Abstract
Introduction
High birth weight (HBW) is an established risk factor for childhood acute lymoblastic leukemia (ALL). The purpose of this study was to evaluate if birth weight (BW) corrected-for-gestational age is a better predictor than BW alone for occurrence of ALL and other malignancies in children.
Materials and Methods
Birth certificate data of 2254 children with cancer who were younger than 5 years old at diagnosis and registered at Texas Cancer Registry during 1995–2003 were compared to 11734 age-matched controls. Multivariable logistic regression was used to compare models with BW corrected-for-gestational age and BW alone.
Results
Compared to children who were appropriate for gestational age (AGA), children who were large for gestational age (LGA) at birth had a 1.66 times (95%CI 1.32–2.10) higher odds of ALL. Similarly, children with a BW≥ 4,000 grams had a 1.5 times (95%CI 1.18–1.89) higher odds for ALL, compared to children who weighed >2,500 grams and <4,000 grams at birth. Using model diagnostics, the model containing BW corrected-for-gestational age was a better predictor than the model with BW alone [Akaike’s Information Criterion (AIC) 4646 vs. 4658, respectively]. Odds ratios were similar for LGA children who were <4,000 grams and LGA children who were ≥4,000 grams (OR=1.5, 95%CI 0.97–2.5 and OR=1.67, 95%CI 1.29–2.16, respectively). BW was not an independent risk factor for acute myeloid leukemia or brain tumors.
Conclusion
BW corrected-for-gestational age is a better predictor than BW alone of risk for ALL. Future studies using BW variable should incorporate gestational age in their analyses.
Keywords: birth weight, childhood acute lymphoblastic leukemia, gestational age
Introduction
High birth weight (HBW) has been reported as a risk factor for childhood cancer by the majority of the studies in the literature1–17. The most consistent relationship has been reported for children with acute lymphoblastic leukemia (ALL), particularly those diagnosed at an age younger than 5 years1;2;4–6;9;18. Interest in the role of the insulin-like growth factor (IGF) family and of other growth factors in cancer etiology has increased over the last several years. IGF and other somatomedins are important in fetal growth and in cord blood are directly associated with birth weight (BW)19–29. One proposed biologic mechanism for leukemia development relates to the interplay of growth factors and their stimulus of hematopoietic progenitor cells17. These and other growth factors may be necessary for the initiation or promotion of malignant transformation in some patients.
If growth factors are involved in the process of oncogenesis, absolute BW alone may not be the most accurate measure of risk. Length of gestation as measured by gestational age is also strongly associated with BW. Most previous studies exploring association between childhood cancer risk and BW have dichotomized BW by using 4,000 grams as the cutpoint. A dichotomous classification of risk based upon a single BW when viewed from the perspective of a growth curve for neonates of various gestational ages would consider virtually all pre-term infants as being in the non-risk group, as they generally weigh less than 4,000 grams (Figure 1). One could expect that in the presence of elevated growth factors, BW at a given gestational age would be high relative to the norm, though not necessarily high as an absolute measure. One potential reason for why a number of studies that did not find an association between childhood leukemia and high birth weight may be due to the lack of appropriate adjustment for gestational age and ultimately a misclassification of at-risk infants to the non-risk category. To better characterize the impact of this proposed biological mechanism on risk of childhood cancer and more accurately identify at-risk children, we explored the relationship between risk and BW using BW corrected-for-gestational age (large for gestational age [LGA], appropriate for gestational age [AGA], small for gestational age [SGA]) as an independent variable in a review of 13,988 childhood cancer cases and controls from the Texas Cancer Registry.
Figure 1.
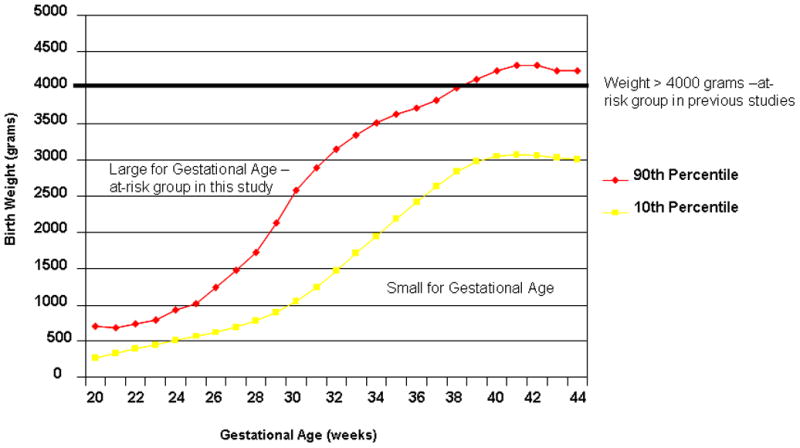
Growth curves comparing at-risk groups for birth weight corrected for gestational age and high birth weight (>4,000 grams).
Materials and Methods
Selection of Cases and Controls
This study was approved by the Texas Cancer Registry (TCR) institutional review board (IRB) for use of the its data, the Texas Department of Health (TDH) IRB for use of birth records from the Bureau of Vital Statistics, and the University of Texas School of Public Health IRB. In this population-based case-control study we identified all children that were singleton births and aged less than five years who were residents of Texas, and had gestational age data, as well as diagnosed with a malignancy between 1995 and 2003, which was registered by the TCR of the TDH. TCR, a population-based registry, was estimated to have documented more than 95% of all childhood cancer cases diagnosed in Texas1. We linked TCR data to birth certificate data using a probabilistic linkage software program AUTOMATCH Generalized Record Linkage System software (Match Ware Technologies, Inc., AUTOMATCH generalized Record Linkage System, Silver Spring, MD, 1992), which matched 2673 of the initially identified 3450 total cases (77%) to their birth records.
The control subjects were drawn from the residual (non-matched) Texas birth files for the study period and frequency-matched at a 5:1 ratio to all cancer cases on birth year only. Thus, of the more than 4.8 million birth records available for the study period, we selected 13,365. No known cancer cases were selected as controls, although there is the possibility that an unmatched case was selected. Subjects were excluded if BW was missing (n = 9); if the BW was less than 500 grams (n = 15); or if conditions diagnosed at birth were likely to cause death during infancy (n = 10). No cases were excluded due to these criteria. As in our selection of cancer cases, twin and triplet births were excluded (n = 339) because of the relatively low BWs in those infants. Finally, we excluded those cases for which gestational age data were missing or implausible (246 cases and 1258 controls), leaving 2254 cases and 11734 control subjects for analysis in our study (Figure 2).
Figure 2.
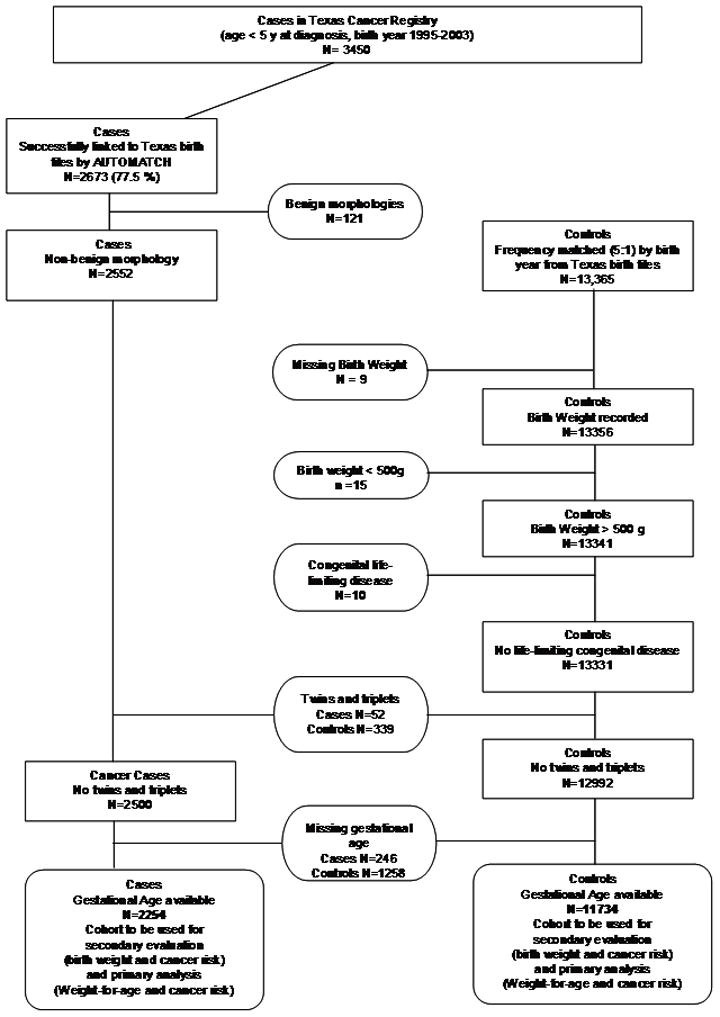
Flowchart of selection of cases and controls for the study.
Data abstraction
We extracted data from the birth certificate files for variables previously reported to be associated with childhood cancer or plausibly associated with BW, gestational age, or childhood cancer. These were the BW, gestational age, ethnicity (recorded on the birth certificate as the race of the mother), and gender of the child; abnormal conditions (e.g., meconium aspiration, presence of acidosis), and congenital abnormalities present in the child; plurality, maternal and paternal age; and the mother’s pregnancy history, medical risk factors such as diabetes (associated with LGA infants), and hypertension (associated with lower BW), previous history of an infant greater than 4,000 grams, and any tobacco or alcohol use during pregnancy. BW (in gram), gestational age (in weeks), and race- and gender-specific growth curve data30 were used to categorize each subject’s weight corrected-for-gestational age as SGA, AGA, or LGA [ Data provided in Appendix 1]. The LGA category comprised children whose weight at the given gestational age was greater than the 90th percentile; the SGA category consisted of those children whose weight at each gestational age in weeks was below the 10th percentile. To facilitate comparison between ours and previous studies, we divided BW into three categories: ≤ 2,500 grams (low BW [LBW]), 2,501 grams to 3,999 grams (normal BW), and ≥ 4,000 grams (HBW).
Statistical analysis
For all analyses cases were grouped into the following categories: all cancers, all leukemias, ALL, acute myeloid leukemia (AML), and all central nervous system (CNS) tumors. We computed descriptive statistics for cases and controls, and then analyzed the differences between them using Pearson’s chi-square test, Fisher’s exact test, or a two sample t-test where appropriate. Unconditional logistic regression analysis was used to calculate odds ratios (OR) and 95% confidence intervals (CIs). Case subgroups by cancer type were compared to the entire control group. Multivariable logistic regression models were constructed for all cancers, all leukemias, ALL, and all CNS tumors by considering factors shown to be significantly associated with the risk of cancer at p<0.25 in the univariate model and factors plausibly associated with biological risk. The final model included only the factors significantly associated with risk at the p≤0.05 level in univariate analyses, biologically plausible risk factors, and year of birth. Measures of goodness of fit were calculated to compare logistic regression models by using Akaike’s Information Criterion (AIC) and Receiver Operating Characteristic (ROC) curve for model selection31. Lower value of AIC and a higher value of ROC suggest an improved model. The Net Reclassification Index (NRI)32 which quantifies overall improvement in model sensitivity and specificity was also used to evaluate whether the model which included BW corrected-for-gestational age improved the classification of cases and controls compared to the model involving BW. A net improvement in risk classification (NRI>0) implies upward reclassification of cases (higher risk) and downward reclassification of the controls (lower risk).
All statistical tests were two-sided and performed with SAS software (Version 9.1, SAS Institute Inc., Cary, NC). We considered a p-value of <0.05 as significant. We also evaluated the agreement between BW and BW corrected-for-gestational age with the kappa statistic33. The kappa statistic quantifies the extent of agreement beyond the expected level of agreement from chance alone. Kappa values range from 1 (perfect agreement), 0 (no agreement) to −1 (perfect disagreement), with values around 0.5 representing fair agreement.
Results
Descriptive analysis
Study characteristics are summarized in Table 1 in 2552 cases and 13331 controls. There were 913 total leukemias, 727 patients with ALL, 124 with AML, and 438 patients with a CNS tumor. In the ALL group, as expected more patients were males, of white or Hispanic ethnicity, had parents of older maternal or paternal age, were LGA weight, BW ≥ 4,000 grams and had a diagnosis of Down syndrome. However, in the CNS group, only males and patients of white ethnicity were overrepresented. In the AML group, higher proportions of patients were males, had parents of older maternal or paternal age, and had a congenital malformation/Down’s syndrome.
Table I.
Birth characteristics of controls and children with any cancer cases and by cancer subtypea
| Controls (n = 13331†) | Total Cases (n = 2552†) | Total leukemia (n = 913) | ALLa (n = 727) | AMLa (n = 124) | CNSa (n = 438) | |
|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Sex | ||||||
| Male | 6788 (50.9) | 1444 (56.6) | 524 (57.4) | 409 (56.3) | 76 (61.3) | 253 (57.8) |
| Female | 6543 (49.1) | 1108 (43.4) | 389 (42.6) | 318 (43.7) | 48 (38.7) | 185 (42.2) |
| Pb | <0.0001 | 0.0002 | 0.0050 | 0.0215 | 0.0048 | |
| Ethnicity | ||||||
| White | 5431 (40.7) | 1180 (46.2) | 387 (42.4) | 322 (44.3) | 45 (36.3) | 234 (53.4) |
| Black | 1568 (11.8) | 224 (8.8) | 63 (6.9) | 34 (4.7) | 19 (15.3) | 48 (11.0) |
| Hispanic | 5931 (44.5) | 1092 (42.8) | 442 (48.4) | 355 (48.8) | 55 (44.4) | 152 (34.7) |
| Other | 401 (3.0) | 56 (2.2) | 21 (2.3) | 16 (2.2) | 5 (4.0) | 4 (0.9) |
| P | <0.0001 | <0.0001 | <0.0001 | 0.5038 | <0.0001 | |
| Maternal age (years) | ||||||
| Mean (SDa) | 26.0 (6.1) | 26.5 (6.2) | 26.8 (6.3) | 26.7 (6.1) | 27.6 (7.3) | 25.8 (6.0) |
| Range | 13–55 | 13–48 | ||||
| P | <0.0001 | <0.0001 | 0.0007 | 0.0139 | 0.6001 | |
| < 35 | 12066 (90.5) | 2271 (89.0) | 799 (87.5) | 645 (88.7) | 97 (78.2) | 401 (91.5) |
| ≥ 35 | 1265 (9.5) | 281 (11.0) | 114 (12.5) | 82 (11.3) | 27 (21.8) | 37 (8.5) |
| P | 0.0175 | 0.0030 | 0.1103 | <0.0001 | 0.4635 | |
| Paternal age (years) | ||||||
| Mean (SD) | 29.0 (6.8) | 29.5 (6.9) | 29.8 (7.1) | 29.8 (7.0) | 30.9 (7.6) | 29.1 (6.9) |
| Range | (14–70) | (15–62) | ||||
| P | 0.0036 | 0.0022 | 0.0049 | 0.0061 | 0.8194 | |
| < 35 | 8971 (67.3) | 1703 (66.7) | 590 (64.6) | 480 (66.0) | 64 (51.6) | 304 (69.4) |
| ≥ 35 | 2293 (17.2) | 517 (20.3) | 207 (22.7) | 160 (22.0) | 39 (31.5) | 79 (18.0) |
| Missing | 2067 (15.5) | 332 (13.0) | 116 (12.7) | 87 (12.0) | 21 (16.9) | 55 (12.6) |
| P | <0.0001 | <0.0001 | 0.0006 | <0.0001 | 0.2412 | |
| Gestational age (weeks) | ||||||
| < 37 | 1301 (9.8) | 253 (9.9) | 83 (9.1) | 65 (8.9) | 15 (12.1) | 40 (9.1) |
| 37–41 | 9408 (70.6) | 1829 (71.7) | 661 (72.4) | 523 (71.9) | 88 (80.0) | 299 (68.3) |
| ≥ 42 | 1574 (11.8) | 261 (10.2) | 99 (10.8) | 81 (11.1) | 13 (10.5) | 58 (13.2) |
| Missing | 1048 (7.9) | 209 (8.2) | 70 (7.7) | 58 (8.0) | 8 (6.4) | 41 (9.4) |
| P | 0.1488 | 0.6793 | 0.8185 | 0.7572 | 0.4874 | |
| Weight corrected for gestational age | ||||||
| SGA | 1343 (10.1) | 235 (9.2) | 72 (7.9) | 49 (6.7) | 14 (11.3) | 44 (10.1) |
| AGA | 9212 (69.1) | 1754 (68.7) | 631 (69.1) | 499 (68.6) | 87 (70.2) | 298 (68.0) |
| LGA (BW<4000 g) | 271 (2.0) | 64 (2.5) | 22 (2.4) | 19 (2.6) | 3 (2.4) | 9 (2.0) |
| LGA (BW≥4000 g) | 852 (6.4) | 201 (7.9) | 85 (9.3) | 76 (10.4) | 5 (4.0) | 33 (7.5) |
| Missing | 1653 (12.4) | 298 (11.7) | 103 (11.3) | 84 (11.6) | 15 (12.1) | 54 (12.3) |
| P | 0.0175 | 0.0023 | <0.0001 | 0.8489 | 0.9195 | |
| Birth weight (g) | ||||||
| ≤ 2500 | 957 (7.2) | 181 (7.1) | 50 (5.5) | 35 (4.8) | 11 (8.9) | 31 (7.1) |
| 2501–3999 | 11068 (83.0) | 2061 (80.8) | 738 (80.8) | 583 (80.2) | 104 (83.9) | 350 (79.9) |
| ≥4000 | 1306 (9.8) | 310 (12.5) | 125 (13.7) | 109 (15.0) | 9 (7.2) | 57 (13.0) |
| P | 0.0015 | 0.0002 | <0.0001 | 0.5195 | 0.0848 | |
| Congenital malformation | ||||||
| No | 13029 (97.7) | 2464 (96.6) | 875 (95.8) | 704 (96.8) | 112 (90.3) | 426 (97.3) |
| Yes | 115 (0.9) | 54 (2.1) | 23 (2.5) | 10 (1.4) | 10 (8.1) | 6 (1.4) |
| Missing | 187 (1.4) | 34 (1.3) | 15 (1.6) | 13 (1.8) | 2 (1.6) | 6 (1.4) |
| P | <0.0001 | <0.0001 | 0.2446 | <0.0001 | 0.5341 | |
| Down syndrome | ||||||
| No | 13326 (100.0) | 2538 (99.4) | 899 (98.5) | 722 (99.3) | 118 (95.2) | 438(100) |
| Yes | 5 (0.0) | 14 (0.6) | 14 (1.5) | 5 (0.7) | 6 (4.8) | |
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
SD, standard deviation; SGA, small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age; BW, birth weight;
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CNS, central nervous system tumors;
P-values derived from Chi-square and Fisher’s exact tests;
Number of cases and controls were calculated before exclusion of twins and triplets.
Univariate logistic regression analyses
Being LGA at birth increased the odds for ALL by more than 50% compared to being AGA (OR=1.57, 95%CI 1.25–1.97, Table 2). Similarly weighing≥ 4,000 grams at birth also increased the odds for ALL (OR=1.59, 95%CI 1.28–1.96). BW corrected-for-gestational age or BW alone was not associated with risk for AML. Having a BW ≥ 4,000 grams was associated with almost a 40% higher odds of a CNS tumor (OR=1.37, 95%CI 1.03–1.83). This relationship was not observed between gestational age corrected BW and risk of CNS tumors. In agreement with previous data, male gender, white and Hispanic ethnicity, older maternal and paternal age, and a Down syndrome diagnosis were associated with a higher odds of ALL. For AML, variables associated with the higher odds included older maternal and paternal age, congenital malformations and Down syndrome. Finally, males, non-Hispanic white ethnicity and congenital malformations were also associated with increased odds of a CNS tumor.
Table II.
Univariate logistic regression of birth characteristics of children with any cancer and specified cancer subtypesa
| Total cases (n= 2500†) | Total leukemia (n= 899) | ALLa (n=717) | AMLa (n=120) | CNSa (n= 430) | |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Sex | |||||
| Maleb | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 0.79 (0.73–0.87) | 0.77 (0.67–0.88) | 0.80 (0.69–0.93) | 0.69 (0.48–0.99) | 0.77 (0.63–0.93) |
| Ethnicity | |||||
| Whiteb | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Black | 0.64 (0.55–0.75) | 0.54 (0.41–0.72) | 0.35 (0.24–0.50) | 1.41 (0.81–2.45) | 0.71 (0.52–0.98) |
| Hispanic | 0.85 (0.77–0.93) | 1.03 (0.89–1.19) | 1.0 (0.85–1.17) | 1.09 (0.73–1.63) | 0.60 (0.49–0.75) |
| Other | 0.66 (0.49–0.87) | 0.74 (0.47–1.16) | 0.68 (0.41–1.13) | 1.53 (0.60–3.88) | 0.24 (0.09– 0.64) |
| Maternal age (years) | |||||
| Continuous (unit = 1 year) | 1.01 (1.01– 1.02) | 1.02 (1.01– 1.03) | 1.02 (1.01–1.03) | 1.04 (1.01–1.07) | 1.00 (0.98– 1.01) |
| Categorical | |||||
| < 35b | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| ≥ 35 | 1.18 (1.03– 1.36) | 1.35 (1.10– 1.67) | 1.22 (0.96– 1.55) | 2.55 (1.64– 3.98) | 0.91 (0.65– 1.29) |
| ≥ 35c | 1.15 (1.00–1.33) | 1.26 (1.02– 1.57) | 1.14 (0.89– 1.46) | 2.32 (1.45– 3.71) | N/A |
| Paternal age (years) | |||||
| Continuous (unit = 1 year) | 1.01 (1.00– 1.02) | 1.02 (1.01–1.03) | 1.02 (1.01– 1.03) | 1.03 (1.01–1.06) | 1.00 (0.99–1.02) |
| Categorical | |||||
| < 35b | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| ≥ 35 | 1.20 (1.07– 1.34) | 1.39 (1.18– 1.64) | 1.34 (1.11– 1.61) | 2.33 (1.55, 3.51) | 1.05 (0.81, 1.35) |
| ≥ 35c | 1.19 (1.06– 1.33) | 1.36 (1.15– 1.61) | 1.31 (1.09– 1.59) | 2.18 (1.43, 3.32) | N/A |
| Gestational age (weeks) | |||||
| Continuous (unit = 1 week) | 0.98 (0.96–0.99) | 0.98 (0.96– 1.01) | 0.99 (0.96– 1.02) | 0.94 (0.89–1.00) | 1.02 (0.98–1.06) |
| Categorical | |||||
| < 37 | 1.01 (0.87–1.17) | 0.94 (0.74– 1.21) | 0.94 (0.72–1.24) | 1.22 (0.68–2.18) | 0.93 (0.65–1.33) |
| 37–41b | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| ≥ 42 | 0.85 (0.74–0.98) | 0.90 (0.72–1.12) | 0.93 (0.73–1.18) | 0.89 (0.49–1.59) | 1.16 (0.87–1.54) |
| Weight-for-Age | |||||
| SGA | 0.94 (0.81– 1.09) | 0.84 (0.65– 1.08) | 0.73 (0.54– 0.98) | 1.13 (0.63–2.04) | 0.98 (0.70– 1.37) |
| AGAb | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| LGA | 1.24 (1.08– 1.43) | 1.39 (1.12– 1.73) | 1.57 (1.25–1.97) | 0.76 (0.37– 1.57) | 1.15 (0.82–1.59) |
| Birth weight (g) | |||||
| Continuous (unit = 1 kg) | 1.13 (1.04– 1.22) | 1.28 (1.12– 1.44) | 1.42 (1.23– 1.63) | 0.82 (0.60– 1.12) | 1.17 (0.98–1.40) |
| Categorical | |||||
| ≤ 2500 | 1.06 (0.88– 1.27) | 0.89 (0.65– 1.22) | 0.80 (0.56– 1.16) | 1.28 (0.64–2.53) | 1.04 (0.69–1.57) |
| 2501–3999b | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| ≥4000 | 1.27 (1.11– 1.45) | 1.44 (1.18– 1.75) | 1.59 (1.28– 1.96) | 0.74 (0.37–1.46) | 1.37 (1.03– 1.83) |
| Congenital malformation | |||||
| Nob | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 2.51 (1.80–3.50) | 3.11 (1.97– 4.91) | 1.68 (0.87– 3.22) | 10.79 (5.49– 21.18) | 1.67 (0.73–3.83) |
| Yesc | 1.92 (1.32–2.79) | 1.28 (0.64– 2.53) | 0.88 (0.36– 2.16) | 4.52 (1.64– 12.50) | 1.75 (0.77–4.01) |
| Down syndrome | |||||
| Nob | 1.0 | 1.0 | 1.0 | 1.0 | |
| Yes | 14.61 (5.26– 40.58) | 41.03 (14.75–114.13) | 18.24 (5.27– 63.15) | 136.66 (41.12– 454.19) | N/A |
SGA, small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age; BW, birth weight; OR, odds ratio point estimate; CI, confidence interval;
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CNS, central nervous system tumors;
Reference group;
Subjects with Down syndrome excluded (no cases of CNS, neuroblastoma, Wilms’ tumor, retinoblastoma, or other cancer);
Number of cases were calculated before exclusion of missing gestational age.
Multivariable analysis
Table 3 shows the results of multivariable analyses for all cancers, all leukemias, ALL, and CNS tumors in 2240 cases and 11729 controls as described before. Subjects with Down syndrome (14 cases and 5 controls) were excluded from all multivariable analyses. Models were reanalyzed after replacing BW for gestational age with BW categories and continuous BW for comparison to previous studies. Because of the associations observed in the univariate analyses, we included the congenital malformations variable only in the all cancers model for multivariable analysis. In the model containing BW corrected-for-gestational age, LGA was associated with a modest increased risk of any cancer (OR = 1.29, 95%CI 1.11–1.49), any leukemia (OR = 1.49, 95%CI 1.20–1.85), and ALL (OR = 1.66, 95%CI 1.32–2.10), independent of all other variables. When BW and gestational age were included in the model individually, BW ≥ 4,000 grams had a 50% increased risk for development of ALL (OR=1.5, 95%CI 1.18–1.89). For the odds of ALL alone, male gender, black ethnicity and birth year were significant variables in the model. Calculated AIC and ROC values for the ALL model containing BW corrected-for-gestational age were 4646.2 and 0.624, respectively compared to 4658.3 and 0.618, respectively for the model with BW alone. This suggested that the model with BW corrected-for-gestational age is a superior model than the one containing BW and gestational age as two separate independent variables. The NRI was 17.4% overall (P<0.001) and 26.7% among ALL cases and −9.2% among controls, implying that the ALL model with BW corrected-for-gestational age had higher sensitivity but lower specificity compared to the model with BW alone. The agreement between the two measures was almost perfect, as shown by a kappa of 0.95 (95% CI 0.91–0.99) for cases and 0.99 (95% CI 0.98–1.00) for the controls. The risk estimates were similar for LGA children who were <4,000 grams and LGA children who were ≥4,000 grams (OR=1.5, 95%CI 0.97–2.5 and OR=1.67, 95%CI 1.29–2.16, respectively). BW ≥ 4,000 grams was not an independent risk factor for development of CNS tumors in these analyses.
Table III.
Multivariate analysis of parental descriptive characteristics and neonatal birth characteristicsb
| Total cases (n = 2240) | Total leukemia (n = 805) | ALL (n = 645) | CNS (n = 377) | |||||
|---|---|---|---|---|---|---|---|---|
| Adjusted OR* | 95 % CI | Adjusted OR* | 95 % CI | Adjusted OR* | 95 % CI | Adjusted OR* | 95 % CI | |
| SGA (vs. AGA) | 0.93 | 0. 80–1.10 | 0.88 | 0.68–1.13 | 0.78 | 0.57–1.05 | 0.98 | 0.70–1.38 |
| LGA (vs. AGA) | 1.29 | 1.11–1.49 | 1.49 | 1.20–1.85 | 1.66 | 1.32–2.10 | 1.14 | 0.82–1.58 |
| P (trend test)† | 0.0016 | 0.0005 | <0.0001 | 0.5128 | ||||
| LGA (<4000 g) | 1.42 | 1.07–1.88 | 1.42 | 0.91–2.21 | 1.5 | 0.97–2.52 | 1.12 | 0.57–2.20 |
| LGA (>4000 g) | 1.25 | 1.06–1.48 | 1.49 | 1.17–1.90 | 1.67 | 1.29–2.16 | 1.15 | 0.79–1.65 |
| Sex (male) | 1.24 | 1.13–1.37 | 1.31 | 1.12–1.52 | 1.25 | 1.06–1.47 | 1.31 | 1.06–1.61 |
| Ethnicity (AA vs. W) | 0.64 | 0.54–0.76 | 0.57 | 0.42–0.76 | 0.39 | 0.26–0.57 | 0.70 | 0.49–0.98 |
| Ethnicity (H vs. W) | 0.85 | 0.77–0.93 | 1.04 | 0.89–1.22 | 1.01 | 0.85–1.20 | 0.60 | 0.48–0.75 |
| Maternal age (≥35 years) | 1.17 | 1.00–1.37 | 1.31 | 1.03–1.67 | 1.18 | 0.89–1.55 | N/A | |
| Congenital malformationa | 2.24 | 1.50–3.34 | ||||||
| Birth year | 0.99 | 0.97–1.01 | 0.94 | 0.91–0.96 | 0.92 | 0.90–0.95 | 0.98 | 0.94–1.01 |
| Secondary analysis | ||||||||
| Birth weight | ||||||||
| (≤ 2500 g)c | 1.04 | 0.83–1.31 | 0.91 | 0.62–1.33 | 0.80 | 0.51–1.25 | 0.93 | 0.56–1.56 |
| (≥ 4000 g)c | 1.19 | 1.03–1.38 | 1.36 | 1.10–1.69 | 1.50 | 1.18–1.89 | 1.14 | 0.83–1.56 |
| P (trend test)† | 0.1777 | 0.0064 | 0.0007 | 0.3672 | ||||
| Gestational age | ||||||||
| (<37 weeks) | 1.04 | 0.88–1.24 | 1.02 | 0.77–1.35 | 1.12 | 0.83–1.53 | 0.99 | 0.67–1.47 |
| (>41 weeks) | 0.86 | 0.74–1.01 | 0.91 | 0.71–1.15 | 0.94 | 0.72–1.23 | 1.07 | 0.78–1.47 |
ALL, acute lymphoblastic leukemia; CNS, central nervous system tumors; OR, odds ratio point estimate; CI, confidence interval; SGA, small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age; AA, African-American; W, White; H, Hispanic;
Subjects with Down syndrome excluded;
All models contained the variable of initial matching, birth year, to control for residual confounding;
OR and 95% CI were generated for all variables shown within the chart except birth weight. Birth weight (continuous, then categorical) were substituted into the model for LGA and SGA to obtain their respective OR and 95% CI. Values for other variables varied less than 5%;
Trend test obtained from the logistic regression model with adjustment for remaining variables;
OR adjusted for all variables containing values within each column.
Discussion
BW is an established risk factor for development of childhood ALL. However, in all of the studies that have examined this relationship, BW was used either as a continuous variable or a categorical one, with gestational age being a covariate. In this population-based case-control study, we explored whether we could increase the predictive role of BW by combining these variables into one (weight for age) in relation to occurrence of pediatric ALL. We found that BW after correction for gestational age is a better predictor of risk for childhood ALL compared to BW alone. Two other groups have reported on recent efforts to refine the BW variable in relation to childhood ALL risk. Milne et al.34 demonstrated a significant improvement in the multivariable model when they used proportion of optimal BW (POBW), a variable calculated by a regression equation including terms for duration of gestation, maternal height, parity and infant sex. The relationship between POBW and ALL risk was strongest for the 0–4 year age group. In contrast to our study, Schüz and Forman35 showed that there was poor agreement between BW alone and BW corrected-for-gestational age in identifying at risk patients (kappa 0.45, 95%CI 0.37–0.53) for development of ALL in a population based sample from Germany, however they did not directly compare the two by comparing two logistic models. By examining BW in the context of each subject’s gestational age, a better proxy measure for fetal growth and, therefore, growth factors, which are thought to be associated with cancer incidence is created. The variability in results in previous studies may be due to misclassification of at risk subjects. In the study by Reynolds et al.13, a decreased risk was seen in subjects with higher gestational age. As evidenced by the growth curve (Figure 1), more children are likely to be misclassified as being at risk with HBW (> 4,000 g) even though they are AGA; and perhaps this explains their finding of a decrease in risk with increasing gestational age.
Gestational diabetes, which is associated with risk for LGA infants, has been reported to be associated with increased risk of cancer (OR=2.83, 95%CI 1.56–5.17)36, but was not found to have an association in our study. Gestational diabetes is frequently misclassified in birth certificate data therefore caution should be used in interpreting the results from this study with respect to gestational diabetes as a risk factor 37;38.
This study had several limitations. The use of birth certificates for a control population offers the benefit of a broadly accessible population-based sample that is likely to be more representative of the study population from which cases would arise39, but the quality of the data is variable and errors or omissions can lead to misclassification or loss of subjects. In particular, gestational age can be difficult to accurately assess and can be improperly recorded. By using this variable to define a risk group instead of BW alone, which is more reliably and accurately measured, further misclassification bias may be introduced; however the misclassification will be differential and will only attenuate the ORs to the null. The variables in birth certificates do not include all suspected risk factors for childhood cancer which limits the ability to control for other potential risk factors. Within a migratory population, a significant proportion of a study population may be born in another state or country and not have an available birth record. As in this study, anyone not born in the Texas is therefore excluded from a study using birth certificates. Third, the linkage between available data sets (birth records and cancer registry data in this study) relies on a probabilistic match through computer software and is imperfect. An increase in matched subjects can be obtained but with a decrease in reliability, so a balance between numbers of subjects and relative certainty of accurate matching must be made. Only 77% of cases could successfully be linked, leading to a loss of one-quarter of all cases.
The strengths of this study include a large sample size and defining a new predictor of risk for childhood ALL in relation to high birth weight with a reduction in misclassification. The growth curve data provided by Alexander30, [Supplemental Appendix 1] which has been used in this study, draws from a national sample of millions of infants of various ethnicities which makes it a likely candidate for a new national standard for neonatal growth curves.
Further biologic studies are necessary to investigate the association between BW corrected-for-gestational age and ALL. An assessment of the implicated growth factors in serum samples from children with ALL, both at birth and at diagnosis, compared to controls can help determine whether differences exist that suggest that these growth factors are part of the causal pathway. Genetic and epigenetic analyses to determine whether polymorphisms with the IGF family contribute to the risk can further strengthen the role of growth factors in oncogenesis. Since fetal growth is associated with maternal nutrition and maternal weight gain during pregnancy interventions during this period could be attempted if maternal anthropometric status including gestation weight gain are implicated 40. Socio-economic status, education, or access to health care may impact the BW corrected-for-gestational age distribution between ethnic groups leading to different rates of childhood cancer among them. Further studies are necessary to determine whether there are interactions between these variables to explain the reduced risk of ALL in African-American children and in females. Prenatal care for all women, which would include close monitoring of weight gain, maintenance of ideal weight gain during the pregnancy, and information and education on diet and physical activity during pregnancy to promote healthy weight gain, could, in addition to improving the overall health of women and infants, lead to a decrease in LGA infants and perhaps those who are at risk for ALL.
This study supports the hypothesis that BW corrected-for-gestational age is a better predictor than HBW alone as an independent risk factor for childhood ALL. To better identify these at-risk infants, a standard neonatal growth curve and more accurate measures of gestational age (via improved access to prenatal care and fetal ultrasound) are necessary. Future studies should explore biological reasons to explain this relation between BW and risk for childhood ALL so that, ultimately, preventive measures can be initiated.
Supplementary Material
Acknowledgments
This research was supported by a cancer prevention fellowship funded by/through NCI training grant R25 CA57730, Robert M. Chamberlain, Ph.D., Principal Investigator, The University of Texas M. D. Anderson Cancer Center.
Footnotes
Conflict of Interest
The authors have no conflict of interest to declare.
References
- 1.Okcu MF, Goodman KJ, Carozza SE, et al. Birth weight, ethnicity, and occurrence of cancer in children: a population-based, incident case-control study in the State of Texas, USA. Cancer Causes Control. 2002;13:595–602. doi: 10.1023/a:1019555912243. [DOI] [PubMed] [Google Scholar]
- 2.Kaye SA, Robison LL, Smithson WA, et al. Maternal reproductive history and birth characteristics in childhood acute lymphoblastic leukemia. Cancer. 1991;68:1351–1355. doi: 10.1002/1097-0142(19910915)68:6<1351::aid-cncr2820680627>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Petridou E, Trichopoulos D, Kalapothaki V, et al. The risk profile of childhood leukaemia in Greece: a nationwide case-control study. Br J Cancer. 1997;76:1241–1247. doi: 10.1038/bjc.1997.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robison LL, Codd M, Gunderson P, et al. Birth weight as a risk factor for childhood acute lymphoblastic leukemia. Pediatr Hematol Oncol. 1987;4:63–72. doi: 10.3109/08880018709141250. [DOI] [PubMed] [Google Scholar]
- 5.Daling JR, Starzyk P, Olshan AF, Weiss NS. Birth weight and the incidence of childhood cancer. J Natl Cancer Inst. 1984;72:1039–1041. [PubMed] [Google Scholar]
- 6.Yeazel MW, Ross JA, Buckley JD, et al. High birth weight and risk of specific childhood cancers: a report from the Children’s Cancer Group. J Pediatr. 1997;131:671–677. doi: 10.1016/s0022-3476(97)70091-x. [DOI] [PubMed] [Google Scholar]
- 7.Westergaard T, Andersen PK, Pedersen JB, et al. Birth characteristics, sibling patterns, and acute leukemia risk in childhood: a population-based cohort study. J Natl Cancer Inst. 1997;89:939–947. doi: 10.1093/jnci/89.13.939. [DOI] [PubMed] [Google Scholar]
- 8.Schuz J, Kaatsch P, Kaletsch U, Meinert R, Michaelis J. Association of childhood cancer with factors related to pregnancy and birth. Int J Epidemiol. 1999;28:631–639. doi: 10.1093/ije/28.4.631. [DOI] [PubMed] [Google Scholar]
- 9.Ross JA, Potter JD, Shu XO, et al. Evaluating the relationships among maternal reproductive history, birth characteristics, and infant leukemia: a report from the Children’s Cancer Group. Ann Epidemiol. 1997;7:172–179. doi: 10.1016/s1047-2797(97)00012-4. [DOI] [PubMed] [Google Scholar]
- 10.Wertelecki W, Mantel N. Increased birth weight in leukemia. Pediatr Res. 1973;7:132–138. doi: 10.1203/00006450-197303000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Cnattingius S, Zack M, Ekbom A, et al. Prenatal and neonatal risk factors for childhood myeloid leukemia. Cancer Epidemiol Biomarkers Prev. 1995;4:441–445. [PubMed] [Google Scholar]
- 12.Cnattingius S, Zack MM, Ekbom A, et al. Prenatal and neonatal risk factors for childhood lymphatic leukemia. J Natl Cancer Inst. 1995;87:908–914. doi: 10.1093/jnci/87.12.908. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds P, Von BJ, Elkin EP. Birth characteristics and leukemia in young children. Am J Epidemiol. 2002;155:603–613. doi: 10.1093/aje/155.7.603. [DOI] [PubMed] [Google Scholar]
- 14.Shaw G, Lavey R, Jackson R, Austin D. Association of childhood leukemia with maternal age, birth order, and paternal occupation. A case-control study. Am J Epidemiol. 1984;119:788–795. doi: 10.1093/oxfordjournals.aje.a113799. [DOI] [PubMed] [Google Scholar]
- 15.Zack M, Adami HO, Ericson A. Maternal and perinatal risk factors for childhood leukemia. Cancer Res. 1991;51:3696–3701. [PubMed] [Google Scholar]
- 16.Savitz DA, Ananth CV. Birth characteristics of childhood cancer cases, controls, and their siblings. Pediatr Hematol Oncol. 1994;11:587–599. doi: 10.3109/08880019409141806. [DOI] [PubMed] [Google Scholar]
- 17.MACMAHON B, NEWILL VA. Birth characteristics of children dying of malignant neoplasms. J Natl Cancer Inst. 1962;28:231–244. [PubMed] [Google Scholar]
- 18.Caughey RW, Michels KB. Birth weight and childhood leukemia: a meta-analysis and review of the current evidence. Int J Cancer. 2009;124:2658–2670. doi: 10.1002/ijc.24225. [DOI] [PubMed] [Google Scholar]
- 19.Ross JA, Perentesis JP, Robison LL, Davies SM. Big babies and infant leukemia: a role for insulin-like growth factor-1? Cancer Causes Control. 1996;7:553–559. doi: 10.1007/BF00051889. [DOI] [PubMed] [Google Scholar]
- 20.Blatt J, Davenport M, Olshan A. The pediatric growth curve as a cancer research tool. J Pediatr. 1999;134:138–140. doi: 10.1016/s0022-3476(99)70404-x. [DOI] [PubMed] [Google Scholar]
- 21.Hedborg F, Holmgren L, Sandstedt B, Ohlsson R. The cell type-specific IGF2 expression during early human development correlates to the pattern of overgrowth and neoplasia in the Beckwith-Wiedemann syndrome. Am J Pathol. 1994;145:802–817. [PMC free article] [PubMed] [Google Scholar]
- 22.Ostlund E, Tally M, Fried G. Transforming growth factor-beta1 in fetal serum correlates with insulin-like growth factor-I and fetal growth. Obstet Gynecol. 2002;100:567–573. [PubMed] [Google Scholar]
- 23.Christou H, Connors JM, Ziotopoulou M, et al. Cord blood leptin and insulin-like growth factor levels are independent predictors of fetal growth. J Clin Endocrinol Metab. 2001;86:935–938. doi: 10.1210/jcem.86.2.7217. [DOI] [PubMed] [Google Scholar]
- 24.Yang SW, Yu JS. Relationship of insulin-like growth factor-I, insulin-like growth factor binding protein-3, insulin, growth hormone in cord blood and maternal factors with birth height and birthweight. Pediatr Int. 2000;42:31–36. doi: 10.1046/j.1442-200x.2000.01167.x. [DOI] [PubMed] [Google Scholar]
- 25.Wiznitzer A, Reece EA, Homko C, et al. Insulin-like growth factors, their binding proteins, and fetal macrosomia in offspring of nondiabetic pregnant women. Am J Perinatol. 1998;15:23–28. doi: 10.1055/s-2007-993893. [DOI] [PubMed] [Google Scholar]
- 26.Yang SW, Kim SY. The relationship of the levels of leptin, insulin-like growth factor-I and insulin in cord blood with birth size, ponderal index, and gender difference. J Pediatr Endocrinol Metab. 2000;13:289–296. doi: 10.1515/jpem.2000.13.3.289. [DOI] [PubMed] [Google Scholar]
- 27.Ben X, Qin Y, Wu S. Placental leptin correlates with intrauterine fetal growth and development. Zhonghua Yi Xue Za Zhi. 2001;81:489–492. [PubMed] [Google Scholar]
- 28.Wiznitzer A, Furman B, Zuili I, et al. Cord leptin level and fetal macrosomia. Obstet Gynecol. 2000;96:707–713. doi: 10.1016/s0029-7844(00)00992-3. [DOI] [PubMed] [Google Scholar]
- 29.Dong M, He J, Wang Z. Study on the relationship between epidermal growth factor and fetal growth retardation. Zhonghua Fu Chan Ke Za Zhi. 1999;34:135–137. [PubMed] [Google Scholar]
- 30.Alexander GR, Kogan MD, Himes JH. 1994–1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J. 1999;3:225–231. doi: 10.1023/a:1022381506823. [DOI] [PubMed] [Google Scholar]
- 31.Akaike H. A new look at the statistical model identification. IEEE Trans Automatic Control. 1974;19:716–723. [Google Scholar]
- 32.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 33.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 34.Milne E, Laurvick CL, Blair E, Bower C, de KN. Fetal growth and acute childhood leukemia: looking beyond birth weight. Am J Epidemiol. 2007;166:151–159. doi: 10.1093/aje/kwm065. [DOI] [PubMed] [Google Scholar]
- 35.Schuz J, Forman MR. Birthweight by gestational age and childhood cancer. Cancer Causes Control. 2007;18:655–663. doi: 10.1007/s10552-007-9011-y. [DOI] [PubMed] [Google Scholar]
- 36.Westbom L, Aberg A, Kallen B. Childhood malignancy and maternal diabetes or other auto-immune disease during pregnancy. Br J Cancer. 2002;86:1078–1080. doi: 10.1038/sj.bjc.6600192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Devlin HM, Desai J, Walaszek A. Reviewing Performance of Birth Certificate and Hospital Discharge Data to Identify Births Complicated by Maternal Diabetes. Matern Child Health J. 2008 doi: 10.1007/s10995-008-0390-9. [DOI] [PubMed] [Google Scholar]
- 38.Lydon-Rochelle MT, Holt VL, Cardenas V, et al. The reporting of pre-existing maternal medical conditions and complications of pregnancy on birth certificates and in hospital discharge data. Am J Obstet Gynecol. 2005;193:125–134. doi: 10.1016/j.ajog.2005.02.096. [DOI] [PubMed] [Google Scholar]
- 39.Ross JA, Spector LG, Olshan AF, Bunin GR. Invited commentary: Birth certificates--a best control scenario? Am J Epidemiol. 2004;159:922–924. doi: 10.1093/aje/kwh137. [DOI] [PubMed] [Google Scholar]
- 40.Romo A, Carceller R, Tobajas J. Intrauterine growth retardation (IUGR): epidemiology and etiology. Pediatr Endocrinol Rev. 2009;6 (Suppl 3):332–336. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


