Summary
Neuroactive steroids with potentiating effects on GABAA channels and inhibitory effects on T-type Ca2+ channels which are located in peripheral sensory neurons are potent modulators of pain perception. The focus of this review is on peripheral anti-nociceptive properties of 5α- and 5β-reduced neuroactive steroids with either selective or combined modulatory action on GABAA and T-type Ca2+ neurotransmission. We report that these neuroactive steroids are very effective in alleviating peripheral nociception in both acute and chronic pain conditions in animal models of pain. We believe that promising animal data warrant the exploration of their usefulness in clinical settings especially considering the fact that chronic pain sufferers are often young and otherwise healthy people.
Keywords: 5 α-reduced neuroactive steroids, 5 β-reduced neuroactive steroids, GABAA channels, T-type calcium channels, thermal and mechanical hyperalgesia, peripheral nociception
Introduction
Neurosteroids are important modulators of a variety of physiological and pathological functions (Mensah-Nyagan et al., 1999; Melcangi et al., 2008; Baulieu, Robel and Schumacker (Eds.), 1999). They alter synaptic transmission by interacting with ionotropic neurotransmitter receptors and/or voltage-dependent Ca2+ or K+ channels as well as by influencing second-messenger pathways (Belelli and Lambert, 2005). Of particular interest for this review is the modulatory effect of neurosteroids on the synaptic ionotropic neurotransmitter receptor, γ-aminobutyric acid (GABAA) and the voltage dependent T-type Ca2+ channel in the pathogenesis and treatment of acute and chronic pain states.
The analgesic properties of endogenously occurring neurosteroids and their synthetic derivatives have been recognized in a variety of behavioral studies (Goodchild et al. 2000; Winter et al. 2003). For example, pain perception during various stages of estrous cycle (Frye et al., 1993; Martinez-Gomez et al., 1994), pregnancy (Gintzler, 1980) and exogenous administration of neurosteroids (McCarthy et al., 1990; Ratka and Simpkins, 1991) has been shown to fluctuate with the high level of analgesia occurring when the plasma level of progesterone and its metabolites is stable and high. Moreover, in states of physiologically elevated levels of neurosteroids (e.g. pregnancy) there is an increased sensitivity to exogenously administered analgesics (Gintzler and Liu, 2001) suggesting that neuroactive steroids are potent modulators of pain perception.
Neurosteroid-induced modulation of GABAA channels in pain pathways
Although it is well established that steroids can alter RNA and protein synthesis by entering the cell, forming steroid-receptor complexes and altering gene expression (Freeman et al., 1993) it is becoming increasingly recognized that some steroids can effectively modulate γ-aminobutyric acid (GABAA) receptor complexes located on neuronal membranes (Majewska, 1992; Hosie et al., 2006). This modulation is based on potent and fairly selective potentiation of GABAA receptor-mediated neurotransmission.
GABAergic potentiation appears to be particularly important in pain modulation. For example, 5α-pregnan-3α-ol-20-one (THP or 3α5αP), the 5α-reduced metabolite of progesterone, which is a potent GABAergic agent that lacks a high affinity for intracellular progestin receptors (Iswari et al., 1986), is also a very potent analgesic endogenous steroid suggesting that neurosteroids as endogenous anti-nociceptive agents could be of therapeutic benefit for the management of pain (Herd et al., 2007; Belelli and Lambert, 2005).
It appears that an important target of neurosteroid anti-nociceptive action is the superficial dorsal horn of the spinal cord which is involved in transmission of the impulses from the periphery to supra-spinal structures (Betz and Laube, 2006). Consequently, it has been shown that certain painful conditions can lead to up-regulated 5α-reduced neurosteroids synthesis in the spinal cord (Kibaly et al., 2008; Patte-Mensah et al., 2004) ultimately causing synaptic inhibition at the level of the spinal substantia gelatinosa (Meyer et al., 2008) and a potent analgesic effect. In addition, exogenous neurosteroids can also modulate GABAA receptor subunit expression and organization (Maguire and Mody, 2007; Shen et al., 2005). For instance, Peng and the colleagues (2009) have shown that exogenously administered progesterone up-regulates the expression of GABAA receptor subunits α2, α3, α4 and δ and is associated with attenuated repetitive stimulation-induced spinal reflex activity in ovariectomized rats. They report that this effect is most likely mediated via progesterone-induced neurosteroid synthesis rather than progesterone-induced receptor activation.
Interestingly, fast inhibitory transmission at the level of spinal cord is also mediated by glycine and strychnine-sensitive glycine (GlyR) receptors. Although the precise mechanism of GlyR and GABAA receptors interaction and their relative contribution in pain transmission is not clear it is becoming increasingly recognized that neurosteroids may also exert their inhibitory effect by potentiating GlyR (Weir et al. 2004). It appears that the inhibitory action in superficial spinal cord layers (lamina II neurons) responsible for neurosteroid-induced hyperalgesia is mainly mediated by GABAA whereas in deeper neuron layers (e.g. lamina V) it is mediated by neurosteroid action on both GABAA and GlyRs (Cronin et al., 2004) suggesting that tonic inhibition in lamina II neurons and/or well balanced tonic inhibition of superficial vs. deeper layers maybe promising options for neurosteroid pain therapy.
Estradiol, a sex hormone that fluctuates during the reproductive cycle in tandem with progesterone, has been shown to potentiate GABAergic neuroactive steroid effects on pain threshold with the efficiency being directly correlated with GABAergic potency of the neuroactive steroid. For instance, the anti-nociceptive effects of THP, 5α-pregnan-3β, 21-diol-20-one and 5α-pregnane-3, 20-dione, the potent modulators of GABAA receptor complex, were more consistently altered by estradiol compared to 4-pregnene-3, 20-dione which has very little effect on GABAA receptors (Frye and Duncan, 1996). Although the exact mechanism of this “priming effect” of estradiol is not clear several studies have shown that estradiol can alter GABA receptor complexes both in vivo and in vitro by increasing the expression of GABA receptors and/or by up-regulating its function (Hamon et al., 1983; Maggi and Perez, 1984; 1986).
Neurosteroid-induced modulation of T-type calcium channels in pain pathways
On the basis of the membrane potential at which they become activated, Ca2+ channels are subdivided into two major classes: high-voltage activated (HVA) or sustained currents and low-voltage activated (LVA) or transient (T-type) Ca2+ currents. T-currents are thought to play a unique role in neuronal excitability (Llinas, 1988; Huguenard, 1996). Major roles for the T-type channels in neurons include promotion of calcium-dependent burst firing, low-amplitude intrinsic neuronal oscillations, promotion of calcium entry and boosting of synaptic signals. The cloning of α1 subunits of T-channels has revealed the existence of at least three subtypes named G (Cav3.1; Perez-Reyes et al., 1998), H (Cav3.2; Cribbs et. al, 1998) and I (Cav3.3; Lee et al., 1999) that are likely to contribute to the heterogeneity of T-type Ca2+ currents observed in native cells (Herrington and Lingle, 1992; Todorovic and Lingle, 1998).
Despite the fact that T-type channels were first described in DRG neurons (Carbone and Lux, 1984) and are present in small and medium dissociated sensory neurons most of which are nociceptors, the role of T-type Ca2+ channels in nociceptive processing remains poorly understood. However, our work and that of others has shown the important role of T-type Ca2+ channels in somatic (Todorovic et al., 2001; Bourinet et al., 2005; Maeda et al., 2009) and visceral peripheral nociception (Kim et al., 2003). These studies and others (Dogrul et al., 2003) are creating interest in exploring the therapeutic potential of T-type Ca2+ current modulation for pain treatment.
I. Acute pain-alleviating action of 5α-reduced neuroactive steroids with modulatory action on T-channels
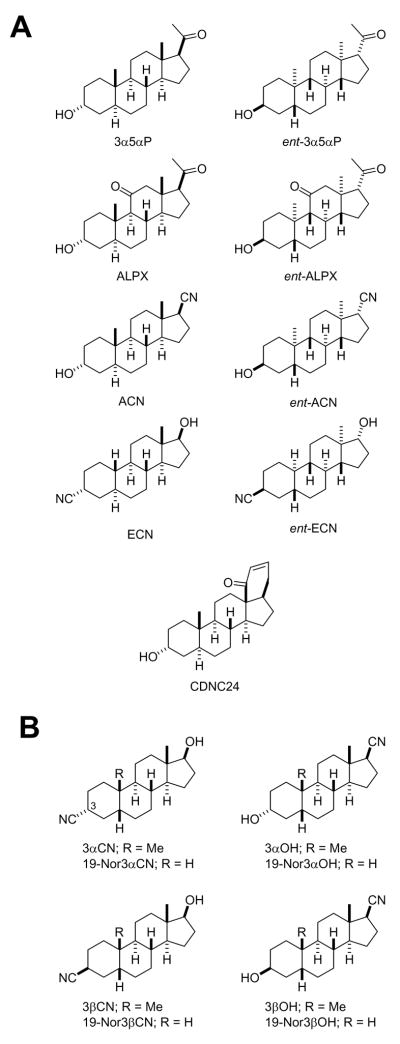
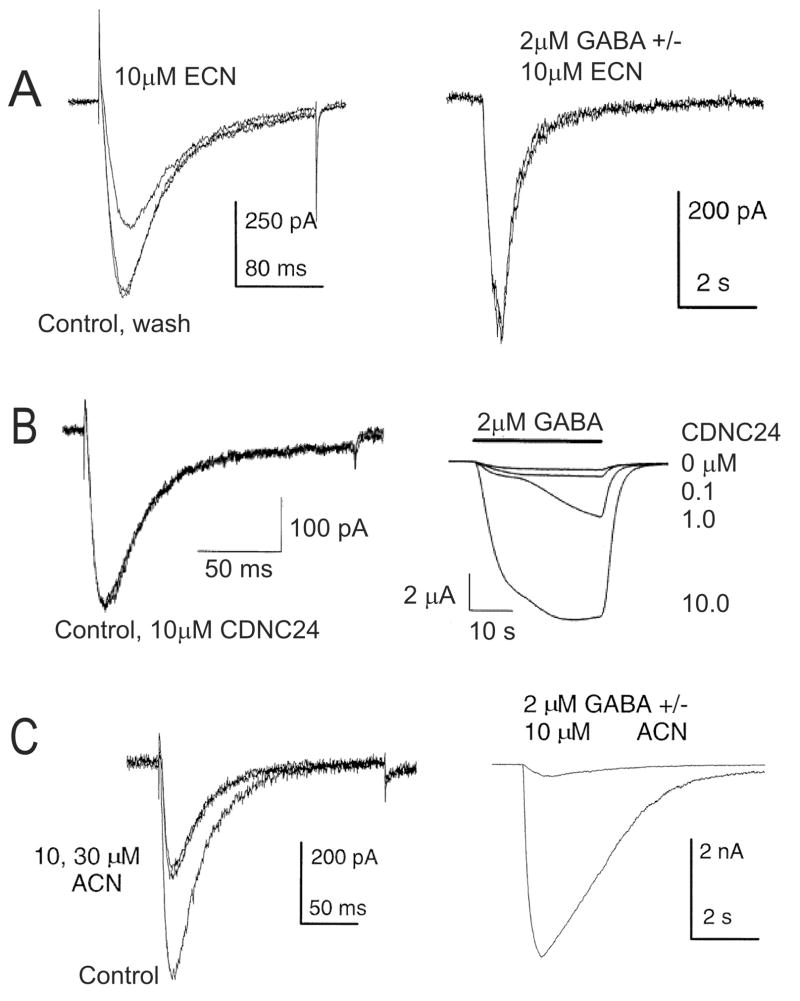
Of special interest for this review is the fact that T-channels might be an important cellular target for a variety of neuroactive steroids. For example, we have previously shown that the novel neuroactive steroid, ECN ((3β,5α,17β)-17-hydroxyestrane-3-carbonitrile, Fig. 1A), is a potent and enantioselective blocker of T-type Ca2+ channels in rat sensory neurons (IC50 300 nM for ECN; IC50 8.8 μM for ent-ECN), and unlike many other neuroactive steroids does not affect GABAA currents in hippocampal neurons (Todorovic et al., 1998) (Fig. 2A). Consequently, ECN and its enantiomer ent-ECN, when locally injected into the peripheral receptive fields of the rat hind paw, have been shown to induce potent peripheral analgesia in-vivo (Pathirathna et al., 2005a). It is noteworthy that their in-vivo analgesic potency mirrored their in-vitro T-channel blocking potency thus signifying the fact that T-channels in the peripheral nerve endings of skin may act as the amplifiers of nociceptive transmission in-vivo (Todorovic et al., 2001).
Figure 1. The chemical structures of 5α (A) and 5β (B) neuroactive steroids discussed in this review.
The chemical names of the neuroactive steroids are as follows: 3α5αP, (3α, 5α)-3-Hydroxypregnan-20-one; ent-3α5αP, (3β,5β,8α,9β,10α,13α,14β,17α)-3-Hydroxypregnan-20-one; ALPX, (3α,5α)-3 Hydroxypregnane-11,20-dione; ent-ALPX, (3β,5β,8α,9β,10α,13α,14β,17α)-3-Hydroxypregnane-11,20-dione; ACN, (3α,5α,17β)-3-Hydroxyandrostane-17-carbonitrile; ent-ACN, (3β,5β,8α,9β,10α,13α,14β,17α)-3-Hydroxyandrostane-17-carbonitrile; ECN, (3β,5α,17β)-17-Hydroxyestrane-3-carbonitrile; ent-ECN, (3α,5β,8α,9β,10α,13α,14β,17α)-17-Hydroxyestrane-3 carbonitrile; CDNC24, (3α,5α)-3-hydroxy-13,24-cyclo-18,21-dinorchol-22-en-24-one; 3αCN, (3α,5β,17β)-17-Hydroxyandrostane-3-carbonitrile; 19-Nor3αCN, (3α,5β17β)-17-Hydroxyestrane-3-carbonitrile; 3αOH, (3α,5β,17β)-3-Hydroxyandrostane-17-carbonitrile; 19-Nor3αOH, (3α,5β,17β)-3-Hydroxyestrane-17-carbonitrile; 3βCN, (3β,5β,17β)-17-Hydroxyandrostane-3-carbonitrile;19-Nor3βCN, (3β,5β17β)-17-Hydroxyestrane-3-carbonitrile; 3βOH, (3β,5β,17β)-3-Hydroxyandrostane-17-carbonitrile;19-Nor3βOH, (3β,5β,17β)-3-Hydroxyestrane-17-carbonitrile.
Figure 2. Differential effects of neuroactive steroid analogues on T-type calcium currents and GABAA-gated currents.
Panels A–C on the left show the effects of selected neuroactive steroids on T-type currents in acutely dissociated DRG cells. Panels A–C on the right show the effects of the same steroids on GABAA-gated currents in cultured hippocampal neurons (A and C) or in xenopus oocytes (panel B).Note that ECN (panel A) inhibited T-type current but had no apparent effects on GABAA currents, while in contrast, CDNC24 (panel B) had no effect on T-type current but potentiated GABAA-gated currents. ACN (panel C) had an effect on both currents as evidenced by the inhibition of T-type current and marked potentiation of GABAA-gated currents. (Reproduced with permission from Todorovic et al., Molecular Pharmacology, 54: 918–927, 1998 – Figs. 2A, 2B, 3A and 9A; Pathirathna et al., Pain, 114: 429–443, 2005- Fig. 8 – top and middle panels).
Interestingly when a variety of other 5α-reduced steroid analogs with either selective potentiating effect on GABAA current (CDNC24) (Pathirathna et 2005a) (Figs. 1A and 2B) or combined effect on GABAA and T-current [ACN (Fig. 2C), ent-ACN, alphaxalone, ent- alphaxalone, 3α5αP and ent-3α5αP] (Fig.1) in-vitro (Todorovic et al., 1998) were tested in-vivo we found that although CDNC 24 was ineffective in causing peripheral analgesia, the neuroactive steroids with a combined effect increased pain thresholds more than ECN alone (Pathirathna et al., 2005a). Furthermore, when ECN was combined with CDNC24, the anti-nociceptive activity of ECN was greatly enhanced, and this effect was GABAA antagonist, bicuculline-sensitive. This intriguing GABAA-T channel interaction was probed further when bicuculline failed to completely block peripheral analgesia induced by alphaxalone, a potent neuroactive steroid with effects on both GABAA receptors and T-channels. It led us to propose that GABAA channels do not contribute to baseline peripheral pain transmission, but can enhance anti-nociception mediated by blockade of T-type Ca2+ channels.
Thus, although there is strong evidence that GABAA receptors play an important role in centrally mediated analgesic effects of neuroactive steroids (Nadeson and Goodchild, 2000) the peripheral analgesic action of 5α-reduced steroids is likely mediated primarily by T-channels and much less by GABAA channels. In support of this view, it has been shown that despite the presence of GABAA channels on small nociceptive fibers in the skin, local application of muscimol, a GABAA agonist, into peripheral receptive fields of sensory neurons did not induce peripheral anti-nociception (Carlton et al., 1999). Similarly, we have shown that local application of CDNC24, a GABAergic neuroactive steroid, lacks peripheral anti-nociceptive effect (Pathirathna et al., 2005a).
Although the precise mechanism for this potentiating effect of GABAA channels in peripheral pain transmission is not clear we consider that perhaps intracellular Ca2+ may have a permissive role in controlling GABAA channel function in peripheral nociceptors, which could be achieved by Ca2+-dependent modulatory pathways that tonically inhibit GABAA channels. Therefore, when voltage-dependent Ca2+ channels are blocked causing a decrease in intracellular Ca2+, GABAA channels may be disinhibited and contribute to peripheral anti-nociception. As a result it appears that steroids with the effects on both GABAA and T channels systems (e.g. ACN, 3α5αP and alphaxalone) have higher analgesic efficacy due to combined blocking effect on T-channels and potentiating effect mediated by GABAA channels (Pathirathna et al., 2005a).
II. Chronic pain-alleviating action of 5α-reduced neuroactive steroids with modulatory action on T-channels
Since neuroactive steroids appear to be potent peripheral analgesics in intact animals an important consideration was whether they would be beneficial in animals with chronic pain. Potential usefulness of neuroactive steroids in treatment of chronic pain would be of great interest in the clinical setting due to the fact that the effective treatment of chronic pain remains a great challenge. Of particular interest is a form of chronic pain referred to as neuropathic pain (NPP) which is caused by the injury to neuronal elements leading to spontaneous firing of peripheral and/or central pain projection fibers. This debilitating form of chronic pain often occurs in young and otherwise healthy people. NPP has been described as a ‘wind-up’ pain due to exaggerated responses to painful stimuli and/or a perception of innocuous tactile or thermal stimuli as painful (Chaplan et al., 1997; Kajander and Bennett, 1992). When the effectiveness of a series of 5α-reduced neuroactive steroids in alleviating thermal and mechanical hyperalgesia was tested in animals with a sciatic nerve injury caused by loose ligation we found that local injections of either ECN or CDNC24 into the peripheral receptive fields of a ligated hind paw were more selective in alleviating thermal nociception in NPP than in sham animals compared to 3α5αP or alphaxalone although the anti-nociceptive effect induced by 3α5αP and alphaxalone was more profound (Pathirathna et al., 2005b). Interestingly, despite the fact that CDNC24 was ineffective as a peripheral analgesic in intact animals (Pathirathna et al., 2005a) it was most selective in alleviating thermal hyperalgesia in NPP animals as shown by a very minimal anti-nociceptive effect in sham animals but a very profound anti-nociceptive effect in NPP animals. This would suggest that, although under normal conditions GABAA modulation per se has a minimal effect on peripheral nociception, under pathological conditions (e.g. caused by the loose ligation of a sciatic nerve), GABAA receptors might be substantially more sensitive to stimulation and as such potentially an important therapeutic target of neuroactive steroids. Indeed, it has been shown that spinal GABAergic modulation is effective in reversing nerve ligation-induced allodynia and hyperalgesia (Hwang and Yaksh, 1997; Malan et al., 2002) and that peripheral nerve injury causes an increase in GABAA receptor-mediated conductance in cutaneous afferent DRG neurons (Oyelese et al., 1997). Moreover, phenotypic changes of GABAA receptor in spinal dorsal horn are shown to be manifested as an up-regulation of α5 subunit (Yang et al., 2004), and this could possibly result in the heightened level of baseline GABA activity.
Our findings have also indicated that a significant portion of the anti-nociceptive action of 3α5αP or alphaxalone in NPP is due to their T channel blocking properties due to the fact that their peripheral nociceptive effect was of a higher magnitude than the one observed with either ECN or CDNC24 and that blocking the GABAergic component of 3α5αP or alphaxalone activity with bicuculline resulted in only a partial decrease in their anti-nociceptive effect. Hence, neurosteroid-induced modulation of T channels could add a substantial therapeutic advantage. Contributing further to this notion is our finding that the peripheral anti-nociception induced by 3α5αP and alphaxalone was of a higher magnitude than the one observed with either ECN or CDNC24 indicating that T channels and GABAA channels may work in concert to control the activation of peripheral nociceptors.
Although the presence of GABAA channels in the peripheral nociceptors has been confirmed (Carlton et al., 1999), the presence of T channels has not yet been established. However, some recent studies report that a knockdown of CaV3.2 channels (CaV3.2 is the most prevalent subtype of T channels in sensory neurons) (Talley et al., 1999) leads to a greatly diminished response to peripheral thermal stimuli in NPP animals (Bourinet et al., 2005) indicating that T channels may play an important role in peripheral nociception. Hence, newly synthesized neuroactive steroids that selectively modulate T channels are a potentially useful pharmacological tool for studying the importance of these channels in nociception (Pathirathna et al., 2005a and b; Todorovic et al., 1998).
The affinity of 5α-reduced neuroactive steroids found to be useful as peripheral analgesics for other cellular targets that might be important in the pathophysiology of NPP (e.g. voltage-gated K+, Na+ and HVA Ca2+ channels and ligand-gated glutamate channels) has been previously studied and it was determined that these targets are either completely insensitive (e.g. ECN, 3α5αP) or significantly less sensitive (e.g. alphaxalone) (Benoit et al., 1988; Nakashima et al., 1998; Todorovic et al., 1998) at the concentrations that caused near maximal effect on GABAA channels (e.g. CDNC24, alphaxalone, 3α5αP) or T channels (e.g. ECN, alphaxalone, 3α5αP) (Pathirathna et al., 2005b).
Of interest in clinical setting is the fact that neuroactive steroids that lack Na+ channel-blocking properties could potentially be useful for regional nerve blocks since a significant analgesia maybe achieved without motor weakness or transient paralysis (commonly observed with presently available local anesthetics), thus achieving a desirable level of comfort while preserving motor function.
III. Acute pain-alleviating action of 5β-reduced neuroactive steroids with modulatory action on T-channels
Our earlier work has shown that another class of newly synthesized neurosteroids, 5β-reduced neuroactive steroids, which are potent blockers of the T-type Ca2+ channels in rat peripheral sensory neurons in-vitro are also very potent anti-nociceptive agents in-vivo (Todorovic et al., 2004). For example, when intact adult rats were injected directly into the peripheral receptive fields of the hind paw we found that compounds having either the 3-cyano and 17β-hydroxyl groups (3αCN, 3βCN, 19-Nor3αCN, and 19-Nor3βCN) or the 3-hydroxyl and 17β-cyano group (3αOH, 3βOH, 19-Nor3αOH, and 19-Nor3βOH) (Fig. 1B) induce significant and dose-dependent peripheral nociception as determined using thermal nociceptive testing (Todorovic et al., 2004). Interestingly, the 19-norsteroids with the 3-cyano, 17β-hydroxy groups were more potent than those with the 19-methyl group, whereas the 19-norsteroids with the 3-hydroxy, 17β-cyano groups were less potent than those with the 19-methyl groups. Again, there was an excellent correlation between the potency of T-current blockade in-vitro and anti-nociceptive potency in-vivo, which further corroborates the notion that T-type Ca2+ channels play an important role in peripheral somatic nociception. It is noteworthy that these 5β-reduced steroids cause almost complete block of neuronal DRG T currents, whereas 5α-reduced neuroactive steroids discussed in this review block T currents only partially (up to 40%; Todorovic et al., 1998).
Conclusions
Neuroactive steroids that are effective modulators of GABAA and/or T-type Ca2+ channels are promising tools for studying the role of these channels in peripheral pain perception. They appear to be very effective in alleviating peripheral nociception in rat models of acute and chronic pain. Our findings regarding the anti-nociceptive effects of locally-injected neuroactive steroids suggest that although their local injection causes significant thermal hyperalgesia in the injected paw, it lacks the systemic nociceptive effect (Todorovic et al., 2004; Pathirathna et al., 2005a; 2005b) indicating that the site of action is most likely on nociceptive nerve endings.
In considering novel 5-reduced steroids as local analgesics, it is important to note that T channels are preferentially located on the smaller size sensory neurons that play an important role in nociceptive transmission but not in other modalities of sensory transmission (e.g., touch, vibration) or motor transmission, making selective and potent blockade of T currents a desirable therapeutic objective -- effective analgesia without causing undesirable motor weakness. The neurosteroids could also be amenable to delivery by direct applications in the form of skin patches or local infiltration (at the site of an acute tissue injury, e.g., thermal coagulation, sunburns) because these agents are highly lipid soluble and should be able to easily access peripheral nerve endings. The effectiveness of neuroactive steroids in modulating pain perception in humans needs to be explored in well-designed, randomized and multi-center clinical studies.
Acknowledgments
Author Disclosure: Our research is supported by Dr. Harold Carron’s endowment (to V.J-T.), NIH R0-1 grant GM075229 (to S.M.T), NIH P0-1 grant GM47969 (to D.F.C.) and funds from Department of Anesthesiology (to V.J-T. and S.M.T.). V.J-T is an Established Investigator of the American Heart Association. The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the r1eport; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baulieu E-E, Robel P, Schumacker M. Neurosteroids: A new regulatory function in the nervous system. In: Baulieu E-E, Robel P, Schumacker M, editors. Contemporary Endocrinology. Humana Press; Totowa, NJ: 1999. [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Benoit E, Carratu MR, Mitolo-Chieppa D. Mechanism of action of a structural analog of alphaxalone on myelinated nerve fibre. Eur J Pharmacol. 1988;158:1–9. doi: 10.1016/0014-2999(88)90246-4. [DOI] [PubMed] [Google Scholar]
- Betz H, Laube B. Glycine receptors: recent insights into their structural organization and functional diversity. J Neurochem. 2006;97:1600–1610. doi: 10.1111/j.1471-4159.2006.03908.x. [DOI] [PubMed] [Google Scholar]
- Bourinet E, Alloui A, Monteil A, Barrere C, Couette B, Poirot O, Pages A, McRory J, Snutch TP, Eschalier A, Nargeot J. Silencing of the Cav3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception. EMBO J. 2005;24:315–324. doi: 10.1038/sj.emboj.7600515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E, Lux HD. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurons. Nature. 1984;310:501–2. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Zhou S, Coggeshall RE. Peripheral GABA(A) receptors: evidence for peripheral primary afferent depolarization. Neuroscience. 1999;93:713–722. doi: 10.1016/s0306-4522(99)00101-3. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997;280:829–838. [PubMed] [Google Scholar]
- Cribbs LL, Lee JH, Yang J, Satin J, Zhang Y, Daud A, Barclay J, Williamson MP, Fox M, Rees M, Perez-Reyes E. Cloning and characterization of alpha1H from human heart, a member of the T-type Ca2+ channel gene family. Circ Res. 1998;83:103–9. doi: 10.1161/01.res.83.1.103. [DOI] [PubMed] [Google Scholar]
- Cronin JN, Bradbury EJ, Lidierth M. Laminar distribution of GABAA-and glycine-receptor mediated tonic inhibition in the dorsal horn of the rat lumbar spinal cord: effects of picrotoxin and strychnine on expression of Fos-like immunoreactivity. Pain. 2004;112:156–163. doi: 10.1016/j.pain.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Dogrul A, Gardell LR, Ossipov MH, Tulunay FC, Lai J, Porreca F. Reversal of experimental neuropathic pain by T-type calcium channel blockers. Pain. 2003;105:159–168. doi: 10.1016/s0304-3959(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Freeman LM, Breedlove SM. Steroid receptors in the central nervous system. In: Conn PM, editor. Methods in neurosciences. Vol. 11 Academic Press; San Diego: 1993. [Google Scholar]
- Frye CA, Cuevas CA, Kanarek RB. Diet and estrous cycle influence pain sensitivity in rats. Pharmacol Biochem Behav. 1993;45:255–260. doi: 10.1016/0091-3057(93)90116-b. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duncan JE. Estradiol benzoate potentiates neuroactive steroids' effects on pain sensitivity. Pharmacol Biochem Behav. 1996;53:27–32. doi: 10.1016/0091-3057(95)00194-8. [DOI] [PubMed] [Google Scholar]
- Gintzler AR. Endorphin mediated increase in pain threshold during pregnancy. Science. 1980;210:193–195. doi: 10.1126/science.7414330. [DOI] [PubMed] [Google Scholar]
- Gintzler AR, Liu NJ. The maternal spinal cord: biochemical and physiological correlates of steroid-activated antinociceptive processes. Prog Brain Res. 2001;133:83–97. doi: 10.1016/s0079-6123(01)33007-8. [DOI] [PubMed] [Google Scholar]
- Goodchild CS, Guo Z, Nadeson R. Antinociceptive properties of neurosteroids I. Spinally-mediated antinociceptive effects of water-soluble aminosteroids. Pain. 2000;88:23–29. doi: 10.1016/S0304-3959(00)00301-8. [DOI] [PubMed] [Google Scholar]
- Hamon G, Goetz C, Euvarard C, Pasqualini C, Le Dafneit M, Kerdelhue B, Cesselin F, Peillon F. Biochemical and functional alterations in central GABA receptors during chronic estrogen treatment. Brain Res. 1983;279:141–152. doi: 10.1016/0006-8993(83)90172-5. [DOI] [PubMed] [Google Scholar]
- Herd MB, Belelli D, Lambert JJ. Neurosteroid modulation of synaptic and extrasynaptic GABAA receptors. Pharmacol Ther. 2007;116:20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Herrington J, Lingle CJ. Kinetic and pharmacological properties of low voltage-activated Ca2+ current in rat clonal (GH3) pituitary cells. J Neurophysiol. 1992;68:213–32. doi: 10.1152/jn.1992.68.1.213. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–9. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- Hwang JW, Yaksh TL. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically-induced neuropathic pain model in the rat. Pain. 1997;70:15–22. doi: 10.1016/s0304-3959(96)03249-6. [DOI] [PubMed] [Google Scholar]
- Iswari S, Colas AE, Karavolas HJ. Binding of 501-dihydroprogesterone and other progestins to female rat anteriorpituitary nuclear extracts. Steroids. 1986;47:189–203. doi: 10.1016/0039-128x(86)90088-7. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Bennett GJ. Onset of a painful peripheral neuropathy in rat: a partial and differential deafferentation and spontaneous discharge in A beta and A delta primary afferent neurons. J Neurophysiol. 1992;68:734–744. doi: 10.1152/jn.1992.68.3.734. [DOI] [PubMed] [Google Scholar]
- Kibaly C, Meyer L, Patte-Mensah C, Mensah-Nyagan AG. Biochemical and functional evidence for the control of pain mechanisms by dehydroepiandrosterone endogenously synthesized in the spinal cord. FASEB J. 2008;22:93–104. doi: 10.1096/fj.07-8930com. [DOI] [PubMed] [Google Scholar]
- Kim D, Park D, Choi S, Lee S, Sun M, Kim C, Shin H-S. Thalamic control of visceral nociception mediated by T-type Ca2+ channels. Science. 2003;302:117–119. doi: 10.1126/science.1088886. [DOI] [PubMed] [Google Scholar]
- Lee JH, Daud AN, Cribbs LL, Lacerda AE, Pereverzev A, Klockner U, Schneider T, Perez-Reyes E. Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. J Neurosci. 1999;19:1912–21. doi: 10.1523/JNEUROSCI.19-06-01912.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–64. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Aoki Y, Sekiguchi F, Matsunami M, Takahashi T, Nishikawa H, Kawabata A. Hyperalgesia induced by spinal and peripheral hydrogen sulfide: evidence for involvement of Cav 3.2 T-type calcium channels. Pain. 2009;142:127–132. doi: 10.1016/j.pain.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Maggi A, Perez J. Progesterone and estrogens in rat brain: Modulation of GABA receptor activity. Eur J Pharmacol. 1984;103:165–168. doi: 10.1016/0014-2999(84)90205-x. [DOI] [PubMed] [Google Scholar]
- Maggi A, Perez J. Estrogen-induced upregulation of gammaaminobutyric acid receptors in the CNS of rodents. J Neurothem. 1986;47:1793–1797. doi: 10.1111/j.1471-4159.1986.tb13090.x. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABAA receptor: relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96:1161–1167. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38:379–95. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- Martinez-Gomez M, Cruz Y, Salas M, Hudson R, Pachecos P. Assessing pain threshold in the rat: Changes with estrus and time of day. Physiol Behav. 1994;55:651–657. doi: 10.1016/0031-9384(94)90040-x. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Caba M, Komisurak BR, Beyer C. Modulation by estrogen and progesterone on the effect of muscimol on nociception in the spinal cord. Pharmacol Biochem Behav. 1990;37:123–128. doi: 10.1016/0091-3057(90)90052-j. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Garcia-Segura LM, Mensah-Nyagan AG. Neuroactive steroids: state of the art and new perspectives. Cell Mol Life Sci. 2008;65:777–97. doi: 10.1007/s00018-007-7403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah-Nyagan AG, Do-Rego JL, Beaujean D, Luu-The V, Pelletier G, Vaudry H. Neurosteroids: expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacol Rev. 1999;51:63–81. [PubMed] [Google Scholar]
- Meyer L, Venard C, Schaeffer V, Patte-Mensah C, Mensah-Nyagan AG. The biological activity of 3 alpha-hydroxysteroid oxido-reductase in the spinal cord regulates thermal and mechanical pain thresholds after sciatic nerve injury. Neurobiol Dis. 2008;30:30–41. doi: 10.1016/j.nbd.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Nadeson R, Goodchild CG. Antinociceptive properties of neurosteroids II. Experiments with Saffan and its components alphaxalone and alphadolone to reveal separation of anaesthetic and antinociceptive effects and the involvement of spinal cord GABA(A) receptors. Pain. 2000;88:31–39. doi: 10.1016/S0304-3959(00)00300-6. [DOI] [PubMed] [Google Scholar]
- Nakashima YM, Todorovic SM, Covey DF, Lingle CJ. The anesthetic steroid (+)-3alpha-hydroxy-5alpha-androstane-17beta-carbonitrile blocks N-, Q-, and R-type, but not L- and P-type, high voltage-activated Ca2+ current in hippocampal and dorsal root ganglion neurons of the rat. Mol Pharmacol. 1998;54:559–568. doi: 10.1124/mol.54.3.559. [DOI] [PubMed] [Google Scholar]
- Oyelese AA, Rizzo MA, Waxman SG, Kocsis JD. Differential effects of NGF and BDNF on axotomy-induced changes in GABA(A)-receptor-mediated conductance and sodium currents in cutaneous afferent neurons. J Neurophysiol. 1997;78:31–42. doi: 10.1152/jn.1997.78.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathirathna S, Brimelow BC, Jagodic MM, Krishnan K, Jiang X, Zorumski CF, Mennerick S, Covey DF, Todorovic SM, Jevtovic-Todorovic V. New evidence that both T-type calcium channels and GABAA channels are responsible for the potent peripheral analgesic effects of 5alpha-reduced neuroactive steroids. Pain. 2005a;114:429–43. doi: 10.1016/j.pain.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Pathirathna S, Todorovic SM, Covey DF, Jevtovic-Todorovic V. 5alpha-reduced neuroactive steroids alleviate thermal and mechanical hyperalgesia in rats with neuropathic pain. Pain. 2005b;117:326–39. doi: 10.1016/j.pain.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Patte-Mensah C, Li S, Mensah-Nyagan AG. Impact of neuropathic pain on the gene expression and activity of cytochrome P450side-chain-cleavage in sensory neural networks. Cell Mol Life Sci. 2004;61:2274–84. doi: 10.1007/s00018-004-4235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng HY, Chen GD, Lee SD, Lai CY, Chiu CH, Cheng CL, Chang YS, Hsieh MC, Tung KC, Lin TB. Neuroactive steroids inhibit spinal reflex potentiation by selectively enhancing specific spinal GABA(A) receptor subtypes. Pain. 2009 doi: 10.1016/j.pain.2008.12.023. in press. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee JH. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- Ratka A, Simpkins JW. Effects of estradiol and progesterone on the sensitivity to pain and on morphine-induced antinociception in female rats. Horm Behav. 1991;25:217–228. doi: 10.1016/0018-506x(91)90052-j. [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases delta GABA(A) receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19:1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic SM, Prakriya M, Nakashima YM, Nilsson KR, Han M, Zorumski CF, Covey DF, Lingle CJ. Enantioselective blockade of T-type Ca2+ current in adult rat sensory neurons by a steroid that lacks gamma-aminobutyric acid-modulatory activity. Mol Pharmacol. 1998;54:918–927. doi: 10.1124/mol.54.5.918. [DOI] [PubMed] [Google Scholar]
- Todorovic SM, Lingle CJ. Pharmacological properties of T-type Ca2+ current in adult rat sensory neurons: effects of anticonvulsant and anesthetic agents. J Neurophysiol. 1998;79:240–252. doi: 10.1152/jn.1998.79.1.240. [DOI] [PubMed] [Google Scholar]
- Todorovic SM, Jevtovic-Todorovic V, Meyenburg A, Mennerick S, Perez-Reyes E, Romano C, Olney JW, Zorumski CF. Redox modulation of T-type calcium channels in rat peripheral nociceptors. Neuron. 2001;31:75–85. doi: 10.1016/s0896-6273(01)00338-5. [DOI] [PubMed] [Google Scholar]
- Todorovic SM, Pathirathna S, Brimelow BC, Jagodic MM, Ko SH, Jiang X, Nilsson KR, Zorumski CF, Covey DF, Jevtovic-Todorovic V. 5beta-reduced neuroactive steroids are novel voltage-dependent blockers of T-type Ca2+ channels in rat sensory neurons in vitro and potent peripheral analgesics in vivo. Mol Pharmacol. 2004;66:1223–35. doi: 10.1124/mol.104.002402. [DOI] [PubMed] [Google Scholar]
- Weir CJ, Ling AT, Belelli D, Wildsmith JA, Peters JA, Lambert JJ. The interaction of anaesthetic steroids with recombinant glycine and GABAA receptors. Br J Anaesth. 2004;92:704–11. doi: 10.1093/bja/aeh125. [DOI] [PubMed] [Google Scholar]
- Winter L, Nadeson R, Tucker AP, Goodchild CS. Antinociceptive properties of neurosteroids: a comparison of alphadolone and alphaxalone in potentiation of opioid antinociception. Anesth Analg. 2003;97:798–805. doi: 10.1213/01.ANE.0000075835.73967.F3. [DOI] [PubMed] [Google Scholar]
- Yang L, Zhang FX, Huang F, Lu YJ, Li GD, Bao L, Xiao HS, Zhang X. Peripheral nerve injury induces trans-synaptic modification of channels, receptors and signal pathways in rat dorsal spinal cord. Eur J Neurosci. 2004;19:871–883. doi: 10.1111/j.0953-816x.2004.03121.x. [DOI] [PubMed] [Google Scholar]