Abstract
Bat ticks, Carios kelleyi, from Iowa were examined for the presence of relapsing fever group borreliae. A novel spirochete was characterized by DNA sequence analysis of polymerase chain reaction amplicons for the 16S rRNA, flaB, and glpQ genes in either triturated tick pools or single ticks. All loci and the concatenated DNA sequence of 3,289 bases identified the Carios bacterium as a relapsing fever spirochete most closely related to, but distinct from, Borrelia turicatae. Spirochetes reactive with a Borrelia-specific monoclonal antibody were observed microscopically in the coxal fluid and salivary glands from one tick. These data confirm the presence of a novel species of relapsing fever spirochete in bat ticks and the potential for new enzootic foci for endemic relapsing fever that warrants further investigation. The name Borrelia johnsonii is proposed for this novel spirochete in honor of Dr. Russell C. Johnson.
Key Words: Borrelia, Relapsing fever, Vector-borne
Introduction
Bats have been occasionally implicated as possible hosts for borrelia for over 100 years. Nicole and Comte (1905, 1906) first found spirochetes in the blood of bats in Tunis, Tunisia, North Africa. The following year, Novy and Knapp (1906) suggested this spirochete be named Spirochaeta vespertilionis, although no extant examples of this bacterium exist. Nájera Angulo (1945) demonstrated that four species of bats captured in Spain were susceptible to infection when inoculated with Spirochaeta hispanica in the blood from infected guinea pigs. Coles (1914) found spirochetes in the blood of two species of bats while studying parasites in many host species in England. In 1952, Heisch reported spirochetes in the blood of one bat collected from a cave near the coast of Kenya, East Africa. In the micrographs accompanying some of these reports, stained preparations of the blood demonstrated spirochetes morphologically consistent for borreliae. In the above cases, no ticks were examined as possible vectors for the spirochetes.
We are unaware of any reports demonstrating spirochetes in bats in the Western Hemisphere. Yet, recently a few studies have found evidence suggesting that the bat tick Carios kelleyi and bats in the United States may be involved in previously unrecognized enzootic cycles for spirochetes closely related to other species known to cause tick-borne relapsing fever. Loftis et al. (2005) utilized polymerase chain reaction (PCR) analysis and DNA sequencing to detect an unidentified Borrelia species in C. kelleyi. The identification of the spirochete as a member of the genus Borrelia was based on a 300-bp sequence of the flagellin gene flaB, which grouped the spirochete closest to Borrelia turicatae and Borrelia parkeri. Gill and coworkers (2008) also used PCR and DNA sequencing to identify another novel species of Borrelia in C. kelleyi, unique from that reported by Loftis et al. (2005), but also most closely related to B. turicatae and B. parkeri. Recent serologic studies using an indirect immunofluorescence assay (IFA) with Borrelia hermsii cells as the antigen showed that 3 of 56 big brown bats, Eptesicus fuscus, in Georgia had possible exposure to borreliae (Reeves et al. 2006).
We have had the opportunity to work with additional specimens of C. kelleyi from Iowa to expand upon the previous findings. Here we report further molecular characterization of the novel Borrelia in C. kelleyi first reported by Loftis et al. (2005) and present additional information regarding this spirochete in ticks.
Materials and Methods
Tick collection and maintenance
C. kelleyi were collected from 2004 to 2007 from a house in Jones County, Iowa. Live ticks were kept in the laboratory at 20°C to 22°C at 85% relative humidity in a glass jar with a saturated KCl2 solution. One attempt was made to feed these ticks on a hatchery-reared Northern bobwhite quail (Colinus virginianus).
Three pooled samples of ticks that contained 18 nymphs, 8 males, and 10 females were washed with 95% ethanol for 5 min, rinsed with BSK-H medium (Sigma-Aldrich, Saint Louis, MO) with phosphomycin (100 μg/mL) and rifampin (50 μg/mL) and triturated in ∼3 mL of fresh BSK-H medium containing the antibiotics using sterile glass grinders. Each triturated tick pool was divided into three aliquots for intraperitoneal inoculation into mice, inoculation into BSK-H medium containing the antibiotics, and DNA purification with a DNAeasy kit (Qiagen Inc., Valencia, CA) for PCR analysis. A few ticks were examined individually (see below).
PCR and DNA sequencing
PCR amplification and DNA sequencing of the Borrelia 16S rRNA, flaB, and glpQ genes from pooled and individual ticks were performed as described (Schwan et al. 2005). Sequences were assembled using the SeqMan program in the Lasergene software package (DNASTAR, Madison, WI).
Animal inoculations
Approximately 0.7 mL of each of the three triturated tick pools was inoculated intraperitoneally into three adult RML mice. Blood samples from the tail vein of the three mice were examined daily by dark-field microscopy at 400× magnification for the presence of spirochetes for 7 days following inoculation. The animals were kept for subsequent serologic testing to detect borrelia-reactive antibodies. An additional mouse was also inoculated intraperitoneally with a culture of B. hermsii DAH (Schwan et al. 2007) at the same time for subsequent serologic comparison with the other mice.
Indirect immunofluorescence assays
Midgut and salivary gland tissues were dissected from five adult C. kelleyi and prepared for antibody staining with monoclonal antibody H9724 (Barbour et al. 1986) and anti-mouse immunoglobulin G-fluorescein isothiocyanate (FITC) (Kirkegaard and Perry, Gaithersburg, MD) as described (Schwan and Hinnebusch 1998). These preparations were viewed with a Nikon Eclipse E800 epifluorescence microscope with a 600× oil immersion lens.
Immunoblot analysis
Whole-cell lysates of B. turicatae, B. parkeri, and B. hermsii were separated by one dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis with Laemmli buffer (Laemmli 1970) as described previously (Schwan et al. 2005). Proteins were blotted onto nitrocellulose membranes with Towbin buffer (Towbin et al. 1979) and examined for reactivity with serum samples from mice inoculated with the triturated pools of C. kelleyi.
Nucleotide sequence accession numbers
Nucleotide sequences have been deposited in the GenBank database with accession numbers EU492388 (16S rRNA; 1,273 bases), EU492387 (flaB; 1,002 bases), and EU492386 (glpQ; 1,014 bases) and designated Borrelia sp. IA-1 for the C. kelleyi spirochete described herein.
Results
PCR and DNA sequencing of tick pools
The flaB primers produced the appropriate-size amplicon with the DNA extracted from the pools of male and female ticks but not from the nymphs. No amplicons were obtained with the 16S rRNA and glpQ primers from any of the pooled samples. DNA sequences included the full-length flaB gene of 1,002 bp. The spirochete flaB sequences from the male and female tick pools were identical and included 300 bp of internal sequences that were identical to the sequence reported previously (AY763104) from three C. kelleyi also from Iowa (Loftis et al. 2005). The sequence alignment showed that this Carios spirochete was most closely related to B. turicatae and B. parkeri with the highest identity value of 98.90% with B. turicatae (Table 1).
Table 1.
DNA Sequence Identity Values (%) for Three Loci in Borrelia sp. nov. Compared to Other North American Species of Relapsing Fever Spirochetes
| Borrelia sp nov. | B. parkeri | B. turicatae | B. hermsii | |
|---|---|---|---|---|
| 16S rRNA | ||||
| Borrelia sp. nov. | — | 99.69 | 99.76 | 99.29 |
| B. parkeri | — | 99.69 | 99.29 | |
| B. turicatae | — | 99.37 | ||
| B. hermsii | — | |||
| glpQ | ||||
| Borrelia sp. nov. | — | 98.13 | 98.13 | 89.71 |
| B. parkeri | — | 98.03 | 89.42 | |
| B. turicatae | — | 89.81 | ||
| B. hermsii | — | |||
| flaB | ||||
| Borrelia sp. nov. | — | 98.50 | 98.90 | 94.03 |
| B. parkeri | — | 98.80 | 93.73 | |
| B. turicatae | — | 93.63 | ||
| B. hermsii | — |
Analysis of single ticks
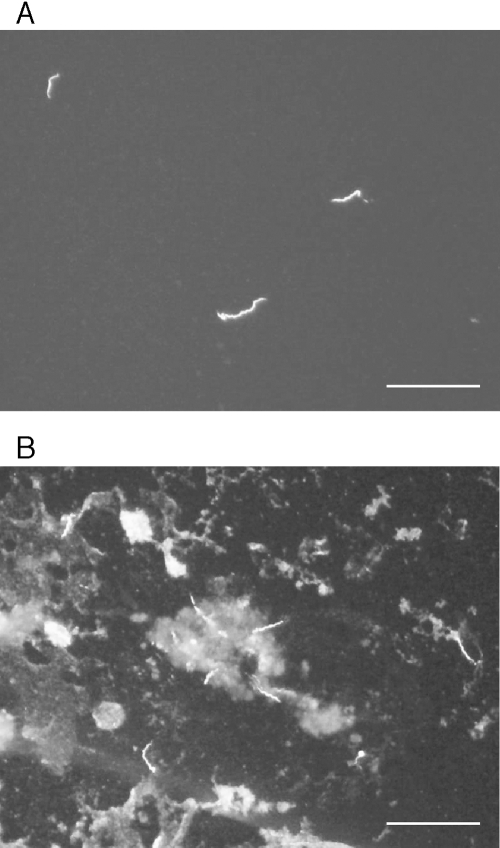
Midgut and salivary gland tissues from five live adult C. kelleyi were removed and fixed for indirect immunofluorescence staining. While preparing to dissect the first specimen, a male tick was placed on its back, and while being probed with forceps, it immediately secreted coxal fluid between the coxae of the first and second right legs. A glass coverslip was lowered to contact only the droplet, lifted, placed on a microscope slide, and examined by dark-field microscopy at 400×. Spirochetes were present in several fields of view. The coverslip was lifted off the microscope slide, and more coxal fluid from the same tick was added to the sample, dried at room temperature, fixed with acetone, and stained with borrelia-reactive monoclonal antibody H9724 and anti-mouse FITC. Numerous spirochetes fluoresced when viewed by epifluorescence microscopy (Fig. 1A) and supported the DNA sequence data that identified these spirochetes as borreliae. The salivary glands from the same tick were squashed, fixed, stained with H9724, and were also seen to be infected with numerous spirochetes that also fluoresced with the antibody (Fig. 1B). No spirochetes were observed in salivary glands and midgut from four other ticks.
FIG. 1.
Borrelia sp. nov. stained with monoclonal antibody H9724 in the coxal fluid (A) and salivary glands (B) from a male Carios kelleyi (scale bars = 20 μm).
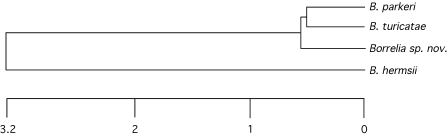
The midgut from the infected tick was not examined by microscopy. DNA was extracted from this organ and examined by PCR. Amplicons of the appropriate size were produced with primers for 16S rRNA, flaB, and glpQ, and DNA sequences were determined for each locus. The flaB sequence was identical to the sequences determined for the spirochetes in the infected male and female tick pools. Like the flaB sequence, the Carios spirochete 16S rRNA and glpQ genes had DNA sequence identity values most closely related to these loci in B. turicatae (Table 1). A phylogram based on a concatenated sequence containing the 16S rRNA, glpQ, and flaB genes totaling 3,289 to 3,301 bp also demonstrated that the Carios spirochete was distinct from other species of North American relapsing fever spirochetes but most closely related to B. turicatae and B. parkeri (Fig. 2).
FIG. 2.
Phylogram comparing the concatenated sequence including the 16S rRNA, flaB, and glpQ genes in the Carios kelleyi spirochete (Borrelia sp. nov.) with Borrelia parkeri RML, Borrelia turicatae 91E135, and Borrelia hermsii DAH. Scale bar represents the number of base substitutions per 100 aligned bases.
We also compared the novel spirochete described here to another spirochete found in bat ticks and partially characterized by Gill and coworkers (2008). We trimmed the glpQ and flaB sequences to match the 368 and 351 base pairs, respectively. These sequences were aligned with the same regions presented for the other bat tick spirochete (Gill et al. 2008) and yielded sequence identity values of 96.5% and 98.0%, respectively. These sequences were also concatenated with the 1,273 base pairs representing the 16S rRNA gene and aligned to yield 98.7% identity between the two spirochetes. Thus, our novel spirochete described herein is less similar to the other bat tick spirochete than it is to B. turicatae and B. parkeri.
Serologic tests of inoculated mice
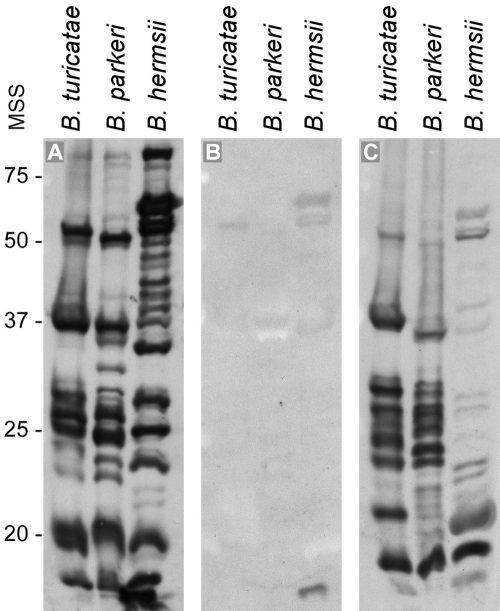
No spirochetes were detected by microscopic examination in the blood of inoculated mice or in the BSK-H medium inoculated with aliquots of the three tick pools. Serologic analysis of serum samples collected 13 months after the three mice were inoculated with aliquots of the tick pools demonstrated individual IFA titers of 1:32, 1:128, and 1:256 when tested with fixed B. hermsii DAH cells as antigen. Immunoblot analysis with these samples diluted to 1:250 demonstrated a robust, diverse, and long-lasting antibody response in the two mice inoculated with the adult tick pools (those with IFA titers of 1:128 and 1:256), with stronger recognition to B. turicatae and B. parkeri than to B. hermsii (one sample shown in Fig. 3). The immunologic responses in the two mice inoculated with the adult C. kelleyi compared to the positive control mouse inoculated with live B. hermsii (Fig. 3A) suggest that mice may be susceptible to infection with this C. kelleyi spirochete. The stronger immunoblot reactivity of the serum samples to B. turicatae and B. parkeri compared to B. hermsii corroborated the DNA sequence analysis, which grouped the bat tick spirochete most closely to B. turicatae.
FIG. 3.
Immunoblot analysis with whole-cell lysates of Borrelia turicatae, Borrelia parkeri, and Borrelia hermsii tested with serum samples (1:250) from three mice. (A) Serum from a mouse inoculated intraperitoneally with live B. hermsii. (B) Serum from a normal uninfected mouse. (C) Serum from a mouse inoculated intraperitoneally with aliquot of triturated pool of adult Carios kelleyi. Positions of molecular size standards (MSS) are shown on left in kilodaltons.
Discussion
Here we further characterize a novel Borrelia species in the bat tick C. kelleyi that is closely related to, but distinct from, other known species of relapsing fever spirochetes in North America. Our identification is based on multilocus DNA sequence typing with 3,289 bases, microscopic visualization of the spirochetes in tick coxal fluid and salivary glands, and reactivity of the spirochetes with a Borrelia genus-specific monoclonal antibody. This spirochete is most closely related to B. turicatae, a spirochete that has long been implicated as a cause of human relapsing fever in parts of western North America (Davis 1940) and Kansas (Davis 1936). Yet, no cultured isolates of B. turicatae have yet been made and typed from humans, although several isolates have been established from clinically ill dogs in Texas and Florida (Breitschwerdt et al. 1994, Schwan et al. 2005, Whitney et al. 2007).
Reports of humans with relapsing fever in the eastern half of the United States are rare. In 1978, Linnemann and coworkers described a protracted case of relapsing fever in a 6½-year-old boy who lived near Cincinnati, Ohio (Linnemann 1978). Scanning electron micrographs demonstrated spirochetes in the blood and urine. Dr. Oscar Felsenfeld, while not a coauthor, was acknowledged by Linnemann for performing multiple serologic tests on the boy’s serum. Felsenfeld included Borrelia recurrentis, B. turicatae, B. parkeri, and B. hermsii in his assays, noting the strongest reactivity with B. turicatae. No records of B. turicatae are known for Ohio, and the only soft tick in the genera Ornithodoros and Carios reported in Ohio is C. kelleyi (Sonenshine and Anastos 1960). Thus, the possibility that the young boy may have been infected with a bat-tick-associated spirochete closely related to B. turicatae is intriguing to consider.
C. kelleyi is widely distributed in the Americas, having been collected from at least 24 of the United States from California to New York, from New Mexico and Texas to Minnesota (Cilek and Knapp 1992, Cooley and Kohls 1941, Cooley and Kohls 1944, Demaree 1986, Dick et al. 2003, Furman and Loomis 1984, Kohls et al. 1965, Lausen 2005, Ritzi et al. 2001, Sonenshine and Anastos 1960, Steinlein et al. 2001, Walker et al. 1998), Alberta and Saskatchewan, Canada (Gregson 1956, Lausen 2005), and Costa Rica (Vargas 1984). Unlike many species of argasid ticks that have fast-feeding larvae, C. kelleyi larvae are slow feeders. One cohort that was fed on a large brown bat, Eptesicus fuscus, required 9 to 20 days (mean 15.4 days) to engorge (Sonenshine and Anastos 1960). This protracted feeding behavior explains why the vast majority of C. kelleyi specimens collected are larvae, which were found attached to captured bats. Such long attachment on hosts that fly likely contributes also to the very wide geographical distribution occupied by these ticks. In our study, 39 ticks were placed on the breast of a quail. Six nymphs fed to repletion and one male and one female partially engorged; no spirochetes were detected microscopically in the bird’s blood for 7 days following the tick feeding. Future work is needed to determine how widespread this novel spirochete is in relation to where C. kelleyi is found and whether birds play any role in either dispersing these ticks or providing an alternate blood meal when bats are absent. Isolation of this novel spirochete in culture will also greatly augment further characterization and experimental work in vivo.
Finally, we propose the provisional name Borrelia johnsonii for this spirochete, to honor Dr. Russell C. Johnson, University of Minnesota, for his many outstanding contributions to the biology of pathogenic spirochetes and for his generous support to so many of us over the years.
Acknowledgments
We thank A. Snyder for collecting the ticks used in our study and Gary Hettrick for help with the figures. This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
References
- Barbour AG. Hayes SF. Heiland RA. Schrumpf ME, et al. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986;52:549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB. Nicholson WL. Kiehl AR. Steers C, et al. Natural infections with Borrelia spirochetes in two dogs from Florida. J Clin Microbiol. 1994;32:352–357. doi: 10.1128/jcm.32.2.352-357.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilek JE. Knapp FW. Occurrence of Ornithodoros kelleyi (Acari: Argasidae) in Kentucky. J Med Entomol. 1992;29:349–351. doi: 10.1093/jmedent/29.2.349. [DOI] [PubMed] [Google Scholar]
- Coles AC. Blood parasites found in mammals, birds and fishes in England. Parasitology. 1914;7:17–61. [Google Scholar]
- Cooley RA. Kohls GM. Further new species of Ornithodoros from bats (Acarina: Argasidae) Public Health Rep. 1941;56:910–914. [Google Scholar]
- Cooley RA. Kohls GM. The Argasidae of North America, Central America and Cuba. American Midland Naturalist. 1944;Monograph No. 1:1–152. [Google Scholar]
- Davis GE. Ornithodoros turicata: the possible vector of relapsing fever in southwestern Kansas. Public Health Rep. 1936;51:1719. [Google Scholar]
- Davis GE. Ticks and relapsing fever in the United States. Public Health Rep. 1940;55:2347–2351. [Google Scholar]
- Demaree HA. Ticks of Indiana. Indiana Dept Natural Resources. Pittman Robertson Bull. 1986;16:1–178. [Google Scholar]
- Dick CW. Gannon MR. Little WE. Patrick MJ. Ectoparasite associations of bats from central Pennsylvania. J Med Entomol. 2003;40:813–819. doi: 10.1603/0022-2585-40.6.813. [DOI] [PubMed] [Google Scholar]
- Furman DP. Loomis EC. The ticks of California (Acari: Ixodida) Bull Calif Insect Surv. 1984;25:1–239. [Google Scholar]
- Gill JS. Ullmann AJ. Loftis AD. Schwan TG, et al. Novel relapsing fever spirochete in bat tick. Emerg Infect Dis. 2008;14:522–523. doi: 10.3201/eid1403.070766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregson JD. The Ixodoidea of Canada. Ottawa: Canada Dept. Agriculture; 1956. [Google Scholar]
- Heisch RB. On spirochaetes observed in the blood of a bat (Megaderma cor Peters) from a Kenya coastal colony. East Afr Med J. 1952;29:327–328. [PubMed] [Google Scholar]
- Kohls GM. Sonenshine DE. Clifford CE. The systematics of the subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae of the western hemisphere and descriptions of three new species. Ann Entomol Soc Amer. 1965;58:331–364. doi: 10.1093/aesa/58.3.331. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lausen CL. First record of hosts for tick Carios kelleyi (Acari: Ixodida: Argasidae) in Canada and Montana. J Med Entomol. 2005;42:497–501. doi: 10.1093/jmedent/42.3.497. [DOI] [PubMed] [Google Scholar]
- Linnemann CC. Tick-borne relapsing fever in the eastern United States. Am J Dis Child. 1978;132:40–42. doi: 10.1001/archpedi.1978.02120260042011. [DOI] [PubMed] [Google Scholar]
- Loftis AD. Gill JS. Schriefer ME. Levin ML, et al. Detection of Rickettsia, Borrelia, and Bartonella in Carios kelleyi (Acari: Argasidae) J Med Entomol. 2005;42:473–480. doi: 10.1093/jmedent/42.3.473. [DOI] [PubMed] [Google Scholar]
- Nájera Angulo L. Receptividad de los murciélagos cavernicolas espanoles. (Miniopterus schreibersii, Myotis myotis, Rhinolophus euryale y Rh. hipposideros minimus) al virus de la fiebre recurrente mediterránea. Bol Real Soc Españ Hist Nat. 1945;43:217–228. [Google Scholar]
- Nicolle C. Comte C. Sur une nouvelle spirillose (Note préliminaire) Compt Rend de la Soc de Biol. 1905;59:200–202. [Google Scholar]
- Nicolle C. Comte C. Sur une spirillose d’un chéiroptère (Vespertillio Kuhli) Ann Inst Paster (Paris) 1906;20:311–320. [Google Scholar]
- Novy FG. Knapp RE. Studies on Spirillum obermeieri and related organisms. J Infect Dis. 1906;3:291–393. [Google Scholar]
- Reeves WK. Streicker DG. Loftis AD. Dasch GA. Serologic survey of Eptesicus fuscus from Georgia, U.S.A. for Rickettsia and Borrelia and laboratory transmission of a Rickettsia by bat ticks. J Vector Ecol. 2006;31:386–389. doi: 10.3376/1081-1710(2006)31[386:ssoeff]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ritzi CM. Ammerman LK. Dixon MT. Richerson JV. Bat ectoparasites from the Trans-Pecos Region of Texas, including notes from Big Bend National Park. J Med Entomol. 2001;38:400–404. doi: 10.1603/0022-2585-38.3.400. [DOI] [PubMed] [Google Scholar]
- Schwan TG. Hinnebusch BJ. Bloodstream- versus tick-associated variants of a relapsing fever bacterium. Science. 1998;280:1938–1940. doi: 10.1126/science.280.5371.1938. [DOI] [PubMed] [Google Scholar]
- Schwan TG. Raffel SJ. Schrumpf ME. Porcella SF. Diversity and distribution of Borrelia hermsii. Emerg Infect Dis. 2007;13:436–442. doi: 10.3201/eid1303.060958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG. Raffel SJ. Schrumpf ME. Policastro PF, et al. Phylogenetic analysis of the spirochetes Borrelia parkeri and Borrelia turicatae and the potential for tick-borne relapsing fever in Florida. J Clin Microbiol. 2005;43:3851–3859. doi: 10.1128/JCM.43.8.3851-3859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE. Anastos G. Observations on the life history of the bat tick Ornithodoros kelleyi (Acarina: Argasidae) J Parasitol. 1960;46:449–454. [PubMed] [Google Scholar]
- Steinlein DB. Durden LA. Gannon WL. Tick (Acari) infestations of bats in New Mexico. J Med Entomol. 2001;38:609–611. doi: 10.1603/0022-2585-38.4.609. [DOI] [PubMed] [Google Scholar]
- Towbin H. Staehelin T. Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MV. Occurrence of the bat tick Ornithodoros (Alectorobius) kelleyi Cooley & Kohls (Acari: Argasidae) in Costa Rica and its relation to human bites. Rev Biol Trop. 1984;32:103–107. [PubMed] [Google Scholar]
- Walker ED. Stobierski MG. Poplar ML. Smith TW, et al. Geographic distribution of ticks (Acari: Ixodidae) in Michigan, with emphasis on Ixodes scapularis and Borrelia burgdorferi. J Med Entomol. 1998;35:872–882. doi: 10.1093/jmedent/35.5.872. [DOI] [PubMed] [Google Scholar]
- Whitney MS. Schwan TG. Sultemeier KB. McDonald PS, et al. Spirochetemia caused by Borrelia turicatae infection in 3 dogs in Texas. Vet Clin Pathol. 2007;36:212–216. doi: 10.1111/j.1939-165x.2007.tb00213.x. [DOI] [PubMed] [Google Scholar]