Abstract
Testicular germ cell tumors (TGCTs) are the most common solid tumors of 15–35 year old men. TGCT patients are frequently cured with cytotoxic cisplatin-based therapy. However, TGCT patients refractory to cisplatin-based chemotherapy have a poor prognosis, as do those having a late relapse. Pluripotent embryonal carcinomas (ECs) are the malignant counterparts to embryonic stem (ES) cells and are considered the stem cells of TGCTs. Here we demonstrate that human EC cells are highly sensitive to 5-aza-deoxycytidine (5-aza-CdR) as compared to somatic solid tumor cells. Decreased proliferation and survival with low nanomolar concentrations of 5-aza-CdR is associated with ATM activation, H2AX phosphorylation, increased expression of p21, and the induction of genes known to be methylated in TGCTs (MGMT, RASSF1A and HOXA9). Notably, 5-aza-CdR hypersensitivity is associated with markedly abundant expression of the pluripotency-associated DNA methyltransferase 3B (DNMT3B) as compared to somatic tumor cells. Knockdown of DNMT3B in EC cells results in substantial resistance to 5-aza-CdR, strongly indicating that 5-aza-CdR sensitivity is mechanistically linked to high levels of DNMT3B. Intriguingly, cisplatin-resistant EC cells retain an exquisite sensitivity to low dose 5-aza-CdR treatment and pretreatment of 5-aza-CdR re-sensitizes these cells to cisplatin-mediated toxicity. This re-sensitization is also partially dependent on high DNMT3B levels. These novel findings indicate that high expression of DNMT3B, a likely byproduct of their pluripotency and germ cell origin, sensitizes TGCT-derived EC cells to low dose 5-aza-CdR treatment.
Keywords: DNMT3B, 5-aza-deoxycytidine, embryonal carcinoma, cisplatin, resistance, testicular cancer
Introduction
Testicular germ cell tumors (TGCTs), the most common solid tumors of adolescent and young men, are thought to derive from transformation of primordial germ cells (PGCs) or early gonocytes (1, 2). TGCTs are classified as seminomas and nonseminomas (1). Within nonseminomas are undifferentiated, pluripotent cells, known as embryonal carcinoma (EC). ECs are proposed to represent the stem cells of TGCTs and to be the malignant counterparts to embryonic stem (ES) cells (1, 2). EC cells can differentiate in vivo as mature teratomas towards extra-embryonic tissues and embryonic tissues (1, 2).
Patients with TGCTs, even those with advanced metastatic disease, are often successfully treated with cisplatin-based chemotherapeutic regimens (3, 4). However, 15–20% of patients are refractory to this treatment and succumb to progressive disease (5). Some TGCT patients, who initially respond to treatment can exhibit a late relapse and have a poor prognosis (4, 5). Testicular cancer survivors have increased incidence of infertility, cardiovascular disease and secondary malignancies (6).
Reasons for the high curability of TGCTs have been elusive. Mouse models of testicular cancer do exist, but they do no recapitulate key features of this human malignancy (1). Mechanisms of inherent or acquired cisplatin resistance in other tumors have not yet provided insights into the exquisite cisplatin-sensitivity of TGCTs (4). That patients with advanced stage TGCTs can be cured implies that the stem cells of TGCTs are effectively targeted with cisplatin-based chemotherapy (1, 4).
To date, DNA methylation inhibitors have been more active in leukemia than in solid tumor cells (7). There is currently little information available on the effects of DNA methylation inhibitors against TGCT cells. In the current study, we establish that TGCT cells are hypersensitive to the DNA methylation inhibitor 5-aza-CdR. This response is associated with remarkably high levels of DNA methyltransferase 3B (DNMT3B). Notably, high DNMT3B expression is validated as functionally important for 5-aza-CdR-mediated hypersensitivity in both cisplatin sensitive and resistant TGCT cells. Indeed, 5-aza-CdR can re-sensitize cisplatin resistant cells to cisplatin-mediated toxicity. Together, these findings indicate that high basal DNMT3B expression in pluripotent EC cells can account for 5-aza-CdR hypersensitivity in cisplatin sensitive and resistant TGCTs.
Materials and methods
Cell culture and drug treatments
All cell lines were cultured in DMEM media with 10% fetal bovine serum (FBS) supplemented with glutamine and antibiotics with the exception of MCF7 cells that were cultured in F12-DMEM. The derivation of the NT2/D1-resistant NT2/D1-R1 cell line was previously described (8, 9). With the exception of colony forming assays, cells were treated with the indicated dosages of 5-aza-CdR for 3 d and drug was replenished each day. For colony forming assay, 3 × 103 cells were plated per well in 24 well plates. The next day, cells were treated with the indicated doses of 5-aza-CdR daily for 3 days and then treated with the indicated doses of 5 aza-CdR every other day for an additional 7 days. Cells were then fixed and stained with Geimsa stain. Cisplatin (Bristol Laboratories) treatments were performed at the concentrations and time points indicated. To assess cell proliferation and survival, Cell-Titre Glo (Promega) assays were performed.
Real-time PCR and Immunoblot analyses
Reverse transcription (RT) was performed on 1 µg RNA using the Taqman RT kit (Applied Biosystems). Twenty ng of the resulting cDNA was used with SYBR green (Applied Biosystems) for quantitative real-time PCR assays utilizing the ddCT method normalized to GAPDH and the ABI Prism Sequence Detection System 7700. Primers are provided in Supplemental Table 1. For Western analyses, cells were lysed in a radioimmune precipitation buffer and separated by SDS-PAGE, as previously described (8, 9). Antibodies to DNMT3B (H-230; sc-20704, Santa Cruz, and Ab2851, Abcam), actin (C-11; sc01615, Santa Cruz), 1981-ATM (Epitomics) and 139-H2AX (Cell Signaling) were employed.
Lentiviral production
Silencing shRNAs targeting human DNMT3B were purchased (Open Biosystems). A pLKO.1-shRNA lentiviral construct was also purchased (pLKO.1, Sigma) and used as a control. Lentiviral stocks were generated from 293T cells and psPAX2 and pMD2G packaging and envelop vectors using standard protocols. Viral stocks were independently added to NT2/D1 and NT2/D1-R1 cells plated at 0.2 × 106 cells/well per 6 well plates. The lentiviral stock was cultured with each of these cells for 24 hours. Cells were then harvested and placed onto 10 cm plates in selection media containing 1.0 µg/ml puromycin. Selection continued for 48 hours, after which no viable cells remained in the mock-transduced control plates. The puromycin-resistant cell pools were passaged for at least 10 days prior to use in these experiments.
Statistics
Where a value for statistical significance is indicated, a two sample two-tailed t-test assuming unequal variances was performed.
Results
Embryonal carcinoma sensitivity to low dose 5-aza-CdR
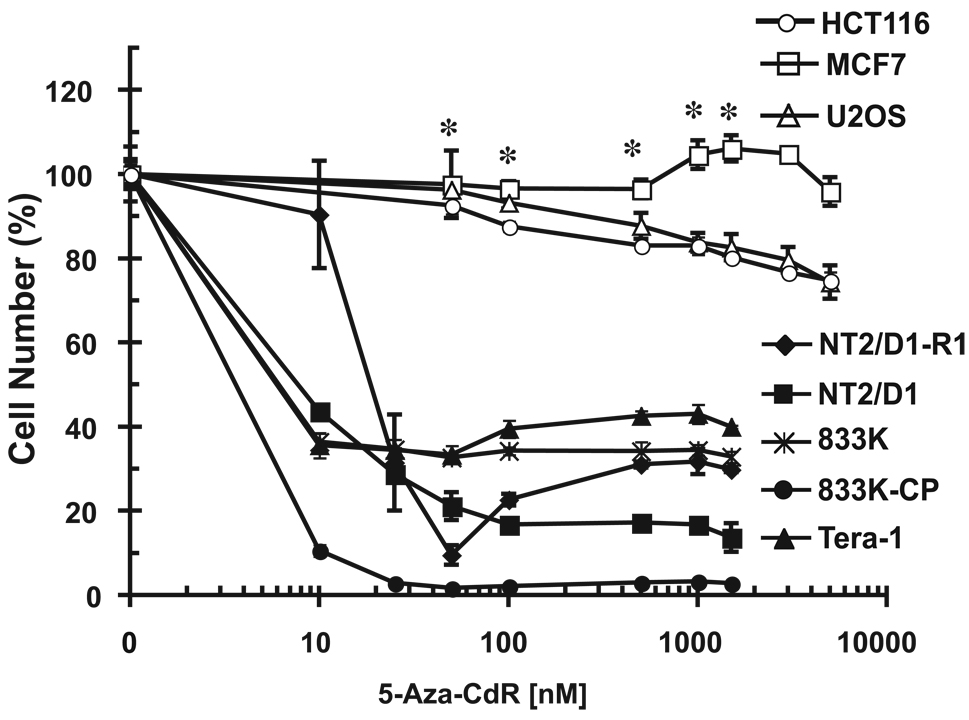
We sought to identify whether different EC cell lines were sensitive to DNA methylation inhibition. Five different EC cell lines, including two that were cisplatin-resistant, NT2/D1-R1 and 833K-CP, as compared to parental NT2/D1 and 833K lines, were highly sensitive to inhibition of cell growth and viability with the DNA methylation inhibitor 5-aza-CdR with IC-50s in the 5–25 nM range (Figure 1). These doses are substantially lower than those typically reported for diverse solid somatic tumors that have IC-50s in the 500 nM to 10 µM range (7, 10). This is in agreement with our somatic tumor cell data as human breast (MCF7), osteosarcoma (U2OS), and colon (HCT116) cancer cells were relatively resistant to 5-aza-CdR treatment at doses as high as 5 µM (Figure 1). Interestingly, the line most sensitive to 5-aza-CdR was the cisplatin resistant line, 833K-CP (also called 833K64-CP10).
Figure 1.
EC cell lines are sensitive to low dose 5-aza-CdR. Indicated doses of 5-aza-CdR were added fresh each day for three days to exponentially growing cultures. Viable cell growth and survival were measured. Data were normalized to no drug treatment. EC cells are NT2/D1, NT2D1/R1, 833K, 833K-CP, and Tera-1. Data are the average of 3 biological replicates. Each line was assayed in at least two independent experiments with similar results. Error bars are S.D. * p < .002 for U2OS, MCF7 and HCT116 versus NT2/D1 cells.
EC cells overexpress DNMT3B compared to somatic cancer cells
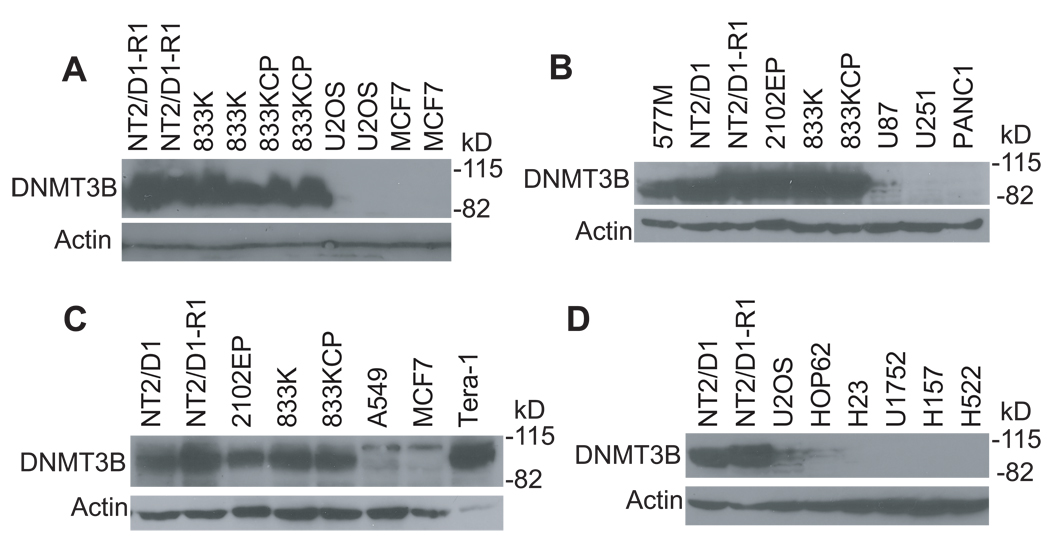
Recent microarray studies indicated that ES and EC cells as well as clinical EC and nonseminomas highly express mRNA for the DNA methyltransferase, DNMT3B, as compared to normal and somatic tumors (11–13). However, this differential expression has not been confirmed or shown at the protein level. We found a striking difference in DNMT3B protein expression in the EC cell lines NT2/D1, NT2/D1-R1, 833K, 833K-CP, Tera-1, 577M and 2102EP as compared to U2OS, MCF7, and lung, pancreatic and glioblastoma cancer cell lines (Figure 2). HCT116 cells also did not express appreciable DNMT3B as compared to EC cells (data not shown). Notably, the high expression of DNMT3B in EC cells could be detected with two distinct DNMT3B antibodies (Figure 2). Densitometry measurements revealed at least a 30-fold increase in DNMT3B expression in the EC cells as compared to somatic tumor cells. Thus, the hypersensitivity of TGCTs to low dose 5-aza-CdR is tightly associated with high expression of DNMT3B in EC cells. It should be noted that there is not an exact correlation between the EC cell lines in regards to the level of DNMT3B and sensitivity to 5-aza-CdR. This is most evident in the NT2/D1-R1 line that is less sensitive to 10 nM 5-aza-CdR as compared to other EC cells yet is one of the highest expressers of DNMT3B. Thus, there appears to be other cell line specific factors, other than DNMT3B, that affect the relative sensitivity of the EC cells to 5-aza-CdR.
Figure 2.
EC cell lines express DNMT3B much more highly than do somatic tumor cell lines. Western analyses for DNMT3B expression in various EC and somatic cancer cell lines. EC cells are NT2/D1, NT2/D1-R1, 833K, 833K-CP, Tera-1, 577M and 2102EP. H23, HOP62, U1752, A549, H522 and H157 are lung cancer cell lines. U87 and U251 are glioblastoma cell lines and PANC1 is a pancreatic cancer cell line. DNMT3B antibody H-230 was used in panel A while DNMT3B antibody ab2851 was used in panels B–D.
Low dose 5-aza-CdR induces H2AX and ATM phosphorylation and tumor suppressor gene expression
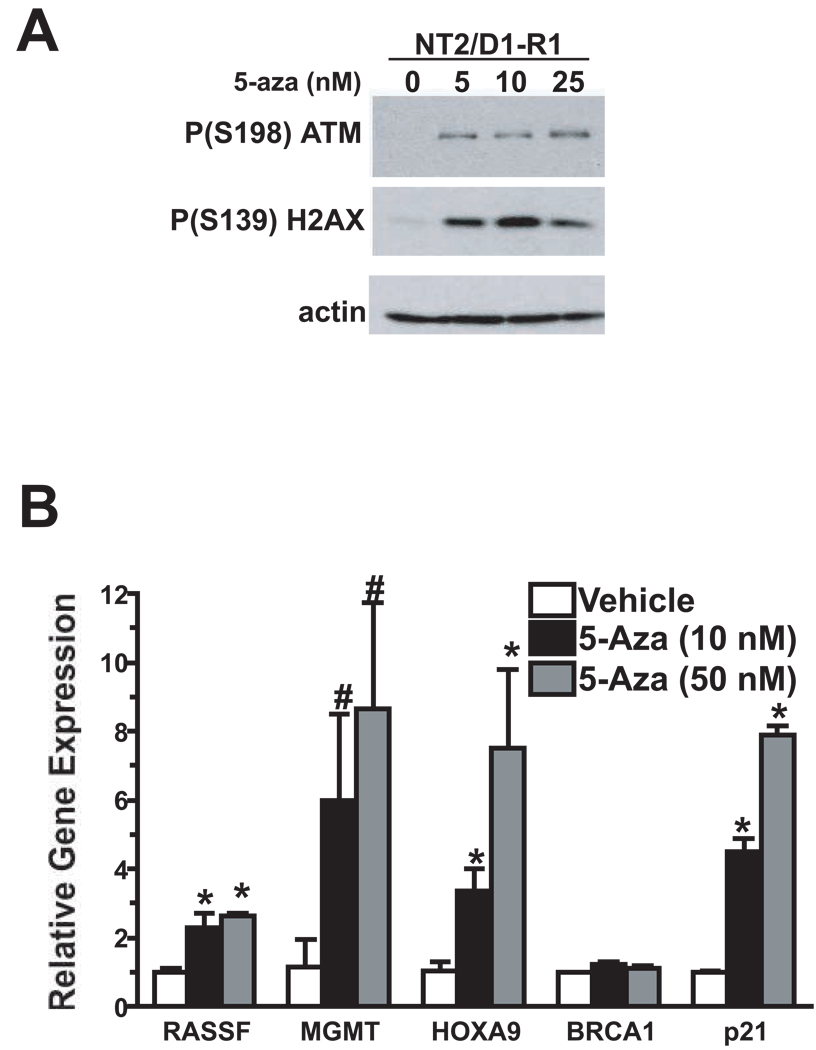
Two mechanisms have been proposed to account for the antitumor effects of 5-aza-CdR, namely, activation of the DNA damage response pathway and re-expression of tumor suppressor genes through DNA demethylation (14–18). We asked whether 5-aza-CdR engaged these mechanisms at the low doses affecting cell proliferation and survival of EC. As shown in Figure 3A, low dose 5-aza-CdR treatment of NT2/D1-R1 cells increased the levels of activated ATM and phosphorylated H2AX, two hallmarks of the DNA damage response previously shown to be induced with high dose 5-aza-CdR in somatic tumor cells (17–19).
Figure 3.
Low dose 5-aza-CdR activates the ATM pathway and induces the expression of methylated genes in EC cells. A) Indicated doses of 5-aza were added fresh each day for three days to NT2/D1-R1 cells. Expression profiles of P(S198)ATM and P(S139)H2AX were assessed. The experiment was repeated with similar results. B) NT2/D1-R1 cells were treated as in A above and expression of the indicated genes assessed by quantitative PCR assays. Average of biological triplicate determinations and error bars are S.D. * p<.01; # p<.05.
To address whether low dose 5-aza-CdR induced the expression of genes known to be methylated in TGCTs, expression of MGMT, RASSF1A, HOXA9, and BRCA1 (20–22), was assessed. Low dose 5-aza-CdR induced the expression of MGMT, RASSF1A, and HOXA9 in NT2/D1-R1 cells. In contrast expression of BRCA1 was not induced by low doses 5-aza-CdR. Low dose 5-aza-CdR was also able to induce the expression of p21. The p21 gene is not known to be commonly methylated in tumor cells but is commonly induced in response to DNA damage (23). Together the data suggest that low doses of 5-aza-CdR can elicit both a DNA damage response and the induction of genes known to be methylated in TGCTs.
Knockdown of DNMT3B in EC cells reverses 5-aza-CdR hypersensitivity
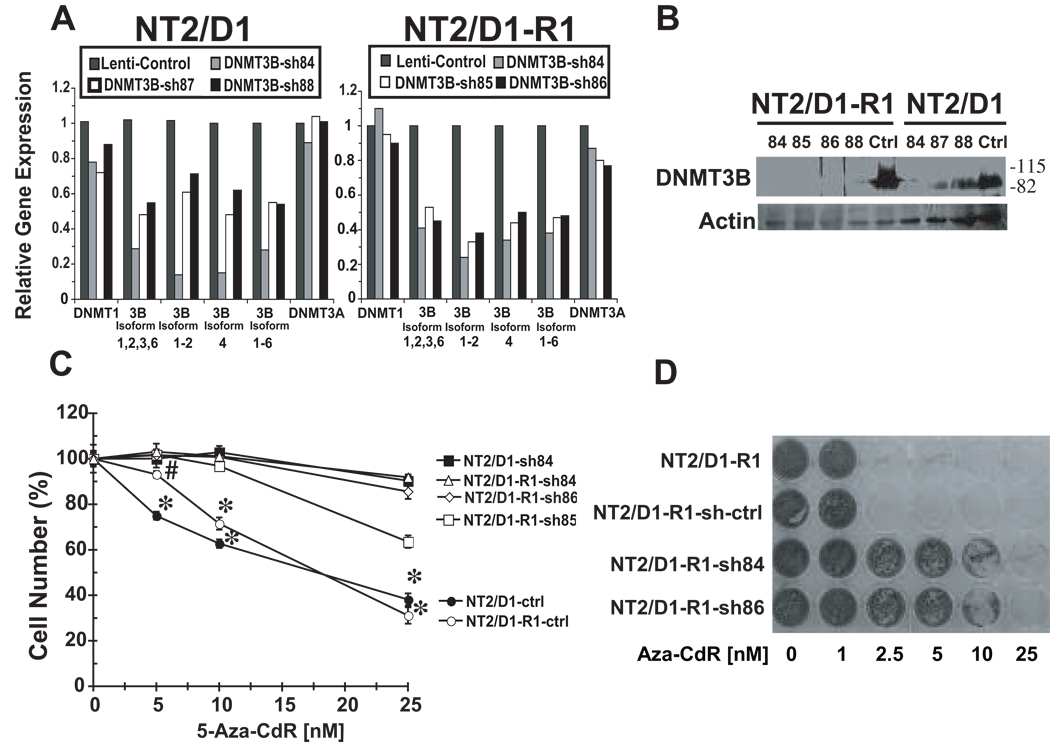
Five different lentiviral shRNAs for DNMT3B were independently used to knockdown DNMT3B. Six potential alternatively spliced isoforms of DNMT3B exist; the most biologically relevant isoforms are variants 1, 2, 3 and 6 (14). Quantitative RT-PCR assays employing isoform-specific primers revealed that these shRNAs (relative to controls) reduced expression of these DNMT3B isoforms (Figure 4A). Furthermore, none of the DNMT3B-specific shRNAs affected levels of DNMT1 or DNMT3A (Figure 4A). DNMT3B-targeting shRNAs also reduced DNMT3B protein in both NT2/D1 and NT2/D1-R1 cells (Figure 4B). Since NT2/D1 cells stably expressing sh84 and NT2/D1-R1 cells stably expressing sh84, sh85 or sh86 had the most efficient knockdown of DNMT3B (Figure 4A and 4B), these cells were tested for 5-aza-CdR sensitivity. Cells expressing DNMT3B-targeting shRNAs exhibited dramatic reduction of 5-aza-CdR sensitivity as compared to control cells (Figure 4C). However, knockdown of DNMT3B by itself had no apparent effect on the growth of NT2/D1 or NT2/D1-R1 cells (data not shown). These results strongly support a functional link between sensitivity of EC cells to 5-aza-CdR and high DNMT3B expression.
Figure 4.
DNMT3B knockdown results in resistance to 5-aza-CdR in EC cells. A) Real time PCR assays of DNMT3B isoforms in control NT2/D1 and in NT2/D1-R1 cells and cells transduced with DNMT3B shRNA lentiviruses. Results 10 days after selection are indicated. Similar results were also obtained in two independent experiments one and two months post-selection indicating that knockdown was maintained (data not shown). B) Western analyses of NT2/D1 and NT2/D1-R1 cells independently transduced with DNMT3B shRNA (sh84, sh85, sh86, sh88, sh84, sh87, and sh88) employing an anti-DNMT3B antibody, H-230. Ctrl is control lentivirus. C) Dose response after 3 day of 5-aza-CdR treatment of lentiviral control NT2/D1 as well as NT2/D1-R1 cells (ctrl) and cells independently transduced with DNMT3B sh84, 85 and 86. Data were the average of biological triplicates and were representative of at least two independent experiments. Error bars are S.D. * p < .002 for control cells versus the respective shRNAs for that cell line., with # p < .02 for NT2/D1-R1 control versus NT2/D1-R1 cells transduced with shRNAs at the 5 nM 5-aza-CdR dose. D) NT2/D1-R1, NT2/D1-R1 control shRNA (sh-ctrl), and NT2/D1-R1 DNMT3B shRNA84 and shRNA86 cells were independently plated in individual wells of 24 well plates (3 × 103 cells per well) and treated with the indicated dosages of 5-aza-CdR for 10 days prior to staining with Geimsa. Fresh 5-aza CdR was added every day for the first 3 days and every other day thereafter. This experiment was repeated with similar results (data not shown).
Chronic low-dose scheduling has been suggested to be the optimal protocol for 5-aza-CdR in the treatment of leukemia (15). Therefore, the effect of DNMT3B expression on the sensitivity of NT2/D1-R1 cells to chronic 5-aza-CdR treatment was assessed. NT2/D1-R1 cells and control shRNA cells demonstrated an even greater sensitivity to a prolonged 10 day treatment with 5-aza-CdR as compared to the 3 day treatment (Figure 4D). These cells were sensitive to 5-aza-CdR at doses as low as 2.5 nM. In contrast, NT2/D1-R1 cells treated with DNMT3B-targeting shRNA demonstrated a dramatic resistance to 5-aza-CdR (Figure 4D).
Restoring cisplatin sensitivity to resistant cells with 5-aza-CdR
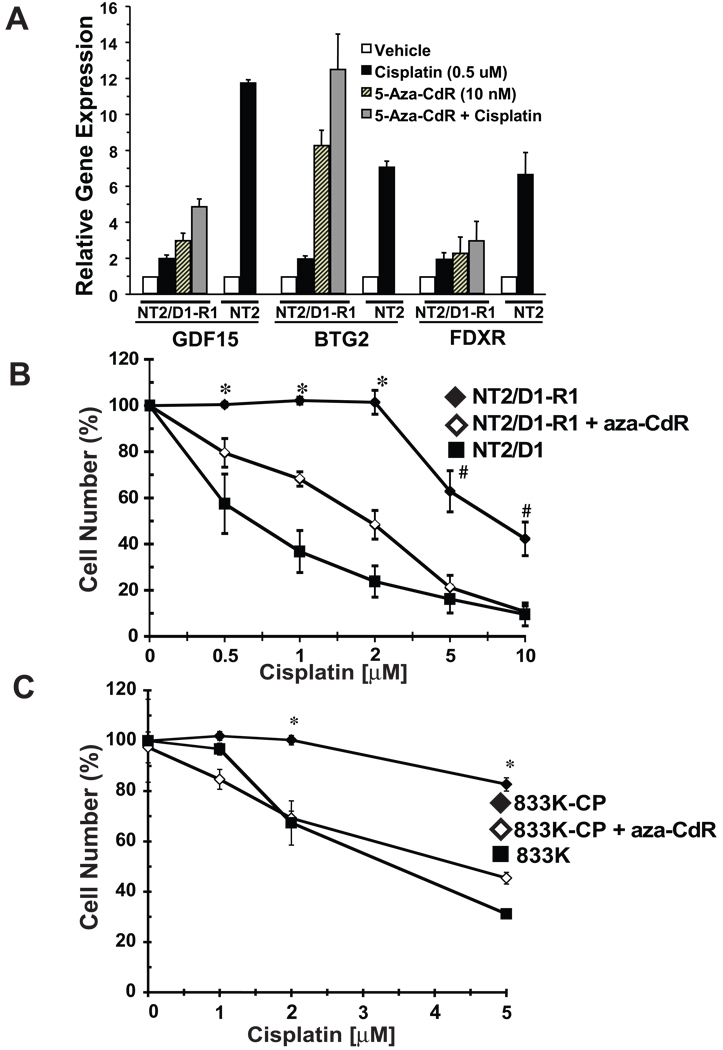
We previously reported that cisplatin causes a global p53-dominant transcriptional response in EC cells (24). Through microarray and other studies we found that the p53 response is repressed in NT2/D1-R1 cells despite having abundant wild-type p53 expression (8, 9, 24, and data not shown). Figure 5A demonstrates that pre-treatment of NT2/D1-R1 cells with low dose (10 nM) 5-aza-CdR for 3 days at least partially restored cisplatin induction of the p53 target genes GDF15 and BTG2 in NT2/D1-R1 cells. Notably, 5-aza-CdR treatment alone substantially induced the expression of GDF15, BTG2, and the p53 target gene, FDXR, in these cells. In contrast, 5-aza-CdR treatment had little or no detected effect on cisplatin induction of FDXR in NT2/D1-R1 cells. This dose of 5-aza-CdR inhibits proliferation of NT2/D1-R1 cells by only about 10% versus controls after 3 days (Figure 1). Viable cells were counted and replated after 5-aza-CdR treatment and allowed to recover for 24 hours before cisplatin treatment. Notably, pretreatment with low dose 5-aza-CdR restored cisplatin growth suppression and toxicity to two separate cisplatin resistant TGCT cell lines, NT2/D1-R1 and 833K-CP (Figure 5B and 5C). 833K-CP cells were pretreated with 2.5 nM 5-aza-CdR, a dose that results in a 10% growth inhibition (Figure 1). Pretreatment with 5-aza-CdR did not alter the cisplatin sensitivity of parental NT2/D1 cells, but did partially increase the cisplatin sensitivity of 833K cells (data not shown). These data indicated that 5-aza-CdR can restore cisplatin cytotoxic response to cisplatin resistant EC cells.
Figure 5.
Pretreatment with low dose 5-aza-CdR restored cisplatin sensitivity to cisplatin resistant EC cells. A) NT2/D1-R1 cells were pretreated with vehicle or 5-aza-CdR (10 nM) for 3 days before replating and a 24-hour recovery period followed by the indicated cisplatin treatments for 6 hours. NT2/D1 (NT2) cells were only treated with cisplatin. Cells were assayed 24 hours later for expression of the indicated p53 target genes by real-time PCR assays. The averages of two biological replicates and error bars were the ranges of the two values. B and C) Cells were pretreated with vehicle or 5-aza-CdR for 3 days before replating and a 24-hour recovery period followed by the indicated cisplatin treatments for 6 hours. Cell viability was assayed 3 days later. For NT2D1-R1 cells, a 10 nM 5-aza-CdR dosage was used. For 833K-CP cells, 2.5 nM 5-aza-CdR was used. Data were the averages of biological triplicates. B) Error bars are standard error of the mean. The p values were calculated for NT2/D1-R1 versus NT2/D1-R1 cells pretreated with 5-aza-CdR, with *p < .005; # p < .05. C) Error bars are S.D, with * p < .005 for 833K-CP versus 833K-CP cells pretreated with 5-aza-CdR.
Relationship between DNMT3B levels and cisplatin sensitivity
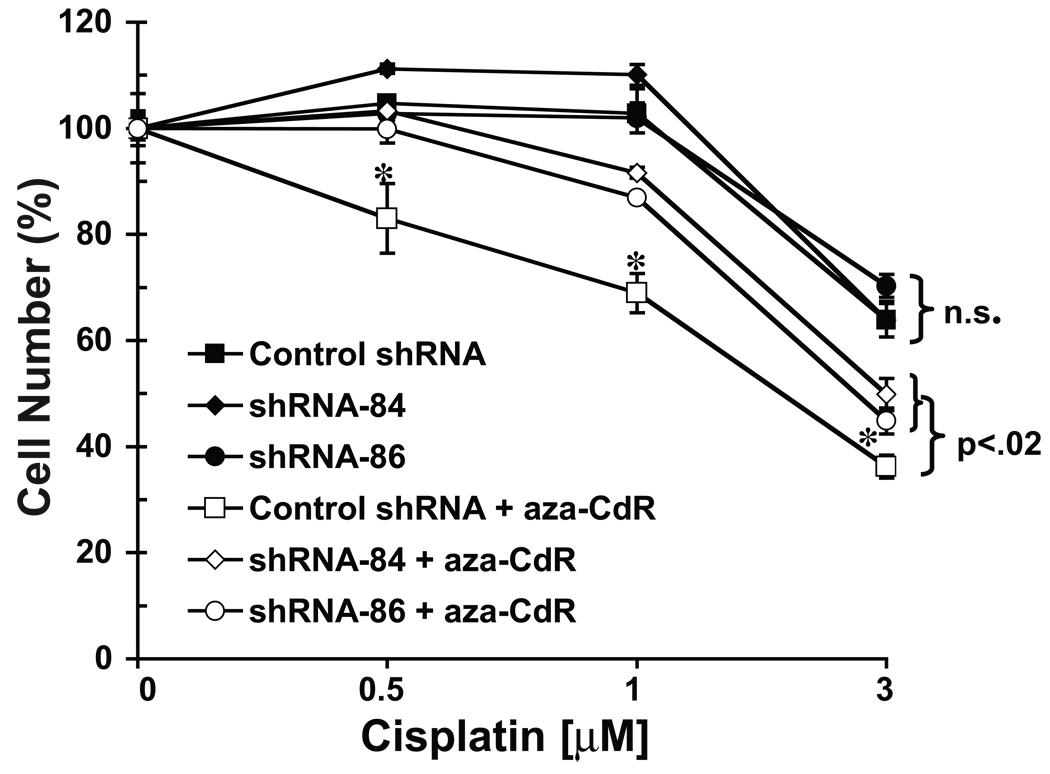
Since DNMT3B knockdown reversed 5-aza-CdR hypersensitivity in cisplatin sensitive and resistant EC cells, it was of interest to investigate whether cisplatin sensitivity itself depended on DNMT3B expression. As shown in Figure 6, DNMT3B knockdown had no appreciable effect on cisplatin sensitivity of the cisplatin resistant EC line NT2/D1-R1. This is in agreement with the finding that both cisplatin sensitive (NT2/D1 and 833K) and resistant (NT2/D1-R1 and 833K-CP) EC cells abundantly express DNMT3B (Figure 2). However, the ability of 5-aza-CdR to sensitize NT2/D1-R1 cells to cisplatin was substantially and significantly repressed by DNMT3B depletion. In summary these data indicate that 5-aza-CdR hypersensitivity of EC cells is dependent on DNMT3B expression regardless of whether the cells are sensitive or resistant to cisplatin. Yet, cisplatin sensitivity per se is not affected by DNMT3B expression.
Figure 6.
NT2/D1-R1 cells retain cisplatin resistance upon DNMT3B knockdown, but the ability of 5-aza-CdR to reverse cisplatin resistance was partially lost. NT2/D1-R1 control shRNA and DNMT3B shRNA-84 and shRNA-86 cells were pretreated with vehicle or 5-aza-CdR (10 nM) for 3 days before replating and a 24-hour recovery period followed by the indicated cisplatin treatments for 6 hour. Cell viability was assayed 3 days later via the Cell-Titer Glo assay. Values represent the averages of biological triplicates and error bars are S.D. N.S., no significant difference in cisplatin resistance (p >.05) for control shRNA cells versus shRNA-84 and shRNA-86 cells, and *, significant change (p < .02) for 5-aza-CdR pretreated control shRNA cells versus 5-aza-CdR pretreated shRNA-84 and shRNA-86 cells. The experiment was independently repeated with similar results (data not shown).
Discussion
Few studies have addressed 5-aza cytidine analog treatment effects in TGCT cells. In the current study, we show that TGCT cells are hypersensitive to the DNA methylation inhibitor, 5-aza-CdR. This response is tightly associated with high levels of DNMT3B protein, which was validated as an important target of 5-aza-CdR-mediated hypersensitivity in cisplatin sensitive as well as resistant TGCTs. Based on these findings, we propose that TGCT cells may be distinctly sensitive to DNA methylation inhibitors due to high basal levels of DNMT3B that are likely a consequence of the PGC origins of EC and their similarities to ES cells, which also are known to express high levels of DNMT3B (11, 12). Indeed, several genomic studies highlighted DNMT3B as a marker of pluripotency (11, 12).
The 5-aza cytidine-related compounds become incorporated into DNA and have been shown to mediate covalent adduct formation with DNMTs (16). The most widely studied and accepted mechanism for the antitumor effects of 5-aza-deoxycytidine is related to DNA demethylation and re-expression of specific tumor suppressor genes (reviewed in 14). However, other studies indicate that DNA-methylation independent effects of 5-aza-CdR are critical, especially for effects mediated through the ATM/ATR/p53-dependent DNA damage checkpoint (16–19,25,26). The degree each mechanism is employed may be cell-context dependent. The studies cited above use doses of 5-aza considerably higher than the doses used here in the treatment of EC cells. We provide evidence that in EC cells, low dose 5-aza-CdR is able to activate the ATM pathway as well as induce the expression of genes known to be methylated in TGCTs (Figure 3).
At first glance it may seem paradoxical that EC cells are hypersensitive to a DNA methylation inhibitor, but are resistant to that inhibitor when one of its targets, DNMT3B, is depleted (Figure 4). This result argues that the actions of 5-aza-CdR in EC cells cannot be explained fully by inhibition of DNMT3B activity and suggests that high basal levels of DNMT3B are needed to efficiently elicit an acute cytotoxic response to 5-aza-CdR.
This finding is not without precedence. Juttermann et al. showed that ES cells and embryos with DNMT1 knockdown were significantly less sensitive to 5-aza-CdR mediated toxicity (16). Oka et al. using DNMT3B/DNMT3A null ES cells also showed that DNMT3B/DNMT3A knockdown results in decreased sensitivity to 5-aza-CdR (27). HCT116 cells that contain a hypomorphic, truncated allele of DNMT1 were shown to have less H2AX phosphorylation and ATM activation in response to 5-aza-CdR, as compared to wild-type HCT116 cells (17). However, cells with the hypomorphic DNMT1 allele were sensitized to 5-aza-CdR cytotoxicity, presumably due to a failure to effectively arrest in G2/M (17). In contrast, our preliminary data has thus far not detected large differences in 5-aza-CdR mediated ATM and H2AX activation in DNMT3B knockdown cells, as compared to wild-type EC cells (data not shown). As DNMTs are known to be auxiliary components of the DNA replication and repair machinery (17,28), it is possible that depleting DNMT3B in the EC context alters downstream responses to 5-aza-CdR-mediated DNA damage that perturbs the balance between cell cycle checkpoint arrest, DNA repair, and apoptosis.
In contrast to 5-aza-CdR sensitivity, cisplatin sensitivity is independent of DNMT3B levels as both cisplatin sensitive and resistant cells express abundant DNMT3B (Figure 2) and knockdown of DNMT3B fails to alter the cisplatin response (Figure 6). Thus the cytotoxic effects of DNMT3B appear not to be engaged by general DNA damaging agents. Rather, it is the combination of 5-aza-CdR and high DNMT3B levels that appears critical for the hypersensitivity of EC cells to 5-aza-CdR.
The methylation status of germ cell tumors has only recently been investigated on a genome-wide level and findings suggest that TGCTs have distinct methylation signatures in comparison to other solid tumors (20,29). Seminomas are prominently hypothmethylated as compared to nonseminomas and somatic tumors (20,29). This signature is likely related to the PGC origins of TGCTs. Male PGCs can undergo complete erasure and re-establishment of DNA methylation during development that is associated with dynamic changes in the expression of the de novo DNA methyltransferase, DNMT3B (30).
5-aza-CdR is FDA approved for the treatment of myelodysplastic syndrome and shows promise for the treatment of certain leukemias. Recent data suggest that 5-aza-CdR is most efficacious when given chronically and at low dosages (15). It has been suggested that less compelling results with 5-aza-CdR in solid tumor trials may have been due in part to the high doses administered and to the monitoring of early rather than later clinical treatment responses (15). Based on these arguments a call to revisit 5-aza-CdR in solid tumors as a single or combined agent has been made (15). In agreement with our data it has been shown that 5-aza-CdR can sensitize somatic tumor cells to cisplatin, but at dosages considerably higher than that shown here for EC cells (31–34).
A number of experimental antitumor drugs were propelled to be successfully tested for treating other solid cancers based primarily on initial studies conducted in testicular cancer (3). We propose that high expression of DNMT3B in EC cells is a consequence of their pluripotent and germ cell origin and results in hypersensitivity to DNA methylation inhibitors. The finding that cisplatin resistant EC cells retain a high degree of sensitivity to low dose 5-aza-CdR and that pretreatment of 5-aza-CdR restores cisplatin cytotoxicity to resistant EC cells is notable since it can be clinically exploited.
Supplementary Material
Acknowledgements
We thank Dr. David Robbins, Dr. Murray Korc, and Dr. Mark Israel (Dartmouth Medical School) for lung cancer, pancreatic cancer, and glioblastoma cell lines, respectively. Supported by NIH and NCI grants R01-CA104312 (MJS), R21 R21CA124817 (MJS), R01-CA111422 (ED), R01-CA087546 (ED) and an American Cancer Society (ACS) Institutional Grant (SJF). ED is an ACS Clinical Research Professor supported by a generous gift from the F.M. Kirby Foundation.
References
- 1.Houldsworth J, Korkola JE, Bosl GJ, Chaganti RS. Biology and genetics of adult male germ cell tumors. J Clin Oncol. 2006;24:5512–5518. doi: 10.1200/JCO.2006.08.4285. [DOI] [PubMed] [Google Scholar]
- 2.Clark AT. The stem cell identity of testicular cancer. Stem Cell Rev. 2007;3:49–59. doi: 10.1007/s12015-007-0002-x. [DOI] [PubMed] [Google Scholar]
- 3.Einhorn LH. Curing metastatic testicular cancer. Proc Natl Acad Sci U S A. 2002;99:4592–4595. doi: 10.1073/pnas.072067999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giuliano CJ, Freemantle SJ, Spinella MJ. Testicular germ cell tumors: A paradigm for the successful treatment of solid tumor stem cells. Curr Cancer Ther Rev. 2006;2:255–270. doi: 10.2174/157339406777934681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Helw L, Coleman RE. Salvage, dose intense and high-dose chemotherapy for the treatment of poor prognosis or recurrent germ cell tumours. Cancer Treat Rev. 2005;31:197–209. doi: 10.1016/j.ctrv.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhary UB, Haldas JR. Long-term complications of chemotherapy for germ cell tumours. Drugs. 2003;63:1565–1577. doi: 10.2165/00003495-200363150-00004. [DOI] [PubMed] [Google Scholar]
- 7.Qin T, Jelinek J, Si J, Shu J, Issa JP. Mechanisms of resistance to 5-aza-2’deoxycytidine in human cancer cell lines. Blood. 2009;113:659–667. doi: 10.1182/blood-2008-02-140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtin JC, Dragnev KH, Sekula D, Christie AJ, Dmitrovsky E, Spinella MJ. Retinoic acid activates p53 in human embryonal carcinoma through retinoid receptor-dependent stimulation of p53 transactivation function. Oncogene. 2001;20:2559–2569. doi: 10.1038/sj.onc.1204370. [DOI] [PubMed] [Google Scholar]
- 9.Kerley-Hamilton JS, Pike AM, Hutchinson JA, Freemantle SJ, Spinella MJ. The direct p53 target gene, FLJ11259/DRAM, is a member of a novel family of transmembrane proteins. Bochim Biophys Acta 20007. 1769:209–219. doi: 10.1016/j.bbaexp.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen L, Kondo Y, Ahmed S, et al. Drug sensitivity prediction by CpG island methylation profile in the NCI-60 cancer cell line panel. Cancer Res. 2007;67:11335–11343. doi: 10.1158/0008-5472.CAN-07-1502. [DOI] [PubMed] [Google Scholar]
- 11.Sperger JM, Chen X, Draper JS, Antosiewicz JE, et al. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc Natl Acad Sci U S A. 2003;100:13350–13355. doi: 10.1073/pnas.2235735100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller FJ, Laurent LC, Kostka D, et al. Regulatory networks define phenotypic classes of human stem cell lines. Nature. 2008;455:401–405. doi: 10.1038/nature07213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skotheim RI, Lind GE, Monni O, et al. Differentiation of human embryonal carcinomas in vitro and in vivo reveals expression profiles relevant to normal development. Cancer Res. 2005;65:5588–5598. doi: 10.1158/0008-5472.CAN-05-0153. [DOI] [PubMed] [Google Scholar]
- 14.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Issa JP. DNA methylation as a therapeutic target in cancer. Clin Cancer Res. 2007;13:1634–1637. doi: 10.1158/1078-0432.CCR-06-2076. [DOI] [PubMed] [Google Scholar]
- 16.Juttermann R, Li E, Jaenisch R. Toxicity of 5-aza-2’-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc Natl Acad Sci U S A. 1994;91:11797–11801. doi: 10.1073/pnas.91.25.11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palii SS, Van Emburgh BO, Sankpal UT, Brown KD, Robertson KD. DNA methylation inhibitor 5-aza-2’-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B. Mol Cell Biol. 2008;28:752–771. doi: 10.1128/MCB.01799-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoglund A, Nilsson LM, Forshell LP, Maclean KH, Nilsson JA. Myc sensitizes p53-deficient cancer cells to the DNA-damaging effects of the DNA methyltransferase inhibitor decitabine. Blood. 2009;113:4281–4288. doi: 10.1182/blood-2008-10-183475. [DOI] [PubMed] [Google Scholar]
- 19.Qin T, Jelinek J, Si J, Shu J, Issa JP. Mechanisms of resistance to 5-aza-2’-deoxycytidine in human cancer cell lines. Blood. 2009;113:659–667. doi: 10.1182/blood-2008-02-140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koul S, Houldsworth J, Mansukhani MM, et al. Characteristic promoter hypermethylation signatures in male germ cell tumors. Mol Cancer. 2002;1:8. doi: 10.1186/1476-4598-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lind GE, Skotheim RI, Fraga MF, Abeler VM, Esteller M, Lothe RA. Novel epigenetically deregulated genes in testicular cancer include homeobox genes and SCGB3A1 (HIN-1) J Pathol. 2006;210:441–449. doi: 10.1002/path.2064. [DOI] [PubMed] [Google Scholar]
- 22.Honorio S, Agathanggelou A, Wernert N, Rothe M, Maher ER, Latif F. Frequent epigenetic inactivation of the RASSF1A tumor suppressor gene in testicular tumors and distinct methylation profiles of seminoma and noseminoma testicular germ cell tumors. Oncogene. 2003;22:461–466. doi: 10.1038/sj.onc.1206119. [DOI] [PubMed] [Google Scholar]
- 23.El Deiry WS, Harper JW, O’Connor PM, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- 24.Kerley-Hamilton JS, Pike AM, Li N, DiRenzo J, Spinella MJ. A p53-dominant transcriptional response to cisplatin in testicular germ cell tumor-derived human embryonal carcinoma. Oncogene. 2005;24:6090–6100. doi: 10.1038/sj.onc.1208755. [DOI] [PubMed] [Google Scholar]
- 25.Link PA, Baer MR, James SR, Jones PA, Karpf AR. P53-inducible ribonucleotide reductase (p53R2/RRM2B) is a DNA hypomethylation-independent decitabine gene target that correlates with clinical response in myelodysplastic syndrome/acute myelogenous leukemia. Cancer Res. 2008;68:9358–9366. doi: 10.1158/0008-5472.CAN-08-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu W, Hileman T, Ke Y, et al. 5-aza-2’-deoxycytidine activates the p53/p21 pathway to inhibit cell proliferation. J Biol Chem. 2004;15:15161–15166. doi: 10.1074/jbc.M311703200. [DOI] [PubMed] [Google Scholar]
- 27.Oka M, Meacham AM, Hamazaki T, Rodic N, Chang L, Terada N. De novo DNA methyltransferases DNMT3A and DNMT3B primarily mediate the cytotoxic effects of 5-aza-2’-deoxycytidine. Oncogene. 2005;24:3091–3099. doi: 10.1038/sj.onc.1208540. [DOI] [PubMed] [Google Scholar]
- 28.Leonhardt H, Page AW, Weier HU, Bestor TH. A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nuclei. Cell. 1992;71:865–873. doi: 10.1016/0092-8674(92)90561-p. [DOI] [PubMed] [Google Scholar]
- 29.Netto GJ, Nakai Y, Nakayama M, et al. Global DNA hypomethylation in intratubular germ cell neoplasia and seminoma, but not in nonseminomatous male germ cell tumors. Mod Pathol. 2008;21:1337–1344. doi: 10.1038/modpathol.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajkova P, Erhardt S, Lane N, Haaf T, El-Maarri O, Reik W, Walter J, Surani MA. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev. 2002;117:15–23. doi: 10.1016/s0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- 31.Qui Y, Mirkin BL, Dwivedi RS. Inhibition of DNA methyltranferase reverses cisplatin induced drug resistance in murine neuroblastoma cells. Cancer Detect Prev. 2005;29:456–463. doi: 10.1016/j.cdp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Mishra MV, Bisht KS, Sun L, et al. DNMT1 as a molecular target in a multimodality-resistant phenotype in tumor cells. Mol Cancer Res. 2008;6:243–249. doi: 10.1158/1541-7786.MCR-07-0373. [DOI] [PubMed] [Google Scholar]
- 33.Plumb JA, Strathdee G, Sludden J, Kaye SB, Brown R. Reversal of drug resistance in human tumor xenografts by 2’-deoxy-5-azacytidine-induced demethylation of the hMLH1 gene promoter. Cancer Res. 2000;60:6039–6044. [PubMed] [Google Scholar]
- 34.Shang D, Liu Y, Matsui M, et al. Demethylation agent 5-aza-2’deoxycytidine enhances susceptibility of bladder transitional carcinoma to cisplatin. Urology. 2008;71:1220–1225. doi: 10.1016/j.urology.2007.11.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.