Abstract
Background:
Recent evidence suggests that epigenetic mechanisms have an important role in the development of addictive behavior. However, little is known about the role of epigenetic mechanisms in the extinction of drug-induced behavioral changes. In this study we examined the ability of histone deacetylase (HDAC) inhibitors to facilitate extinction and attenuate reinstatement of cocaine-induced conditioned place preference (CPP).
Methods:
C57BL/6 mice were subject to cocaine-induced CPP using 20mg/kg dose. To facilitate extinction, mice were administered an HDAC inhibitor following non-reinforced exposure to the conditioned context. To measure persistence, mice were subject to a reinstatement test using 10mg/kg dose of cocaine.
Results:
We demonstrate that HDAC inhibition during extinction consolidation can facilitate extinction of cocaine-induced CPP. Animals treated with an HDAC inhibitor extinguished cocaine-induced CPP both more quickly and to a greater extent than did vehicle-treated animals. We also show that the extinction of context-drug associated memories via HDAC inhibition modulates extinction learning such that reinstatement behavior is significantly attenuated. Acetylation of histone H3 in the nucleus accumbens following extinction was increased by HDAC inhibition.
Conclusions:
This study provides the first evidence that modulation of chromatin modification can facilitate extinction and prevent reinstatement of drug-induced behavioral changes. These findings provide a potential novel approach to the development of treatments that facilitate extinction of drug-seeking behavior.
Introduction
A key open question in the field of substance abuse is how drugs act on the brain to modulate long-lasting effects that produce drug seeking behavior and increase the risk of relapse. One potential mechanism that may produce these long-lasting effects is stable changes in cellular function leading to stable changes in neuronal plasticity. There is accumulating evidence from several different fields of research that such cellular changes are mediated by gene expression that establish transcription profiles for specific cellular functions (1, 2) . One mechanism by which gene expression may be regulated for long-lasting cellular functions is via chromatin modification and remodeling. Chromatin is the DNA-protein complex that packages genomic DNA. The enzymes that regulate chromatin with respect to histone modifications at specific promoters have been shown to be involved in gene expression changes potentially required for long-lasting changes in neuronal plasticity involved in substance abuse (3-6) as well as long-term memory (7).
There are numerous chromatin modifications carried out by a number of histone modifying enzymes to regulate access to DNA (8) and one of the best studied chromatin modifications is acetylation of histones. Histone acetyltransferases (HATs) add acetyl groups to relax chromatin structure, while histone deacetylases (HDACs) remove acetyl groups, generally resulting in transcriptional silencing (8). Administration of cocaine leads to an increase in histone acetylation at promoters of genes implicated in the development of drug seeking (4, 5, 9, 10). Cocaine increases acetylation mediated by the transcriptional coactivator CREB binding protein (CBP), a potent HAT, and cbp mutant mice show decreased sensitization to chronic cocaine (5). Inhibition of HDACs produces a hyper-acetylated state and enhances several behavioral effects of cocaine (4, 10). Together, these findings demonstrate that HATs and HDACs regulate changes in transcription profiles underlying the development of substance abuse.
Treatments for substance abuse often incorporate extinction techniques, in which the patient learns that the environmental cues or behavioral responses no longer produce the substance of abuse (11). Many recent studies have demonstrated that extinction in a variety of tasks, including substance abuse, can be enhanced pharmacologically (12, 13). We and others have demonstrated that HDAC inhibition can facilitate extinction of contextual fear conditioning (14, 15), but little is known about the role of chromatin modification in the extinction of drug-induced behavioral changes. One of the challenges that any approach to enhancing extinction faces is that the behavioral changes that occur during extinction may be quickly reversed by, for example, re-exposure to the drug of abuse. Thus, approaches to enhancing extinction need to focus on methods that not only enhance the rate of extinction, but also prevent the reinstatement of further drug seeking after an episode of relapse.
In the present study, we examined the effects of HDAC inhibition on extinction of cocaine-induced conditioned place preference (CPP) in mice. In this paradigm, an association is formed between environmental cues and drug, leading to a preference for the drug-paired context. We demonstrate that systemic administration of an HDAC inhibitor following exposure to the previously cocaine-paired context facilitates extinction of cocaine-induced CPP and reduces reinstatement of CPP after subsequent cocaine exposure. Furthermore, acetylation of histone H3 in the nucleus accumbens, a brain region implicated in cue-elicited drug craving and drug-seeking (16-20), may mediate these effects. Our study extends the findings that HDAC inhibitors enhance extinction learning and provides the first evidence that modulation of chromatin modification can facilitate extinction of drug-induced behavioral changes.
Materials and Methods
Animals
Male C57BL/6J mice (8 weeks old) obtained from Jackson Laboratories (Bay Harbor, ME) had access to food and water ad libitum. Lights were maintained on a 12 hr light/dark cycle, with all procedures performed during the light portion of the cycle. All experiments were conducted according to National Institutes of Health guidelines for animal care and use and were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
Place Preference Apparatus
Conditioning took place in a three-chamber apparatus consisting of two larger compartments (12.5cm × 17cm) separated by a smaller compartment (12.5cm × 11.5cm) with guillotine doors. The two larger compartments were distinguished by different visual, tactile, and olfactory cues. One compartment had white walls with a bar floor above pine shavings. The other compartment had checkered walls with a grid floor above cedar shavings. The middle compartment had two gray walls as well as a checkered wall and a white wall leading into the corresponding compartment and had a solid gray PVC floor above home cage bedding. Guillotine doors, patterned to match the outer compartments, separated the three compartments and were raised on test days. The CPP apparatuses were placed on an open bench located in a separate isolated room with fluorescent lighting.
The time spent in each chamber of the CPP apparatus and total distance traveled was tracked automatically from MPEG video using EthoVision 3.1 software (EthoVision 3.1; Noldus Technology, Leesburg, VA; see 21). MPEG videos were recorded using digital video cameras mounted above the CPP chambers.
Cocaine Conditioned Place Preference
Experiment 1: Non-confined Extinction
All mice were handled for 3 consecutive days for 1 min each day prior to the experiment (days 1-3; see Figure 1A). Baseline preferences were assessed by placing the animals in the center compartment of the place preference apparatus with the guillotine doors open, allowing free access to all compartments for 15 min (day 4). Time spent in each compartment was recorded. Conditioning took place over the next 4 days with the guillotine doors closed, confining animals to a specific compartment for 30 min (days 5-8). Because time spent in each compartment during the initial preference test did not differ significantly, an unbiased paradigm was used such that half of the animals were injected with cocaine-HCl (20 mg/kg, i.p.; Sigma) prior to placement in the checkered compartment, and half were injected with cocaine prior to placement in the white compartment (CS+). The next day, mice were injected with 0.9% saline (1.0 ml/kg, i.p.) prior to placement in the alternate compartment (CS−). Injections were alternated for subsequent conditioning sessions.
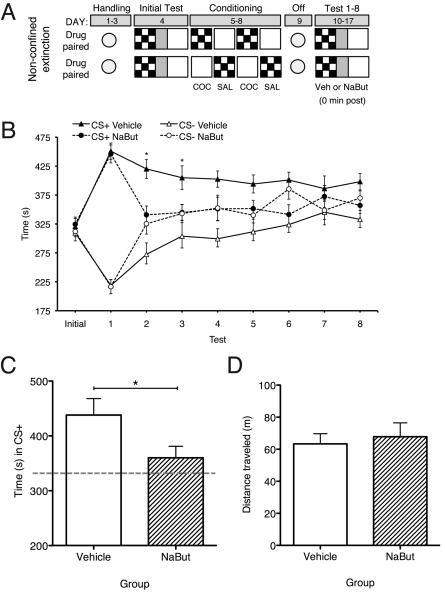
Figure 1.
HDAC inhibition significantly facilitates non-confined extinction and attenuates reinstatement of cocaine-CPP. (A) Schematic representation of non-confined extinction procedure. (B) Cocaine-CPP expression indicated by mean ± S.E.M. of time in CS+ vs. CS−. NaBut treated animals (n=10) exhibited significantly facilitated extinction as compared to vehicle treated animals (n=14). (C) NaBut treated mice (n=7) exhibited significantly attenuated cocaine-primed reinstatement (time in CS+ during test) as compared to vehicle treated mice (n=8). Gray dashed line indicates times in CS+ during initial test. (D) Treatment (NaBut, n=7; Vehicle, n=8) did not affect exploration in test apparatus indicated by mean ± S.E.M. of distance traveled during reinstatement test. * P < 0.05, compared between groups.
Forty-eight hours after the last conditioning session, preference (15 min, Test 1; day 10) was assessed in all animals as described above in a drug-free state. This marked the beginning of 8 non-confined extinction sessions (days 10-17). Immediately following Test 1, animals received an injection of either sodium butyrate (NaBut, 1.2 g/kg, i.p.; Upstate, Charlottesville, VA) dissolved in distilled water or distilled water alone (vehicle, 1.0 ml/kg, i.p.) and were returned to their home cage. For the next 7 days, mice continued to receive one preference test (15 min, non-confined extinction session) per day followed by either NaBut or vehicle injection (see Figure 1A).
Twenty-four hours after Test 8, animals achieving our a priori extinction criterion (a preference for the cocaine-paired compartment equal to or less than their initial preference) received a reinstatement test (Vehicle, n = 8; NaBut, n = 7) where they received a priming injection of cocaine (10 mg/kg, i.p.) immediately prior to a 15-min preference test.
The dose of 1.2 g/kg was chosen based on our previous studies on HDAC inhibition in synaptic plasticity, extinction of fear, and long-term memory (25, 15, 45). Lower doses of 100 and 300 mg/kg of NaBut failed to facilitate extinction of memory for conditioned fear (14).
Experiment 2: Confined Extinctionß
A different set of mice was conditioned as in Experiment 1 (see Figure 2A). Forty-eight hours after the last conditioning session, preference (Test 1) was assessed in all animals as described above in a drug-free state. Animals were divided into two groups matched on Test 1 preference to undergo confined extinction sessions. On alternating days, animals received drug-free confined exposures in either the previously drug-paired or -unpaired compartment for 3 min. Half of the animals received an injection of NaBut (1.2 g/kg, i.p.) and half received vehicle (water, 1.0 ml/kg, i.p.) immediately after exposure to the previously drug-paired compartment. All animals received a vehicle injection immediately after exposure to the drug-unpaired compartment. All animals received a preference test (Test 2) in a drug free state 24 h after two days of confined extinction sessions (see Figure 2A).
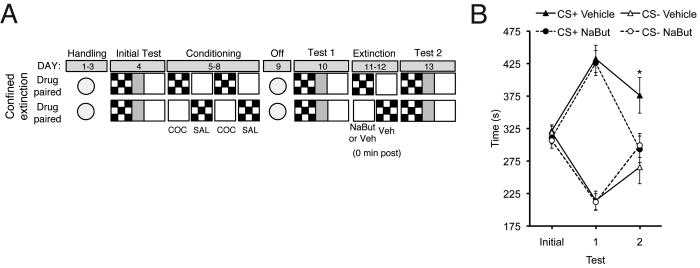
Figure 2.
HDAC inhibition facilitates confined extinction of cocaine-CPP. (A) Schematic representation of confined extinction procedure. (B) Cocaine-CPP expression indicated by mean ± S.E.M. of time in CS+ vs. CS−. NaBut treated animals (n=10) exhibited significantly facilitated extinction as compared to vehicle treated animals (n=10). * P < 0.05, compared between groups.
Experiment 3: Reinstatement after confined extinction
Similarly to Experiment 2, mice were divided into two groups (NaBut and Vehicle) after Test 1. On alternating days mice received 4 non-reinforced exposures in each of the previously drug-paired and -unpaired compartments (see Figure 4A). Half of the animals received an injection of NaBut and half received vehicle immediately after each exposure to the previously drug-paired compartment and all animals received a vehicle injection immediately after exposure to the drug-unpaired compartment. All animals received a preference test (Test 2) in a drug free state 24 h after a total of 8 confined extinction sessions (see Figure 4A). Twenty-four hours after Test 2, animals received a reinstatement test as described in Experiment 1.
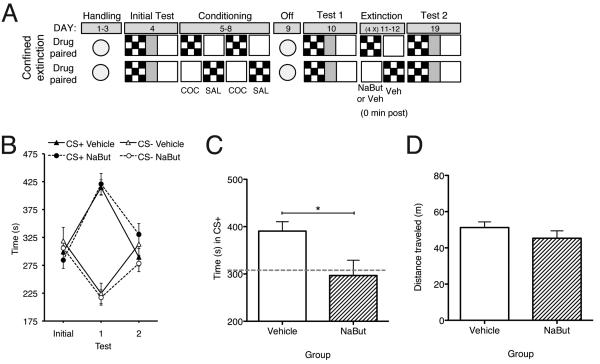
Figure 4.
HDAC inhibition significantly attenuates reinstatement of cocaine-CPP. (A) Schematic representation of extended confined extinction procedure (8 days total). (B) Cocaine-CPP expression indicated by mean ± S.E.M. of time in CS+ vs. CS−. NaBut treated animals (n=6) exhibited similar extinction of CPP as vehicle treated animals (n=6). (C) NaBut treated mice (n=6) exhibited significantly attenuated cocaine-primed reinstatement (time in CS+ during test) as compared to vehicle treated mice (n=6). Gray dashed line indicates times in CS+ during initial test. (D) Treatment (NaBut, n=6; Vehicle, n=6) did not affect exploration in test apparatus indicated by mean ± S.E.M. of distance traveled during reinstatement test. * P < 0.05, compared between groups.
Experiment 4: Immunohistochemistry
Similarly to experiment 2, a different set of mice was divided into two groups (NaBut or Vehicle) after Test 1. One day after Test 1, all mice were exposed to the previously drug-paired compartment, treated with either vehicle or sodium butyrate immediately after, and were euthanized 30 minutes later. Mice were anesthetized deeply with sodium pentobarbital (100 mg/kg, i.p.) and perfused transcardially with ice-cold PBS, pH 7.4, followed by ice-cold 4% paraformaldehyde in PBS, pH 7.4. The brains were removed, postfixed overnight at 4°C, and then transferred to 30% sucrose for 48hr at 4°C. Brains were frozen and mounted on cryostat chucks using OCT. Coronal sections were cut at a thickness of 20 μm and collected at the level corresponding to nucleus accumbens (NAc; AP: +1.25 mm from bregma). Immunoflourescence was performed as described (25) using acetyl-HistoneH3 (AcH3lys14) primary antibody (1:1000, Millipore).
Data Analysis
The primary dependent variable was time spent in the cocaine paired compartment (CS+). Extinction of CPP data was analyzed using repeated measures analysis of variance with Test as a within-subjects variable, and Treatment as a between-subjects factor, followed by Bonferroni post hoc comparisons. Specific group comparisons were made using Student's t tests. P-values < 0.05 were considered statistically significant.
Results
HDAC inhibition facilitates non-confined extinction and reduces reinstatement of cocaine-induced conditioned place preference
To examine the ability of HDAC inhibitors to facilitate extinction of cocaine-induced conditioned place preference (CPP), we administered systemic injections of sodium butyrate (NaBut, 1.2 g/kg, i.p.) or vehicle (water, 1.0 ml/kg, i.p.) immediately after 8 non-confined extinction sessions of CPP (see Figure 1A). As shown in Figure 1B, mice showed a significant preference for the cocaine-paired compartment (CS+) following cocaine CPP training when compared to initial preference, as shown by a repeated measures ANOVA [22] on time in CS+ (effect of Test: F(1, 22) = 64.60, p < 0.01; effect of Treatment: F(1,22) = 0.001, p = 0.98; interaction Test X Treatment: F(1,22) = 0.08, p = 0.79). During subsequent drug-free preference tests, which served as extinction sessions, a repeated measures ANOVA (time in CS+) revealed a main effect of Test (F(7, 154) = 4.42, p < 0.01) and Treatment (F(1, 154) = 8.99, p < 0.01) with no reliable interaction of Test X Treatment (F(7, 154) = 1.02, p = 0.42). Bonferroni post hoc comparisons revealed that on Test 2 and 3, vehicle-treated mice spent significantly more time in the cocaine-paired compartment than NaBut-treated mice (Test 2: t22 = 2.98, p < 0.05; Test 3: t22 = 2.30, p < 0.05). These results indicate that NaBut facilitated extinction of cocaine seeking.
To investigate the lasting effects of extinction, animals that achieved an a priori extinction criterion (see Methods) by the last extinction session (Vehicle, −11.96±31.71 (difference between CS+ and CS−), n=8; NaBut, 6.56±27.85, n =7; t13 = 0.46, p = 0.65) received a reinstatement test, consisting of an injection of 10 mg/kg of cocaine followed by a preference test. Mice treated with Vehicle following extinction spent significantly more time in the previously drug-paired compartment compared to NaBut treated mice (Vehicle, 438.2±30.1 sec; NaBut, 360.2±21.03 sec; t13 = 1.99, p < 0.05), indicating a reduction in reinstatement of CPP in NaBut treated mice (Figure 1C). Moreover, there was no effect of treatment on locomotor activity in the test apparatus (t13=0.42, p = 0.68; Figure 1D).
HDAC inhibition facilitates confined extinction of cocaine-CPP
To investigate the generality of the effect of NaBut on extinction, we next examined whether NaBut would facilitate extinction when mice were confined to the previously drug-paired compartment (confined extinction; see Figure 2A). Following conditioning, all mice developed a preference for the cocaine-paired (CS+) compartment (effect of Test: F(1, 18) = 57.51, p < 0.01; effect of Treatment: F(1,18) = 0.27, p = 0.61; interaction Test X Treatment: F(1,18) = 0.02, p = 0.88; Figure 2B). The day after one session of confined extinction followed by NaBut or Vehicle, mice received another preference test (Test 2). A repeated measures ANOVA (time in CS+) revealed main effects of Test (F(1,18) = 14.52, p < 0.01) and Treatment (F(1,18) = 5.31, p < 0.05) with no reliable interaction of Test X Treatment (F(1,18) = 2.38, p = 0.14). Bonferroni post hoc analysis revealed a significant difference between treatments on Test 2 (t18 = 2.63, p < 0.05), indicating that mice treated with NaBut (289.2±18.8 sec) spent significantly less time in the drug-paired compartment than mice treated with vehicle (376.0±27.4 sec) after a single confined extinction session, indicating that NaBut facilitates extinction of cocaine-induced CPP.
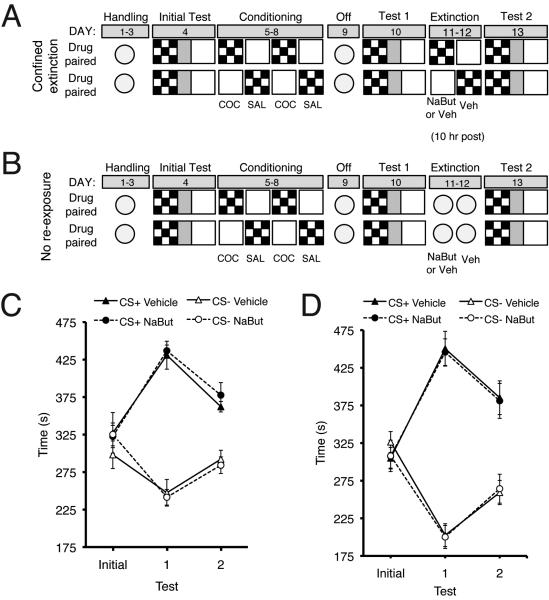
Effects of HDAC inhibition are temporally defined and dependent on re-exposure to the previously conditioned context
In contrast to the effects observed when NaBut is administered immediately after the extinction session, NaBut administered 10 hr post extinction session (see Figure 3A) did not decrease preference for the cocaine-paired compartment as compared to the Vehicle treated group (Figure 3C). Similarly, mice treated with NaBut without re-exposure to the CPP apparatus (see Figure 3B) did not differ from mice treated with Vehicle (Figure 3D). The apparent decrease in preference during Test 2 in Figure 3D was likely due to the effects of the first test, which itself was an extinction session, coupled with generalized extinction associated with handling, transport into the experimental room, and injection procedure. To address the possibility that NaBut was producing an aversion to the previously drug-paired compartment, a drug-unpaired group was injected with either NaBut or Vehicle immediately after confinement to one compartment. These animals did not show a change in preference for that compartment when tested 24 hr after the last injection (Vehicle: Test 1, 333.8±16.0 sec: Test 2, 342.4±17.27 sec; NaBut: Test 1, 309.9±20.6 sec: Test 2, 327.2±35.0 sec). Details for the methods used in experiments shown in Figure 3 are described in Supplemental Information.
Figure 3.
NaBut does not facilitate extinction of cocaine-CPP when administered either 10 hrs post confined extinction or without re-exposure to CPP apparatus. (A) Schematic representation of procedure for experiment shown in Figure 3C examining the effect of NaBut delivered 10 hrs post confined extinction. (B) Schematic representation of procedure for experiment shown in Figure 3D examining the effect of no re-exposure. (C) Mice were treated with either NaBut or vehicle 10 hrs post confined extinction. NaBut treated animals (n=8) exhibited similar extinction to vehicle treated animals (n=9). (D) Mice were not re-exposed to the CPP chamber during extinction. NaBut treated animals (n=10) exhibited similar extinction to vehicle treated animals (n=10).
From these experiments we obtained three results: 1) Extinction and NaBut administration must be temporally contiguous to enhance extinction; 2) The ability of NaBut to facilitate extinction is contingent on exposure to the previously conditioned context; 3) NaBut alone does not change compartment preference. Together, with the results from Figure 2B, these results indicate that HDAC inhibition facilitates extinction of cocaine seeking.
HDAC inhibition attenuates reinstatement of cocaine-CPP
To examine reinstatement following the confined extinction protocol we subjected animals to an extended extinction paradigm, in which all mice received confined extinction sessions over a total of 8 days (see Figure 4A). Similar to our previous results, conditioning resulted in a significant preference (Test 1) for the previously cocaine-paired compartment (F(1, 10) = 84.63, p < 0.01) with no group difference (F(1, 10) = 0.88, p = 0.37) or reliable interaction of Test X Treatment (F(1,10) = 0.02, p = 0.88) (Figure 4B). Following our extended confined extinction protocol mice were subjected to another preference test (Test 2). There was no effect of Treatment (F(1,10) = 0.20 , p = 0.65), but there was a significant effect of Test (F(1,10) = 88.44 p < 0.01). Importantly, preference on Test 2 was eliminated in both Vehicle and NaBut treated mice, indicating that all subjects achieved extinction (Figure 4B).
To investigate the persistence of extinction, mice subjected to the extended extinction protocol were given a reinstatement test. In response to a cocaine-priming injection, mice treated with vehicle following confined extinction sessions spent significantly more time in the previously drug-paired compartment compared to those treated with NaBut (t10 = 2.48, p < 0.05; Figure 4C). Furthermore, there was no effect of treatment on locomotor activity (t10 = 1.15, p = 0.27; Figure 4D). These results corroborate our findings in Figures 1C and 1D, and provide an additional demonstration that HDAC inhibition during extinction significantly attenuates reinstatement without influencing exploration in the test apparatus.
HDAC inhibition following extinction of cocaine-CPP increases histone acetylation in NAc
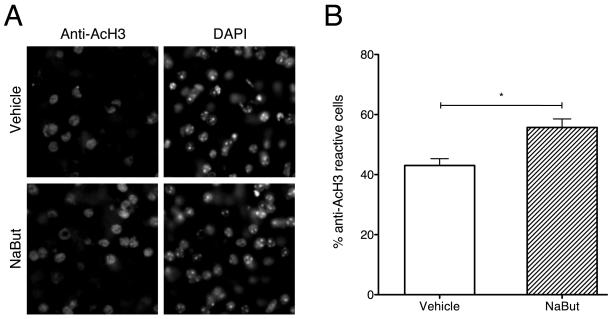
To analyze the effect of HDAC inhibition on histone acetylation during extinction, histone H3 lysine14 acetylation was analyzed in the NAc using immunofluorescence. We chose acetylation of histone H3 lysine 14 (AcH3lys14) because this site has been shown to be regulated in other forms of plasticity and memory processes (23-25). The percent of AcH3ly14 immunoreactive cells was significantly higher in the NaBut treated group compared to the vehicle treated group when examined 0.5 hr after Test 1 (the first extinction trial) (t9 = 3.40, p < 0.01; Figure 5). These results indicate that a single systemic administration of the HDAC inhibitor NaBut after extinction increases histone acetylation in the NAc.
Figure 5.
HDAC inhibition significantly increases histone H3 lysine14 acetylation in nucleus accumbens following extinction of cocaine-CPP. (A) NAc acetylated histone H3 lysine14 immunoreactivity in vehicle and NaBut treated mice following extinction of cocaine-CPP. DAPI was used as a nuclear stain. (B) Quantification of the percentage of immunoreactive cells to total cells counted. NaBut treated mice (n=6) have a significantly higher percentage of acetylated histone H3 lysine14 immunoreactive cells than vehicle treated mice (n=5) following a single extinction session. * P < 0.01, compared between groups.
Discussion
In this study, we demonstrate that HDAC inhibition during extinction consolidation can facilitate extinction of cocaine-induced conditioned place preference (CPP). Whether animals were subjected to non-confined extinction, in which they are free to move about all chambers (CS−, middle, or CS+) of the apparatus, or forced extinction, in which they are confined to the previously conditioned CS+ context, those treated with the HDAC inhibitor sodium butyrate extinguished cocaine-induced CPP both more quickly and to a greater extent than did vehicle-treated animals. We also show that the extinction of context-drug associated memories via HDAC inhibition modulates extinction learning such that reinstatement behavior is significantly attenuated. Control experiments indicate that the effects of HDAC inhibition on extinction are temporally restricted and dependent on re-exposure to the previously conditioned context. Together, these results are the first to demonstrate that HDAC inhibitors could serve as a potent therapeutic agent for the extinction of context-drug associated memories.
Chromatin modification via histone acetylation has been shown to be a critical factor in mechanisms underlying cellular and behavioral responses to cocaine (2). The first ground-breaking study demonstrated that alterations in histone acetylation directly affected cocaine-dependent expression of specific genes as well as the behavioral responses to cocaine (4). For example, systemic delivery of the HDAC inhibitor TSA prior to conditioning or site-specific delivery of the HDAC inhibitor SAHA into the nucleus accumbens (NAc) prior to CPP testing significantly increased the effects of cocaine in CPP (4, 10). These results suggest that HDAC inhibition facilitates gene expression involved in the formation of context-drug associated memories similar to observations showing that HDAC inhibition administered immediately after training (during consolidation) enhances context-shock associated memories (25). Conversely, over-expression of HDAC4 or HDAC5 in the NAc decreased cocaine-induced CPP (4, 10). These results indicate that increases in histone acetylation are required for cocaine-induced gene expression. This idea is well supported by a study showing that CREB-binding protein (CBP) heterozygous knockout mice have decreased sensitivity to cocaine-induced locomotor sensitization, which correlates with decreased CBP occupancy at the fosB promoter and decreased histone H4 acetylation (5). The role of CBP and its HAT activity in the acquisition of cocaine-induced CPP remains to be understood.
The role of the transcription factor CREB in drug reward mechanisms has been examined in numerous studies (29, 26, 27). When activated, CREB can recruit CBP to acetylate histones and thereby facilitate target gene activation (28). The transcription factor ΔFosB—a splice variant of FosB—accumulates with repeated exposure to cocaine and overexpression of ΔFosB in the NAc increases the rewarding effects of cocaine (29). By recruiting HATs, HDACs, and the chromatin remodeling protein SWI-SNF to gene promoters, ΔFosB differentially regulates transcription of target genes specified in drug-induced behaviors (4, 5, 9, 10). Together, these studies provide several lines of evidence of how chromatin modifications can alter gene expression required for cocaine-induced neural and behavioral plasticity.
Many recent studies have examined pharmacological treatments that may enhance extinction in several preparations, including cocaine-induced CPP. Enhancements in extinction generally have been inferred from one of two behavioral effects (30). One effect is on the rate of extinction; enhancements in extinction are revealed through faster losses of conditioned behavior in drug-treated compared to vehicle-treated animals. The second effect is on the persistence of extinction; enhancements in extinction are revealed through weakened reinstatement, spontaneous recovery, or contextual renewal of the conditioned behavior. Often, rate or persistence, but not both, may be affected by a pharmacological treatment (e.g., 31, 32). In our experiments, sodium butyrate increased: one, the rate of extinction, as reflected in rapid loss of preference after a single extinction trial; and two, the persistence of extinction, eliminating reinstatement of a preference that occurred following a post-extinction priming injection of cocaine in vehicle-treated mice. These combined effects on rate and persistence are consistent with the idea that sodium butyrate enhanced extinction of the cocaine-induced place preference.
In addition to actions on enhancements in learning and memory mechanisms that occur during extinction, facilitated behavioral extinction effects have been taken as evidence for impairments in the reconsolidation of the original memory (e.g., 37). Because either impaired reconsolidation or enhanced extinction would result in a loss of behavior, studies have appealed to both processes to explain the behavioral effects (e.g., 13, 35, 38-41). Persistent effects, like those we observed, have been taken as evidence for actions on reconsolidation rather than extinction (42), but certainly any facilitated extinction effect should also result in persistent effects, weakening spontaneous recovery, reinstatement, and renewal (e.g., 13, 43, 45, 46, 48). Indeed, there are many demonstrations starting with (44) that unmasking phenomena associated with extinction are a function of amount of extinction – more extinction causes less recovery, reinstatement, and renewal.
Whether one appeals to extinction or reconsolidation processes to explain the loss of behavior often depends on assumptions about what the pharmacological treatment is doing at the cellular and molecular level. The challenge from a molecular perspective is to determine what HDAC inhibition does that would result in facilitated extinction. Studies that have shown facilitated acquisition by HDAC inhibition have suggested that HDAC inhibitors increase access to transcription factors, thereby increasing transcription and translation necessary for memory consolidation (4). Similar consolidation mechanisms have been shown to operate during extinction, so one possibility is that increasing accessibility to transcription factors promotes some aspect of the memory that develops during extinction. Our results indicate that acetylation of histone H3 in the accumbens may be an important site of action for these effects.
In summary, we demonstrate for the first time that HDAC inhibition during extinction consolidation can facilitate extinction of cocaine-induced CPP. We also show that HDAC inhibition modulates long-term extinction such that reinstatement behavior is significantly attenuated. A growing body of evidence clearly indicates that the fundamental mechanisms of regulating gene expression via chromatin modification (one type of epigenetic regulation) are involved in long-term synaptic plasticity and long-term memory processes as well as persistent drug-induced behavioral responses (2, 7). Our increasing understanding of these epigenetic mechanisms will provide key answers to basic processes in memory and drug addiction, how these processes may impact one another, and hopefully provide insight into designing improved treatments for memory disorders and drug addiction.
Supplementary Material
Acknowledgements
We thank Chris Cunningham and John Marshall for helpful discussions and critical reading of this manuscript. This work was supported by NIDA (R01DA025922, multi-PI to M.A.W. and K.M.L.), NIMH (R01MH081004, to M.A.W.; R01MH077111, to K.M.L.), the Ramon y Cajal program of the Ministerio de Ciencia e Innovacion (to C.S-S.), a Fundacion Alicia Koplowitz fellowship (to C.S-S.), and the Conselleria d'Educacio de la Generalitat Valenciana (to C.S-S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89(1):121–45. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14(8):341–50. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroeder FA, et al. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry. 2007;62(1):55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48(2):303–14. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Levine AA, et al. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc Natl Acad Sci U S A. 2005;102(52):19186–91. doi: 10.1073/pnas.0509735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsankova NM, et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9(4):519–25. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 7.Barrett RM, Wood MA. Beyond transcription factors: the role of chromatin modifying enzymes in regulating transcription required for memory. Learn Mem. 2008;15(7):460–7. doi: 10.1101/lm.917508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Renthal W, et al. Delta FosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine exposure. J Neurosci. 2008;28(29):7344–9. doi: 10.1523/JNEUROSCI.1043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renthal W, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56(3):517–29. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 11.Heather N, Bradley BP. Cue exposure as a practical treatment for addictive disorders: why are we waiting? Addict Behav. 1990;15(4):335–7. doi: 10.1016/0306-4603(90)90043-w. [DOI] [PubMed] [Google Scholar]
- 12.Botreau F, Paolone G, Stewart J. d-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172(1):173–8. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Paolone G, Botreau F, Stewart J. The facilitative effects of D: -cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berl) 2009;202(13):403–9. doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- 14.Bredy TW, et al. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14(4):268–76. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lattal KM, Barrett RM, Wood MA. Systemic or Intrahippocampal Delivery of Histone Deacetylase Inhibitors Facilitates Fear Extinction. Behav Neurosci. 2007;121(5):1125–1131. doi: 10.1037/0735-7044.121.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calu DJ, Schoenbaum G. Cocaine-paired cues activate aversive representations in accumbens neurons. Neuron. 2008;13(57):633. doi: 10.1016/j.neuron.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carelli RM, Ijames SG. Nucleus accumbens cell firing during maintenance, extinction, and reinstatement of cocaine self-administration behavior in rats. Brain Res. 2000;866(12):44–54. doi: 10.1016/s0006-8993(00)02217-4. [DOI] [PubMed] [Google Scholar]
- 18.Carelli RM, Ijames SG. Selective activation of accumbens neurons by cocaine-associated stimuli during a water/cocaine multiple schedule. Brain Res. 2001;907(12):156–61. doi: 10.1016/s0006-8993(01)02604-x. [DOI] [PubMed] [Google Scholar]
- 19.Childress AR, et al. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156(1):11–8. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant S, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93(21):12040–5. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pham J, et al. Automated scoring of fear-related behavior using EthoVision software. J Neurosci Methods. 2009;178(2):323–6. doi: 10.1016/j.jneumeth.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan Z, et al. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111(4):483–93. doi: 10.1016/s0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- 24.Levenson JM, et al. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279(39):40545–59. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 25.Vecsey CG, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007;27(23):6128–40. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlezon WA, Jr., et al. Regulation of cocaine reward by CREB. Science. 1998;282(5397):2272–5. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 27.Walters CL, Blendy JA. Different requirements for cAMP response element binding protein in positive and negative reinforcing properties of drugs of abuse. J Neurosci. 2001;21(23):9438–44. doi: 10.1523/JNEUROSCI.21-23-09438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14(13):1553–77. [PubMed] [Google Scholar]
- 29.Nestler EJ. Review. Transcriptional mechanisms of addiction: role of DeltaFosB. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3245–55. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lattal KM. Extinction and the erasure of memories. Psychol Science Agenda. 2007;21(34):16–18. [Google Scholar]
- 31.Groblewski PA, Lattal KM, Cunningham CL. Effects of d-Cycloserine on Extinction and Reconditioning of Ethanol-Seeking Behavior in Mice. Alcohol Clin Exp Res. 2009 doi: 10.1111/j.1530-0277.2009.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behav Neurosci. 2006;120(5):1159–62. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]
- 33.Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47(6):873–84. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Thanos PK, et al. D-cycloserine accelerates the extinction of cocaine-induced conditioned place preference in C57bL/c mice. Behav Brain Res. 2009;199(2):345–9. doi: 10.1016/j.bbr.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernardi RE, Lattal KM, Berger SP. Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuroreport. 2006;17(13):1443–7. doi: 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- 36.Fricks-Gleason AN, Marshall JF. Post-retrieval beta-adrenergic receptor blockade: effects on extinction and reconsolidation of cocaine-cue memories. Learn Mem. 2008;15(9):643–8. doi: 10.1101/lm.1054608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milton AL, Lee JL, Everitt BJ. Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on {beta}-adrenergic receptors. Learn Mem. 2008;15(2):88–92. doi: 10.1101/lm.825008. [DOI] [PubMed] [Google Scholar]
- 38.Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009;10(3):224–34. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- 39.Davis M, et al. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60(4):369–75. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 40.Pavlov IP, Anrep G.V.e. Conditioned reflexes; an investigation of the physiological activity of the cerebral cortex. Oxford university press; Humphrey Milford: 1927. pp. xv–430. London. [Google Scholar]
- 41.Bouton ME, Vurbic D, Woods AM. D-cycloserine facilitates context-specific fear extinction learning. Neurobiol Learn Mem. 2008;90(3):504–10. doi: 10.1016/j.nlm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stafford JM, Lattal KM. Direct comparisons of the size and persistence of anisomycin-induced consolidation and reconsolidation deficits. Learn Mem. 2009 doi: 10.1101/lm.1452209. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tronel S, Milekic MH, Alberini CM. Linking new information to a reactivated memory requires consolidation and not reconsolidation mechanisms. PLoS Biol. 2005;3(9):e293. doi: 10.1371/journal.pbio.0030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber M, Hart J, Richardson R. Effects of D-cycloserine on extinction of learned fear to an olfactory cue. Neurobiol Learn Mem. 2007;87(4):476–82. doi: 10.1016/j.nlm.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 45.Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci U S A. 2009;106:9447–9452. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.