Abstract
Apart from the well known inhibitory effects of estradiol on food intake, meal size, and body weight in female rats that have been documented over the past thirty years, a more recent report presents the opposite finding; that a large dose of estradiol can increase food intake and weight gain in gonadally intact female rats presented with a palatable diet. The purpose of the present experiment was to further examine this hypothesis by evaluating the ability of estradiol to influence feeding behavior in ovariectomized rats presented with diets that differ in flavor and fat content. Female rats were given a cyclic regimen of estradiol benzoate treatment (5.0 or 20.0 µg) or the oil vehicle and were presented with the standard chow diet or a diet with a higher fat content and chocolate flavor. Food intake, meal size, and meal number were monitored three days after the first injection of estradiol or oil. Compared to the chow diet, food intake increased when animals had access to the chocolate/fat diet during the vehicle treatment condition. Both doses of estradiol significantly decreased food intake, meal size, and body weight gain when animals were presented with either the standard chow diet or the chocolate/fat diet. These findings indicate that estradiol does not stimulate the intake of a palatable diet in ovariectomized rats, and suggest that previous results showing that estradiol enhanced eating and weight gain stemmed from a disruption of the hypothalamic-pituitary-gonadal axis when intact females received a large dose of exogenous estradiol.
Keywords: estradiol, palatable food, meal size, body weight
1. Introduction
In addition to their effects on reproductive physiology and behavior, ovarian hormones (e.g., estradiol) also influence regulatory processes such as the control of food intake and body weight [1, 2]. For example, in a wide rage of mammalian species, estradiol produces an inhibitory effect on food intake [3–7], an effect that is mediated in large part by a decrease in meal size [6, 8, 9]. In female rats, withdrawal of estradiol following ovariectomy causes a significant increase in food intake and meal size, and a concomitant increase in body weight compared to gonadally intact females [8, 10, 11, 12]. Treating ovariectomized rats with physiological doses of estradiol reduces food intake and body weight and restores meal patterns to those seen in intact cycling rats [8].
Apart from these well established effects of estradiol on feeding behavior and body weight, data from a more recent study suggest that estradiol has the opposite effect on food intake; namely that it increases the intake of a palatable food (chocolate cake mixture). In that experiment, a pharmacological dose of estradiol valerate (EV, 1500 µg/kg) administered to gonadally intact female rats increased the intake of the chocolate cake mixture relative to controls when animals were presented with that diet 11 days after the EV injection [13]. The effects of EV on ovarian cyclicity were not evaluated. According to the authors, the anorectic effect of estradiol reported in the studies cited above results from the use of experimental procedures that lead to neophobia and malaise following the initial exposure to estradiol, an effect that presumably gets associated with that diet [13].
The following experiment was therefore conducted to further explore the hypothesis that estradiol can increase the consumption of a palatable diet and evaluate the physiological significance of this effect. To test this hypothesis, we treated ovariectomized rats with cyclic regimens of nearly physiological and supraphysiological doses of estradiol and measured food intake and meal patterns when the animals were presented with diets that differed in flavor and fat content. Examining changes in ingestive behavior follow estradiol replacement in ovariectomized rats avoids the potential confound of a disruption of the hypothalamic-pituitary-gonadal axis that can occur when intact females are treated with exogenous estradiol [14]. To minimize the potential effect of neophobia and the likelihood that females would acquire an estradiol-induced aversion to the diets that might mask an increase in food intake, animals had access to each diet for two weeks before the onset of estradiol treatment.
2. Materials and methods
2.1 Animals, housing, and ovariectomy
Ten female Long-Evans rats (Blue Spruce) obtained from Harlan (Indianapolis, IN) served as subjects in these experiments. Animals were approximately 50 days of age upon arrival and were individually housed in stainless steel cages (18 × 18 × 23 cm) in a windowless colony room. The room was maintained at 20 +/− 3 deg. C with a 12:12 hr light-dark cycle (lights on at 0:400 hr). A white noise generator (Lafayette Instruments, Lafayette, IN) was used to mask any outside noise. Pelleted rodent chow (Mazuri, Brentwood, MO) and tap water were available ad libitum.
Following a one-week acclimation period to their new environment, the rats were ovariectomized via a single midline abdominal incision under ketamine (75 mg/kg; Ketaset, Fort Dodge, IA) and xylazine (5 mg/kg, Xyla-ject, Phoenix Scientific, St. Joseph, MO) anesthesia. Animals were given a subcutaneous injection of butorphanol (0.5 mg/kg, Torbugesic, Fort Dodge, IA) after surgery as an analgesic. One week after surgery, animals were transferred to the test cages described below. All procedures were approved by the Niagara University Institutional Animal Care and Use Committee and were consistent with the ethical standards of the American Psychological Association.
2.2 Spontaneous meal patterns and diet types
Animals were individually housed in soundproof, Plexiglas cages (43.2 × 43.2 × 19 cm) equipped with computer-controlled food dispensers (Med Associates, St. Albans, VT). Rodent food pellets that differed in flavor and fat content (45 mg, NOYES Precision Pellets, Research Diets, Inc., New Brunswick, NJ) were dispensed in response to the activation of a photosensor placed above each food cup. The food pellets used were the standard rodent diet (3.66 kcal/gm, 5% of calories from fat) and the chocolate/fat diet (3.73 kcal/gm, artificially chocolate flavored, 20% of calories from fat). Animals were allowed free access to food throughout the study. Tap water was provided by a water bottle adjacent to the food cup and refilled daily. The Plexiglas cages were connected via an interface (Med Associates, St. Albans, VT) to an IBM-compatible computer. The software provided with this system (MED-PC IV) records the number of pellets dispensed and converts the data into spontaneous feeding patterns (e.g., time and bouts of feeding, amount consumed during each interval). A meal was defined as any feeding bout of at least 0.2 g that is separated from other feeding bouts by at least 15 minutes. Using these criteria, recorded meals account for 96% of daily food intake in female rats [15]. The data collected were saved to disk for subsequent analyses. The raw data were converted into Excel files using MED-PC 2 Excel and translated into meal size and meal number with the Tongue Twister program (T.A. Houpt, Florida State University, Tallahassee, FL) on a Macintosh G4 computer. Animals were given a two-week acclimation period in the test cages with each type of diet (chocolate/fat pellets for one week, standard chow pellets for one week) before the onset of experimental treatments.
2.3 Treatment protocol
At 09:00 hr each day the MED-PC system was shut down for maintenance and data collection. During this time animals were weighed to the nearest gram on an electronic balance and water bottles were weighed to the nearest tenth of a gram to determine intake for that 23-hr period and then refilled. The MED-PC system was then restarted at 10:00 hr.
Following the acclimation period described above, animals received a subcutaneous injection of 5.0 µg estradiol benzoate (EB), (Sigma, St. Louis, MO, dissolved in 0.1 ml sesame oil), 20.0 µg EB, or the oil vehicle at 09:30 on Tuesday and Wednesday in a within-subjects design. This cyclic pattern of estradiol replacement produces behavioral and neurochemical changes associated with estrus without neuroendocrine and behavioral carryover effects that accompany continuous hormone replacement paradigms [16]. Following an initial treatment series with the oil vehicle, the hormone condition was reversed weekly for all subjects so that all rats received alternating treatment with oil and both doses of estradiol in combination with the standard chow diet and the chocolate/fat diet over a 6 week period. Data were analyzed with the SPSS statistical software package (SPSS Inc., Chicago, IL) using a 2 × 3 ANOVA for repeated measures. When the ANOVA detected significant effects (p < 0.05), differences between individual means were compared with the Tukey-Kramer honestly significant difference test and considered significant when p < 0.05.
3. Results
3.1. 23-h food and water intake
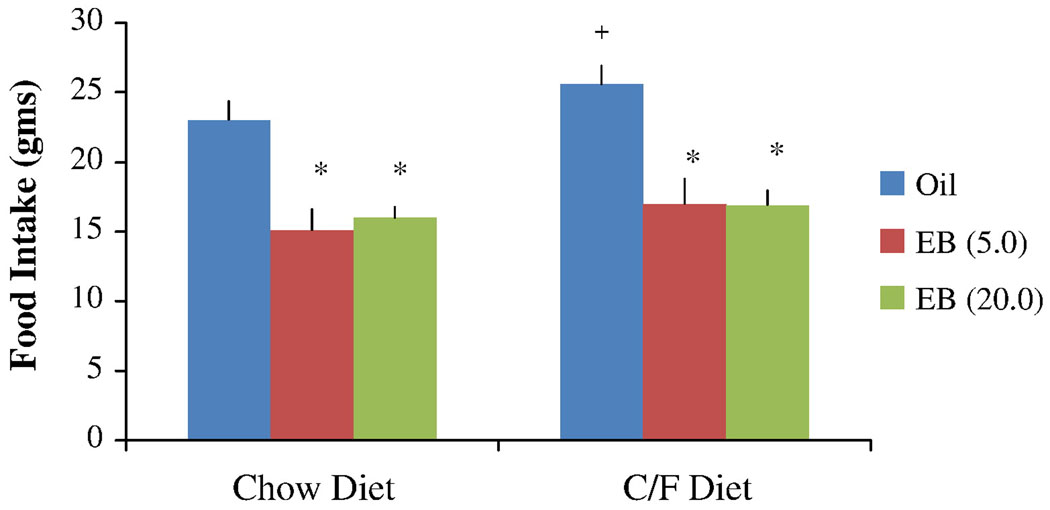
The data collected during the 72 hr period following the first EB or oil injection were analyzed, as this time point models behavioral estrus in the cyclic hormone replacement regimen that was used [15]. Estradiol treatment decreased intake of both diets (Figure 1). The ANOVA revealed significant main effects of hormone (F [2, 16] = 15.96, p < 0.001) and diet type (F [1, 9] = 5.58, p < 0.05) on 23 hr food intake. The hormone-diet interaction was not statistically significant (p > 0.05). Post hoc tests indicated that both doses of EB significantly decreased intake of the standard and chocolate/fat diet compared to intake during treatment with the oil vehicle. There was no significant difference between the 2 doses of EB. Although food intake was higher when animals were presented with the chocolate/fat diet compared to the standard diet, only the chocolate/fat vs. standard diet comparison during oil treatment was statistically significant. There were no significant main effects of hormone or diet type on water intake during the course of the experiment, and the hormone-diet interaction was not statistically significant.
Figure 1.
Effects of oil and EB injections (5.0 or 20.0 µg) on 23-h intake of the standard chow diet and the chocolate/fat (C/F) diet. The data were obtained three days after the first oil or EB injection. Bars represent means ± SE intakes. * Significantly different from oil treatment (P < 0.01); and + significantly different from chow diet (P < 0.05).
3.2 Meal patterns
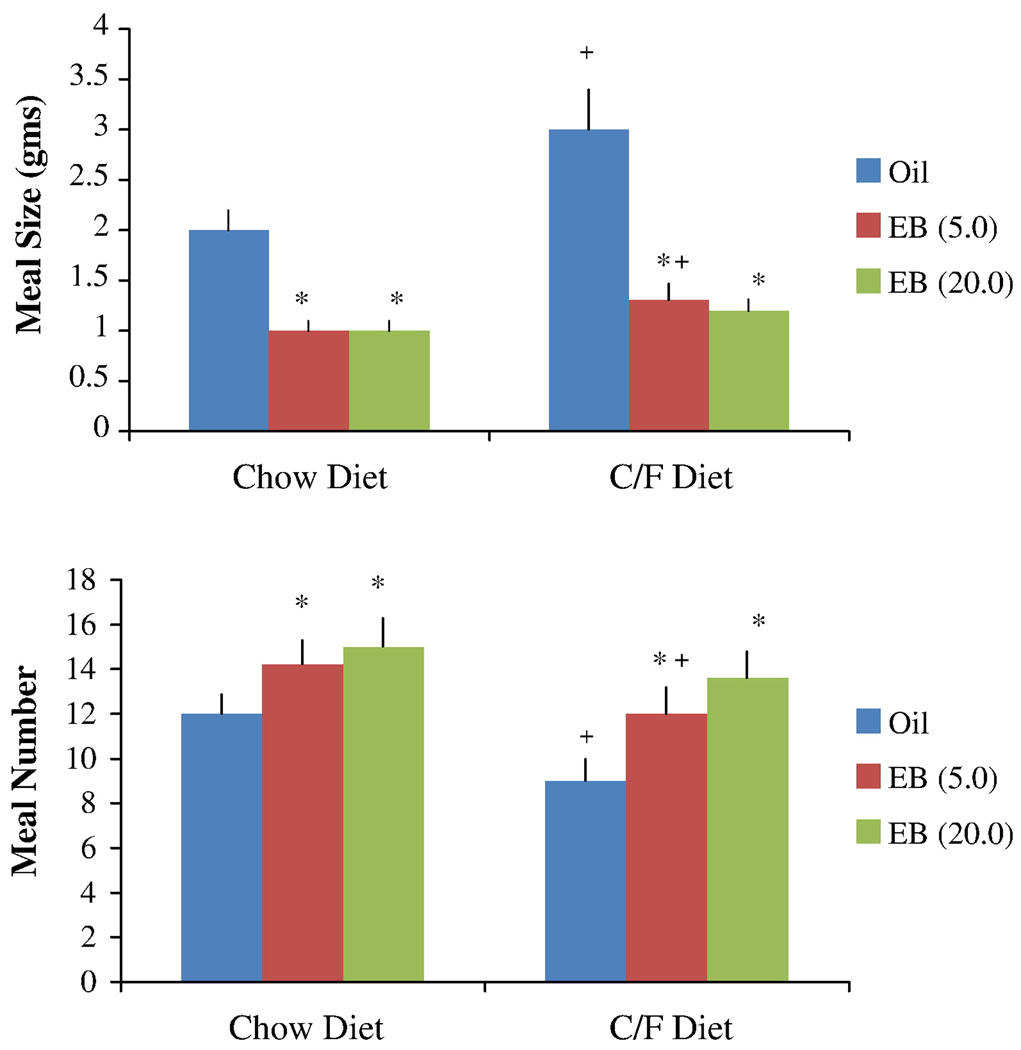
Estradiol treatment decreased meal size and increased meal number of both diets (Figure 2). Analyses of the meal pattern data revealed significant main effects of hormone (F [2, 16] = 23.18, p < 0.001), diet type (F [1, 9] = 14.54, p < 0.01), and a hormone by diet interaction (F [2, 16] = 8.87, p < 0.05) on meal size. In comparison to the data obtained during oil treatment, post hoc tests indicated that meal size was significantly smaller following EB treatments in both diet conditions. Compared to the standard diet, meal size was larger when animals were given the chocolate/fat diet, although this effect was only significant for the oil condition and the 5.0 µg dose of EB. There were no significant differences in meal size between the 2 doses of EB. The ANOVA also revealed main effects of hormone (F [2, 16] = 19.22, p < 0.001) and diet type (F [2, 8] = 6.81, p < .05) on meal number. The hormone-diet interaction was not statistically significant. Post hoc tests indicated that both doses of EB significantly increased meal number relative to oil treatment regardless of diet type. Compared to the standard diet, animals given the oil vehicle and the 5.0 µg dose of EB decreased meal frequency when they were on the chocolate/fat diet.
Figure 2.
Effects of oil and EB injections (5.0 or 20.0 µg) on average meal size (upper panel) and meal number (lower panel) following exposure to the standard chow diet and the chocolate/fat (C/F) diet. The data were obtained from the 23-h period three days after the first oil or EB injection. Bars represent means ± SE meal size and meal number. * Significantly different from oil treatment (P < 0.05); and + significantly different from chow diet (P < 0.05).
3.3 Body weight
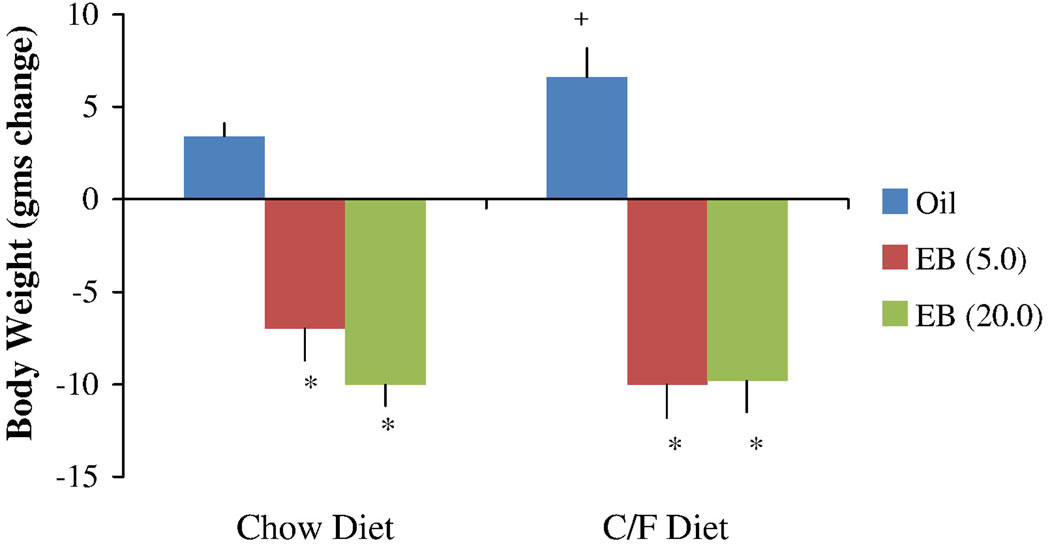
Estradiol treatment decreased body weight gain on both diet types (Figure 3). The ANOVA revealed a significant main effect of hormone (F [2, 16] = 27.78, p < 0.001) and a hormone-diet interaction (F [2, 16] = 11.54, p = 0.001) on the change in body weight during the 3-day period following the first EB or oil injection. The main effect of diet type was not statistically significant. In comparison to the data obtained during oil treatment, post hoc tests indicated that body weight gain was reduced following EB treatments in both diet conditions. Compared to the standard diet, body weight gain was greater when animals were given the chocolate/fat diet, but this effect was only significant for the oil condition. There were no significant differences in body weight change between the 2 doses of EB
Figure 3.
Effects of oil and EB injections (5.0 or 20.0 µg) on body weight following exposure to the standard chow diet and the chocolate/fat (C/F) diet. The data were obtained from the 23-h period three days after the first oil or EB injection. Bars represent means ± SE gms change from body weight value on the first day of oil or EB treatment. * Significantly different from oil treatment (P < 0.01); and + significantly different from chow diet (P < 0.05).
4. Discussion
The main goal of this experiment was to determine if estradiol treatment could increase the intake of a palatable diet in female rats as has been previously reported [13]. Ovariectomized rats received a nearly physiological (5.0 µg) or supraphysiological (20.0 µg) regimen of estradiol or vehicle treatment, and food intake and meal patterns during the 72-hour period following the first EB or oil injection were analyzed. In support of the hypothesis that estradiol exerts an inhibitory effect on food intake and meal size, both doses of EB significantly decreased food intake, meal size, and body weight gain when animals were presented with either the standard chow diet or the chocolate/fat diet. These findings indicate that a cyclic pattern of estradiol replacement does not enhance the intake of a palatable diet in ovariectomized rats, and that this failure of estradiol to do so was not overcome by treating the animals with a larger dose of EB.
In the present study, the chocolate/fat diet, a formula that is sweeter and higher in fat than the standard chow diet, did lead to an increase in food intake in oil- treated ovariectomized rats. This enhancement of food intake by the chocolate/fat diet was mediated by an increase in meal size. In comparison to the data obtained during treatment with the oil vehicle, both doses of estradiol significantly decreased food intake and meal size when animals were presented with the chocolate/fat diet. The present findings are in agreement with a number of prior experiments demonstrating that peripheral treatment with estradiol decreases food intake in ovariectomized rats and guinea pigs [4, 6, 8, 12]. The fact that a 2-day period of EB treatment significantly reduced body weight gain is also consistent with previous reports showing that estradiol replacement decreases body weight in ovariectomized rats [16, 17, 8].
The fact that the inhibitory effect of estradiol on the intake of both diets used in this experiment was mediated by a decrease in meal size is consistent with several previous studies examining the effects of ovarian hormones on meal patterns in ovariectomized and gonadally intact female rats [6, 8, 9, 20]. This effect of estradiol on food intake and meal size has been shown to result from an interaction with cholecystokinin (CCK) satiety systems [17, 20, 21]. According to this model, estradiol lowers food intake and meal size by augmenting a CCK-induced neural message that occurs during the consumption of meal [9]. Consistent with this hypothesis, pretreatment with the CCK1 receptor antagonist devazepide attenuates the effects of estradiol on food intake in intact [20] and ovariectomized rats [22], and direct placement of estradiol in hypothalamic [23] and hindbrain sites [24] enhances the effects of CCK on feeding and c-Fos expression. The ability of estradiol to suppress intake of the chocolate/fat diet in the present study suggests that the effects of estradiol on feeding are not limited to the standard chow diet used in the experiments described above, and that estradiol can also decrease meal size for a sweeter, higher fat food. This finding is in agreement with previous work showing that estradiol treatment decreases fat intake in ovariectomized rats during a feeding protocol that models binge eating [25].
It is unlikely that the failure of estradiol to increase intake of the chocolate/fat diet was the result of neophobia or malaise following the initial exposure to estradiol, as has been previously suggested [13]. First, animals were exposed to each diet for two weeks before the onset of estradiol treatment, thus allowing for the diminishment of a neophobic response that can occur when rats are presented with a novel food. Secondly, previous research has shown that estradiol can decrease food intake in female rats without producing a conditioned taste aversion (and presumably malaise) to that diet [26]. The fact that we analyzed the data collected 3 days after initial exposure to estradiol also makes it unlikely that the inhibitory effect of EB on the intake of the familiar, chocolate/fat diet was the result of persistent malaise. In addition, the findings that the acute effects of estradiol on food intake can be attenuated by administering a CCK1 receptor antagonist [22] does not support the hypothesis that the estrogenic suppression of food intake stems from malaise. It is quite likely that several procedural differences between the present experiment and the study by Boswell et al. [13] accounted for the discrepant findings. For example, Boswell et al. [13] treated gonadally intact female rats with a supraphysiological dose of estradiol valerate (1500 µg/kg) and measured intake of the chocolate cake mixture for an 8-day period beginning 11 days after the estradiol or vehicle injection. In the present experiment, ovariectomized animals received EB replacement to avoid the potential confound of the disruption of the hypothalamic-pituitary-gonadal axis that can occur when intact females are treated with exogenous estradiol. We also analyzed food intake and body weight data collected 3 days after initial treatment with EB or oil, whereas Boswell et al. [13] analyzed data collected over an 8 day period beginning 11 days after the EV or vehicle injection. In the absence of estrous cycle data, there is no way to know whether animals in the study by Boswell et al. [13] were displaying regular ovarian cycles during the period between the EV injection and data collection. It is quite likely that treating intact female rats with a pharmacological dose of EV led to a disruption of ovarian cycles and a concomitant increase in food intake and body weight similar to what is seen in female rats when ovarian cycles cease during pseudopregnancy or following ovariectomy [27, 28]. Thus, the fact that EV treated females consumed more of the chocolate cake mixture than vehicle treated animals during the 19 day period following the injection may simply reflect the fact that EV antagonized the effects of endogenous estradiol normally secreted during the ovarian cycle. In that sense, estradiol did not “enhance” the intake of the chocolate cake mixture relative to vehicle treated animals, but instead the feeding behavior of the EV-treated females resembled the behavior of female rats during pseudopregnancy or after ovariectomy when ovarian cycles cease and food intake increases.
In summary, the results of the present experiment provide evidence that a cyclic regimen of both nearly physiological and supraphysiological doses of estradiol decrease food intake and meal size of a palatable diet in ovariectomized rats. These findings do not support the hypothesis that estradiol enhances the intake of palatable foods that has been previously reported [13], and suggest that the effects of estradiol on feeding in that experiment stemmed from a disruption of ovarian cyclicity when intact females were treated with a pharmacological dose of estradiol valerate. We conclude that a cyclic regimen of treatment with close-to-physiological, or supraphysiological doses of estradiol decrease rather than increase intake of a sweet, high fat food just as estradiol decreases intake of regular chow. Whether or not chronic, long-term exposure to estradiol can enhance the intake of a palatable diet in ovariectomized females remains to be investigated.
Acknowledgements
This work was supported in part by NIH R15-HD053382.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wade GN. Sex hormones, regulatory behaviors, and body weight. In: Rosenblatt J, Hinde R, Shaw E, Beer C, editors. Advances in the study of behavior. Vol 6. New York: Academic Press; 1976. pp. 201–279. [Google Scholar]
- 2.Shi H, Clegg DJ. Sex differences in the regulation of body weight. Physiol Behav. 2009;97:199–204. doi: 10.1016/j.physbeh.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forbes JM. Effects of estradiol 17-β on voluntary food intake in sheep and goats. J Clin Endocrinol. 1972;VIII:52–57. [PubMed] [Google Scholar]
- 4.Czaja JA, Goy RW. Ovarian hormones and food intake in female guinea pigs and rhesus monkeys. Horm Behav. 1975;6:329–337. doi: 10.1016/0018-506x(75)90003-3. [DOI] [PubMed] [Google Scholar]
- 5.ter Haar MB. Circadian and estrual rhythms in food intake in the rat. Horm Behav. 1972;3:213–220. doi: 10.1016/0018-506x(72)90034-7. [DOI] [PubMed] [Google Scholar]
- 6.Blaustein JD, Wade GN. Ovarian influences on meal patterns of female rats. Physiol Behav. 1976;17:201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- 7.Gong EJ, Garrel D, Calloway DH. Menstrual cycle and voluntary food intake. Am J Clin Nutr. 1989;49:252–259. doi: 10.1093/ajcn/49.2.252. [DOI] [PubMed] [Google Scholar]
- 8.Geary N, Asarian L. Cyclic estradiol treatment normalizes body weight and meal size in ovariectomized rats. Physiol Behav. 1999;67:141–147. doi: 10.1016/s0031-9384(99)00060-8. [DOI] [PubMed] [Google Scholar]
- 9.Eckel LA. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol Behav. 2004;82:35–41. doi: 10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Holt H, Keeton RW, Vennesland B. The effect of gonadectomy on body structure and body weight in albino rats. Am J Physiol. 1936;114:515–522. [Google Scholar]
- 11.Tarttelin MF, Gorski RA. The effects of ovarian steroids on food and water intake and body weight in the female rat. Acta Endocrinol (Copenhagen) 1973;72:551–557. doi: 10.1530/acta.0.0720551. [DOI] [PubMed] [Google Scholar]
- 12.Wade GN. Some effects of ovarian hormones on food intake and body weight in female rats. J Comp Physiol Psychol. 1975;88:183–191. doi: 10.1037/h0076186. [DOI] [PubMed] [Google Scholar]
- 13.Boswell KJ, Reid LD, Caffalette CA, Stitt KT, Klein LA, Lacroix AM, Reid ML. Estradiol increases consumption of a chocolate cake mix in female rats. Pharmacol Biochem Behav. 2006;84:84–93. doi: 10.1016/j.pbb.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Brawer JR, Naftolin F, Martin J, Sonnenschein C. Effects of a single injection of estradiol valerate on the hypothalamic arcuate nucleus and on reproductive function in the female rat. Endocrinology. 1978;103:501–511. doi: 10.1210/endo-103-2-501. [DOI] [PubMed] [Google Scholar]
- 15.Eckel LA, Langhans WL, Kahler A, Campfield LA, Smith FJ, Geary N. Chronic administration of OB protein decreases food intake by selectively reducing meal size in female rats. Am J Physiol. 1998;44:R186–R198. doi: 10.1152/ajpregu.1998.275.1.R186. [DOI] [PubMed] [Google Scholar]
- 16.Schumacher M, Coirini H, Pfaff DW, McEwen BS. Light-dark differences in behavioral sensitivity to oxytocin. Behav Neurosci. 1991;105:487–492. doi: 10.1037//0735-7044.105.3.487. 1991. [DOI] [PubMed] [Google Scholar]
- 17.Geary N, Trace D, McEwen B, Smith GP. Cyclic estradiol replacement increases the satiety effect of CCK-8 in ovariectomized rats. Physiol Behav. 1994;56:281–289. doi: 10.1016/0031-9384(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 18.Gray JM, Greenwood MRC. Time course of changes in food intake and lipid metabolism during treatment of ovariectomized rats with ovarian steroids. Am J Physiol. 1982;245:E132–E137. [Google Scholar]
- 19.Butera PC, Doerflinger AL, Roberto F. Cyclic estradiol treatment enhances the effects of interleukin-1β on food intake in female rats. Brain Behav Immun. 2002;16:275–281. doi: 10.1006/brbi.2001.0621. [DOI] [PubMed] [Google Scholar]
- 20.Eckel LA, Geary N. Endogenous cholecystokinin’s satiating action increases during estrus in female rats. Peptides. 1999;20:451–456. doi: 10.1016/s0196-9781(99)00025-x. [DOI] [PubMed] [Google Scholar]
- 21.Butera PC, Bradway DM, Cataldo NJ. Modulation of the satiety effect of cholecystokinin by estradiol. Physiol Behav. 1993;53:1235–1238. doi: 10.1016/0031-9384(93)90387-u. [DOI] [PubMed] [Google Scholar]
- 22.Butera PC. CNS steroids and the control of feeding. In: Stone TW, editor. CNS neurotransmitters and neuromodulators: neuroactive steroids. New York: CRC Press; 1996. pp. 211–235. [Google Scholar]
- 23.Butera PC, Xiong M, Davis RJ, Platania SP. Central implants of dilute estradiol enhance the satiety effect of CCK-8. Behav Neurosci. 1996;110:823–830. doi: 10.1037//0735-7044.110.4.823. [DOI] [PubMed] [Google Scholar]
- 24.Thammacharoen S, Lutz TA, Geary N, Asarian L. Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2008;149:1609–1617. doi: 10.1210/en.2007-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Z, Geary N, Corwin RL. Ovarian hormones inhibit fat intake under binge-type conditions in ovariectomized rats. Physiol Behav. 2008;95:501–507. doi: 10.1016/j.physbeh.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flanagan-Cato LM, Grigson PS, King JL. Estrogen-induced suppression of intake is not mediated by taste aversion in female rats. Physiol Behav. 2001;72:549–558. doi: 10.1016/s0031-9384(01)00411-5. [DOI] [PubMed] [Google Scholar]
- 27.Brobeck JR, Wheatland M, Strominger JL. Variations in the regulation of energy exchange associated with estrous, diestrous, and pseudopregnancy in rats. Endocrinology. 1947;40:65–73. doi: 10.1210/endo-40-2-65. [DOI] [PubMed] [Google Scholar]
- 28.Wade GN, Zucker I. Hormonal and developmental influences on rat saccharin preferences. J Comp Physiol. 1969;69:291–300. doi: 10.1037/h0028208. [DOI] [PubMed] [Google Scholar]