies of morphine indicate that a proinflammatory response opposes morphine analgesia, and microglial and astrocyte activation markers are upregulated following repeated morphine (Song and Zhao, 2001; Hutchinson et al., 2009a). Behaviorally, blocking morphine-induced release of proinflammatory molecules prolongs acute morphine analgesia and attenuates morphine-induced hyperalgesia (Johnston, 2004; Hutchinson et al., 2008a). Given the structural similarity between morphine and M3G, we hypothesized that M3G may induce glial activation and release of proinflammatory molecules, and that this effect may be involved in enhanced pain in response to both M3G and morphine.
It has largely been assumed that morphine-mediated effects on microglia and astrocytes were due to μ opioid receptor activity, the same mechanism as the analgesic effects of morphine on neurons. However, a growing body of evidence suggests that a novel, non-classical opioid receptor is primarily responsible. In contrast to morphine's analgesic activity, glial activation following morphine is nonstereoselective, and there is a similar lack of stereoselectivity in naloxone inhibition of morphine-induced glial activation (Hutchinson et al., 2009c). One candidate receptor for nonstereoselective morphine-induced glial activation is toll-like receptor 4 (TLR4). TLR4 is expressed predominantly by microglia in the CNS and, when activated by a wide range of endogenous or exogenous molecules, leads to microglial activation and release of proinflammatory products including interleukin-1 (IL1, Lehnardt et al., 2003). TLR4 is activated by opioids in a nonstereoselective manner (Hutchinson et al., 2009c), and inhibited by both (+)- and (−)-naloxone (Hutchinson 2008b), consistent with observations of morphine-induced glial activation.
This study seeks to investigate a potential non-neuronal component to M3G-enhanced pain. We present evidence that inhibitors of TLR4 signaling can block M3G-induced TLR4 activity in vitro. We then employ a range of pharmacological approaches to explore mechanisms underlying M3G-induced pain enhancement and define the effect of M3G on mRNA levels in the dorsal spinal cord reflective of a proinflammatory process.
Materials and Methods
Animals
Adult, male, pathogen-free Sprague-Dawley rats (Harlan Labs, Madison, WI) were used for all in vivo experiments. Rats (350–400 g at time of experiments) were housed in temperature (23±3 °C) and light (12 h:12 h light:dark) controlled rooms with water and food given ad libitum. All habituation and behavioral testing procedures were performed during the light phase of the daily cycle. All procedures were approved by the University of Colorado-Boulder Institutional Animal Care and Use Committee. Unless otherwise noted, each experimental group was comprised of 6 rats.
Drugs
M3G, minocycline, (−)-naloxone and glucuronic acid were purchased from Sigma (St. Louis, MO). (+)-Naloxone was obtained from the National Institute on Drug Abuse. IL1 receptor antagonist (IL1ra) was purchased from Amgen (Thousand Oaks, CA). 17-DMAG was purchased from Tocris Biosciences (Ellisville, MO). Endotoxin-free, sterile 0.9% saline (Abbott Laboratories, North Chicago, IL) was used as a vehicle for all drugs in behavioral studies (Experiments 3–6, 8). M3G, saline, (+)-naloxone, (−)-naloxone, minocycline, 17-DMAG and IL1ra were confirmed to be endotoxin-free. Where appropriate, doses are reported as a free base concentration.
All drugs administered in vivo were given intrathecally over lumbosacral spinal cord in Experiments 3–6, 8 and 11. M3G was administered at a dose of 0.75 μg, based on pilot studies. Minocycline was administered as 2 successive doses (100 μg followed by 33.3 μg), with the first dose prior to M3G and the second dose concurrent with M3G, as described in the experimental procedures below. This dosing regimen was based on Ledeboer et al. (2005), which showed that minocycline is more effective at blocking microglial activation than reversing it. (+)-Naloxone and (−)-naloxone were given at a dose of 20 μg, consistent with Hutchinson et al. (2008b), which showed a significant reversal of neuropathic pain by this dose of (+)-naloxone. IL1ra was administered at a dose of 100 μg, a dose that potentiates morphine analgesia by reducing the proinflammatory response by glia and mitigating the neuroexcitatory effects of IL1 (Hutchinson et al., 2008a). In Experiment 4, a second dose of IL1ra was given 60 min into the 125 min time course to obviate the short half-life of IL1ra (Milligan et al., 2005). 17-DMAG was given at 4 μg, a dose which suppresses neuropathic pain (Hutchinson et al., 2008d). In Experiment 5, IL1ra and (+)-naloxone were each co-administered with M3G and readministered 1.5 h into the experiment due to their short half-lives. For the same reason, the second mechanical sensitivity testing time point was moved from 4 h to 3 h between Experiment 3a and Experiment 5. In Experiment 11, glucuronic acid was administered in equimolar concentration to the M3G dose, at a dose of 0.38 μg and (+)- naloxone was again administered at a dose of 20 μg.
Intrathecal catheter implantation and injections
Acute intrathecal injections were used to administer drugs in Experiment 3a. Acute indwelling intrathecal catheters were used to administer drugs in Experiments 3b, 4, 6 and 8. Chronic indwelling intrathecal catheters were used in Experiments 5 and 11. Catheter implantations via the L5/L6 intervertebral approach and drug microinjections were performed based on Milligan et al. (1999). For each of the 3 procedures, rats were briefly anaesthetized under isoflurane anesthesia. An 18-gauge needle was placed between L5 and L6 into the intrathecal space and served as a guide. Sterile polyethylene-10 tubing was threaded rostrally through the guide so to terminate over the lumbosacral enlargement.
For acute injections in Experiment 3a, the catheters were pre-loaded with drugs at the intrathecal end and the remainder filled with sterile saline. Upon insertion, the drug was injected with a 10.5 μl flush to ensure complete drug delivery, and the needle and catheter then removed.
For acute indwelling lumbosacral catheters, used in Experiment 3b, 4, 6 and 8 the 18-gauge needle was removed after catheter placement and the tubing secured to the superficial musculature of the lower back with 3–0 silk suture. The tubing was then threaded subcutaneously to exit the nape of the neck. Catheters were 90 cm in length, pre-loaded with drugs at the intrathecal end and the remainder filled with sterile saline. This allowed remote injection of drug during behavioral testing without disturbing the animal, and the injection of a small void volume (10.5 μl) that ensured delivery of drugs. Drug administration occurred 2 h after intrathecal catheter placement.
Chronic indwelling lumbosacral catheters used in Experiment 5 and 11 were 45 cm in length and heat sealed at the external end until used for drug injections. Rats were allowed to recover from this catheter placement for 7 d before behavioral testing and drug administration. Catheters were filled with sterile saline and the appropriate drug dose in 1 μl, followed by 3 μl of saline. Injections had a total volume of 50 μl, due to the void volume of the catheter that preceded the drug into the intrathecal space.
Behavioral Testing
All testing was conducted by an experimenter blind to group assignment. Separate rats were used for assessing thermal and mechanical response thresholds. Rats received at least four 1 h habituations to the appropriate testing apparatus and environment prior to baseline testing, as in our previous studies (Hutchinson et al. 2008b). There were six rats in each experimental group.
Hargreaves test for thermal hyperalgesia
Thermal testing measured withdrawal latency to radiant heat applied separately to the tail and the plantar surface of each hind paw in a modified Hargreaves test (Hargreaves et al., 1988). Baseline latencies prior to drug administration were calculated as the average of two latencies, measured 15 min apart. Following drug administration, latencies to withdrawal from each paw and the tail were measured at 10 min intervals for 125 min. The intensity of the heat source was adjusted such that pre-drug latencies to withdrawal were 8–10 s, with a 20 s cut off to avoid tissue damage. This allowed both analgesia and hyperalgesia to be measured. The percent maximum possible effect (%MPE) was calculated for each testing point from each rat as: %MPE=[test latency- baseline latency]/[cut-off - baseline latency] x100, as originally established by Grumbach (1966).
von Frey test for low threshold mechanical allodynia
The response threshold to light touch on both hind paws was measured using calibrated microfilaments (von Frey hairs; Stoelting, Wood Dale, IL, USA), as described in Milligan et al. (2000). Briefly, a logarithmic range of hairs from 0.406–15.136 g force were used, allowing both analgesia and allodynia to be measured, following the standard up-down procedures previously described (Chaplan et al., 1994). Baseline thresholds were taken as the average of two tests from each paw. Responses were fitted to a Gaussian integral psychometric function using a maximum-likelihood fitting method as described in Milligan et al. (2000).
Cellular and Molecular Analyses
In vitro assay for TLR4 signaling
A human embryonic kidney-293 (HEK 293) cell line was used that was stably transfected by Invivogen to over-express human TLR4 and co-receptor molecules (MD-2, CD14) (293-htlr4a-md2cd14; here referred to as HEK-TLR4). In addition, these cells stably express an optimized alkaline phosphatase reporter gene under the control of a promoter inducible by transcription factors, such as NF-κB and AP-1, activated as part of the TLR4 signaling cascade. Secreted alkaline phosphatase (SEAP) protein is produced as a consequence of TLR4 activation.
HEK-TLR4 cells were grown at 37 °C (5% CO2; VWR incubator model 2300) in 10 cm dishes (Greiner Bio-One, CellStar 632171; Monroe, NC, USA) in normal supplement selection media (DMEM media [Invitrogen, Carlsbad, CA, USA] supplemented with 10% fetal bovine serum [Hyclone; Logan, UT, USA], HEK-TLR4 selection [Invivogen]; Penicillin 10,000 U/ml [Invitrogen]; streptomycin 10 mg/ml [Invitrogen], Normocine [Invivogen], and 200 nM L-Glutamine [Invitrogen]). The cells were then plated for 48 h in 96 well plates (Microtest 96 well plate, flat bottom, Becton Dickinson; 5 × 103 cells/well) with the same media. After 48 h, supernatants were removed and replaced with 180 μl artificial cerebrospinal fluid (sterile aCSF; 124 mM NaCl, 5 mM KCl, 0.1 mM CaCl2 2H20, 3.2 mM MgCl2 6H20, 25 mM NaHCO3, 10 mM glucose, pH 7.4) to model in vivo CNS conditions. Drugs under test were added in concentrations indicated and incubated for 24 h. Supernatants (15 μl) were then collected from each well for immediate assay.
SEAP levels in the supernatants were assayed using the Phospha-Light System (Applied Biosystems) according to the manufacturer's instructions. This chemiluminescent assay incorporates Tropix CSPD chemiluminescent substrate. The 15 μl test samples were diluted in 45 μl of 1x dilution buffer, transferred to 96-well plates (Thermo, Walthma, MA, USA), and heated at 65°C in a water bath (Model 210, Fisher Scientific, Pittsburgh, PA, USA) for 30 min, then cooled on ice to room temperature. Assay buffer (50 μl/well) was added and, 5 min later, reaction buffer (50 μl/well) is added and allowed to incubate for 20 min at room temperature. The light output is then measured in a microplate luminometer (Dynex Technologies, #IL213.1191, Chantilly, VA, USA).
In vitro assay for microglial cell IL1 production
A murine microglial cell line, BV-2 (gifted by Rona Giffard, Stanford University) were grown in macrophage serum free media (Invitrogen, Carlsbad, CA) in 75 cm2 Primaria-treated flasks (Falcon, BD Biosciences, San Jose, CA) with no supplementation at 5% CO2 and 37 °C. 200 000 cells/well were plated in 6-well Primaria-treated plates (Falcon, BD Biosciences, San Jose, CA) for 48 h, in 1.6 ml of the same media, before drug administration.
Enzyme-linked Immunosorbant Assay (ELISA) for IL1 protein
Cell lysates from BV-2 cells and CSF samples were assayed for IL1 protein content using commercially available mouse (BV-2 cell lysates) and rat (CSF samples) IL1β kits (R&D Systems, Minneapolis, MN). Procedures were conducted according to manufacturer's directions. CSF samples were diluted 1:10 due to small sample volumes, as described in O'Connor et al. (2004). The rat IL1 kit has a minimum detection threshold of <5 pg/ml and an inter- and intra-assay variability of <10%. The mouse IL1 kit had a minimum detection threshold of 6 pg/ml.
mRNA isolation and real-time polymerase chain reaction (RT-PCR) quantification
Total RNA was isolated as described in Johnston et al. (2004). Samples were DNase treated (DNA-free kit; Ambion), followed by cDNA synthesis. Amplification of cDNA was performed using the QuantiTect SYBR Green PCR Kit (Qiagen) in iCycler iQ 96 well PCR plates (Bio-Rad) on a MyiQ Single Color Real-Time PCR Detection System (Bio-Rad), as previously described (Johnston et al., 2004). SYBR Green 1 fluorescence (PCR product formation) was monitored in real time using the MyiQ Single Color Real-Time PCR Detection System (Bio-Rad). Threshold for detection of PCR product was set in the log-linear phase of amplification and the threshold cycle (CT, the number of cycles to reach threshold of detection) was determined for each reaction. The levels of the target mRNAs were quantified, using blinded procedures, relative to the level of the housekeeping gene β-actin using the comparative ΔΔCT method. In the event that an individual mRNA level was more than two standard deviations above the group when not included, it was considered an outlier and excluded.
The following targets were investigated and expressed relative to the levels of the housekeeping gene β-actin (BC063166; forward: AGAGGCATCCTGACCCTGAA; reverse: GCTCATTGTAGAAAGTGTGGT): IL1 (M98820; forward: GAAGTCAAGACCAAAGTGG; reverse: TGAAGTCAACTATGTCCCG), Tumor Necrosis Factor-α (TNF) (D00475; forward: CTTCAAGGGACAAGGCTG; reverse: GAGGCTGACTTTCTCCTG), TLR4 (NM_019178; forward: CAGAGGAAGAACAAGAAGC; reverse: CCAGATGAACTGTAGCATTC), and CD11b (NM_012711, forward: CTGGGAGATGTGAATGGAG, reverse: ACTGATGCTGGCTACTGATG). Primers were purchased from Proligo (Boulder, CO, USA).
in silico Docking simulations
in silico Docking simulation methods previously outlined were employed to examine the docking of M3G and (+)-naloxone to TLR4 and MD-2 (Hutchinson et al., 2009c). Briefly, the complexed human TLR4 and MD-2 pdb file was obtained from RCSB Protein Data Bank database (PDBID: 3fxi). Modified pdb files were inputted into AutoDock 4.0 (http://autodock.scripps.edu), hydrogens added, and resaved in pdbqt format. M3G and (+)-naloxone structures were gathered using PubChem isomeric SMILES then converted to .pdb using a structure file generator (http://cactus.nci.nih.gov/services/translate/). All dockings were executed with Lamarkian genetic algorithms.
Statistics
GraphPad Prism (version 5 for Windows, San Diego, CA) software was used for all statistical analyses. Two-way repeated-measures ANOVAs with Bonferroni post-hoc tests when appropriate were used to determine statistical significance between behavioral measures. One-way ANOVAs with appropriate Bonferroni post hocs were used to confirm that there were no baseline differences on behavioral measures. Independent sample t-tests were used to determine statistical significance on the mRNA tests. For all analyses, p<0.05 was considered significant.
Experimental Procedures
Experiment 1: Can the in vitro increase in TLR4 signaling seen with M3G be blocked by a TLR4 antagonist?
M3G (10 μM) was applied to HEK-TLR4 cells as described above. This concentration was determined by pilot studies to produce a robust effect without cytotoxicity. Lipopolysaccharide from Rhodobacter sphaeroides (LPS-RS, Invivogen, San Diego, CA) lacks functional TLR4 activity and acts as a competitive TLR4 antagonist. LPS-RS was applied to the HEK-TLR4 cells at the same time as the M3G at concentrations of 0.0, 0.01, 0.1, 1.0 ng/ml. Cells were incubated for 24 h, then supernatants removed and assessed for SEAP activity, indicative of TLR4 activity.
Experiment 2: Does M3G induce IL1 protein in mouse BV-2 microglial cells?
BV-2 cells were plated in 6-well Primaria-treated plates with 200,000 cells per well. After 48 h, fresh media and M3G (final concentrations of: 10 nM, 100 nM, 1 μM, 10 M in the well) or vehicle was added (n=6 per condition). 24 h after drug administration the supernatants were collected and the cells lysed in 200 μl of sonication buffer (cold Iscoves culture medium containing 5% fetal calf serum and a cocktail enzyme inhibitor: 100 mM amino-n-caproic acid, 10 mM EDTA, 5 mM benzamidine–HCl, and 0.2 mM phenylmethylsulfonyl fluoride). Sonicated samples were centrifuged at 14,000 rpm at 4 °C for 10 min. Supernatants of the cell lysate and the cell culture supernatants were immediately measured for IL1 protein using the mouse ELISA described above.
Experiment 3: Does M3G produce enhanced pain responses?
Experiment 3a
Rats received either 0.75 μg M3G or equivolume (1 μl) saline vehicle, via an acute intrathecal injection, as described above. Both injections had a saline flush for a total injection volume of 12.5 μl. Rats were tested for mechanical allodynia 1 d prior to injection (baseline; BL) and again 0.75 and 4 h post-injection.
Experiment 3b
Drug dosing was identical to that described in Experiment 3a, above. Rats were tested for thermal hyperalgesia just prior to injection (BL) and again from 5–125 min post injection.
Experiment 4: Is M3G induced hyperalgesia blocked by inflammatory and glial modulators?
All rats received 0.75 μg M3G (in 1 μl) coadministered with one of four glial or inflammatory modulators or equivolume (1 μl) saline vehicle. All injections were followed by a saline flush and had a total injection volume of 12.5 μl. Behavioral testing for thermal sensitivity occurred prior to (BL) and 5–125 min post-M3G and coadministered drug. Drugs separately coadministered with M3G were: 100 μg IL1ra, 20 μg (+)-naloxone, 20 μg (−)-naloxone, or 100 μg minocycline administered 2 h prior to 33.3 μg minocycline coadministered with M3G as discussed above.
Experiment 5: Is M3G induced allodynia blocked by inflammatory and glial modulators?
All rats received 0.75 μg M3G (in 1 μl) coadministered with one of 4 glial or inflammatory modulators or equivolume (1 μl) saline vehicle. An additional 6 rats received only equivolume vehicle injections. All injections were followed by a saline flush for a total volume of 50 μl. Behavioral testing for mechanical allodynia occurred prior to (BL), 0.75 and 3 h post-M3G and coadministered drug. IL1ra (100 μg) was coadministered with M3G and again (100 μg) 1.5 h post-M3G administration due to its shorter half-life. Similarly, (+)-naloxone (20 μg) and (−)-naloxone (20 μg) were both coadministered with M3G and administered again 1.5 h after M3G administration due to short half lives. Minocycline was given 0.75 h (100 μg) prior to M3G and coadministered (33.3 μg) with M3G, as discussed above.
Experiment 6: Is M3G induced hyperalgesia reversed by IL1ra?
Rats received either 0.75 μg M3G (in 1 μl) or equivolume saline vehicle, via an acute indwelling intrathecal injection. Both injections had saline flush for a total injection volume of 12.5 μl. Behavioral testing occurred prior to (BL) and then 5–55 min after injection. At this time, either IL1ra or equivolume (12.5 μl) saline vehicle was administered, and response thresholds recorded for an additional 5–65 min post-IL1ra.
Experiment 7: Does the HSP90 inhibitor 17-DMAG block M3G-induced TLR4 signaling in vitro?
M3G (10 μM) was applied to HEK-TLR4 cells as described above and identical to the procedure for Experiment 1. This concentration of M3G was determined by pilot studies to produce a robust effect without cytotoxicity. 17-DMAG, a HSP90 inhibitor, was applied to the HEK-TLR4 cells at the same time as the M3G at concentrations of 0.0, 0.01, 0.1, 1.0 ng/ml. Cells were incubated for 24 h, then supernatants removed and assessed for SEAP activity, indicative of TLR4 activation.
Experiment 8: Does the HSP90 inhibitor 17-DMAG block M3G-induced hyperalgesia?
Rats received 0.75 μg M3G (in 1 μl) with either 4 μg 17-DMAG or equivolume (1 μl) saline vehicle. All injections were followed by a saline flush and had a total injection volume of 12.5 μl. Behavioral testing for thermal sensitivity occurred prior to (BL) and 5–125 min post-M3G and coadministered drug.
Experiment 9: Is there an increase in IL1 CSF protein level or transcription of glial activation markers in the dorsal spinal cord following intrathecal M3G?
M3G (0.75 μg, n=6) or equivolume saline vehicle (n=5) was administered via acute intrathecal injection, as in Experiment 3a. While cell surface marker evidence of glial activation can take 6–8 h to develop (Milligan, 2001) mRNA and proinflammatory cytokine protein expression changes can be detected much more rapidly, in a time frame consistent with M3G's in vivo half-life following a single injection. Sixty min after acute intrathecal M3G injection, rats were deeply anaesthetized with sodium pentobarbital (50 mg/kg) and lumbrosacral CSF was collected via acute intrathecal puncture and immediately flash frozen in liquid nitrogen. Following CSF collection, rats were transcardially perfused with chilled saline. The lumbrosacral enlargement of the spinal cord was exposed by laminectomy and a 1 cm section dissected and moved to an ice chilled glass plate. The meninges were removed and the dorsal half of the spinal cord dissected. The dorsal section was then flash frozen in liquid nitrogen. Samples were stored at −80 °C until RT-PCR or ELISA analysis.
Experiment 10: Does M3G dock in silico the TLR4/MD-2 complex?
Initially, the in silico docking of ligands to the entire TLR4 MD-2 dimer complex was conducted (AutoGrid center set 3.438, −7.805, 2.034; 126 grid points expanding in all directions; GA running number of 100, Max Evals 5 × 106 and 1.0 Å spacing). These data demonstrated that the two ligands docked with human MD-2 independent of human TLR4 interactions. Therefore, all the ligands were docked to MD-2 alone with greater resolution (AutoGrid center set 27.991, 0.851, 19.625; 126 grid points expanding in all directions; GA running number of 100, Max Evals 5 × 106 and 0.375 Å spacing). The lowest energy and highest interaction docking conformation was visualized for (+)- naloxone and M3G. The (+)-naloxone conformation was then saved as a combined MD-2/(+)-naloxone pdf file and the M3G in silico docking was repeated on the new combined MD-2/(+)-naloxone complex to determine the change in docking owing to the presence of already docked (+)-naloxone.
Experiment 11: Does glucuronic acid alone induce pain behaviors and is this a TLR4-dependent mechanism?
Rats received 0.38 μg glucuronic acid (in 1 μl) intrathecally, a dose equimolar to the M3G dose used previously, coadministered with either (+)-naloxone (20 μg in 1 μl) or equivolume saline. All injections were followed by a saline flush for a total volume of 50 μl. Behavioral testing for mechanical allodynia occurred prior to (BL), 0.75 and 3 h following glucuronic acid administration. (+)-Naloxone (20 μg) was coadministered with glucuronic acid and administered again (20 μg) 1.5 h post-M3G administration due to its shorter half-life.
Results
In all studies employing the von Frey test, no differences between right and left hind paws were detected. Thus, results are reported as the average of the response thresholds recorded for both paws. In response to the Hargreaves test, both hind paws and tail latencies to withdrawal showed identical patterns of results. As consistent data were obtained across both hind paws and the tail, only tail flick responses are reported for simplicity.
Experiment 1: M3G-induced TLR4 activation is blocked by a TLR4 antagonist
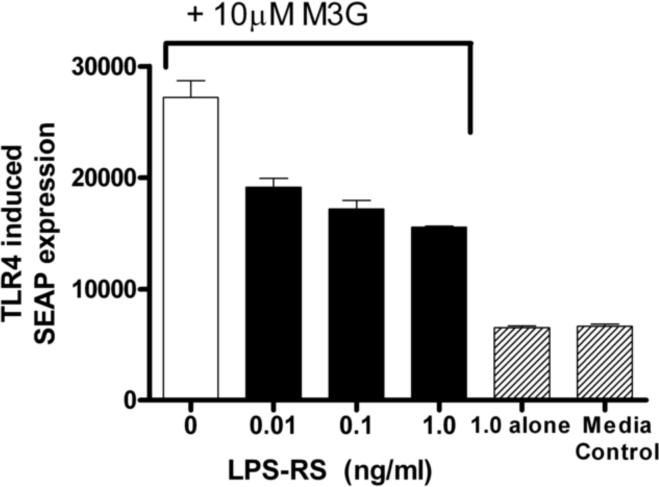
Previous studies have shown that M3G increases TLR4-dependent SEAP expression in HEK-TLR4 cells compared to media control, an effect dose-dependently blocked by (+)-naloxone (Hutchinson et al., 2009c). HEK cells with the SEAP reporter gene but without TLR4 overexpression did not increase SEAP expression (Hutchinson et al., 2009c). Here, the M3G-induced TLR4-dependent SEAP expression was dose-dependently suppressed by LPS-RS, a classical TLR4 competitive antagonist (F5,6= 110, p<0.05, Figure 1), providing further evidence that M3G can signal through TLR4. Post-hoc analyses showed that LPS-RS significantly reduced SEAP TLR4 signaling at concentrations of 0.01, 0.1 and 1.0 ng/ml. LPS-RS alone, without coadministration of M3G, did not produce any increase in SEAP expression above media control.
Figure 1.
M3G causes a significant increase in HEK-TLR SEAP reporter protein which is significantly reduced by the TLR4 antagonist LPS-RS at all three concentrations (F(5,6)= 110, p<0.05). Error bars represent standard error of the mean.
Experiment 2: Mouse BV-2 microglial cells produce IL1 in response to M3G in vitro
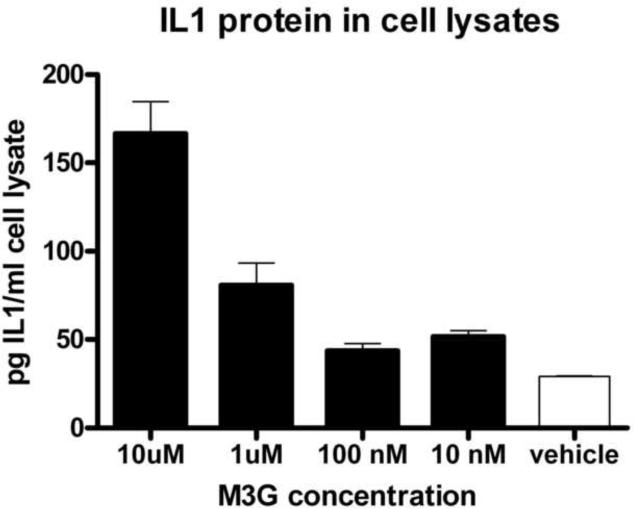
Given that TLR4 is expressed on microglial cells, IL1 is an end product of TLR4 activation, and that both activated microglial cells and IL1 can each contribute to enhanced pain, we explored whether M3G could produce a proinflammatory response in mouse BV-2 microglial cells. These cells were stimulated with 10 μM, 1 μM, 100 nM, 10 nm or 0 μM M3G for 24 h. M3G dose dependently increase IL1 protein (Figure 2, F(4,25)=28.9, p<0.05). Post-hoc test showed significant increases in IL1 protein in response to 10 μM (t=9.53, p<0.05) and 1 μM M3G (t=3.57, p<0.05) compared to vehicle. There was no increase in IL1 protein in the cell culture supernatants, suggesting that IL1 is produced following M3G stimulation in vitro but not released under these experimental conditions.
Figure 2.
M3G induces a significant increase in IL1 protein production in the mouse BV2 microglial cell line at concentrations of 10 μM and 1 μM (F(4,25)=28.89, p<0.05). Error bars represent standard error of the mean.
Experiment 3: Intrathecal M3G induces thermal hyperalgesia & mechanical allodynia
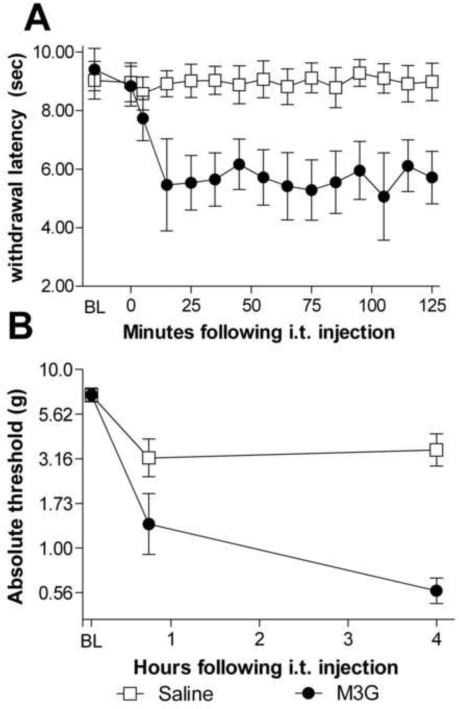
To confirm that M3G induces exaggerated pain responses under our laboratory conditions, we examined both thermal (Hargreaves test) and mechanical (von Frey test) sensitivities following acute intrathecal injection of M3G compared to an equivolume saline injection. On the Hargreaves test, there was no difference on baseline latencies to withdrawal from radiant heat prior to drug administration (t10=0.53, p>0.05). Following M3G administration, there was a rapid and reliable decrease in latencies compared to saline controls (Figure 3A, F(1,10)=43.3, p<0.05).
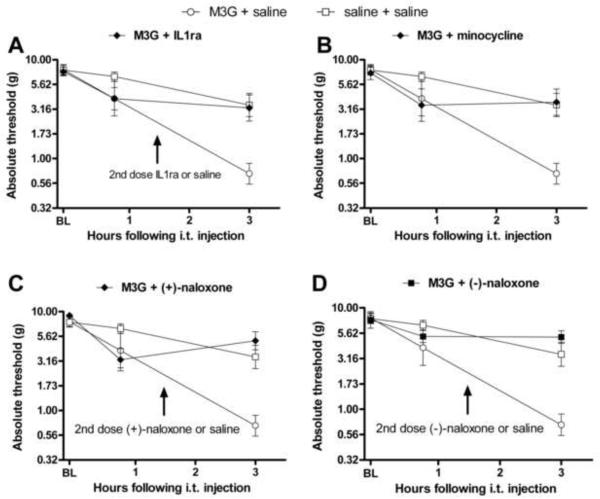
Figure 3.
Intrathecal M3G causes the rapid onset of robust thermal hyperalgesia (A) and tactile allodynia (B). Intrathecal M3G (0.75 μg) induced significant decrease in withdrawal latencies from a radiant heat source (main effect of drug treatment F(1,10)=43.3, p<0.05, A). The M3G group had a baseline withdrawal latency mean of 9.4 s and the saline group had a baseline withdrawal mean of 8.9 s. The same dose of M3G produced a significant increase in sensitivity to calibrated pressure stimuli 4 h following injection (main effect of drug treatment F(1,10)=26.2, p<0.05, B). Error bars represent standard error of the mean.
On the von Frey test, there was no difference between groups on baseline response to calibrated pressure stimuli prior to intrathecal injection (t10=0.03, p>0.05), but a robust decrease in tactile thresholds in rats given M3G compared to those receiving saline (Figure 3B, F(1,10)=26.2, p<0.05). Post-hoc analysis revealed a significant decrease in response threshold in rats receiving M3G compared to saline 4 h post-M3G administration (t10=5.78, p<0.05).
Experiment 4: M3G induced hyperalgesia is blocked by glial and inflammatory inhibitors
As Experiment 3 clearly showed that intrathecal M3G rapidly and potently produced hyperalgesia, the question arises as to the mechanisms underlying that effect. The induction of IL1 production in microglial cells in Experiment 2 suggests that proinflammatory microglial activation may be involved. Here, we explore whether modulating glial activation or proinflammatory molecules will affect this M3G-induced hyperalgesia. To investigate this, we tested a range of glial and inflammatory modulators to determine if they could block M3G hyperalgesia. As detailed below, all glial activation and inflammatory inhibitors tested blocked M3G hyperalgesia when coadministered with M3G. There were no baseline differences between any of the groups tested (F(5, 30)=0.67, p>0.05). When all M3G, saline, and coadministered blocker groups were analyzed, there was a significant main effect of drug treatment on behavioral responses (F(5,30)=13.04 p<0.05). Post-hoc analyses were used to delineate what treatment and time points were significantly different, as reported below.
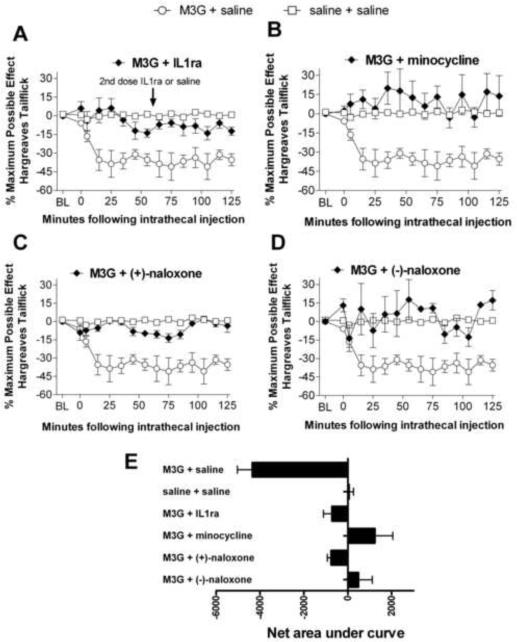
IL1 is a proinflammatory cytokine known to be induced by TLR4 signaling and upregulated in the CNS following morphine administration (Johnston, 2004; Hutchinson et al., 2008a). Blocking the effect of IL1 with its receptor antagonist (IL1ra), while having no effect in the absence of morphine, increases the duration of morphine analgesia, suggesting that IL1-induced neuroexcitation opposes the pain-suppressive effects of morphine (Hutchinson et al., 2008a). Coadministration of M3G + IL1ra suppressed M3G-induced hyperalgesia (Figure 4A). The area under the curve was significantly smaller for M3G + IL1ra than M3G + saline, indicative of reduced hyperalgesia when IL1ra was also administered (Figure 4E). Post hoc analysis showed the M3G + saline group had significantly faster (p<0.05) withdrawal latencies than M3G + IL1ra at 15, 25, 35, and 75 min following intrathecal injection. There were no significant differences between M3G + IL1ra and the saline + saline control group, indicative of a complete blockade of M3G induced hyperalgesia by IL1ra.
Figure 4.
M3G-induced hyperalgesia is blocked by several inflammatory and glial mediators. Acute intrathecal M3G (0.75) coadministered with one of: IL1ra (100 μg coadministered with M3G, 100 μg at 65 min, baseline latency of 8.1 ± 0.33 s, A), minocycline (100 μg 120 min prior to M3G and 33.3 μg with M3G, baseline latency of 8.8 ± 0.69 s, B), (+)-naloxone (20 μg, baseline latency of 8.8 ± .51 s, C), (−)-naloxone (20 μg, baseline latency of 9.7 ± 0.90 s, D), blocks the rapid onset of M3G-induced hyperalgesia (main effect of drug F(5,30)=13.04 p<0.05). Area under the curve analyses of these time courses showed M3G + saline had a significantly larger area under the curve than saline + saline, M3G + IL1ra, M3G + minocycline, M3G + (+)-naloxone, M3G + (−)-naloxone (F(5, 30)=13.1, p<0.05, E). Saline and M3G groups are repeated on each graph to facilitate visual comparisons. Error bars and ranges represent standard error of the mean.
Minocycline has been shown to reduce microglial activation, proinflammatory cytokines and neuropathic pain following inflammatory nerve injury and gp120-induced spinal glial activation (Ledeboer et al., 2005). Minocycline alone has no effect on basal pain thresholds (Ledeboer et al., 2005). Coadministration of minocycline (100 μg) with M3G did not significantly attenuate pain behaviors (n=4, data not shown). However, a dosing paradigm where minocycline (100 μg) was given 2 h prior to the M3G and an additional 33.3 μg of minocycline successfully suppressed M3G-induced hyperalgesia (Figure 4B). This result is consistent with prior work showing that minocycline is more effective when given prior to the inflammatory stimulus (Ledeboer, 2005). The area under the curve was significantly smaller for M3G + minocycline than M3G + saline, indicating reduced hyperalgesia when minocycline was administered along with the M3G (Figure 4E). Post-hoc analyses showed a significant decrease in withdrawal latencies between M3G + saline relative to M3G + minocycline groups at every time point from 15–125 min following intrathecal injection. There were no significant differences in withdrawal latencies between M3G + minocycline and saline + saline groups, indicative of a complete blockade of M3G induced hyperalgesia by minocycline.
(+)-Naloxone has been shown to block TLR4 signaling by the classic TLR4 agonist LPS and by morphine in both HEK-TLR4 and RAW macrophages (Hutchinson et al., 2008b; Hutchinson et al., 2009c), reduce proinflammatory cytokine induction and glial activation following nerve injury, and reverse TLR4-dependent neuropathic pain (Hutchinson et al., 2008b). Coadministration of (+)-naloxone with M3G significantly reduced M3G-induced hyperalgesia (Figure 4C). The area under the curve was significantly smaller for M3G + (+)-naloxone than M3G + saline (Figure 4E). Post-hoc analysis showed a significant decrease in withdrawal latencies in the group administered M3G + vehicle relative to M3G + (+)-naloxone at 25, 35, 95 and 105 min following intrathecal injection. No significant differences were observed between M3G coadministered with (+)-naloxone and saline controls, indicative of a complete blockade of M3G induced hyperalgesia by minocycline.
(−)-Naloxone is the opioid-active, clinically employed isomer of naloxone, which blocks the effect of opioids on classical opioid receptors. However, M3G does not have any activity at those receptors (Labella, 1979), so its hyperalgesic effects are not accounted for via such actions. In contrast, both the (+) and (−) isomers of naloxone are able to block TLR4 activation and subsequent proinflammatory response with similar efficacy (Hutchinson et al., 2008b). We predicted that (−)-naloxone would be similarly effective to (+)-naloxone in blocking M3G-induced hyperalgesia. Coadministration of M3G + (−)-naloxone blocked M3G-induced hyperalgesia (Figure 4D). The area under the curve was significantly smaller for M3G + (−)-naloxone than M3G + saline (Figure 4E). Post-hoc analysis showed a significant decrease in withdrawal latencies in the group administered M3G + vehicle relative to M3G + (−)-naloxone at 15, 25, 35, 45, 55, 65, 75, 115 and 125 min following intrathecal injection. There were no significant differences between M3G coadministered with (−)-naloxone and saline controls, indicative of a complete blockade of M3G induced hyperalgesia by (−)-naloxone.
Experiment 5: M3G-induced allodynia is blocked by glial and inflammatory inhibitors
The mechanisms of allodynia and hyperalgesia are not identical (Zimmermann, 2001). Given the success of glial and inflammatory modulators in reducing M3G induced hyperalgesia, we investigated these molecules on M3G induced allodynia. Rationales for the drugs selected for test are as described in Experiment 4, above. Coadministered IL1ra, minocycline, (+)-naloxone and (−)-naloxone were all able to block M3G-induced allodynia. There were no baseline differences between any of the experimental groups (F(5, 28)=.33, p>0.05). When all M3G, saline, and coadministered blocker groups were analyzed there was a significant main effect of drug treatment on behavioral responses (F(5,28)=2.73 p<0.05, Figure 5). Post-hoc analyses were used to determine what treatments and time points were significantly different from M3G + saline controls, and are summarized below.
Figure 5.
M3G-induced allodynia is blocked by glial and immune mediators (F(5,28)=2.73 p<0.05). Acute intrathecal M3G (0.75 μg) produced allodynia which was blocked by any one of: IL1ra (100 μg coadministered with M3G, 100 μg at 1.5 h, A), minocycline (100 μg 1 h prior to M3G, 33.3 μg with M3G, B), (+)-naloxone (20 μg coadministered with M3G, 20 μg at 1.5 h, C) or (−)-naloxone (20 μg coadministered with M3G, 20 μg at 1.5 h, D). Saline and M3G groups are repeated on each graph to facilitate visual comparisons. Error bars represent standard error of the mean.
Coadministration of IL1ra with M3G resulted in the blockade of M3G-induced allodynia. Post-hoc analysis showed that M3G + IL1ra group had significantly decreased sensitivity to mechanical stimuli compared to M3G + saline 3 h following intrathecal administration, but not at 45 min following injection (Figure 5A). There were no significant differences between M3G coadministered with IL1ra and saline controls.
Coadministration of minocycline with M3G resulted in the blockade of M3G-induced allodynia. Post-hoc analysis showed that M3G + minocycline group had significantly decreased sensitivity to mechanical stimuli compared to M3G + saline 3 h following intrathecal administration, but not at 45 min following injection (Figure 5B). There were no significant differences between M3G coadministered with minocycline and saline controls.
Coadministration of (+)-naloxone with M3G resulted in the blockade of M3G-induced allodynia. Post-hoc analysis showed that M3G + (+)-naloxone group had significantly decreased sensitivity to mechanical stimuli compared to M3G + saline 3 h following intrathecal administration, but not at 45 min following injection (Figure 5C). There were no significant differences between M3G coadministered with (+)-naloxone and saline controls, indicative of a complete blockade of M3G hyperalgesia.
Coadministration of (−)-naloxone with M3G resulted in the blockade of M3G-induced allodynia. Post-hoc analysis showed that M3G + (−)-naloxone group had significantly decreased sensitivity to mechanical stimuli compared to M3G + saline 3 h following intrathecal administration, but not at 45 min following injection (Figure 5D). There were no significant differences between M3G coadministered with (−)-naloxone and saline controls, indicative of a complete blockade of M3G hyperalgesia.
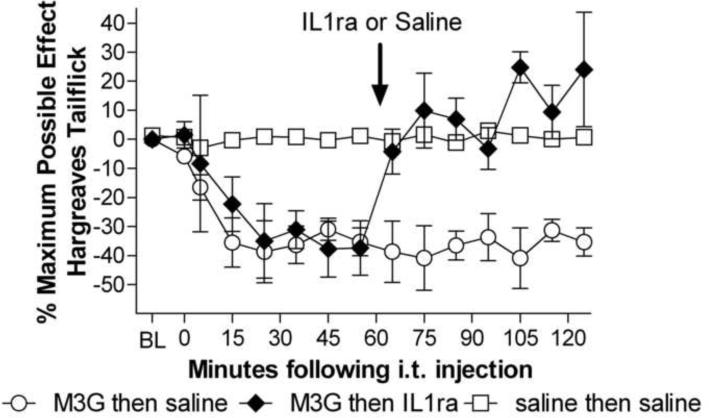
Experiment 6: IL1ra reverses established M3G hyperalgesia
Given the effectiveness of IL1ra in blocking M3G induced hyperalgesia and allodynia, we investigated whether continuing IL1 release was necessary for the maintenance of M3G induced hyperalgesia. IL1ra completely reversed established M3G hyperalgesia. There were no baseline differences between M3G + vehicle, M3G + IL1ra, and vehicle + vehicle groups (F(2,14)=0.14, p>0.05). There was a significant main effect of drug treatment (F(2,14) = 14.81, p<0.05, Figure 6) and a significant interaction between drug treatment and time (F(2,14)= 4.71 p<0.05). Post-hocs test showed that there were no significant differences between the M3G + later IL1ra and M3G + saline groups prior to IL1ra administration. The M3G + later IL1ra group had significantly shorter withdrawal latencies prior to IL1ra administration when compared to saline at 25, 45, 55 min following intrathecal M3G, prior to intrathecal IL1ra. Following IL1ra administration 60 min after M3G administration, the M3G + IL1ra group's withdrawal latencies rapidly increased to become significantly longer than M3G+saline at 65– 85 and 105–125 min following M3G administration. The withdrawal latencies for the M3G + IL1ra groups following IL1ra were indistinguishable from saline + saline withdrawal latencies, indicating that IL1ra was able to fully reverse M3G-induced allodynia.
Figure 6.
Established M3G-induced hyperalgesia is reversed by IL1ra. Intrathecal M3G (0.75 μg) produced hyperalgesia that was significantly reversed by intrathecal IL1ra (100 μg, baseline latency 9.0 ± 0.82 s) given 60 min following M3G (interaction F(2,14)= 4.71 p<0.05). Reversal was maintained for the remainder of the test. Error bars and ranges represent the standard error of the mean.
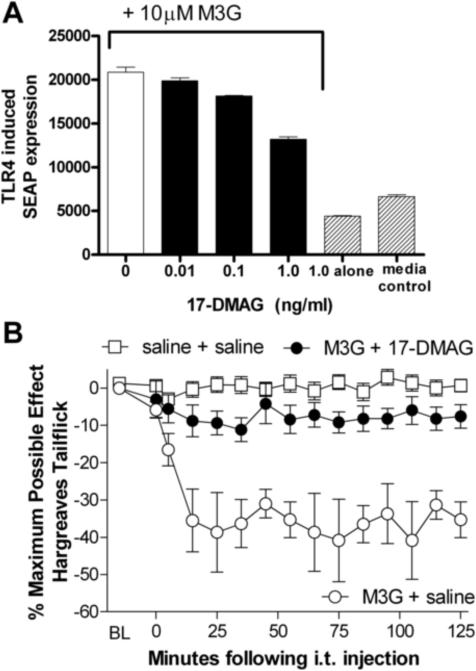
Experiment 7: M3G-induced TLR4 activity is blocked by the HSP90 inhibitor 17-DMAG in vitro
TLR4 is activated in models of spinally mediated pain, but producing pain with TLR4 activation alone using LPS has been unexpectedly difficult. Recent evidence suggests that HSP90 is able to function as a second signal to enhance TLR4 activation (Hutchinson et al., 2008d; Hutchinson et al., 2009b). We tested the HSP90 inhibitor, 17-DMAG, to determine if it would significantly reduce M3G-induced TLR4 activity in vitro. The M3G-induced SEAP signal was significantly blocked by 17-DMAG, (F(3,8)= 94.6, p<0.05, Figure 7A). Post-hoc analyses showed that 17-DMAG reduced M3G-induced TLR4 activity, as measured by SEAP expression, at concentrations of 0.1 (t=6.34, p<0.05) and 1 ng/ml (t=17.8 p<0.05). 17-DMAG without M3G also reduced SEAP expression compared to media control (t=4.64, p<0.05), but a 2 (0 vs 1.0 ng/ml 17-DMAG) × 2 (0 vs 10 μM M3G) ANOVA indicated that the efficacy of 17-DMAG in reducing SEAP expression was greater when M3G was present. This finding suggests that hsp90 may be involved in TLR4 signaling under basal conditions as well as during TLR4 activation.
Figure 7.
The HSP90 inhibitor 17-DMAG significantly reduces M3G-induced TLR4 activation, measured by SEAP activity in TLR4-HEK cells (main effect of 17-DMAG: F(3,8)= 94.6, p<0.05, A). 17-DMAG alone reduced TLR4-induced SEAP signal, but the effect of 17-DMAG depended on whether M3G was present or absent (F(1,7) =57.1, p<0.05). Intrathecal M3G (0.75 μg)-induced hyperalgesia is blocked by coadministration of 17-DMAG (4 μg, F(2,14)=24.28, p<0.05, baseline latency of 8.9 ± 0.36 s, B). Error bars represent standard error of the mean.
Experiment 8: M3G-induced hyperalgesia is blocked by the HSP90 inhibitor 17-DMAG
Experiment 7 documented that the HSP90 inhibitor 17-DMAG was able to block M3G-induced TLR4 signaling in vitro. To determine if HSP90 was involved in M3G-induced hyperalgesia, we tested whether 17-DMAG could block M3G-induced hyperalgesia. 17-DMAG at this intrathecal dose was able to enhance morphine analgesia, presumably by removing anti-nociceptive morphine-induced glial activation, while having no effect on pain thresholds in the absence of morphine (Hutchinson et al., 2008d; Hutchinson et al., 2009b). Experiment 8 was run concurrently with Experiment 4 so the same control groups are used here. There were no significant baseline differences between M3G+ saline, M3G+17-DMAG and saline+saline groups prior to drug administration (F(2,14)=.215, p>.05). Coadministration of the HSP90 inhibitor 17-DMAG with M3G resulted in a blockade of M3G-induced hyperalgesia (F(2,14)=24.28, p<0.05, Figure 7B). The area under the curve was significantly smaller for M3G + 17-DMAG than M3G + saline (F=23.5, p<0.05). Post-hoc analysis showed a significant decrease in withdrawal latencies in the group administered M3G + vehicle compared to M3G + 17-DMAG at 15, 25, 35, 45, 55, 65, 75, 85, 95, 105, 115 and 125 min following intrathecal injection. There were no significant differences between M3G coadministered with 17-DMAG and saline controls. The relatively complete blockade here, compared to the partial blockade seen in in vitro Experiment 7 may be, at least in part, explained by the short 2-hour time course of behavioral measurement, rather than the 24 time point for the SEAP assay.
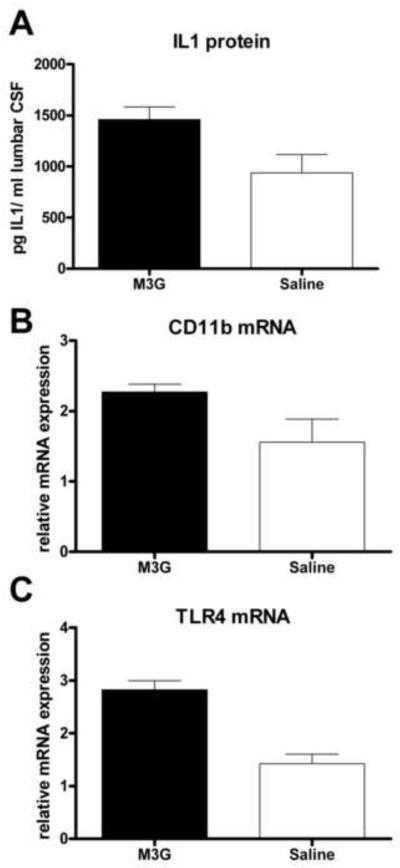
Experiment 9: M3G increases the levels of glial activation marker mRNAs and proinflammatory molecules
Given the success of microglial and inflammatory inhibition in blocking M3G-induced allodynia and hyperalgesia, we examined the changes in IL1 protein expression in the CSF and proinflammatory and glial activation marker gene transcription in the dorsal spinal cord. Our behavioral data showed a rapid onset of allodynia that can be blocked with IL1ra, indicative of rapid IL1 release following M3G administration. In support of this, intrathecal M3G (0.75 μg) caused an increase in IL1 protein in the lumbrosacral CSF in rats given M3G compared to saline vehicle (t9=2.461, p<0.05, Figure 8A).
Figure 8.
M3G significantly upregulates IL1 protein in the CSF and the mRNA levels of glial activation markers in the dorsal spinal cord. Intrathecal M3G (0.75 μg) significantly increased IL1 protein in the CSF (t9=2.461, p<0.05, A) and mRNA levels of CD11b (t8= 2.45, p<0.05, B) and TLR4 (t8= 5.44, p<0.05, C) compared to equivolume saline vehicle controls. Error bars represent standard error of the mean.
We then analyzed dorsal spinal cord (meninges removed) 60 min after 0.75 μg intrathecal M3G administration for changes in mRNA expression relative to the housekeeping gene β-actin using RT-PCR. There was no difference between treatment groups in the level of β-actin mRNA (t9= 0.66, p>0.05)
Our behavioral data suggested that microglia were activated by M3G, and previous research has shown that the microglial activation marker CD11b is upregulated following chronic morphine administration (Hutchinson et al., 2009a). Supporting and extending those results, we found a significant upregulation of CD11b mRNA 60 min following intrathecal M3G (t8= 2.45, p<0.05, Figure 8B). TLR4 mRNA is increased following glial activation in both acute (Raghavendra et al., 2004) and chronic pain models (Tanga et al., 2004). Again supporting and extending those results, here we found a significant increase in TLR4 mRNA 60 min following intrathecal M3G (t8= 5.44, p<0.05, Figure 8C), consistent with an increase in proinflammatory activation of glia. At the time point sampled, the proinflammatory cytokines IL1 and TNF showed no significant changes at an mRNA level between rats administered intrathecal M3G and those administered intrathecal saline.
Experiment 10: M3G docks in silico to MD-2 in a (+)-naloxone sensitive manner
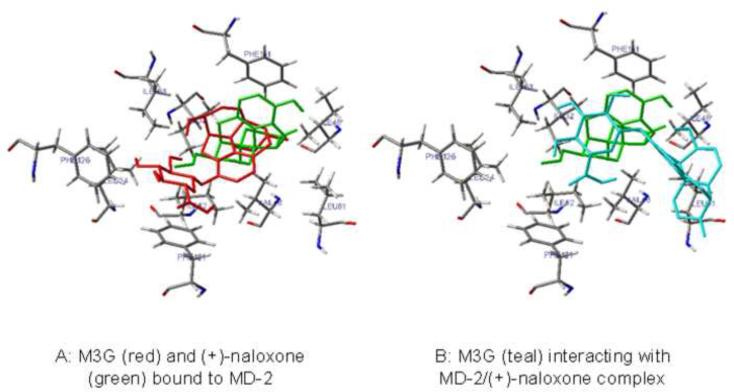
Experiments 1–9 provide substantial evidence for a TLR4-dependent pro-inflammatory response to M3G in the central nervous system. Therefore, to further validate these in vitro and in vivo experimental data, an in silico docking analysis was conducted using the recently published high-resolution crystalline structure of the dimer of human TLR4 and MD-2 (Park et al., 2009) and the in silico docking software suite AutoDock 4. In addition, the possible interaction of (+)-naloxone with M3G in silico was examined. Using AutoDock 4, 100 independent docking simulations were run for each ligand with the entire dimer of human TLR4 and MD-2. M3G and (+)-naloxone both docked with the LPS binding cleft of MD-2, independent of interactions with TLR4. Current docking simulations have higher confidence in predicting the binding site than calculating the binding energies. Therefore, the in silico docking was repeated with MD-2 alone at higher resolution (0.375 Å), M3G and (+)-naloxone docked to the same pocket of MD-2 (Figure 9). Interestingly, the glucuronic acid portion of M3G interacted closely with MD-2 residues F121 and F126 previously identified as important for TLR4 activation by LPS (Teghanemt et al., 2008). When M3G was docked in silico again to MD-2 with (+)-naloxone already in place, M3G docking was modified substantially such that it no longer interacted with these pivotal MD-2 residues (Figure 9).
Figure 9.
M3G (red) and (+)-naloxone (green) both bind to the LPS-binding pocket on MD-2 in an in silico model (A). When M3G (teal) binding was modeled to a MD-2/(+)-naloxone complex, M3G no longer interacted with the critical LPS-binding residues in MD-2 (B).
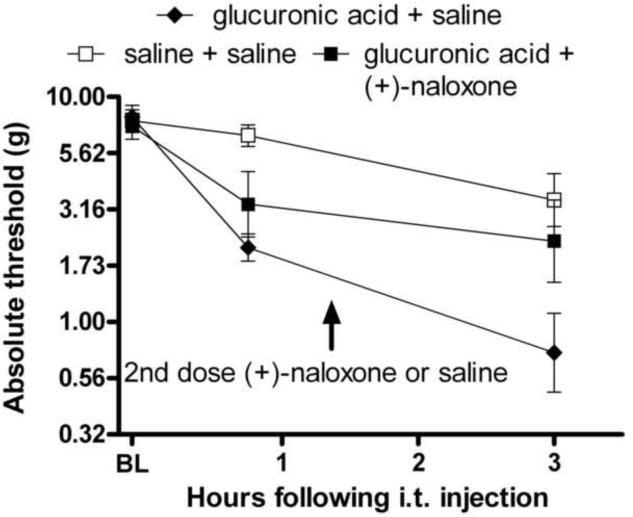
Experiment 11: Glucuronic acid produced enhanced pain sensitivity, and that change is blocked by a TLR4 inhibitor
Based on the results from Experiment 10, we would predict that glucuronic acid alone may be capable of inducing TLR4 activation, and that (+)-naloxone could block this effect. To test whether the glucuronide portion of the molecule is capable of producing enhanced pain in vivo through TLR4-dependent processes, glucuronic acid (0.38 μg) was given intrathecally, coadministered with either (+)-naloxone (20 μg) or saline. As discussed in Experiment 3, (+)-naloxone is the opioid-inactive isomer of naloxone which has been shown to block TLR4 activation. Glucuronic acid produced significant allodynia 0.75 and 3 h following intrathecal injection (Figure 10, F(3,20)=4.23, p<0.05). This allodynia was blocked by (+)-naloxone (20 μg) 3 h following glucuronic acid administration.
Figure 10.
Glucuronic acid causes enhanced pain which is blocked by the TLR4 antagonist (+)-naloxone. Intrathecal glucuronic acid (0.38 μg) produced robust allodynia 0.75 and 3 h following administration (main effect of drug treatment F(3,20)=4.23, p<0.05). Coadministered (+)-naloxone blocked that allodynia 3 h after administration.
Discussion
The present series of studies explored the mechanistic basis of hyperalgesia and allodynia induced by intrathecal administration of the morphine metabolite M3G. We present the first evidence that M3G can activate microglial cells in vitro to induce IL1 release, and that M3G induced pain enhancement is mediated by, at minimum, the proinflammatory cytokine, IL1, as IL1ra prevented and/or reversed M3G-induced hyperalgesia and allodynia and was increased in the CSF 60 min following intrathecal M3G. Additionally, intrathecal minocycline, a compound known to inhibit activation of microglia and other monocyte-derived cells, prevented M3G-induced hyperalgesia and allodynia. TLR4, a receptor in the spinal cord principally expressed on microglia in the central nervous system, appears to be an important link. First, M3G induced TLR4 signaling in vitro, an effect which was blocked both by the TLR4 competitive antagonist, LPS-RS, and by inhibition of the TLR4 cosignaling molecule, HSP90 with 17-DMAG. Behaviorally, the recently discovered inhibitors of TLR4 signaling (Hutchinson et al., 2008b; Hutchinson et al., 2009c), (+)- and (−)-naloxone, were each able to reduce M3G-induced pain enhancement, an effect duplicated by the HSP90 inhibitor 17-DMAG. Providing further evidence for a proinflammatory response to M3G, mRNA for TLR4 and the microglial/macrophage activation marker CD11b were both upregulated 60 min following intrathecal M3G. Finally, in silico modeling confirmed the in vitro and in vivo inhibition of M3G effects by (+)-naloxone, demonstrating M3G docked to MD-2 and interacted closely with pivotal residues on MD-2 which may facilitate TLR4/MD-2 signaling. Importantly, the in silico docking was (+)-naloxone sensitive agreeing with in vitro and in vivo data. The action of glucuronic acid alone was tested in vivo and found to produce marked allodynia which was also blocked by (+)-naloxone, again suggesting a role for TLR4.
All previous studies of M3G have sought a direct action of M3G on neurons to explain its observed effects. The present study provides the first evidence that spinal microglia/macrophages likely contribute to the effects of M3G. Because M3G has no affinity for classical opioid receptors (Labella et al., 1979), M3G must act via a different receptor to create pain enhancement. Our data support TLR4 as a receptor activated by M3G. TLR4 is a pattern-recognition receptor that is expressed predominantly by peripheral and central immunocompetent cells of monocyte origin, including macrophages and microglia. There is little to no evidence of TLR4 expression by spinal cord astrocytes, neurons, or endothelial cells under basal conditions (Miyake, 2007). Further, fluorescently tagged LPS binds to microglia but not to neurons (Lehnardt et al., 2003), although neurons can express and up-regulate TLR4 in response to ischemia (Tang et al., 2007, Tang et al., 2008). TLR4 signaling activation following complexing of the required accessory molecules CD14 and MD-2, induces proinflammatory responses by macrophages following exposure to bacterial lipopolysaccharide (LPS) and other `danger signals'. Like macrophages, TLR4 activation on microglia induces a proinflammatory state, including the release of proinflammatory cytokines, such as IL1, that are well-documented to enhance pain and neuroexcitation (Lehnardt et al., 2003; Milligan and Watkins, 2009).
The present studies expand on prior work indicative of TLR4 signaling activation by both morphine and M3G, but not by the second major metabolite of morphine, M6G. In vitro studies of HEK-TLR4 cells found significant increases in TLR4-dependent reporter protein expression in response to (+)-morphine or (−)-morphine, compared to media control (Hutchinson, 2009c). Intriguingly, M3G, but not M6G, also increased TLR4 signaling by these cells (Hutchinson et al., 2009c and the present report). These HEK-TLR4 signaling data are consistent with in silico TLR4/MD-2 in silico docking data which indicate that M3G and morphine both optimally interact with the same “pocket” on the 3-dimensional TLR4/MD-2 complex, whereas M6G does not bind there in the same fashion (Hutchinson et al., 2009c). Indeed, TLR4 signaling induced by M3G in HEK-TLR4 cells was significantly greater than that induced by equimolar morphine (Hutchinson et al., 2009c), suggestive that in vivo TLR4 signaling following morphine administration may increase as morphine is metabolized to M3G.
The in silico and in vivo data strongly support a role for the glucuronide group in producing enhanced pain following M3G administration owing to the close interaction with two MD-2 residues previously found to be pivotal to LPS TLR4 signaling LPS (Teghanemt et al., 2008). The implications of TLR4 signaling activation by M3G's interaction with these and other residues in the absence of LPS deserves further investigation as it has implications for TLR4 signaling activation by other endogenous small molecules, environmental or pharmacological xenobiotics. For example, other 3-glucuronide opioid metabolites have been shown to neuroexcitatory or pain enhancing properties, including hydromorphone-3-glucuronide (Wright et al., 2001) and normorphone-3-glucuronide (Smith and Smith, 1998). The studies presented here would predict that glial and inflammatory activity could contribute to these responses. However, there may also be a contribution of the morphine moiety to the pain response, as other morphine-3 conjugates can also produce hyperactive motor behavior (Labella et al, 1979) and tactile sensitivity (Yaksh et al., 1986, Yaksh et al., 1988). Further studies are necessary to fully elucidate the structure-function relationship of these molecules with TLR4/MD-2.
The use of (+)-naloxone and (−)-naloxone as inhibitors of TLR4 signaling in the present studies is based on recent in vitro, in vivo, and in silico studies. In silico receptor/ligand computer modeling studies reveal that both (+)-naloxone and (−)- naloxone dock in the same TLR2/MD-2 binding pocket as does morphine (Hutchinson et al., 2009c). In such computer modeling studies, activators and inhibitors of TLR4 signaling are predicted to dock similarly, as the software does not allow for global conformation change. Conformational change upon agonist binding is necessary to facilitate signal transduction (agonism) versus inhibition (antagonism). Notably, a recently solved TLR4/ligand complex crystal structure showed that activators and inhibitors of TLR4 signaling occupy the same binding site in the TLR4/MD-2 association (Park et al., 2009). In vitro studies support that naloxone inhibits TLR4 signaling nonstereoselectively as: (a) (+)-naloxone and (−)-naloxone each block LPS signaling both in HEK-TLR4 cells and in RAW274.7 macrophages, as does the classic TLR4 antagonist, LPS-RS; (b) (+)-naloxone blocks morphine signaling both in HEK-TLR4 cells and in RAW274.7 macrophages, as does the classic TLR4 antagonist, LPS-RS; and (c) while (+)-naloxone potentiates morphine analgesia in wild-type mice, it fails to potentiate morphine analgesia in TLR4 knockout mice (which do not express opioid receptors) (Hutchinson et al., 2008b; Hutchinson et al, 2009c). This same lack of stereoselectivity for naloxone actions was also observed in the blockade of M3G-induced hyperalgesia in the present study, consistent with a TLR4-dependent mechanism. Were M3G action to involve classical opioid receptors, only (−)-naloxone would antagonize the effect while (+)-naloxone would be ineffective, which was not the results observed. Thus, our findings support M3G action on a non-classical opioid receptor, found principally on microglia and blocked nonstereoselectively by naloxone, all of which is consistent with M3G action on TLR4/MD-2.
TLR4 proinflammatory activation of microglia, however, appears to require a second signal in vitro and in vivo. Very recent evidence suggests that heat shock protein 90 (HSP90) can act as a TLR4 co-signaling molecule in vitro and in vivo, and blocking HSP90 function significantly increases and prolongs morphine analgesia consistent with the effects observed by direct TLR4 antagonism (Hutchinson et al., 2008d; Hutchinson et al., 2009b; Triantafilou et al. 2001). To ascertain if HSP90 was needed as a second signal to facilitate M3G-induced TLR4 activation, 17-DMAG, an HSP90 inhibitor, was tested both in vitro and in vivo. 17-DMAG did significantly attenuate M3G-induced TLR4 activity in vitro and was able to block M3G-induced hyperalgesia, suggestive of HSP90 involvement. While 17-DMAG can also apparently enhance HSP70 following chronic administration, the direction of effects observed here following acute administration are not congruent with such a mechanism. Given previous evidence for a role of HSP90 in TLR4-dependent signaling in vitro and in vivo, this provides another line of evidence for TLR4 involvement in M3G-induced hyperalgesia.
M3G induces other behaviors in addition to enhanced pain, including wet-dog shakes, tonic-clonic seizures, myoclonus and ataxia in rats (Bartlett, 1994). The mechanism(s) by which M3G induces such behaviors is not known, although several neuronally based mechanisms have been investigated without resolving the question. All of the reported M3G-induced behaviors, including enhanced pain, are indicative of increased neuronal excitation. Our data are supportive of a TLR4, IL1 and, likely, microglial component to the neuroexcitation leading to enhanced pain, and suggest that parallel investigations for other M3G-induced behaviors is warranted especially in light of recent advances in the epilepsy seizure literature implicating TLR4 and IL1 in epileptogenesis (Rodgers et al., 2009).
M3G effects are very potent, requiring only 0.75 μg to markedly enhance pain, while greater doses of morphine are needed to produce analgesia (Hutchinson et al., 2008a). Given the presence of M3G in the CNS following morphine administration and the potency of M3G, the natural metabolic formation of M3G may be a significant endogenous counter-regulator, or opponent process, to morphine analgesia. Recent research supports this possibility. In rats, the decline in morphine analgesia following a single acute injection can be prolonged by an injection of IL1ra, evidence that proinflammatory mechanisms may reduce the analgesic actions of morphine (Hutchinson et al., 2008a). In the same study, inhibiting other proinflammatory cytokines with soluble TNF receptor and interleukin-6 neutralizing antibody, as well as inhibiting microglia with minocycline enhanced and prolonged morphine analgesia (Hutchinson et al., 2008a). Further, blocking TLR4 activation enhances morphine analgesia in rats (Hutchinson et al., 2009c). Given the evidence for M3G-induced inflammatory molecules presented here, it is reasonable to suspect that M3G-induced inflammation could contribute to the inflammatory opposition of morphine analgesia.
Such a conclusion would support prior work, using pharmacological modeling, which concluded that the time course of morphine analgesia was optimally accounted for only when including M3G concentrations as an anti-analgesic component (Mazoit et al., 2007). This study was based on the finding in humans that the analgesic profile of morphine does not parallel its CSF or plasma concentrations (Mazoit et al., 2007). This provided further evidence that active metabolites of morphine may play an important role in modulating morphine analgesia. In this model, based on the results of a study of 50 pain patients, the CSF time to maximum concentration of morphine was 30 min, while M6G had a time to maximum concentration of 4 h and M3G 10 h. Analgesia was maximal around 4 h following i.v. morphine administration, such that analgesia is waning as M3G levels continue to rise (Mazoit et al., 2007). Resolving the anti-analgesic effects of M3G could improve the analgesic efficacy of morphine. As presented here, M3G and morphine both activate TLR4, M3G more potently, and produce proinflammatory glial activation that may oppose the analgesic effects of morphine. This proinflammatory glial activation is effectively blocked by (+)-naloxone in rats. Because (+)-naloxone does not reduce morphine's analgesic effects, it may be an effective way to reduce TLR4 signaling and consequent proinflammatory glial activation and anti-analgesia following morphine administration.
In summary, converging lines of evidence support glial activation and release of proinflammatory molecules as necessary for M3G-induced pain behaviors. Blocking the proinflammatory cytokine IL1, microglial activation, or TLR4 activity were each able to completely block M3G-induced allodynia and/or hyperalgesia. Increases in mRNA levels of proinflammatory activation markers and production and release of IL1 by M3G both in vitro and in vivo further support this hypothesis. Taken together, these data provide evidence for a substantial TLR4-dependent glial component to M3G-induced effects including enhanced pain and gene activation and suggest that the clinical efficacy of opioids for pain control could be enhanced by selectively blocking TLR4 activation by drugs like (+)-naloxone.
Acknowledgements
This work was supported by an International Association for the Study of Pain International Collaborative grant, American Australian Association Merck Company Foundation Fellowship, National Health and Medical Research Council CJ Martin Fellowship (ID 465423; M.R.H.) and NIH Grants DA015642, DA024044, and DE017782. This work was partially supported by the by the NIH Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism. We thank Avigen (Alameda, CA, USA) for the gift of the HEK293-TLR4 cell line.
List of abbreviations
- aCSF
artificial CSF
- BL
baseline
- CNS
central nervous system
- CSF
cerebrospinal fluid
- ELISA
Enzyme-linked immunosorbant assay
- HEK-TLR4
HEK 293 cells stably transfected with TLR4 and the co-receptor proteins, CD14 and MD-2
- HSP90
heat shock protein 90
- IL1
interleukin-1β
- IL1ra
interleukin-1 receptor antagonist
- LPS-RS
lipopolysaccharide from Rhodobacter sphaeroides
- M3G
morphine-3-glucuronide
- mRNA
messenger ribonuclear acid
- RT-PCR
reverse transcription - polymerase chain reaction
- SEAP
secreted alkaline phosphatase
- TLR4
toll-like receptor 4
- TNF
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbot FV, Palmour RM. Morphine-6-glucuronide: analgesic effects and receptor binding profile in rats. Life Sci. 1988;43:1685–1695. doi: 10.1016/0024-3205(88)90479-1. [DOI] [PubMed] [Google Scholar]
- Andersen G, Christrup L, Sjøgren P. Relationships among morphine metabolism, pain and side effects during long-term treatment: an update. J Pain Symptom Manage. 2003;25:74–91. doi: 10.1016/s0885-3924(02)00531-6. [DOI] [PubMed] [Google Scholar]
- Bartlett SE, Dodd PR, Smith MT. Pharmacology of morphine and morphine-3-glucuronide at opioid, excitatory amino acid, GABA and glycine binding sites. Pharmacol Toxicol. 1994a;75:73–81. doi: 10.1111/j.1600-0773.1994.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Bartlett SE, Cramond T, Smith MT. The excitatory effects of morphine-3-glucuronide are attenuated by LY274614, a competitive NMDA receptor antagonist, and by midazolam, an agonist at the benzodiazepine site on the GABAA receptor complex. Life Sci. 1994b;54:687–694. doi: 10.1016/0024-3205(94)00552-4. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative Assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Christrup LL. Morphine Metabolites. Acta Anaesthesiol Scand. 1997;41:116–122. doi: 10.1111/j.1399-6576.1997.tb04625.x. [DOI] [PubMed] [Google Scholar]
- Dale O, Thoner J, Nilsen T, Tveita T, Borchgrevink PC, Klepstad P. Serum and cerebrospinal fluid morphine pharmacokinetics after single doses of intravenous and intramuscular morphine after hip replacement surgery. Eur J Clin Pharmacol. 2007;63:837–842. doi: 10.1007/s00228-007-0329-x. [DOI] [PubMed] [Google Scholar]
- Frenk H, Watkins LR, Miller J, Mayer DJ. Nonspecific convulsions are induced by morphine but not D-Ala2-methionine-enkephalinamide at cortical sites. Brain Res. 1984;299:51–59. doi: 10.1016/0006-8993(84)90787-x. [DOI] [PubMed] [Google Scholar]
- Foley TE, Greenwood BN, Koch LG, Britton SL, Fleshner M. Elevated central monoamine receptor mRNA in rats bred for high endurance capacity: implications for central fatigue. Behav Brain Res. 2006;174:132–142. doi: 10.1016/j.bbr.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Grumbach L, Chenov HI. The prediction of analgesic activityin man by animal testing. In: Knighton RS, Dunke PR, editors. Pain. Little Brown; Boston: 1966. pp. 163–182. [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hemstapat K, Monteith GR, Smith D, Smith MT. Morphine-3-glucuronide's neuro-excitatory effects are mediated via indirect activation of N-methyl-D-aspartic acid receptors: mechanistic studies in embryonic cultured hippocampal neurones. Anesth Analg. 2003;97:494–505. doi: 10.1213/01.ANE.0000059225.40049.99. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, Martin D, Poole S, Judd CM, Maier SF, Watkins LR. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008a;22:1178–1189. doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008b;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Chao LW, Kearney JJ, Zhang Y, Berkelhammer DL, Loram LC, Rozeske RR, Bland ST, Maier SF, Gleeson TT, Watkins LR. Minocycline suppresses morphine-induced respiratory depression, suppresses morphine-induced reward, and enhances systemic morphine-induced analgesia. Brain Behav Immun. 2008c doi: 10.1016/j.bbi.2008.07.008. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Rezvani N, Sholar PW, Kearney JJ, Shridhar M, Zhang Y, Maier SF, Watkins LR. Inhibition of spinal HSP90 acutely reverses chronic constriction injury induced allodynia & potentiates intrathecal morphine analgesia. Proc. Society for Neuroscience. 2008d2008 [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, Maier SF, Watkins LR, Johnson KW. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast) Brain Behav Immun. 2009a;23:240–250. doi: 10.1016/j.bbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Sholar PW, Kearney JJ, Zhang Y, Maier SF, Watkins LR. Evidence for a role of heat shock protein-90 (HSP90) in TLR4 mediated pain enhancement. 2009b. In invited revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Shridhar M, Evans J, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR. Evidence that opioids may have toll like receptor 4 and MD-2 effects. Brain Beh Immun. 2009c doi: 10.1016/j.bbi.2009.08.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353–7365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CD, Rios GR, Assouline JA, Tephly TR. Expression of UDP-glucuronosyltransferases (UGTs) 2B7 and 1A6 in the human brain and identification of 5-hydroxytryptamine as a substrate. Arch Biochem Biophys. 1999;365:156–162. doi: 10.1006/abbi.1999.1155. [DOI] [PubMed] [Google Scholar]
- Labella FS, Pinsky C, Havlicek V. Morphine derivatives with diminished opiate receptor potency show enhanced central excitatory activity. Brain Res. 1979;174:264–271. doi: 10.1016/0006-8993(79)90849-7. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoit JX, Butscher K, Samii K. Morphine in postoperative patients: pharmacokinetics and pharmacodynamics of metabolites. Anest Analg. 2007;105:70–78. doi: 10.1213/01.ane.0000265557.73688.32. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Hinde JL, Mehmert KK, Maier SF, Watkins LR. A method for increasing the viability of the external portion of lumbar catheters placed in the spinal subarachnoid space of rats. J Neurosci Methods. 1999;90:81–86. doi: 10.1016/s0165-0270(99)00075-8. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861:105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- Milligan ED, O'Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci. 2001;21:2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Langer SJ, Sloane EM, He L, Wieseler-Frank J, O'Connor K, Martin D, Forsayeth JR, Maier SF, Johnson K, Chavez RA, Leinwand LA, Watkins LR. Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci. 2005;21:2136–2148. doi: 10.1111/j.1460-9568.2005.04057.x. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol. 2007;19:3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- O'Connor KA, Holguin A, Hansen MK, Maier SF, Watkins LR. A method for measuring multiple cytokines from small samples. Brain Behav Immun. 2004;18:274–280. doi: 10.1016/j.bbi.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–5. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–73. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Rodgers KM, Hutchinson MR, Northcutt A, Maier SF, Watkins LR, Barth DS. The cortical innate immune response increases local neuronal excitability leading to seizures. Brain. 2009;132:2478–86. doi: 10.1093/brain/awp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjøgren P, Thunedborg LP, Christrup L, Hansen SH, Franks J. Is development of hyperalgesia, allodynia and myoclonus related to morphine metabolism during long-term administration? Six case histories. Acta Anaesthesiol Scand. 1998;42:1070–1075. doi: 10.1111/j.1399-6576.1998.tb05378.x. [DOI] [PubMed] [Google Scholar]
- Smith GD, Smith MT. The excitatory behavioral and antianalgesic pharmacology of normorphine-3-glucuronide after intracerebroventricular administration to rats. J Pharmacol Exp Ther. 1998;285:1157–62. [PubMed] [Google Scholar]
- Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res [Suppl] 2001;39:281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci. 2007;104:13798–803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SC, Lathia JD, Selvaraj PK, Jo DG, Mughal MR, Cheng A, Siler DA, Markesbery WR, Arumugam TV, Mattson MP. Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid beta-peptide and the membrane lipid peroxidation product 4-hydroxynonenal. Exp Neurol. 2008;213:114–21. doi: 10.1016/j.expneurol.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanga FY, Raghavendra R, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int. 2004;45:397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA. 2005;102:5856–5961. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teghanemt A, Re F, Prohinar P, Widstrom R, Gioannini TL, Weiss JP. Novel roles in human MD-2 of phenylalanines 121 and 126 and tyrosine 131 in activation of Toll-like receptor 4 by endotoxin. J Biol Chem. 2008;283:1257–1266. doi: 10.1074/jbc.M705994200. [DOI] [PubMed] [Google Scholar]
- Triantafilou K, Triantafilou M, Dedrick RL. A CD-14 independent LPS receptor cluster. Nat Immunol. 2001;2:338–45. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
- Wright AW, Nocente ML, Smith MT. Hydromorphone-3-glucuronide: biochemical synthesis and preliminary pharmacological evaluation. Life Sci. 1998;63:401–11. doi: 10.1016/s0024-3205(98)00288-4. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Harty GJ, Onofrio BM. High doses of spinal morphine produce a nonopiate receptor-mediated hyperesthesia: clinical and theoretical implications. Anesthesiology. 1986;64:590–597. doi: 10.1097/00000542-198605000-00008. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Harty GJ. Pharmacology of allodynia in rats evoked by high dose intrathecal morphine. J Pharmacol Exp Ther. 1988;244:501–507. [PubMed] [Google Scholar]
- Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]