Abstract
Background
The cystine-glutamate exchanger is down-regulated after chronic cocaine, resulting in reduced extracellular levels of nucleus accumbens glutamate. The importance of cocaine-induced loss of glutamate homeostasis is revealed by N-acetylcysteine restoring cystine-glutamate exchange and attenuating reinstatement to cocaine-seeking. Another regulator of extracellular glutamate is the glial glutamate transporter GLT-1. We hypothesized that cocaine self-administration reduces GLT-1, and that GLT-1 up-regulation inhibits cocaine-seeking.
Methods
We measured [3H] glutamate uptake and protein expression of GLT-1 and xCT, the catalytic subunit of the cystine-glutamate exchanger, following cocaine self-administration and 3 weeks of extinction training. We also examined the affect of ceftriaxone (previously shown to increase GLT-1) and N-acetylcysteine treatment on the expression of GLT-1 and xCT. Ceftriaxone was also tested for the capacity to inhibit cue- and cocaine-induced relapse.
Results
Cocaine self-administration reduced glutamate uptake and the expression of both GLT-1 and xCT. Ceftriaxone restored GLT-1 and xCT levels and prevented cue- and cocaine-induced reinstatement of drug-seeking. NAC also restored GLT-1 and xCT levels.
Conclusion
These results indicate that glutamate transport and cystine-glutamate exchange may be co-regulated, and provide further evidence that targeting glutamate homeostasis is a potential method for treating cocaine relapse.
Keywords: cocaine, accumbens, cystine-glutamate exchange, glutamate uptake, GLT-1, self-administration
Introduction
Relapse to cocaine-seeking in the animal model of reinstatement is strongly associated with changes in the extracellular levels of glutamate in the nucleus accumbens core, namely decreased basal levels of glutamate in the NAcc core as well as enhanced extracellular glutamate levels in response to a cocaine challenge (1). Glutamate is released into the extracellular space from synaptic and nonsynaptic sources, and is eliminated via a family of glutamate uptake transporters (2). The balance between synaptic and nonsynaptic glutamate release and elimination is termed glutamate homeostasis, which modulates synaptic activity and plasticity by controlling the stimulation of iontotropic and metabotropic glutamate receptors (3, 4). The cystine-glutamate exchanger, which exchanges one extracellular cystine molecule for one intracellular glutamate molecule (5), accounts for the majority of nonsynaptic extracellular glutamate in the nucleus accumbens (6) and its activity is down-regulated after chronic cocaine (7, 8). The nutritional supplement N-acetylcysteine restores the function of the cystine-glutamate exchanger, increases the basal levels of extracellular glutamate in the accumbens after withdrawal from cocaine and thereby attenuates reinstatement to cocaine-seeking in animals (3, 7, 8) and cocaine cue-reactivity in humans (9).
In addition to the cystine-glutamate exchanger, sodium-dependent glutamate transport into glia is a critical regulator of extracellular glutamate concentrations; this type of transport is maintained primarily by the major glial glutamate transporter, GLT-1 (EAAT2) (2). GLT-1 is responsible for 90% of total brain glutamate uptake (10). Given the apparent imbalance in glutamate homeostasis associated with withdrawal from cocaine self-administration, we postulated that cocaine also induces changes in glutamate transport. Recently, this hypothesis was supported by mathematical modeling of glutamate homeostasis at glutamatergic synapses in the nucleus accumbens that predicted both cystine-glutamate exchange and sodium-dependent uptake would be reduced by cocaine self-administration (11). Thus, it is possible that these two mechanisms of glutamate transport, glutamate uptake and cystine-glutamate exchange, are co-regulated. Supporting this possibility, Bannai (12) reported that cystine-glutamate exchange is stimulated by increased activity of sodium-dependent glutamate transport. Additionally, down-regulated transporter activity leaves excess glutamate in the extrasynaptic space, which has been found to inhibit the cystine-glutamate exchanger from exporting glutamate (13). The catalytic subunit of the cystine-glutamate exchanger is the protein xCT (5), and nicotine self-administration was recently shown to reduce the levels of both xCT and GLT-1 in the nucleus accumbens (14).
Here, we measured glutamate uptake and levels of GLT-1 and xCT protein expression in nucleus accumbens tissue of rats which had self-administered cocaine and underwent 3 weeks of extinction training. The β-lactam antibiotic ceftriaxone increases the expression and activity of GLT-1 (15, 16) and we tested its ability to attenuate the reinstatement of cocaine-seeking and elevate GLT-1 levels following cocaine self-administration. We investigated the possibility of co-regulation of the two glutamate transport systems by measuring the effects of ceftriaxone treatment on xCT expression and conversely, the effect of N-acetylcysteine treatment on GLT-1 expression in the nucleus accumbens of rats withdrawn from cocaine self-administration.
Methods and Materials
Rats were trained to self-administer cocaine for two weeks and then underwent three weeks of extinction training. Glutamate uptake was measured in nucleus accumbens tissue (see Supplement for detailed description of uptake methods). A separate set of animals received ceftriaxone (200 mg/kg IP), N-acetylcysteine (100 mg/kg IP), or vehicle (saline) daily for the last 7 days of extinction training and were euthanized via rapid decapitation. The nucleus accumbens was dissected and a membrane sub-fraction was generated. Western blotting was performed on this tissue for GLT-1 and xCT proteins (for detailed description of the membrane sub-fractionation and western blotting procedures, see Supplement).
Animals self-administered cocaine for 2 hr/day for two weeks followed by extinction training for 3 weeks and until lever pressing reached 25% of self-administration levels. During extinction, animals were treated with either ceftriaxone (200 mg/kg IP) or vehicle for 5–12 days and then tested for both cue- and cocaine-primed reinstatement of the drug-seeking response. To ensure that ceftriaxone was not producing sedation, a separate group of animals was injected with ceftriaxone (200 mg/kg IP) for 7 days and then tested for basal locomotion and the locomotor response to a saline injection (see Supplement for detailed description of the behavioral methods).
Results
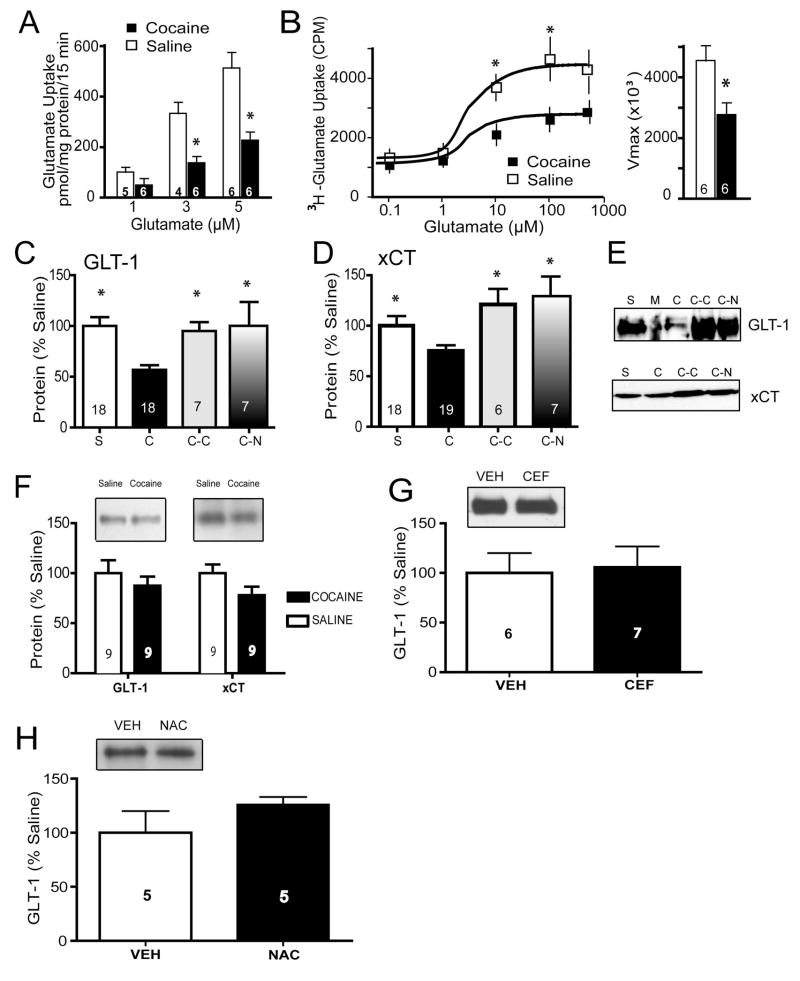
Sodium-dependent glutamate uptake in tissue slices from the accumbens was reduced in cocaine-trained compared to saline controls over a physiological range of glutamate concentrations (1, 3, 5 μM; Fig. 1A). This was confirmed in a separate set of cocaine and saline subjects showing that reduced uptake resulted from decreased Vmax, without a difference in binding affinity (Kd= 2.6±0.9 μM [cocaine], =2.4±0.9 μM [saline]; Fig 1B). Consistent with decreased Vmax, we observed significant down-regulation of GLT-1 expression in the accumbens membrane subfraction following cocaine self-administration (Fig. 1C), but not in the prefrontal cortex (Fig. 1F). In the same samples used to measure GLT-1, reduced expression of xCT was also measured in the accumbens (Fig. 1D), but not prefrontal cortex (although the level of xCT trended down in the cocaine animals; Fig. 1F). Both N-acetylcysteine and ceftriaxone treatment alone up-regulated the expression of both xCT and GLT-1 (Fig. 1C–E). Of note, this ceftriaxone treatment regimen did not up-regulate GLT-1 expression in drug-naïve rats (Fig. 1G). Similarly, treatment with N-acetylcysteine failed to upregulate GLT-1 in drug-naïve rats (Fig. 1H).
Figure 1.
Cocaine self-administration reduces glutamate transporter expression and function. A) [3H] glutamate uptake was significantly decreased in accumbens tissue slices following cocaine self-administration. A two-way ANOVA revealed significant main effects of cocaine treatment (F(1,26)=11.36, p=0.002) and glutamate concentration (F(2,26)=14.73, p<0.001); *p<0.05). B) The Vmax of glutamate uptake was reduced in cocaine subjects with no change in Kd. A two-way ANOVA revealed a significant effect of cocaine treatment (F(1,49)=11.21, p=0.001) and concentration of glutamate (F(4,49)=13.61, p<0.001). * p<0.05). C–E) Cocaine self-administration followed by 3 weeks of extinction training significantly decreased GLT-1 and xCT protein expression, and chronic treatment with N-acetylcysteine or ceftriaxone restored levels of both proteins. One-way ANOVA’s confirmed an effect of group for both GLT-1 (F(3,47)=5.34, p=0.003) and xCT (F(3,45)=4.47, p=0.008). *p<0.05 compared to cocaine. S=Saline; C=Cocaine; C-C=Cocaine+Ceftriaxone; C-N=Cocaine+N-acetylcysteine; M=Marker Ladder. F) Membrane fraction levels of GLT-1 and xCT were not changed in the prefrontal cortex following cocaine self-administration. G) Seven days of ceftriaxone treatment (200 mg/kg IP) had no effect on accumbens GLT-1 levels in naïve rats. H) Seven days of N-acetylcysteine treatment (100 mg/kg IP) had no effect on accumbens GLT-1 levels in naïve rats.
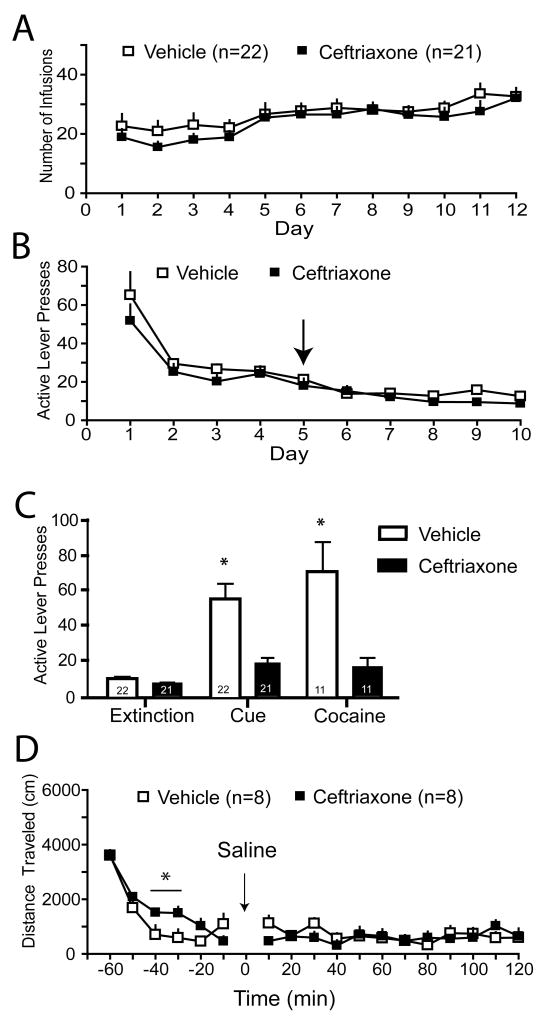
There was no difference in the number of cocaine infusions attained over the course of the self-administration period for the ceftriaxone- and vehicle-treated subjects (Fig. 2A; for inactive lever presses see Fig. S1C); likewise, self-administration levels were no different between groups used for the uptake assay, western blots, or reinstatement tests (Fig. S1A,B). Ceftriaxone treatment had no effect on extinction levels of lever pressing (Fig. 2B), however the lever-pressing behavior was largely extinguished by Day 5, when ceftriaxone administration began. Ceftriaxone treatment significantly attenuated both cue- and cocaine-primed reinstatement (Fig. 2C). Importantly, ceftriaxone potentiated spontaneous motor activity in an unhabituated open field test, indicating a lack of nonspecific sedation (Fig. 2D). This increase in locomotion was most likely indicative of an enhanced response to a novel environment, a phenomenon which would not influence reinstatement behavior since reinstatement was measured in a familiar environment (operant chamber).
Figure 2.
Ceftriaxone inhibits cue-and cocaine-primed reinstatement of cocaine-seeking. A) The mean number of cocaine infusions (0.2 mg/infusion) self-administered (no ceftriaxone was administered at this time). B) The mean number of active lever presses during extinction training; ceftriaxone or vehicle administration began on Day 5 (arrow). C) Animals treated with ceftriaxone (200 mg/kg IP) during extinction training displayed less active lever pressing in response to both cues previously paired with cocaine infusion and a priming injection of cocaine (15 mg/kg IP). A two-way ANOVA indicated significant effects of Treatment (F(1,3)=35.19, p<0.001), Test condition (F(2,3)=17.78, p<0.001), and interaction (F(3,103)= 8.180, p=0.005). * p <0.05. D) Ceftriaxone treatment did not reduce locomotor activity. A two-way ANOVA revealed effects of Time (F(1,17)=23.84, p<0.001 and a Time × Group interaction (F(1,17)=2.01, p=0.04. * p<0.05.
Discussion
In summary, the Vmax of glutamate uptake was decreased in the nucleus accumbens of rats with a history of cocaine self-administration and 3 weeks of extinction training (Fig 1B). The decreased uptake was associated with reduced expression of the glutamate transporter GLT-1 (Fig 1C). The FDA-approved, β-lactam antibiotic ceftriaxone increases glutamate uptake and the transcription of GLT-1(15, 16), and we showed that it restores nucleus accumbens levels of both GLT-1 and xCT in rats trained to self-administer cocaine (Fig 1C,D). Moreover, another compound known to inhibit cocaine-seeking, N-acetylcysteine, also restored GLT-1 and xCT levels in the accumbens (Fig. 1C,D). These results point to a co-regulation of expression of the proteins responsible for the two glutamate transport systems.
The restoration of these proteins critical for glutamate homeostasis by ceftriaxone resulted in an attenuation of both cue- and cocaine-primed reinstatement (Fig. 2C). This reduction in drug-seeking behavior was not due to a non-specific affect of ceftriaxone on locomotor behavior (Fig. 2D). Since it was previously shown that decreased levels of extracellular glutamate in the accumbens core predisposes rats to the reinstatement of cocaine-seeking (1, 7), it seems paradoxical that ceftriaxone-induced up-regulation of sodium-dependent glutamate transport prevented reinstatement. However, ceftriaxone treatment increased the expression of both GLT-1 and xCT (Fig. 1C,D), indicating that the increase in uptake was offset by an increase in the efflux of nonsynaptic glutamate via enhanced cystine-glutamate exchange. It is possible that the attenuation of reinstatement observed here is due solely to the restoration of xCT expression, an idea that is supported by the previous finding that by normalizing basal glutamate levels in the accumbens, N-acetylcysteine restores glutamatergic tone on mGluR2/3 receptors, thus preventing the overflow of glutamate that drives cocaine-primed reinstatement (17). However, it is likely that the up-regulation of GLT-1 observed here is an essential factor in the attenuation of reinstatement, serving to dampen the overflow of extracellular glutamate during reinstatement. In fact, a computer model of the accumbens synapse following cocaine self-administration requires a down-regulation in sodium-dependent glutamate uptake in addition to a decrease in basal levels of glutamate and mGluR2/3 function to account for the increase in extracellular glutamate observed during reinstatement (11). It is important to note that this study was conducted in animals undergoing extinction training, which may have contributed to the observed changes in glutamate homeostasis. However, this seems unlikely given that daily noncontingent cocaine administration is also known to alter measures of glutamate homeostasis, such as lowering basal extracellular glutamate content and reducing cystine-glutamate exchange (7). In conclusion, these data indicate that the restoration of glutamate homeostasis by normalizing glutamate uptake and cystine-glutamate exchange with ceftriaxone inhibits cocaine-seeking in animals, and poses drugs regulating glutamate homeostasis as potential therapies in human addiction.
Supplementary Material
Acknowledgments
This research was supported by DA 026010 awarded to Lori Knackstedt, and DA 015369 and DA 12513 awarded to Peter Kalivas.
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 3.Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulholland PJ, Carpenter-Hyland EP, Hearing MC, Becker HC, Woodward JJ, Chandler LJ. Glutamate transporters regulate extrasynaptic NMDA receptor modulation of Kv2.1 potassium channels. J Neurosci. 2008;28:8801–8809. doi: 10.1523/JNEUROSCI.2405-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McBean GJ. Cerebral cystine uptake: a tale of two transporters. Trends Pharmacol Sci. 2002;23:299–302. doi: 10.1016/s0165-6147(02)02060-6. [DOI] [PubMed] [Google Scholar]
- 6.Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 8.Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27:13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, et al. Is cocaine desire reduced by N-acetylcysteine? . Am J Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- 10.Haugeto O, Ullensvang K, Levy LM, Chaudhry FA, Honore T, Nielsen M, et al. Brain glutamate transporter proteins form homomultimers. J Biol Chem. 1996;271:27715–27722. doi: 10.1074/jbc.271.44.27715. [DOI] [PubMed] [Google Scholar]
- 11.Pendyam S, Mohan A, Kalivas PW, Nair SS. Computational model of extracellular glutamate in the nucleus accumbens incorporates neuroadaptations by chronic cocaine. Neuroscience. 2009;158:1266–1276. doi: 10.1016/j.neuroscience.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem. 1986;261:2256–2263. [PubMed] [Google Scholar]
- 13.Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron. 1989;2:1547–1558. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- 14.Knackstedt LA, Larowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, et al. The Role of Cystine-Glutamate Exchange in Nicotine Dependence in Rats and Humans. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, et al. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem. 2008;283:13116–13123. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 17.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.