Abstract
Estradiol is a potent steroid of both gonadal and neuronal origin that exerts profound and enduring effects on the brain as it develops. Differences in estradiol production in males and females underlie the establishment of many sexually dimorphic brain characteristics. Two paradigm shifts in the understanding of estradiol and its actions have expanded the view from one of slow narrowly controlled nuclear transcription to include rapid effects initiated at the membrane and inducible by locally synthesized steroid. A survey of estradiol actions reveals regional specificity underlying opposing effects such that estradiol induces cell death in one region but prevents it in another or promotes synaptogenesis in one region but retards it in the other. Similarly, estradiol is neuroprotective or neurodamaging and enhances excitation or dampens excitation, depending on the model and neurotransmitter under study. Understanding the diverse actions of estradiol in different brain regions under differing conditions is essential to harnessing the tremendous therapeutic potential of this endogenous naturally occurring and efficacious neural modulator.
Keywords: preoptic area, hypothalamus, sex differences, sex behavior, neuroprotection, prostaglandins
Steroid hormones are among the most powerful and enduring signaling molecules in the body. When transported via the circulation, steroids travel great distances from the site of synthesis in an endocrine organ to a distant target organ, one of which is the brain. Alternatively, steroids can act as local autocrine or paracrine signals that impact only the microenvironment, including within the brain. The half-life of steroids is several-fold greater than that of other blood-borne signaling molecules, such as insulin, which disappear within minutes to hours. Estradiol is the final end product of 6 to 7 enzymatic conversions of the precursor cholesterol, and it is the most potent steroid, being active at concentrations in the femtomolar range. The critical p450scc enzyme, CYP19, also called aromatase or estrogen synthase, is the rate-limiting step in estradiol synthesis from androgen precursors and is a nodal point of regulation. The fetal environment is replete with estradiol originating in the maternal circulation and placenta, and from synthesis directly in fetal neurons. Steroid binding globulins, such as α-fetoprotein, are essential and poorly understood regulators of steroid access to the fetal brain and may serve to both protect and selectively deliver estradiol to specific neuronal populations (see Bakker and others 2006; McCarthy 2008; Pfaff and Keiner 1973).
Two paradigm shifts in the past decade have altered our views on mechanisms of steroid action in the brain. The first paradigm shift focuses on the receptor. Estradiol binds to 1 of 2 receptor isoforms, ERα and ERβ, both of which are members of the nuclear receptor transcription factor superfamily. As transcription factors, ER act directly at the genome as part of a transcription complex that recognizes hormone responsive elements in promoter regions to either induce or repress gene expression (Beato and Klug 2000). This capacity to directly regulate protein synthesis contributes to the potent and enduring effects of steroids on developing systems, including the brain. However, the same transcription factor receptors also colocalize to neuronal membranes, particularly in caveoli, specialized microdomains at which a variety of signal transduction proteins are localized. Additional uniquely membrane-associated ER have also been detected (Toran-Allerand 2004; Toran-Allerand and others 2002). Within these specialized domains, ER directly interact with cellular kinases, most prevalently the MAP kinase family and PI3 kinase, as well as membrane receptors such as mGluR (Kuo and others 2009). These interactions are rapid and capable of inducing the full cascade of cellular responses attendant to these signaling systems. A permissive role of rapid membrane-mediated estradiol effects followed by enduring genomic effects of estradiol has been demonstrated for some endpoints but not others. Regardless, this dual action of estradiol at both the membrane and the nucleus greatly expands the repertoire of cellular endpoints modulated by this potent steroid (Fig. 1).
Figure 1.
Estradiol effects are slow, estradiol effects are rapid. Estradiol binds to its cognate estrogen receptor (ER), either ERα or ERβ, both of which are members of the nuclear receptor transcription factor family and interact directly with the transcriptional complex and the genome, frequently at estrogen response elements (ERE) to alter gene expression. The resultant changes in protein synthesis are slow and often enduring. Estradiol can also activate receptors located at the membrane (mER), which initiate signal transduction cascades that are relatively rapid and transient, but may nonetheless lead to enduring effects via changes in gene expression.
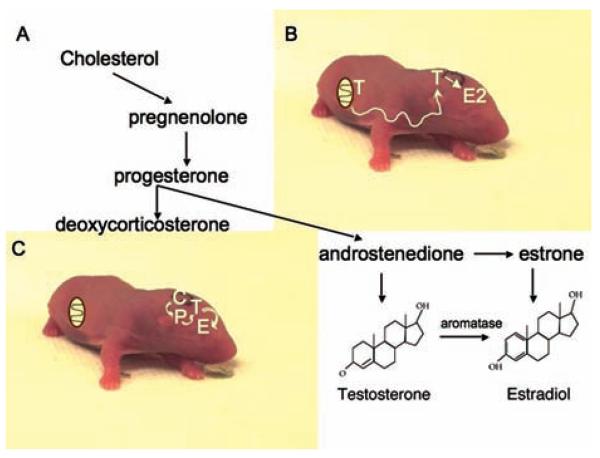
The second paradigm shift involves the site of estradiol synthesis. Estradiol is frequently referred to as a gonadal steroid or a sex steroid, in recognition of the production of estradiol by the ovary. However, as noted before, neurons are capable of directly converting androgens to estrogens via aromatization and this capacity has been recently expanded to include astrocytes (Garcia-Segura and others 2003). Aromatase activity is highest in the immature brain, particularly in brain regions directly relevant to reproduction. This provides for the conversion of testicularly derived androgens into estrogens to mediate the process of sexual differentiation of the brain during a restricted developmental window called the sensitive period. The locally produced estradiol organizes the brain along a masculine phenotype and provides the neuroanatomical substrate that will be activated by androgens to promote male sexual behavior and physiology in adulthood. These 2 concepts together are codified as the organizational/activational hypothesis and the aromatization hypothesis of sexual differentiation (see for review McCarthy 2008). Although the aromatization hypothesis involves estradiol synthesized locally in neurons, it is still considered a gonadal steroid because the precursor, androgens, are synthesized in the gonad and gain access to the brain secondarily. A critically important result is that males have higher brain levels of estradiol than females, and this has been demonstrated for the hypothalamus, the major brain region undergoing sexual differentiation (Amateau and others 2004). In contrast, recent evidence indicates estradiol can also be synthesized de novo from cholesterol entirely within select regions of the brain. All of the synthetic enzymes required to complete the conversion of cholesterol to estradiol have been identified in the brain, and in some instances measurement of brain estradiol levels exceed those in circulation (Hojo and others 2004; Prange-Kiel and others 2003; Rune and others 2002). To date 2 brain regions have convincingly been demonstrated to synthesize estradiol, the hippocampus and the cerebellum (see for review Dean and McCarthy 2008). In both cases the neurosteroid is important to adult neuroplasticity. We have indirect evidence that estradiol is also synthesized de novo in the developing hippocampus and possibly cortex. The amount of estradiol measured in neonatal hippocampus and cortex is the same in males and females, and this may be the result of de novo local estradiol synthesis (Amateau and others 2004). This has led us to postulate that during development the ultimate function of brain estradiol synthesis outside the reproductively important hypothalamus is to reduce, not produce, sex differences in the brain (McCarthy and Konkle 2005). As reviewed below, estradiol is a potent endogenous regulator of many developmental processes. Females would be deprived of this important modulator if the only source is the fetal testis inasmuch as the fetal ovary remains quiescent until well into postnatal life. By synthesizing estradiol locally within only select regions of the brain, females can maximize the beneficial effects while avoiding the masculinizing effects on reproductively relevant brain regions. To state it more simply, when estradiol is operating in the guise of a gonadal steroid its site of action and endpoints are directly relevant to reproduction and largely restricted to the hypothalamus. But, when estradiol is a neurosteroid both its site of action and endpoints are more directly relevant to cognition and emotionality (Fig. 2).
Figure 2.
Estradiol is a gonadal steroid; estradiol is a neurosteroid. A, All steroids are synthesized via the progressive modification and reduction of the precursor cholesterol. Estradiol is the finished product of as little as 6 or 7 enzymatic reactions, with aromatization from testosterone being the penultimate conversion. B, Estradiol is a gonadal steroid because it is synthesized primarily in the adult ovary, but is also made in the neonatal male brain from testicularly derived androgen. C, Estradiol can also be a neurosteroid, meaning it is made in the brain without dependence on precursors from the periphery. All of the necessary steroidogenic enzymes have been identified in the brain and evidence suggests estradiol can be made in some regions de novo from cholesterol, thus fitting the definition of a classic neurosteroid.
Estradiol Is a Gonadal Steroid
In the rodent, the principal action of estradiol aromatized from testicular androgens during development is to masculinize the brain for the optimization of adult reproductive physiology and behavior. This end is achieved via multiple independent yet related mechanisms in distinct brain regions. The result is a coordinated whole but assures a high degree of individual phenotypic variability because each endpoint can vary independently.
Estradiol both Prevents and Initiates Naturally Occurring Cell Death in the Developing Brain
Among the first sex differences discovered in the brain was the relative size of a particular subregion or nucleus. The initial discovery that song control nuclei in male canaries were much larger than the corresponding region in female canaries was followed up with reports of similar but smaller magnitude sex differences in the mammalian brain and spinal cord (see for review Morris and others 2004). There are several ways in which a brain region can become larger in one sex. These include differences in the amount of cell birth, death, density, migration, and/or differentiation. All of these parameters have been investigated to varying degrees, but in the clearest examples of volumetric sex differences the principal mechanism is differential cell death. Males and females generate the same number of neurons but in response to estradiol (or androgen) action the cells are either protected from a natural course of apoptosis, as in the sexually dimorphic nucleus of the pre-optic area (SDN-POA), or estradiol actually induces cell death, as in the anteroperiventricular nucleus (AVPV). Both these nuclei are centrally involved in reproduction and respond to estradiol derived from testicularly synthesized androgens during the natural course of brain sexual differentiation (for review see Simerly 2002). The principal nucleus of the bed nucleus of the stria terminalis (pBNST) also integrates stimuli relevant to reproductive behavior and is larger in males due to greater cell death in females. The cellular mechanisms of estradiol effects on cell death are only beginning to be identified but appear to involve classical Bax/Bcl-2 signaling. Bax is a pro-death gene of the Bcl-2 family that is a central regulator of apoptosis in neural development The elimination of Bax via null mutation in genetically modified mice eliminates the sex difference in both the AVPV and the pBNST by increasing the numbers of neurons in both nuclei compared with wild-type mice of the same sex (Forger and others 2004; Fig. 3). However, sex differences in specific neuronal phenotypes, such as vasopressin neurons in the pBNST and dopaminergic neurons of the AVPV, are not eliminated by Bax deletion, suggesting there are multiple mechanisms by which sex differences in these nuclei are established (for review see Forger 2006; Forger 2009; Forger and others 2004).
Figure 3.
Estradiol prevents cell death; estradiol promotes cell death. Naturally occurring cell death, or apoptosis, is an integral part of brain development. Differences in the amount of cell death contribute to sex differences in the size of particular brain regions or subnuclei. A, In laboratory rats, the sexually dimorphic nucleus of the preoptic area (SDN-POA) is 5 to 7 times larger in males than females because the neurons in this region die in females during a postnatal sensitive period. Treating newborn females with estradiol early in life prevents the cell death and results in a male-sized SDN for the remainder of the animal's life (reprinted from Todd and others 2005, with permission from Elsevier). In the anteroperiventricular (AVPV) nucleus, also found in the POA, females have more neurons than males because of estradiol-induced cell death in males. Cell death in the AVPV is mediated by the classic Bax signaling pathway (reprinted with permission from Forger and others, Copyright 2004, National Academy of Sciences, USA). B, The opposite effects of estradiol on apoptosis in the 2 brain regions are evident quantitatively in the number of dying cells detected during the first few days of postnatal life (redrawn from Arai and others 1996, with permission from Elsevier). The mechanisms by which estradiol has opposite effects on cell death in closely associated regions of the developing brain remain unknown but appear to involve the classic Bax/Blc2 caspase 3 signaling pathway.
Estradiol both Promotes and Prevents Synaptogenesis in the Developing Brain
The morphology of dendrites can be profoundly different in males and females, but the direction of the sex difference varies by brain region. Estradiol acting as a gonadal steroid during the perinatal sensitive period drives the sex difference in at least 3 of these brain regions, and of considerable interest is that the cellular mechanism by which estradiol acts is distinct in each region. The arcuate nucleus is a periventricular structure located at the midline of the ventral hypothalamus. It contains a variety of neuronal phenotypes, many of which are critical to the regulation of the anterior pituitary and its role in reproduction, stress, energy, and metabolism homeostasis. The density of dendritic spine synapses versus axosomatic synapses on arcuate neurons is markedly different in males and females, and the morphology of neighboring astrocytes is equally profoundly different (Mong and others 1999; Mong and McCarthy 2002). In the laboratory rat these sex differences are established within the first few postnatal days and maintained throughout life. During the perinatal sensitive period, estradiol increases levels of the amino acid transmitter GABA by up-regulating the rate-limiting enzyme glutamic acid decarboxylase (GAD 65/67). GABA acts on GABA-A receptors of astrocytes and induces process growth and branching, resulting in an increased stellate morphology (Mong and others 2002). The increased astrocyte complexity is inversely correlated with dendritic complexity such that males have fewer spine synapses per unit of dendrite than females (Fig. 4). Whether the astrocytes actively impair synapse formation by forming a physical barrier between pre- and postsynaptic partners, or whether there is an additional signaling pathway depressing synapse formation, is currently unknown.
Figure 4.
Estradiol promotes synaptogenesis; estradiol prevents synaptogenesis. An important component of estradiol-induced masculinization of the developing brain is establishment of synaptic patterns that will endure into adulthood and subserve the neural networks of hormonally regulated behavior and physiology. A, In the preoptic area and ventromedial hypothalamus, 2 brain regions critical to the control of sexual behavior, estradiol organizes the synaptic pattern by promoting the development and stabilization of dendritic spine synapses. The mechanism by which this is achieved is fundamentally different in the 2 regions, but involves glutamate and ionotropic glutamate receptors in both cases. B, The arcuate nucleus exerts regulatory control over the anterior pituitary gland and in this region estradiol acts to suppress the formation of dendritic spine synapses. Astrocytes in this region are also differentiated by estradiol, exhibiting a more complex and stellate morphology than those not exposed to estradiol during this sensitive period of development.
There is also a relationship between astrocyte morphology and neuronal morphology in the medial preoptic nucleus (mPOA), except in this brain region more complex astrocytes are positively correlated with dendritic complexity. Neurons in the male mPOA have significantly more dendritic spines per unit area than female mPOA neurons and astrocytes in the same region have longer processes that branch more frequently (Amateau and McCarthy 2002a, 2002b). This sex difference is also established by estradiol functioning as a gonadal steroid, but the cellular mechanism is entirely distinct from that in the arcuate nucleus. In the mPOA, estradiol induces an up-regulation of the prostaglandin synthetic enzymes, cycloxygenase 1 and 2 (COX 1/2), resulting in a 7-fold increase in PGE2 levels within the mPOA (Amateau and McCarthy 2004). PGE2 induces the formation of dendritic spines following binding to EP2 and EP4 receptors and activation of PKA (Wright and others 2008). Glutamate, released from either the astrocytes or the neurons, is an additional essential component of the signaling cascade, with activation of both the ionotropic AMPA/kainate and the metabotropic mGluR receptors required (Fig. 4). The anatomical changes induced by this cellular cascade dictate the expression of masculine sexual behavior in adulthood. The discovery that a prostaglandin, in particular PGE2, exerts a permanent organizational effect on synaptic patterning of the male brain was indeed a surprise and is one of the first demonstrations that this normally inflammatory mediator also mediates processes essential to phenotypic variability in the developing brain. COX 1 and 2 inhibitors are among the most widely prescribed and over-the-counter medications available. These findings illustrate the need to further understand prostaglandins in the context of brain development and highlight the benefits gained by the study of naturally occurring sex differences in the brain.
The ventromedial nucleus (VMN) of the hypothalamus is a third brain region essential to reproductive endpoints and that possesses sexually dimorphic neuronal morphology determined by gonadally derived androgens being aromatized to estradiol. Here we have a completely new mechanism that was again unexpected. As discussed briefly above, the ER is a transcription factor and in both the arcuate and mPOA nuclei the effects of estradiol are initiated by an increase in transcription of a target gene, GAD in the arcuate and COX 1 and 2 in the mPOA. In the VMN, however, the cellular processes establishing enduring sex differences in dendritic morphology begin with the rapid activation of PI3 kinase and increased glutamate release from presynaptic terminals. The estradiol-induced gluta-mate release does not require synthesis of new proteins and is initiated within as little as 1 h of hormone exposure. The released glutamate leads to activation of postsynaptic NMDA and AMPA receptors, which in turn leads to activation of MAP kinase, which leads to the growth and stabilization of excitatory dendritic spine synapses. The establishment of new dendritic spines does require the synthesis of new proteins, but this is independent of the activation of the ER (Schwarz and others 2008). Thus, the process of sexual differentiation of the synaptic pattern is not cell autologous, but instead requires cell-to-cell communication (Fig. 5). This same concept, that the effects of estradiol are not cell autologous but instead require cell-to-cell communication, also applies to the organizational changes observed in the arcuate and mPOA (Schwarz and McCarthy 2008). An important consequence of this mode of transmission of estradiol signal is the coordination of phenotypic change among groups of cells without need for an ER in each one. This means that instead of individual neurons differentiating in a needle-in-a-haystack scenario, there are instead coordinated changes across an entire region. The functional significance of this population mode of differentiation as opposed to individual cells differentiating is unclear, but may be important to the establishment of neural networks relevant to the control of complex motivated behaviors.
Figure 5.
Estradiol acts within neurons; estradiol acts between neurons. Heterogeneity in estrogen receptor (ER) expression within populations of neurons is a potential source of cellular specificity in hormonal influence. If only neurons expressing ER responded to estradiol with morphological changes, this would be cell autologous sexual differentiation. If estradiol acts within one cell to transmit signals to other cells and thereby organize their morphology, this would indicate heterologous sexual differentiation. Several lines of evidence support the latter, with estradiol initiating signaling cascades in one cell that secondarily influence morphology of neighboring cells. A, In some instances, cell-to-cell communication involves neuron-to-neuron communication. In the ventromedial nucleus, estradiol activates ER, presumably at the membrane, which activates PI3 kinase and promotes release of glutamate. Glutamate then activates postsynaptic NMDA and AMPA receptors, causing calcium influx, activation of MAP kinase, production of spinophilin, and construction of new dendritic spines. There is no requirement for ER in the postsynaptic cell (Schwarz and others 2008). B, In the arcuate nucleus, estradiol activates nuclear estrogen receptors in neurons, resulting in increased expression of GAD, the rate-limiting enzyme in GABA synthesis. GABA is released from the neuron and acts on neighboring astrocytes, which cannot make GABA and do not appear to have estrogen receptors, resulting in increased astrocyte stellation. The increased complexity of the astrocytes is inversely correlated with the density of dendritic spines on neurons in the region (Mong and others 2002).
The VMN of the hypothalamus is also the site of another unique mechanism of estradiol-induced differentiation. In all of the mechanisms previously discussed, activation of ER leads to an increase in gene transcription and/or kinase activation, but there is one instance where estradiol decreases important proteins. Focal adhesion kinase (FAK) and the associated protein, paxillin, were first identified for their role in cell metastasis but are now recognized as negative regulators of neurite growth and branching (Rico and others 2004). During the perinatal sensitive period for sexual differentiation, estradiol down-regulates both FAK and paxillin and this appears to be an important component of the increase in dendritic branching observed in response to estradiol in this brain region (Speert and others 2007). How the decrease in FAK and paxillin levels is achieved is unknown, as is whether the decrease is related to the same mechanisms inducing formation of dendritic spines. Coordinating these 2 processes, dendritic growth and spine formation in response to estradiol, is an important future goal.
Each of these distinct actions of estradiol reviewed here, the induction of GAD, COX 1 and 2, Bax, glutamate release, and down-regulation of FAK and paxillin, subserve the goal of sexual differentiation of the brain, in particular the establishment of the male phenotype. Administering estradiol to neonatal females mimics the naturally occurring process in males in which testicularly derived androgens are locally aromatized to estradiol within the hypothalamus or preoptic area, and thus estradiol is acting as gonadal steroid and altering reproductive function. Recent years have revealed another source of estradiol, the brain itself acting as an endocrine organ that synthesizes steroids de novo from cholesterol. In these instances, the steroids are called “neurosteroids” in recognition of both the site of synthesis and target organ.
Estradiol Is a Neurosteroid
The synthesis of steroids is not unlike that of peptides in that it begins with a large precursor molecule, in this case cholesterol, which is progressively trimmed and modified to produce a series of biologically active compounds. This is achieved via a progressive series of enzymatic reactions, and estradiol is essentially the last stop on the synthetic pathway before further modifications become part of the degradative pathway (although interest is growing in the potential biological activity of the so-called metabolites). Unlike peptides, steroids are produced on demand and are never stored. The principle regulatory site in estradiol synthesis is the aromatase enzyme, the activity of which is regulated by calcium and possibly afferent input. The notion that steroidogenesis can be induced in a tightly controlled temporal and physical domain raises steroids to a level above long-distance blood-borne messengers and suggests similarities to other rapidly acting neuromodulators, including neurotransmitters (Balaban 1996; Balthazart and Ball 1998; Balthazart and Ball 2006). We are only at the beginning stages of elucidating the function of locally synthesized estradiol. A role in synaptic plasticity is apparent in the adult brain (Balthazart and others 2006; Rune and others 2002), but has not been demonstrated in the developing brain. There is, however, evidence that estradiol is synthesized de novo in the developing brain (Nunez and McCarthy 2008).
Estradiol Is Neuroprotective and Neurodamaging in the Developing Brain
The potential for estradiol as a therapeutic neuroprotective agent in the aging brain has been of intense interest for over a decade. The relative benefits versus costs of hormone replacement therapy (HRT) is controversial. What appeared to be an iron clad conclusion of beneficial effects of estradiol based on retrospective population-based studies was completely reversed by the prospective Women's Health Initiative (WHI), a double-blind placebo-controlled study that was terminated early because of detrimental effects of HRT on cardiovascular outcomes, including stroke. The finding of negative effects of HRT was in marked contrast to a large body of basic science research on animal models (Wise 2003), and as a result the pendulum has since swung back closer to center with the revelation that some aspects of the WHI study design were flawed and that the treatments used were complex mixtures of conjugated estrogens and medroxyacetate progestin, not estradiol and progesterone, which are used in animal studies. Thus the jury remains out on the relative merits of HRT as protective against neurodegeneration; and although research on this topic has slowed considerably, it remains ongoing.
In comparison with the aging brain, the perinatal period is an additional life phase that is characterized by a high risk for brain injury because of fetal hypoxia/ischemia subsequent to stroke, preeclampsia, placental compromise, or birth trauma. This period of life is also characterized by extraordinarily high steroid levels both of maternal origin and produced by the fetal gonads and adrenals. In humans, the major period of fetal gonadal steroid production is during the second and third trimester and extending briefly into postnatal life. In rats, the fetal testis is active during the last 4 to 5 days of gestation and briefly after birth. The 1-week-old rat is considered roughly representative of the newborn human, making the newborn rat more analogous to a prenatal or premature human. The relative role of maternal/placental derived steroids and those made directly by the fetus remain poorly understood, but it is undeniable that a premature infant is deprived of steroids relative to the term infant. Thus understanding whether steroids benefit or exacerbate damage following insult is vitally important. Moreover, gender differences in outcomes following injury remain unexplained and gonadal steroids may be an important piece of the puzzle (Fig. 6).
Figure 6.
Estradiol is neuroprotective; estradiol is neurodamaging. The developing brain is subject to injury following hypoxia/ischemia that may occur because of maternal pre-eclampsia, prematurity, stroke, or traumatic birth. There are multiple models of perinatal brain damage, and estradiol has opposing effects depending on the source of the damage. A, In a model involving glutamate-induced excitoxicity, estradiol is potently neuroprotective, preventing cell death in part by reducing mGluR receptors and depressing the release of calcium from intracellular stores. The number of TUNEL-positive cells is significantly reduced by estradiol alone and the increased death induced by glutamate is prevented by pretreatment with estradiol (graph redrawn and image reprinted from Hilton and others 2006, European Journal of Neuroscience, with permission from Wiley-Blackwell.). B, Early in development GABA is a major source of excitation via membrane depolarization and influx of calcium via voltage-gated calcium channels. In a model involving GABA-induced excitoxicity, estradiol exacerbates the damage by increasing the number of dying cells over muscimol (a GABA-A agonist), resulting in reduced hippocampal volume and impaired learning and memory (graph redrawn and image reprinted from Nunez and McCarthy, 2003, Endocrinology, with permission from The Endocrine Society.).
Estradiol Enhances Depolarizing GABA Actions and Is Neurodamaging
Understanding how estradiol influences pediatric brain damage must incorporate the unique parameters of the immature brain, and this begins with an appreciation for the profoundly different role of GABA. In the adult, GABA is the predominant inhibitory neurotransmitter that dampens excitability by combined hyperpolarization and shunting inhibition. In the neonate, however, GABA is the predominant source of excitation via membrane depolarization sufficient to open voltage-gated calcium channels and periodically induce action potentials. These diametrically opposed effects of GABA are mediated by the GABA-A receptor, a chloride ionophore, and are achieved by regulation of the transmembrane chloride gradient. In mature neurons, intracellular chloride levels are low and the driving force for ion movement is inward. In immature neurons, the opposite is true, intracellular chloride levels are high and ion fluxes out of the cell upon receptor opening, thereby depolarizing the membrane. The difference in gradient is a function of electrically neutral energy-dependent cotransporters that exchange chloride for sodium and potassium. The transporter that moves chloride into neurons, NKCC1, is expressed at high levels in immature neurons and is gradually replaced by KCC2, which transports chloride out of cells as maturation progresses (Rivera and others 1999; Staley 1996; Figure 7).
Figure 7.
Estradiol enhances excitation; estradiol dampens excitation. The neuroprotective versus neurodamaging effects of estradiol can both be traced to the impact of the steroid on intracellular free calcium. A, Pretreatment with estradiol reduces the percentage of glutamate-induced calcium in cultured hippocampal neurons by ~30%. A pseudo-colored image shows the relative amount of calcium after glutamate in neurons treated with vehicle (top) versus neurons treated with physiological levels of estradiol for 24 to 48 h prior (reprinted with permission from Hilton and others 2006). B, Activation of GABA-A receptors in immature hippocampal neurons also invokes calcium influx and prior treatment of neurons with the same estradiol regimen markedly enhances the amount of calcium as well as the number of neurons that respond to GABA with membrane depolarization. The top panel shows a representative trace (blue bar equals muscimol application, a GABA-A agonist) and the bottom panel is pseudo-colored images showing calcium responses across time (reprinted from Nunez and others 2005, European Journal of Neuroscience, with permission from Wiley-Blackwell.).
The most salient consequence of depolarizing GABA responses is the influx of calcium via L-type voltage-gated calcium channel. The cellular signaling cascades initiated enhance cell survival, differentiation, and synaptic integration (Ganguly and others 2001). Estradiol markedly enhances depolarizing GABA responses by amplifying the magnitude of individual calcium transients produced by GABA-A receptor activation, increasing the number of neurons in a given population that respond to GABA with depolarization and extending the developmental time period during which GABA responses are depolarizing (Nunez and others 2008; Perrot-Sinal and others, 2001). Thus, GABA is an important trophic factor in the developing brain and estradiol is capable of enhancing those cellular responses considered central to a trophic effect (Perrot-Sinal and others 2003). However, GABA is also released extracellularly following hypoxia/ischemia in the immature brain, and excessive calcium influx is excitoxic. Thus, GABA is also centrally positioned to induce cell death following insult to the developing brain, and estradiol would be predicted to make that damage worse. We have found the latter to be the case, with exogenous administration of a GABA-A receptor agonist combined with prior exposure to estradiol resulting in enhanced cell death in the immature hippocampus. Damage is prevented by blocking the L-type calcium channels, confirming the source of cell death as excitoxicity due to excessive free intracellular calcium (Nunez and McCarthy 2003). Thus, in a model in which excessive calcium is induced via the GABA-A receptor, estradiol enhances neuronal vulnerability and increases the number of cells lost. But what about glutamate? Does estradiol also impact on glutamate-mediated excitoxicity?
Estradiol Dampens Glutamate-Mediated Excitation and Is Neuroprotective
The majority of research on pediatric brain damage that uses the rodent model focuses on pups that are at least 4, and more commonly 7, days old. This is in large part because prior to that time the classic NMDA and AMPA glutamate receptors are not fully functional, or at least do not function the same as in the adult, and therefore do not mediate excitoxicity when stimulated. We recently discovered that there is still glutamate-mediated excitoxicity in very young neurons, but interestingly the critical receptor is the mGluR receptor (type I) and the source of the calcium is intracellular stores of the endoplasmic reticulum. Estradiol completely prevents glutamate-induced cell death in neonatal hippocampal neurons by down-regulating mGluR1 and mGluR5 and decreasing the amount of calcium released from the endoplasmic reticulum (Hilton and others 2006).
Specific effects of estradiol on either glutamate or GABA-mediated signaling is of interest, but the critical question is how these divergent effects are integrated into a coherent whole. Rodent models of pediatric brain damage are difficult, with no one best approach generally agreed upon. The Rice-Vannuci model involves a combination of carotid artery ligation and hypoxia in 1-week-old rat pups to produce substantial damage to the cortex and hippocampus. Pretreatment with estradiol provides considerable protection in this model (Nunez and others 2007). How estradiol provides protection in this model is unknown, but it nonetheless suggests that further attention should be given to the potential therapeutic benefits of estradiol in pediatric brain damage.
Estradiol Promotes Cell Genesis in the Developing Hippocampus
When considering the difference between male and female brains, most attention is given to whether a particular region is larger in one sex over the other. The most celebrated and probably the most intensely investigated sex difference in the brain is the sexually dimorphic nucleus (SDN) of the preoptic area (POA), named for the fact that it is 5 to 7 times larger in males. There are multiple ways in which a brain region can become larger in one sex, including sex difference in cell birth, death, migration, density, and differentiation. In the case of the SDN and several other well-characterized volumetric sex differences in the rodent brain and spinal cord, it has been definitively determined that cell death is the primary, if not only variable underlying the difference. Thus there was no evidence for sex differences in cell genesis, that is, until the recent discovery that there are significantly more new cells born in newborn male hippocampus compared with female. In an attempt to understand the source of this sex difference, females were treated with androgens or estrogens and both steroid hormones increased the number of new cells born to that of males (Zhang and others 2008). More new cells does not necessarily mean more new neurons; nor does it mean the new cells will survive in the long term. Determining the final fate of these cells is ongoing, but preliminary results reveal an estradiol-induced increase in astrocytes, a cell type now known to play a critical role in the development and maintenance of synapses. Additional important information yet to be determined is whether estradiol is the factor mediating the observed sex difference in cell genesis, and whether that estradiol is derived via aromatization of testicular androgens or whether the estradiol is synthesized as a neurosteroid locally within the hippocampus to stimulate cell genesis. Finally, whether estradiol genuinely stimulates cell proliferation, enhances the survival of new cells, or both, remains unclear. Regardless, these findings further reveal the tantalizing therapeutic potential of estradiol in the developing brain.
Summary and Conclusions
Estradiol is a naturally occurring multifaceted signaling molecule of tremendous potency. Despite decades of intense scrutiny, we continue to discover surprising and unexpected sources and mechanisms of estradiol action. Understanding when, where, and how estradiol acts is fundamental on several levels, including our ability to exploit the beneficial effects of this hormone and to recognize the potential deleterious effects of estrogen mimetics in the environment. The observation that estradiol exerts unique and often opposing effects in different brain regions at different times in development, in males versus females and in a healthy versus damaged brain, substantially increases the challenge in fully comprehending estrogen actions. Future research will require an integrated view that incorporates the myriad of possible cellular effects with the state of the organism in which the effects are occurring. A comprehensive view will enhance our ability to generate designer estrogens that will activate only those responses desired in specific regions and at specific times so that we can fully harness the therapeutic potential of this amazing molecule.
Financial Disclosure/Funding
The author disclosed receipt of the following financial support for the research and/or authorship of this article: research presented in this review was supported by grants RO1MH52716 and R01NS050525.
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no potential conflicts of interests with respect to their authorship or the publication of this article.
References
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–17. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostglandin-E2. J. Neurosci. 2002a;22:8586–96. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. Sexual differentiation of astrocyte morphology in the developing rat preoptic area. J Neuroendocrinol. 2002b;14:904–10. doi: 10.1046/j.1365-2826.2002.00858.x. [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. Induction of PGE(2) by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–50. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- Arai Y, Sekine Y, Murakami S. Estrogen and apoptosis in the developing sexually dimorphic preoptic area in female rats. Neurosci Res. 1996;25:403–7. doi: 10.1016/0168-0102(96)01070-x. [DOI] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9:220–6. doi: 10.1038/nn1624. and others. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. New insights into the regulation and function of brain estrogen synthase (aromatase) Trends Neurosci. 1998;21:243–9. doi: 10.1016/s0166-2236(97)01221-6. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–9. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Cornil CA, Taziaux M, Charlier TD, Baillien M, Ball GF. Rapid changes in production and behavioral action of estrogens. Neuroscience. 2006;138:783–91. doi: 10.1016/j.neuroscience.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Beato M, Klug J. Steroid hormone receptors: an update. Hum Reprod Update. 2000;6:225–36. doi: 10.1093/humupd/6.3.225. [DOI] [PubMed] [Google Scholar]
- Dean SL, McCarthy MM. Steroids, sex and the cerebellar cortex: implications for human disease. Cerebellum. 2008;7:38–47. doi: 10.1007/s12311-008-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138:929–38. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Forger NG. Control of cell number in the sexually dimorphic brain and spinal cord. J Neuroendocrinol. 2009;21:393–9. doi: 10.1111/j.1365-2826.2009.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, De Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci U S A. 2004;101:13666–71. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–32. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Veiga S, Sierra A, Melcangi RC, Inigo A. Aromatase: a neuroprotective enzyme. Prog Neurobiol. 2003;71:31–41. doi: 10.1016/j.pneurobio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Hilton GD, Nunez JL, Bambrick L, Thompson SM, McCarthy MM. Glutamate-mediated excitotoxicity in neonatal hippocampal neurons is mediated by mGluR-induced release of Ca++ from intracellular stores and is prevented by estradiol. Eur J Neurosci. 2006;24:3008–16. doi: 10.1111/j.1460-9568.2006.05189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–70. doi: 10.1073/pnas.2630225100. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J, Hariri OR, Bondar G, Ogi J, Micevych P. Membrane estrogen receptor-alpha interacts with metabotropic glutamate receptor type 1a to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology. 2009;150:1369–76. doi: 10.1210/en.2008-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Konkle AT. When is a sex difference not a sex difference? Front Neuroendocrinol. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:1464–72. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, McCarthy MM. Ontogeny of sexually dimorphic astrocytes in the neonatal rat arcuate. Dev Brain Res. 2002;139:151–8. doi: 10.1016/s0165-3806(02)00541-2. [DOI] [PubMed] [Google Scholar]
- Mong JA, Nunez JL, McCarthy MM. GABA mediates steroid-induced astrocyte differentiation in the neonatal rat hypothalamus. J Neuroendocrinol. 2002;14:1–16. doi: 10.1046/j.1365-2826.2002.00737.x. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–9. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Nunez J, Yang Z, Jian Y, Grandys T, Mark I, Levison SW. 17Beta-estradiol protects the neontal brain from hypoxia-ischemia. Exp Neurol. 2007;208:269–76. doi: 10.1016/j.expneurol.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, Aberdeen GW, Albrecht ED, McCarthy MM. Impact of estradiol on GABA- and glutamate-mediated calcium responses of fetal baboon (papio anubis) hippocampal and cortical neurons. Endocrinology. 2008;149:6433–43. doi: 10.1210/en.2007-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, Bambrick LL, Krueger BK, McCarthy MM. Prolongation and enhancement of gamma-aminobutyric acid receptor mediated excitation by chronic treatment with estradiol in developing rat hippocampal neurons. Eur J Neurosci. 2005;21:3251–61. doi: 10.1111/j.1460-9568.2005.04175.x. [DOI] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Estradiol exacerbates hippocampal damage in a model of preterm brain injury. Endocrinology. 2003;144:2350–9. doi: 10.1210/en.2002-220840. [DOI] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Resting intracellular calcium concentration, depolarizing GABA and possible role of local estradiol synthesis in the developing male and female hippocampus. Neuroscience. 2008;158:623–34. doi: 10.1016/j.neuroscience.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Auger AP, McCarthy MM. Excitatory GABA-induced pCREB in developing brain is mediated by L-type Ca+2 channels and dependent on age, sex and brain region. Neuroscience. 2003;116:995–1003. doi: 10.1016/s0306-4522(02)00794-7. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Davis AM, Gregerson KA, Kao JPY, McCarthy MM. Estradiol enhances excitatory gamma-aminobutyric acid-mediated calcium signaling in neonatal hypothalamic neurons. Endocrinology. 2001;143:2238–43. doi: 10.1210/endo.142.6.8180. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151:121–58. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Wehrenberg U, Jarry H, Rune GM. Para/autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus. 2003;13:226–34. doi: 10.1002/hipo.10075. [DOI] [PubMed] [Google Scholar]
- Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF. Control of axonal branching and synapse formation by focal adhesion kinase. Nat Neurosci. 2004;7:1059–69. doi: 10.1038/nn1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation [see comments] Nature. 1999;397:251–5. doi: 10.1038/16697. and others. [DOI] [PubMed] [Google Scholar]
- Rune GM, Wehrenberg U, Prange-Kiel J, Zhou L, Adelmann G, Frotscher M. Estrogen up-regulates estrogen receptor alpha and synaptophysin in slice cultures of rat hippocampus. Neuroscience. 2002;113:167–75. doi: 10.1016/s0306-4522(02)00152-5. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Liang S-L, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58:584–98. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, McCarthy MM. Steroid-induced sexual differentiation of the brain: multiple pathways, one goal. J Neurochem. 2008;105:1561–72. doi: 10.1111/j.1471-4159.2008.05384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–36. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Speert DB, Konkle AT, Zup SL, Schwarz JM, Shiroor C, Taylor ME. Focal adhesion kinase and paxillin: novel regulators of brain sexual differentiation? Endocrinology. 2007;148:3391–401. doi: 10.1210/en.2006-0845. and others. [DOI] [PubMed] [Google Scholar]
- Staley K, Smith R, Schaack J, Wilcox C, Jentsch TJ. Alteration of GABA-A receptor function following gene transfer of the CLC-2 chloride channel. Neuron. 1996;17:543–51. doi: 10.1016/s0896-6273(00)80186-5. [DOI] [PubMed] [Google Scholar]
- Todd BJ, Schwarz JM, McCarthy MM. Prostaglandin-E2: a point of divergence in estradiol-mediated sexual differentiation. Horm Behav. 2005;48:512–21. doi: 10.1016/j.yhbeh.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Minireview: a plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–74. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM. Estrogens: protective or risk factors in brain function? Prog Neurobiol. 2003;69:181–91. doi: 10.1016/s0301-0082(03)00035-2. [DOI] [PubMed] [Google Scholar]
- Wright CL, Burks SR, McCarthy MM. Identification of prostaglandin E2 receptors mediating perinatal masculinization of adult sex behavior and neuroanatomical correlates. Dev Neurobiol. 2008;68:1406–19. doi: 10.1002/dneu.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-M, Konkle ATM, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]