Abstract
CCK-1 receptor deficient Otsuka Long Evans Tokushima Fatty (OLETF) rats are hyperphagic, which leads to subsequent obesity and diabetes. Additionally, they have increased sham intake and enhanced preference for sucrose solutions relative to control, Long Evans Tokushima Otsuka (LETO) rats. To determine the effects of oil on ingestion, we first measured real feeding of various concentrations of oil emulsions (12.5, 25, 50, 75, 100%) in rats that were fed ad libitum. Secondly, to isolate the orosensory compontent of oils from post-ingestive consequences, as well as determine the contribution of energy status, we measured sham-feeding in OLETF and LETO rats using one-bottle acceptance tests while non-deprived and overnight food deprived. Finally, to assess the orosensory effects of nutritive and non-nutritive oils, we used two-bottle preference tests in sham fed OLETF and LETO rats. We found that real feeding resulted in increased intake of high oil concentrations for OLETF rats relative to LETO rats. Similarly, OLETF rats consumed significantly more of higher concentration corn oils than LETO while non-deprived sham feeding. Conversely, OLETF rats overconsumed low concentration corn oil compared to LETO during overnight deprived sham feeding tests. In 2-bottle sham feeding preference tests, both non-deprived OLETF and LETO rats preferred corn to mineral oil. Collectively, these results show that increased oil intake in OLETF rats is driven by both peripheral deficits to satiation and altered orosensory sensitivity.
Keywords: oil intake, hyperphagia, fat, taste
1. Introduction
Substantial amount of evidence has placed the high amounts of fatty foods within the western diet as a cause for the obesity epidemic [1]. High-fat diets, due to their high palatability and energy density, stimulate voluntary energy intake leading to obesity in both animals [2] and humans [3]. Although fat ingestion is guided by orosensory and post-ingestive factors, the orosensory properties are sufficient for discrimination and ingestion independently of postingestive factors [4]. The orosensory properties of fats stimulate ingestion and this occurs in deprived and sated sham-feeding animals for nutritive (corn oil) as well as non-nutritive (mineral oil) stimuli [5, 6]. Thus, ingestion of fats occurs in the absence of feedback with regard to energy status. Also, orosensory stimuli, such as fatty flavors, are rewarding, and promote approach behavior and operant learning even when postoral feedback is minimized [4, 7]. In some obese rat models, hyperphagia for palatable foods is related to the positive feedback from orosensory properties [8]. For example, we recently showed that increased food intake in obese Otsuka Long Evans Tokushima Fatty (OLETF) rats can be attributed, at least in part, to altered orosensory functions. Specifically, we found increased preference for high concentrations of sweet solutions [9], altered neural coding for sweet taste [10], and heightened reward sensitivity [11].
The OLETF rat, a rodent model for obesity and non-insulin dependent diabetes mellitus (NIDDM) carries a natural single point deletion of the CCK-1R [12, 13]. They are hyperphagic compared to LETO controls and gradually become obese [14] during their life span. The underlying cause(s) of hyperphagia in this rat model has not been fully revealed. Whereas most deficits can be explained by the absence of the CCK-1R and alterations in peripheral and central CCK signaling [15, 16], additional non-CCK deficits have been shown to be responsible for OLETF rat’s chronic hyperphagia, including impairments in hypothalamic peptide signaling [15, 17-19], and dopamine functions [9, 20-22]. As well as having deficits in peripheral satiation and enhanced orosensory stimulation, the OLETF has altered levels of dopamine, which positively correlate in reward with palatable sources [11, 22-24].
Similar to other obese rodent models [8, 25], OLETF rats have an increased preference and intake of highly rewarding or preferred foods, especially fat. For example, OLETF rats have decreased intestinal sensitivity to both intestinal infusion of oils [26] as well as solid high fat foods [27]. However, whether OLETF rats prefer fats more than LETO rats based on oral stimulatory effects is not known. Physiological need-states, such as food deprivation [28, 29] or limited access to palatable food (e.g. sweet or fatty meals [30]) results in increased intake when the stimuli become available again (i.e., “reward sensitization”) (see [31] for a review). For example, relative to lean controls, OLETF rats overeat on meals that are normally preferred when food deprived (fat [27]; sugars [9]).
Therefore, to examine the relationship of the orosensory component with feedback inhibition from the gastrointestinal tract, we employed real feeding of increasing oil emulsion concentrations (12.5, 25, 50, 75, and 100%). Further, to examine the orosensory acceptance of oils in various feeding states in the pre-diabetic OLETF compared to LETO controls, we sham fed OLETF and LETO rats corn oil emulsions under both non-deprived and food deprived conditions. Finally, to determine preference for a nutritive to non-nutritive oil between the two strains, we performed brief access (30 min) two bottle preference tests with 100% emulsions of corn and mineral oil.
2. Methods
2.1 Animals
OLETF and LETO male rats were obtained from the Tokushima Research Institute, Otsuka Pharmaceutical, Tokushima, Japan. Rats were housed individually in wire floored, hanging steel cages in a temperature controlled vivarium on a 12:12 light:dark cycle (lights on at 0600 hours). Rats were provided ad libitum access to pelleted rat chow (Purina 5001) and water, except the night before food deprived sham feeding tests described below. Rats were handled for a minimum of one week during the acclimation period before experimental protocol began. All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the Pennsylvania State University.
2.2 Surgical Procedure: Gastric Cannulation
Following overnight fasting, rats were anaesthetized with a combination of ketamine (50mg/kg), xylazine (5mg/kg) and acepromazine (1mg/kg). Gastric cannulation was performed as described previously [32]. Briefly, after anesthesia, rats were surgically fit with chronic gastric cannulae on the non-glandular portion of the stomach. A puncture wound was made in the lateral section of the rat’s torso through which the cannula exited. Bard mesh was then used to secure the cannula to the abdominal wall. Rats were given two weeks recovery from the surgery before any feeding experiments begun.
2.3 Real feeding of oil emulsions
Rats (n = 13, 6 OLETF, 7 LETO) with average weights of 567 ± 14 g and 475 ± 15 g, respectively were allowed ad libitum access to chow until presentation of oils. At 0900 h, chow was removed from the cages, rats were weighed, placed back into their cages, and presented with burettes filled with 12.5, 25, 50, 75 and 100% oil emulsions. To achieve the desired concentrations of oils, 0.75 ml of Tween-80 (Sigma) was added to every 100 ml mixture of oil and tap water. All oil concentrations were tested at least twice and intake were manually recorded by an individual every 5 min for 60 min. Tests were conducted every other day.
2.4 Sham feeding of oil emulsions in the non-deprived condition
A separate group of rats (n = 13, 6 OLETF and 7 LETO) weighing 601 ± 30 g and 477 ± 13 g, respectively were equipped with chronic gastric cannulae and fed ad libitum chow throughout the experiment. Rats underwent an acclimation period of three trials that consisted of 60 min sham feeding 25% corn oil in the morning (0900) after overnight (1700 – 0900) water and food deprivation. During testing, at 0900 h, rats were weighed and their stomachs lavaged with warm water until the drained contents were clear and devoid of any food particles. Rats were then fitted with sham feeding tubes and placed in mesh wire floored Plexiglas sham feeding boxes. Each concentration of corn oil (12.5, 25, 50, 75, 100%) were presented to all rats a minimum of two times in both ascending and descending order of concentration. Intake of oil emulsions was individually recorded every 5 min for 60 min. Sham feeding tests took place every other day. In all sham feeding tests, gastric drainage was collected in plastic containers placed beneath each sham feeding box. Great care was taken to ensure patency of drainage tubes and flow of oil freely through the tubes. In the event gastric drainage did not occur while rats were sham feeding, a connector tube attached to a syringe was used to verify eventual blockage and clear the tube promptly. This becomes important, particularly when high concentrations of oils are used, which in combination with gastric mucus, form viscous, foam like secretions.
2.5 Sham feeding of oil following overnight food deprivation
Rats (n = 12, 5 OLETF, 7 LETO) weighing 440 ± 7 g and 361 ± 8 g, respectively were sham fed after overnight (16-hr) food deprivation. Before tests begun, rats were trained to sham feed oil following a 2-h water deprivation. After training, rats were sham fed the oil emulsions as described above.
2.6 Oil preference tests
Seventeen naïve rats (8 OLETF and 9 LETO) with average weights of 574 ± 21 g and 458 ± 6 g, respectively, equipped with gastric cannulas received ad libitum access to rat chow, except during two-bottle preference testing. For training, rats were sham fed with either 100% corn or 100% mineral oil for 30 min in blocks of two days for a total of 8 days: 4 cycles × 2 days. After training, preference tests were conducted for two consecutive days. Rats received concomitant access to burettes containing 100% corn and 100% mineral oil and sham intake was measured for 30 min. To avoid side preference, burette positions were alternated for each test.
2.7 Oral Glucose Tolerance Test (OGTT)
A subset of overnight food deprived rats from each experimental condition (sham feeding and real feeding; n = 12, 6 per strain) were tested for blood glucose levels after an oral gavage of a glucose load (2 g/kg) using 8-French tubing as described previously [9]. Tail blood was collected at 0, 30, 60, 90, and 120 minutes post glucose administration. Rats with blood glucose over 300 mg/dl at any time post gavage or over 200 mg/dl at 120 minutes post gavage were considered diabetic [33]. Blood was analyzed using a glucometer (Lifescan, One-Touch Basic).
2.8 Statistical Analyses
For 60-min intakes, two-way repeated measures ANOVA (rmANOVA) was performed with strain and oil concentration as main factors. Separate one-way ANOVA was run for each oil concentration to reveal differences between strains. Also, one-way rmANOVA was used to analyze all cumulative 5-min intakes in both OLETF and LETO groups. There was no significant effect of the presentation order of oil concentrations (ascending vs descending) between strains, therefore data was pooled for statistical analysis. To determine the effect of food deprivation, oil intake of OLETF was calculated as percent difference from LETO oil intake for each condition and subjected to two-way ANOVA with oil concentration and deprivation as main factors. Total intake of both oil emulsions (corn and mineral) were used to calculate preference percentage according to the following formula: % preference = [volume of oil type (ml) × 100/total volume of corn and mineral oil (ml)]. Post hoc tests were conducted using Bonferonni pair wise tests. OGTT data was analyzed by one-way rmANOVA, with strain as the main factor. All statistics were computed using Statistical Analysis Software (SAS, version 9.1.3, SAS Institute, Cary, NC). Data were expressed as means ± SE. In all analyses, significance was set at α < 0.05.
3. Results
3.1 Real feeding of oil emulsions
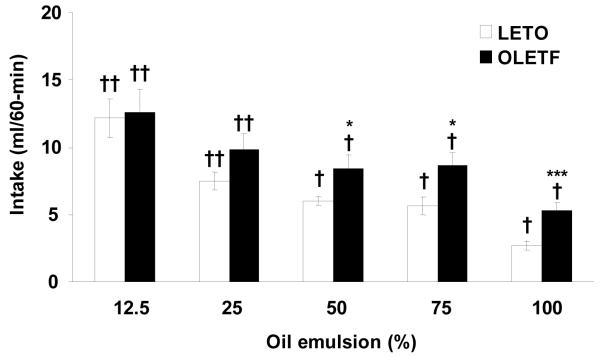
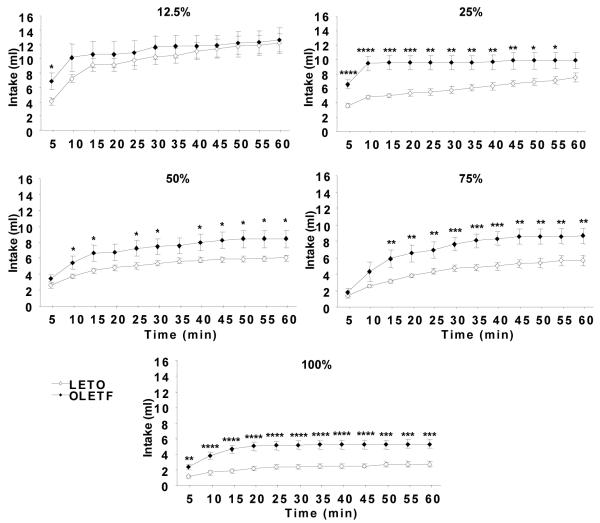
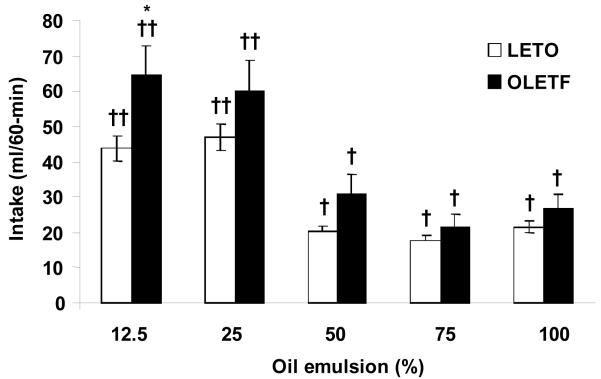
ANOVA revealed that OLETF rats consumed significantly more 50, 75, and 100% oil emulsions during the 60 min test compared to LETO (OLETF: 50%: 8.41 ± 1.02 ml, P < 0.05; 75%: 8.66 ± 0.91 ml, P < 0.05; 100%: 5.31 ± 0.57 ml, P < 0.001; LETO: 50%: 6.00 ± 0.33 ml; 75%: 5.64 ± 0.64 ml; 100%: 2.73 ± 0.35 ml). No strain differences in intake were noted for 12.5 and 25% oil emulsions (P < 0.05). Within strain analysis showed that both strains consumed significantly more of the lower (12.5 and 25%) than higher (50, 75, and 100%) oil concentrations (Fig. 1). Analysis of 5-min intake intervals detected significant differences between strains starting at 10, 15, and 5 minutes for the 50 (P < 0.05), 75 (P < 0.01), and 100% (P < 0.01) oils, respectively. This difference in oil consumption between strains remained at the same level of significance until the end of the test session. At 12.5 and 25% oils, OLETF drank significantly more than LETO at the beginning of the test, but the difference was not maintained throughout the duration of the session (Fig. 2).
Fig. 1.
Real feeding of oil in OLETF and LETO rats. OLETF consumed significantly more 50, 75, and 100% oil solutions compared to LETO. There were no strain differences in consumption of 12.5 and 25% oil. Within strains, both OLETF and LETO consumed significantly more 12.5 and 25% oil compared to other concentrations tested (50 – 100%). Data are expressed as means ± SEM. * P < 0.05, *** P < 0.001 denotes statistical significance between strains. † denotes statistical significance within strain, P < 0.01.
Fig. 2.
Sixty-minute cumulative real feeding in OLETF and LETO rats. OLETF drank significantly more oil at higher concentrations (50 – 100%) than LETO starting at 5 to 15 minutes after presentation of oils. This significant difference was maintained throughout the 60-min feeding test. At 12.5 and 25% oils, OLETF drank significantly more oil than LETO at the beginning of the test session, but this difference was not maintained by the end of the test. Data are expressed as means ± SEM. * P < 0.05, ** < 0.01, *** < 0.001, **** < 0.0001 denotes statistical significance between strains.
3.2 Sham feeding in non-deprived condition
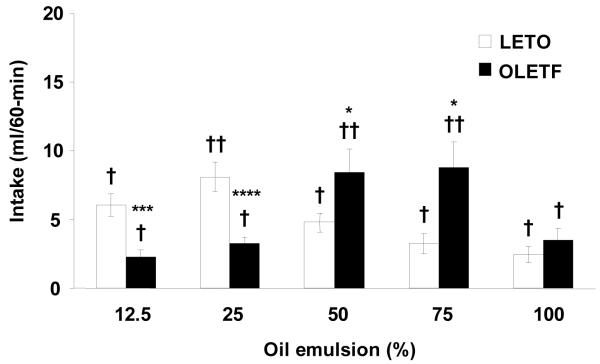
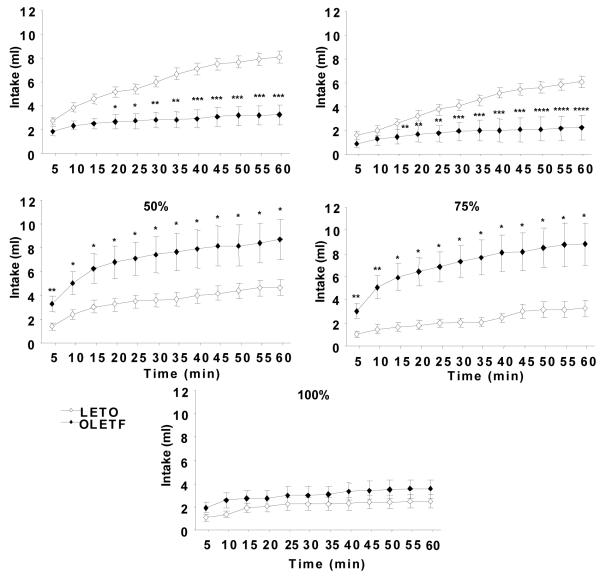
Beginning five minutes after oil presentation, OLETF consumed significantly more of the 50 and 75% concentrations than LETO (P < 0.01 for each). The difference between strains remained significant for the remainder of the test, which resulted in OLETF (50%: 8.47 ± 1.67 ml, P < 0.05; 75%: 8.80 ± 1.86 ml, P < 0.05) overconsuming both 50 and 75% oils over 60-min relative to LETO (50%: 4.79 ± 0.69 ml; 75%: 3.25 ± 0.69 ml). In contrast, OLETF consumed significantly less oil than LETO at the 12.5 and 25% concentrations (OLETF: 12.5%: 2.27 ± 0.50 ml, P < 0.001; 25%: 3.24 ± 0.48 ml, P < 0.0001; LETO: 12.5%: 6.06 ± 0.81 ml; 25%: 8.07 ± 1.05 ml) (Fig. 3). The difference between strains was evident at 20 minutes for the 12.5% (P < 0.05) and 10 minutes at the 25% oil (P < 0.05 for each) (Fig. 4). Feeding 100% oil resulted in no significant differences in oil consumption between strains over 60-min. Within strains, OLETF rats consumed significantly more of the 50 and 75% oil concentrations compared to 12.5, 25%, and 100% concentrations (P < 0.01 for each concentration), while LETO consumed significantly more 25% oil concentration compared to all other concentrations tested (P < 0.01).
Fig. 3.
Sham intake of oils in non-deprived OLETF and LETO rats. Compared to LETO rats, OLETF consumed significantly more of the 50 and 75% oil and less of the 12.5 and 25% oil concentrations. Within strain, OLETF consumed significantly more 50 and 75% oil compared to other concentrations while LETO consumed more 25% oil concentration compared to all other concentrations tested. Data are expressed as means ± SEM. * P < 0.05, *** P < 0.001, **** P < 0.0001 denotes differences between strains. †P <0 .01 denotes statistical significance within strains.
Fig 4.
Sixty-minute cumulative sham intake in non-deprived OLETF and LETO rats. OLETF drank significantly less of the lower oil concentrations (12.5 and 25%) compared to LETO starting within the first 5 to 20 minutes of the test session. The difference between strains remained at a plateau level during the second half of the feeding test. At higher oil concentrations (50 – 75%), OLETF drank significantly more oil than LETO starting at 5 minutes after the test began and consumption remained significantly different throughout the remainder of the 60-min test. Data are expressed as means ± SEM. * P < 0.05, ** < 0.01, *** < 0.001, **** < 0.0001 denotes statistical significance between strains.
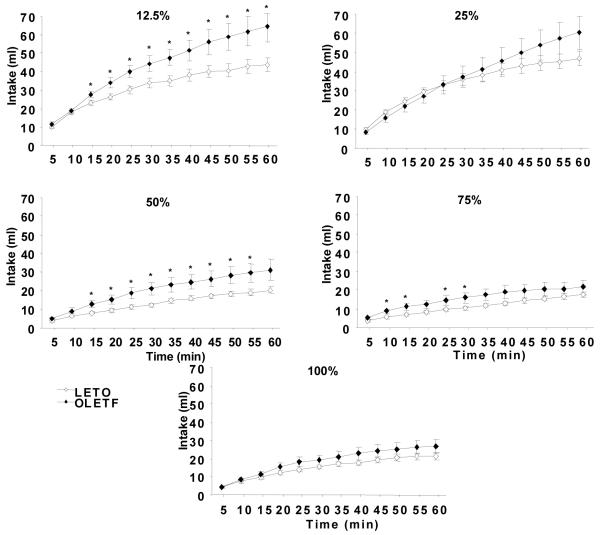
3.3 Sham feeding after overnight deprivation
Exposure to low oil concentrations after overnight food deprivation resulted in OLETF consuming significantly more 12.5% oil beginning at 15 minutes (P < 0.05) compared to LETO. This difference remained significant throughout the 60-min testing period (OLETF: 64.51 ± 8.30 ml vs. LETO: 43.81 ± 3.62 ml; P < 0.05) (Fig. 5). At higher oil concentrations (50 – 100%), although there were similar trends in intake between strains with OLETF consuming slightly more oil, no significant differences in 60-min oil intake were detected (Fig. 6). Within strains, both OLETF and LETO consumed significantly more 12.5 and 25% oil concentrations compared to 50, 75, and 100% concentrations (P < 0.01 for each).
Fig. 5.
Sham oil intake in overnight food deprived OLETF and LETO rats. OLETF rats consumed significantly more 12.5% oil than LETO. At all other oil concentrations (25 – 100%) there were no significant differences between OLETF and LETO. Both strains consumed significantly more 12.5 and 25% oil compared to all concentrations tested (50 – 100%). Data are expressed as means ± SEM. * P < 0.05 denotes statistical significance between strains. † P < 0.01 denotes statistical significance within strains.
Fig 6.
Sixty-minute cumulative sham intake in overnight food deprived OLETF and LETO rats. OLETF consumed significantly more 12.5% oil than LETO starting at 15 minutes of the test session. At higher oil concentrations (50 – 75%) OLETF consumed more than LETO during the feeding tests, but the difference between strains was not significant at the completion of the test. During exposure to 100% oil, both strains consumed similar volumes of oil. Data are expressed as means ± SEM. * denotes statistical significance between strains. * P < 0.05.
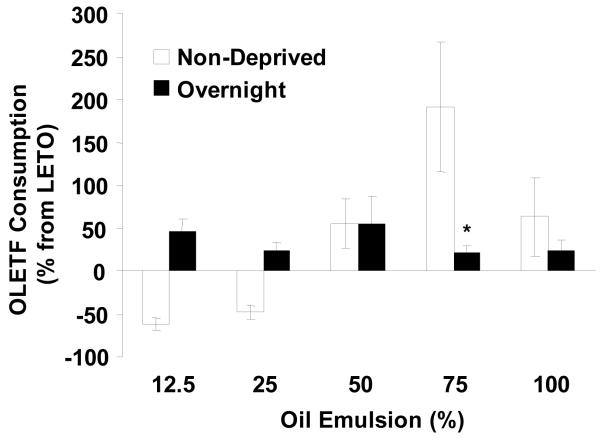
3.4 Comparisons across conditions
When oil consumption was expressed as percent change from LETO oil consumption, OLETF rats were found to consume significantly more 75 % oil when non deprived (191.45 ± 75.20%) compared to overnight food deprivation (21.13 ± 7.62%, P < 0.05) (Fig. 7). When non-deprived, OLETF rats drank less of low oil concentrations (12.5 and 25%) oil compared to the deprived condition; however, this was not found to be significant.
Fig. 7.
Difference in oil intake (% from LETO) across sham feeding conditions. OLETF rats consumed significantly more 75% oil in non-deprived compared to the deprived condition. There was a trend for decreased consumption of low concentration oils (12.5 and 25%) in the non-deprived relative to the deprived condition, but this was not significant. Data are expressed as means ± SEM. * P < 0.05 denotes statistical significance from non-deprived condition,
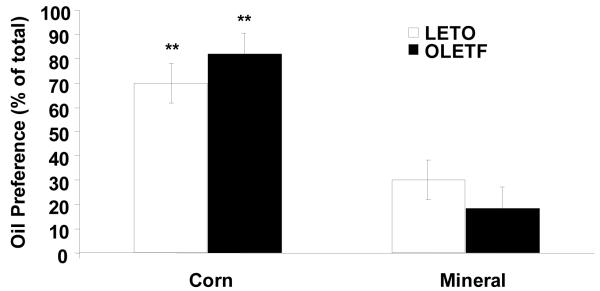
3.5 Preference for corn and mineral oil
Repeated measures ANOVA revealed that both OLETF (corn: 81.7 ± 8.7%; mineral: 18.3 ± 8.7% of total intake, P < 0.01) and LETO (corn: 69.8 ± 8.1 %; mineral: 30.2 ± 8.1 % of total intake, P < 0.01) rats preferred 100% corn oil to mineral oil in non-deprived sham feeding preference tests (Fig. 8). There were no differences in preference for oils between strains.
Fig. 8.
Corn and mineral oil preference after brief access two-bottle tests in non-deprived OLETF and LETO rats. Both OLETF and LETO rats preferred corn oil to mineral oil. Data are expressed as means ± SEM. ** P < 0 .01 denotes significant difference from mineral oil.
3.6 Oral Glucose Tolerance Test (OGTT)
Baseline (fasting) blood glucose levels between the OLETF and LETO strains were not significantly different at any time-point post-gavage (0 min: P = 1.0; 30 min: P = 0.18; 60 min: P = 0.32; 90 min: P = 1.0; 120 min: P = 1.0). Results of the oral glucose tolerance tested performed at the end of the study showed that OLETF rats were prediabetic because they did not show elevated blood glucose levels indicative of NIDDM [33].
4. Discussion
The present study shows that obese OLETF rats feed significantly larger amounts of corn oil at higher concentrations compared with LETO rats irrespective of whether oil was real or sham fed. This increased consumption of high oil concentrations was significantly higher when rats were non-deprived compared to when deprived before the test. Further, these differences in oil consumption between strains during the feeding tests were evident as early as 5 minutes following exposure to oil. Nevertheless, while acceptance of oils differed between strains and conditions, we found that both OLETF and LETO preferred nutritive (corn) oil to non-nutritive (mineral) oil. Collectively, these results demonstrate, for the first time, that OLETF rats express increased avidity to oils and consume more oil at high concentrations compared to LETO rats. These effects are more pronounced when they are fed ad libitum.
Intake and acceptance of oils has been shown to be stimulated by orosensory mechanisms and inhibited through gastrointestinal feedback [34]; although gut feedback also has a stimulatory effect on repeated consumption [6]. To examine the contribution of both oral and postoral feedback on oil consumption in the OLETF and LETO rats we first used real feeding of different oil concentrations. The results revealed that OLETF and LETO rats consumed similar amounts of low (12.5 and 25%) oil concentrations; however at moderate (50%) and high (75 and 100%) oil concentrations, OLETF rats significantly increased oil consumption compared to LETO rats. These data indirectly support our previous findings [26], as well as Schwartz et. al [27] showing that OLETF rats are less sensitive to the intraintestinal satiating effects of lipids. Schwartz et. al previously found that duodenal infusion of fats decreased fatty food intake significantly less in OLETF than LETO rats. Here, we show that OLETF rats overconsume oils relative to LETO when post-ingestive feedback is present.
To further dissociate the oral component of oil intake from the post-oral influences, we used a sham feeding preparation and measured increasing oil concentration intake. In our study, the non-deprived OLETF rats’ oil intake was higher at 50 and 75% compared to LETO’s; however, LETO drank higher amounts of 12.5 and 25% oil concentrations compared to OLETF. These results suggest a right-shift in the preference curve in OLETF vs. LETO rats that is consistent with the OLETF’s well documented increased appetite when feeding, non-deprived, on a high fat diet. Whether this reflects increased or decreased sensitivity to oral fat will be discussed below.
To determine whether changes in oil consumption based on orosensory characteristics are driven by caloric needs, we subjected OLETF and LETO rats to an overnight food deprivation challenge. Previous work from Mindell et al. [5] using deprived rats showed that sham oil intakes in one-bottle acceptance tests are dependent upon concentration, with the highest intakes occurring from 12.5 to 50% oil concentrations. Similarly, our results show that when deprived overnight, OLETF rats significantly increase their intake of low oil concentrations compared to LETO rats. Thus, deprivation results in an increase in sensitivity to oil, pushing the rats to drink more of the low concentrations. OLETF rats have altered homeostatic energy regulation, and overcompensate when food deprived [35]. The fact that OLETF’s intake of both low and high oil concentrations were increased after deprivation, compared to non-deprived condition suggests that their intake was driven by energy deficit as well as orosensory factors. Another important finding is that, after overnight deprivation, sham-feeding OLETF rats showed a pattern of eating similar to LETO controls (Fig. 5), eating more of the lower compared to the higher concentrations of oils. The decline in sham feeding intakes as oil concentrations increased suggests that oil palatability declined as concentration increased, a pattern opposite to that obtained with other palatable stimuli, such as sucrose or sweeteners. Although it is possible that our concentrations used may have prevented us from seeing a shift at lower concentrations that are readily consumed [36], our findings of a peak oil consumption in the LETO are consistent with previous results over a thirty minute period [5].
Analysis of five minute cumulative intake intervals shows that the differences in real and sham intake between strains in the fed, non-deprived condition were driven primarily by palatability. This is based on the rationale that if sham feeding declined early in the test sessions, this would support a palatability explanation, but if intakes declined only late in the session it would support a satiation (oral or postingestive) explanation. Indeed, when real fed, OLETF rats drank more oil during the first 5 to 15 min of the test session after which their intake plateaued. This suggests that their higher intake is primarily due to increased palatability of high concentrations of oils and/or decreased gastrointestinal feedback consistent with their hyperphagia.
When sham-feeding low concentrations (12.5 and 25%), non-deprived OLETF consumed significantly less oil than LETO during the first 10 to 20 minutes. The difference between strains increased slightly until 30 minutes and remained constant during the second half of the sham feeding test. Exposure to higher oil concentrations (50, 75%) resulted in OLETF drinking significantly more oil during the early period (5–20 min) after which the difference between strains remained constant. Sham-feeding 100% oil resulted in both strains drinking similar amounts during the first 5 minutes and a subsequent linear trend in intake for the remainder of the test. These results suggest that OLETF rats exhibit a decrease in sensitivity to oils in the absence of intestinal feedback.
When animals were overnight food deprived, the pattern of intake changed. At low concentrations (12.5 and 25%), OLETF and LETO continually increased oil intake throughout the 60 min period, however, OLETF consumed significantly more of the 12.5% oil than LETO. At higher oil concentrations (50 – 75%) there were similar increases in intakes between OLETF and LETO, with OLETF consuming more oil during the first 15 minutes. The difference in cumulative intake between strains remained relatively unchanged over the second 30 minutes of the feeding tests. This suggests that after overnight deprivation, OLETF rats increase oil intake to compensate for the energy deficit, thus demonstrating the ability of detecting the caloric value of oil in the absence of postingestive feedback. Furthermore, the observation that oil intake trends at high concentrations were similar in OLETF and LETO rats in the deprived, but not the non-deprived condition, suggests that food deprivation alters OLETF rats’ oral capacity to detect oils. Thus, it appears that oral sensitivity to fats in OLETF is increased following an overnight deprivation. While there are currently limited studies examining the effect of food deprivation on taste sensitivity to fatty stimuli, prior research shows that during deprivation or need-states, preference for palatable stimuli is increased [30].
It is worth noting that although the overall results from our sham feeding tests show that changes in sensitivity to oils occur primarily early on in the test session, hence pointing to a palatability explanation, gastric and intestinal feedback should not be excluded. It is known that a gastric fistula does not necessarily block all food absorption [37], and that as much as 25% of sham fed oil could be absorbed [38].
To compare cumulative 60-min oil intake exclusively within the OLETF strain across conditions, we normalized their consumption relative to LETO rats. We found that non-deprived OLETF rats drank significantly more of the 75% oil compared to the overnight deprivation condition. These findings show that OLETF rats’ orosensory sensitivity to oil is decreased when fed compared to fasted, resulting in increased intake of high oil concentrations. Thus, when fed, OLETF rats’ increased consumption of high oil concentration may lead to a higher susceptibility to caloric overconsumption resulting from fatty foods. Together, the present findings from both real and sham feeding studies, in concert with previous data, show that when fed, decreased oral sensitivity and peripheral satiation deficits observed in OLETF rats contribute to an increased consumption of high fat foods.
Several factors involving pathways of taste including fatty acid receptors or taste-cells responsive to fatty acids in the oral cavity may be responsible for decreased oral sensitivity to oils in OLETF rats. Although there are no studies examining mechanisms of fat detection in the OLETF rats, another rodent model of hyperphagia and obesity on a HF diet expresses less taste cells responsive to fatty acid induced depolarization than lean controls [39]. Additionally, functional differences in fatty acid responses of DRK channels in obesity prone and obesity resistant rat strains have been reported [40] suggesting differences in chemoreception of fats in the taste of obese versus lean phenotypes. Thus, a decreased detection of fats in the oral cavity may be responsible for the overconsumption of highly fatty foods by OLETF rats. It is also important to note that these current findings are similar to our previous work showing that OLETF rats have a decreased oral sensitivity to sugar, drinking significantly less of a 0.03 M, but more of a 1.0 M sucrose solution than LETO controls [9]. Thus, it appears that an altered oral sensitivity in the OLETF is more generalized to palatable nutrients and may be partly responsible for hyperphagia in this strain. Although the underlying cause of decreased sensitivity to peripheral satiation signals are most likely the reason for passive overconsumption of fats in intact OLETF rats, the increased acceptance of oils at high concentrations during sham feeding is unlikely to be caused by satiation signal deficits. Administration of exogenous CCK inhibits sham feeding in rodents [41, 42], but is easily reversed by a CCK-1R antagonist [43, 44]. However, administration of the antagonist alone does not increase sham intake [45]. Further, there is a weak correlation between the CCK-1R and orosensory properties of palatable foods [46, 47]. Together, these observations suggest that orosensory deficits in addition to the lack of a functional CCK-1R in OLETF rats are most likely to be responsible for their increased consumption of high oil concentrations relative to LETO rats.
Another possible candidate responsible for altered sensitivity to oils in OLETF rats appears to be changes in reward functions. In outbred Sprague-Dawley rats, palatable food sources are associated with an increased release of dopamine in brain areas associated with reward [48, 49]. Additionally, obese humans and rodents have alterations in the release or reuptake of dopamine as well as binding of dopamine receptors [50-52]. In the absence of obesity, both intermittent [53] and long-term [54] exposure to palatable foods modifies dopamine receptor binding in brain areas involved in reward. CCK also has been shown to increase central release of dopamine through CCK-1R activation [55]. Work from our laboratory shows that OLETF rats have a compromised central dopamine system characterized by both altered receptor binding [23] and dopamine release [22]. Additionally, OLETF rats exhibit increased lick responses to palatable sweets even when briefly exposed to the stimulus [56], and demonstrate altered neural responses in the pontine taste relay responsible for oral sucrose exposure [10]. Furthermore, they are more sensitive to suppression of sham sucrose intake after administration of D2 dopamine receptor antagonism, demonstrating that OLETF rats’ increased preference of highly palatable foods is mediated by the dopamine system [24]. Together, these data suggest that similar to increased preference for sucrose, the overconsumption of oil in OLETF rats may be partly due to their differential sensitivity of the reward system to palatable foods. However, this hypothesis needs to be tested.
In an attempt to elucidate possible differences in the detection of oils and their orosensory properties between strains, we employed two bottle preferences tests of 100% corn and mineral oil. We found that when non-deprived, both strains were able to discriminate corn oil from mineral oil, with each preferring corn oil. While this result is similar to what has been reported previously in the literature [5, 57], the specific underlying mechanisms are largely unknown. However, several possible explanations have been offered that include, but are not limited, to olfaction, lipolytic activity in the oral cavity, and gustation. Previously, olfaction was thought to control discrimination of oils; however, rats are able to detect solid fats mixed with chow even after they are made anosmic [5]. Though tacticle mechanisms are correlated to detection of fattiness of foods in humans [58], scant data exists in rodent models [59]. Taste appears to be another pathway as well in controlling oil intake in the absence of gastrointestinal feedback. The fatty acid transporter CD36, is located on taste receptor cells [60, 61] and correlates with increased preference for fats [62]. However, while tantalizing, this evidence is not yet conclusive. Additional research shows fatty acids can depolarize taste cells co-localized with CD36 [40, 60]. Further, lingual lipase, which is present in rodents and infants, hydrolyzes triglycerides to free fatty acids in the oral cavity [63]. Although this may be attractive in explaining orosensory sensitivity to oils, orlistat, a lipase inhibitor, added to corn oil does not abolish preference from a plain corn oil emulsion [63]. Thus, these supporting data are far from complete to implicate it as primary controlling mechanisms of oil intake during sham feeding.
In summary our study shows that although OLETF and LETO rats exhibit equal preference for corn over mineral oil, the OLETF overconsumes high corn oil concentrations relative to LETO when either real or sham fed. This increase of consumption is more pronounced when rats are non-deprived than when food deprived. Collectively, these data provide strong support that OLETF rats’ overconsumption of oils results from both decreased intestinal sensitivity as well as an altered orosensory sensitivity to oil which is dependent on concentration of oil and the feeding condition of the animal.
5. Acknowledgements
The authors wish to thank Otsuka Pharmaceutical Co. (Tokushima, Japan) for the generous donation of the OLETF and LETO animals used to perform this research. This research was supported by National Institute of Diabetes & Digestive & Kidney Diseases Grant DK-065709.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Price RA, Charles MA, Pettitt DJ, Knowler WC. Obesity in Pima Indians: large increases among post-World War II birth cohorts. Am J Phys Anthropol. 1993;92:473–9. doi: 10.1002/ajpa.1330920406. [DOI] [PubMed] [Google Scholar]
- [2].Gao J, Ghibaudi L, van Heek M, Hwa JJ. Characterization of diet-induced obese rats that develop persistent obesity after 6 months of high-fat followed by 1 month of low-fat diet. Brain Res. 2002;936:87–90. doi: 10.1016/s0006-8993(02)02493-9. [DOI] [PubMed] [Google Scholar]
- [3].Lissner L, Levitsky DA, Strupp BJ, Kalkwarf HJ, Roe DA. Dietary fat and the regulation of energy intake in human subjects. Am J Clin Nutr. 1987;46:886–92. doi: 10.1093/ajcn/46.6.886. [DOI] [PubMed] [Google Scholar]
- [4].Sclafani A. How food preferences are learned: laboratory animal models. Proc Nutr Soc. 1995;54:419–27. doi: 10.1079/pns19950011. [DOI] [PubMed] [Google Scholar]
- [5].Mindell S, Smith GP, Greenberg D. Corn oil and mineral oil stimulate sham feeding in rats. Physiol Behav. 1990;48:283–7. doi: 10.1016/0031-9384(90)90314-t. [DOI] [PubMed] [Google Scholar]
- [6].Ackroff K, Vigorito M, Sclafani A. Fat appetite in rats: the response of infant and adult rats to nutritive and non-nutritive oil emulsions. Appetite. 1990;15:171–88. doi: 10.1016/0195-6663(90)90018-4. [DOI] [PubMed] [Google Scholar]
- [7].Warwick ZS, Weingarten HP. Determinants of high-fat diet hyperphagia: experimental dissection of orosensory and postingestive effects. Am J Physiol. 1995;269:R30–7. doi: 10.1152/ajpregu.1995.269.1.R30. [DOI] [PubMed] [Google Scholar]
- [8].Greenberg D, Weatherford SC. Obese and lean Zucker rats differ in preferences for sham-fed corn oil or sucrose. Am J Physiol. 1990;259:R1093–5. doi: 10.1152/ajpregu.1990.259.6.R1093. [DOI] [PubMed] [Google Scholar]
- [9].De Jonghe BC, Hajnal A, Covasa M. Increased oral and decreased intestinal sensitivity to sucrose in obese, prediabetic CCK-A receptor-deficient OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R292–300. doi: 10.1152/ajpregu.00481.2004. [DOI] [PubMed] [Google Scholar]
- [10].Kovacs P, Hajnal A. Altered pontine taste processing in a rat model of obesity. J Neurophysiol. 2008;100:2145–57. doi: 10.1152/jn.01359.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hajnal A, Acharya NK, Grigson PS, Covasa M, Twining RC. Obese OLETF rats exhibit increased operant performance for palatable sucrose solutions and differential sensitivity to D2 receptor antagonism. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1846–54. doi: 10.1152/ajpregu.00461.2007. [DOI] [PubMed] [Google Scholar]
- [12].Funakoshi A, Miyasaka K, Jimi A, Kawanai T, Takata Y, Kono A. Little or no expression of the cholecystokinin-A receptor gene in the pancreas of diabetic rats (Otsuka Long-Evans Tokushima Fatty = OLETF rats) Biochem Biophys Res Commun. 1994;199:482–8. doi: 10.1006/bbrc.1994.1254. [DOI] [PubMed] [Google Scholar]
- [13].Funakoshi A, Miyasaka K, Shinozaki H, Masuda M, Kawanami T, Takata Y, Kono A. An animal model of congenital defect of gene expression of cholecystokinin (CCK)-A receptor. Biochem Biophys Res Commun. 1995;210:787–96. doi: 10.1006/bbrc.1995.1728. [DOI] [PubMed] [Google Scholar]
- [14].Moran TH, Katz LF, Plata-Salaman CR, Schwartz GJ. Disordered food intake and obesity in rats lacking cholecystokinin A receptors. Am J Physiol. 1998;274:R618–25. doi: 10.1152/ajpregu.1998.274.3.R618. [DOI] [PubMed] [Google Scholar]
- [15].Moran TH. Unraveling the obesity of OLETF rats. Physiol Behav. 2008;94:71–8. doi: 10.1016/j.physbeh.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Moran TH, Bi S. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos Trans R Soc Lond B Biol Sci. 2006;361:1211–8. doi: 10.1098/rstb.2006.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bi S, Ladenheim EE, Schwartz GJ, Moran TH. A role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R254–60. doi: 10.1152/ajpregu.2001.281.1.R254. [DOI] [PubMed] [Google Scholar]
- [18].Moran TH, Lee P, Ladenheim EE, Schwartz GJ. Responsivity to NPY and melanocortins in obese OLETF rats lacking CCK-A receptors. Physiol Behav. 2002;75:397–402. doi: 10.1016/s0031-9384(01)00667-9. [DOI] [PubMed] [Google Scholar]
- [19].Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, Bi S. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. J Neurosci. 2009;29:179–90. doi: 10.1523/JNEUROSCI.4379-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Feifel D, Shilling PD, Kuczenski R, Segal DS. Altered extracellular dopamine concentration in the brains of cholecystokinin-A receptor deficient rats. Neurosci Lett. 2003;348:147–50. doi: 10.1016/s0304-3940(03)00767-5. [DOI] [PubMed] [Google Scholar]
- [21].De Jonghe BC, DiMartino C, Hajnal A, Covasa M. Brief intermittent access to sucrose differentially modulates prepulse inhibition and acoustic startle response in obese CCK-1 receptor deficient rats. Brain Res. 2005;1052:22–7. doi: 10.1016/j.brainres.2005.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Anderzhanova E, Covasa M, Hajnal A. Altered basal and stimulated accumbens dopamine release in obese OLETF rats as a function of age and diabetic status. Am J Physiol Regul Integr Comp Physiol. 2007;293:R603–11. doi: 10.1152/ajpregu.00301.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hajnal A, Margas WM, Covasa M. Altered dopamine D2 receptor function and binding in obese OLETF rat. Brain Res Bull. 2008;75:70–6. doi: 10.1016/j.brainresbull.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hajnal A, De Jonghe BC, Covasa M. Dopamine D2 receptors contribute to increased avidity for sucrose in obese rats lacking CCK-1 receptors. Neuroscience. 2007;148:584–92. doi: 10.1016/j.neuroscience.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Okada S, York DA, Bray GA, Mei J, Erlanson-Albertsson C. Differential inhibition of fat intake in two strains of rat by the peptide enterostatin. Am J Physiol. 1992;262:R1111–6. doi: 10.1152/ajpregu.1992.262.6.R1111. [DOI] [PubMed] [Google Scholar]
- [26].Covasa M, Ritter RC. Attenuated satiation response to intestinal nutrients in rats that do not express CCK-A receptors. Peptides. 2001;22:1339–48. doi: 10.1016/s0196-9781(01)00461-2. [DOI] [PubMed] [Google Scholar]
- [27].Schwartz GJ, Whitney A, Skoglund C, Castonguay TW, Moran TH. Decreased responsiveness to dietary fat in Otsuka Long-Evans Tokushima fatty rats lacking CCK-A receptors. Am J Physiol. 1999;277:R1144–51. doi: 10.1152/ajpregu.1999.277.4.R1144. [DOI] [PubMed] [Google Scholar]
- [28].Sakai RR, Fine WB, Epstein AN, Frankmann SP. Salt appetite is enhanced by one prior episode of sodium depletion in the rat. Behav Neurosci. 1987;101:724–31. doi: 10.1037//0735-7044.101.5.724. [DOI] [PubMed] [Google Scholar]
- [29].Sakai RR, Frankmann SP, Fine WB, Epstein AN. Prior episodes of sodium depletion increase the need-free sodium intake of the rat. Behav Neurosci. 1989;103:186–92. doi: 10.1037//0735-7044.103.1.186. [DOI] [PubMed] [Google Scholar]
- [30].Hagan MM, Holguin FD, Cabello CE, Hanscom DR, Moss DE. Combined naloxone and fluoxetine on deprivation-induced binge eating of palatable foods in rats. Pharmacol Biochem Behav. 1997;58:1103–7. doi: 10.1016/s0091-3057(97)00318-3. [DOI] [PubMed] [Google Scholar]
- [31].Carr KD. Feeding, drug abuse, and the sensitization of reward by metabolic need. Neurochem Res. 1996;21:1455–67. doi: 10.1007/BF02532386. [DOI] [PubMed] [Google Scholar]
- [32].Yox DP, Ritter RC. Capsaicin attenuates suppression of sham feeding induced by intestinal nutrients. Am J Physiol. 1988;255:R569–74. doi: 10.1152/ajpregu.1988.255.4.R569. [DOI] [PubMed] [Google Scholar]
- [33].Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–8. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- [34].Greenberg D, Smith GP. The controls of fat intake. Psychosom Med. 1996;58:559–69. doi: 10.1097/00006842-199611000-00004. [DOI] [PubMed] [Google Scholar]
- [35].Bi S, Moran TH. Response to acute food deprivation in OLETF rats lacking CCK-A receptors. Physiol Behav. 2003;79:655–61. doi: 10.1016/s0031-9384(03)00173-2. [DOI] [PubMed] [Google Scholar]
- [36].Davis JD, Kung TM, Rosenak R. Interaction between orosensory and postingestional stimulation in the control of corn oil intake by rats. Physiol Behav. 1995;57:1081–7. doi: 10.1016/0031-9384(95)00008-7. [DOI] [PubMed] [Google Scholar]
- [37].Sclafani A, Nissenbaum JW. Is gastric sham feeding really sham feeding? Am J Physiol. 1985;248:R387–90. doi: 10.1152/ajpregu.1985.248.3.R387. [DOI] [PubMed] [Google Scholar]
- [38].Reed DR, Tordoff MG, Friedman MI. Sham-feeding of corn oil by rats: sensory and postingestive factors. Physiol Behav. 1990;47:779–81. doi: 10.1016/0031-9384(90)90095-l. [DOI] [PubMed] [Google Scholar]
- [39].Gilbertson TA, Liu L, Kim I, Burks CA, Hansen DR. Fatty acid responses in taste cells from obesity-prone and -resistant rats. Physiol Behav. 2005;86:681–90. doi: 10.1016/j.physbeh.2005.08.057. [DOI] [PubMed] [Google Scholar]
- [40].Gilbertson TA, Liu L, York DA, Bray GA. Dietary fat preferences are inversely correlated with peripheral gustatory fatty acid sensitivity. Ann N Y Acad Sci. 1998;855:165–8. doi: 10.1111/j.1749-6632.1998.tb10560.x. [DOI] [PubMed] [Google Scholar]
- [41].Kraly FS, Carty WJ, Resnick S, Smith GP. Effect of cholecystokinin on meal size and intermeal interval in the sham-feeding rat. J Comp Physiol Psychol. 1978;92:697–707. doi: 10.1037/h0077501. [DOI] [PubMed] [Google Scholar]
- [42].Antin J, Gibbs J, Holt J, Young RC, Smith GP. Cholecystokinin elicits the complete behavioral sequence of satiety in rats. J Comp Physiol Psychol. 1975;89:784–90. doi: 10.1037/h0077040. [DOI] [PubMed] [Google Scholar]
- [43].Brenner L, Ritter RC. Peptide cholesystokinin receptor antagonist increases food intake in rats. Appetite. 1995;24:1–9. doi: 10.1016/s0195-6663(95)80001-8. [DOI] [PubMed] [Google Scholar]
- [44].Brenner LA, Ritter RC. Intracerebroventricular cholecystokinin A-receptor antagonist does not reduce satiation by endogenous CCK. Physiol Behav. 1998;63:711–6. doi: 10.1016/s0031-9384(97)00519-2. [DOI] [PubMed] [Google Scholar]
- [45].Reidelberger RD, Heimann D, Kelsey L, Hulce M. Effects of peripheral CCK receptor blockade on feeding responses to duodenal nutrient infusions in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R389–98. doi: 10.1152/ajpregu.00529.2002. [DOI] [PubMed] [Google Scholar]
- [46].Eckel LA, Ossenkopp KP. Cholecystokinin reduces sucrose palatability in rats: evidence in support of a satiety effect. Am J Physiol. 1994;267:R1496–502. doi: 10.1152/ajpregu.1994.267.6.R1496. [DOI] [PubMed] [Google Scholar]
- [47].Gosnell BA, Hsiao S. Effects of cholecystokinin on taste preference and sensitivity in rats. Behav Neurosci. 1984;98:452–60. doi: 10.1037//0735-7044.98.3.452. [DOI] [PubMed] [Google Scholar]
- [48].Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R31–7. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- [49].Liang NC, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1236–9. doi: 10.1152/ajpregu.00226.2006. [DOI] [PubMed] [Google Scholar]
- [50].Huang XF, Zavitsanou K, Huang X, Yu Y, Wang H, Chen F, Lawrence AJ, Deng C. Dopamine transporter and D2 receptor binding densities in mice prone or resistant to chronic high fat diet-induced obesity. Behav Brain Res. 2006;175:415–9. doi: 10.1016/j.bbr.2006.08.034. [DOI] [PubMed] [Google Scholar]
- [51].Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- [52].Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding YS, Wong C, Ma Y, Pradhan K. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–43. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–52. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- [54].South T, Huang XF. High-fat diet exposure increases dopamine D2 receptor and decreases dopamine transporter receptor binding density in the nucleus accumbens and caudate putamen of mice. Neurochem Res. 2008;33:598–605. doi: 10.1007/s11064-007-9483-x. [DOI] [PubMed] [Google Scholar]
- [55].Crawley JN. Cholecystokinin-dopamine interactions. Trends Pharmacol Sci. 1991;12:232–6. doi: 10.1016/0165-6147(91)90558-a. [DOI] [PubMed] [Google Scholar]
- [56].Hajnal A, Covasa M, Bello NT. Altered taste sensitivity in obese, prediabetic OLETF rats lacking CCK-1 receptors. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1675–86. doi: 10.1152/ajpregu.00412.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Elizalde G, Sclafani A. Fat appetite in rats: flavor preferences conditioned by nutritive and non-nutritive oil emulsions. Appetite. 1990;15:189–97. doi: 10.1016/0195-6663(90)90019-5. [DOI] [PubMed] [Google Scholar]
- [58].Mela DJ. Sensory assessment of fat content in fluid dairy products. Appetite. 1988;10:37–44. doi: 10.1016/s0195-6663(88)80031-x. [DOI] [PubMed] [Google Scholar]
- [59].Hamilton CL. Rat’s Preference for High Fat Diets. J Comp Physiol Psychol. 1964;58:459–60. doi: 10.1037/h0047142. [DOI] [PubMed] [Google Scholar]
- [60].Gaillard D, Laugerette F, Darcel N, El-Yassimi A, Passilly-Degrace P, Hichami A, Khan NA, Montmayeur JP, Besnard P. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 2008;22:1458–68. doi: 10.1096/fj.07-8415com. [DOI] [PubMed] [Google Scholar]
- [61].Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–84. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1823–32. doi: 10.1152/ajpregu.00211.2007. [DOI] [PubMed] [Google Scholar]
- [63].Kawai T, Fushiki T. Importance of lipolysis in oral cavity for orosensory detection of fat. Am J Physiol Regul Integr Comp Physiol. 2003;285:R447–54. doi: 10.1152/ajpregu.00729.2002. [DOI] [PubMed] [Google Scholar]