Abstract
The vulnerability to mood disorders, impulsive-aggression, eating disorders, and suicidal behavior varies greatly with gender, and may reflect gender differences in central serotonergic function. We investigated the relationships of gender, mood, impulsivity, aggression and temperament to 5HT2A receptor binding in 21 healthy subjects using [18F]altanserin and PET neuro-imaging. Binding potentials in pre-defined Regions of Interest (ROI) were calculated using the Logan graphical method, corrected for partial volume effects, and compared by gender with age co-varied. SPM analysis was used for voxel level comparisons. Altanserin binding (BPp) was greater in male than female subjects in 9 ROIs: hippocampus (HIP) and Lt. HIP, lateral orbital frontal cortex (LOF) and Lt.LOF, left medial frontal cortex (Lt.MFC), left medial temporal cortex (Lt. MTC), left occipital cortex (Lt. OCC), thalamus (THL) and Lt. THL. Differences in Lt. HIP and Lt. MTL remained significant after Bonferroni correction. Gender differences were noted in the co-variation of psychological traits with BPp values in specific ROIs. Among males alone, aggression was negatively correlated with BPp values in Lt. LOF and Lt. MFC, and Suspiciousness positively correlated in LOF, Lt. LOF and Lt. MFC. Among female subjects alone, Negativism was positively correlated with BPp values in HIP, and Verbal Hostility in Lt. HIP. Altanserin binding in Lt. MTC was positively correlated with Persistence, with no significant gender effect. Gender differences in 5HT2A receptor function in specific ROIs may mediate expression of psychological characteristics such as aggression, suspiciousness and negativism. Future studies of 5HT2A receptor function and its relationship to behavior should control for gender.
Keywords: [18F]altanserin binding, PET neuroimaging, aggression, impulsivity, temperament
1. INTRODUCTION
Dysregulation of central serotonergic function is implicated in the etiology of mood disorders, impulsive-aggression, eating disorders, attempted and completed suicide (Oquendo and Mann, 2000; Kaye et.al., 2001; Frank et.al., 2002). Vulnerability to these disorders varies greatly with gender, and may reflect underlying gender differences in central serotonergic function.
We have been studying the central serotonin-2A receptor (5HT2A) and its relationship to suicidal behavior in Borderline Personality Disorder, a diagnostic group at high risk for impulsive-aggression, suicide attempts and completion (Soloff et.al., 2007). Dysregulation of central 5HT2A receptor function has been demonstrated in patients with major depressive disorder (MDD) (Biver et.al., 1997; Yatham et.al., 2000; Messa et.al., 2003; Meyer et.al., 2003; Mintun et.al., 2004; Bhagwagar et.al., 2006), borderline personality disorder (BPD) (Soloff et.al., 2007), and among subjects with attempted or completed suicide (van Heeringen et.al., 2003; Audenaert et.al., 2000; Rosel et.al., 1998, 2000; Arango et.al., 1995, 1997). Although vulnerability to these disorders may vary with gender, inadequate attention has been paid to possible gender effects on 5HT2A binding in these disorders, or in healthy subjects. The few studies which have examined gender effects on 5HT2A binding in healthy subjects have reported inconsistent results.
Biver et.al., (1996) used the selective 5HT2A receptor antagonist [18F]altanserin, and positron emission tomography (PET) neuroimaging, to assess regional binding potential (BP) measures in a sample of 22 healthy subjects (11 males and 11 females) with an age range of 23 – 64 years (mean 36.1 yrs. for males, 38.6 yrs. for females). They found increased BP values in males compared to females, most pronounced in left and right frontal cortices and in the cingulate cortex. Citing gender differences in earlier platelet studies of [3H] imipramine binding sites, these researchers suggested that diminished 5HT2A receptor binding in women compared to men was related to greater presynaptic serotonergic activity and compensatory post-synaptic down regulation in women. Other studies, using 123 I-5-I-R91150 SPET (Baeken et.al., 1998), or [18F]altanserin (Meltzer et.al., 1998; Adams et.al., 2004; Frokjaer et.al., 2008) found no gender differences in 5HT2A BP values among healthy subjects. However, these studies reported significant negative correlations between age and 5HT2A binding in cortical regions, an effect not examined by Biver et.al.(1996).
Baeken et.al. (1998) studied 26 healthy subjects (13 male and 13 female) with an age range of 23 – 60 years (mean 38.2 yrs. for males, 38.9 yrs. for females) and found a 42% decrease in binding over a 40 year time span. Meltzer et.al. (1998) found a 61.3% age-related decrease in BP values comparing healthy elderly subjects (6 female, 3 male) with a mean age of 69 years, (range 61 – 76 yrs.) to younger subjects (6 female, 3 male) with a mean age of 23 years (range 18 –29 yrs.) Adams et.al.(2004) reported an age-related decrease in binding potentials of 4% - 6% per decade in a sample of 52 healthy subjects with an age range of 21–79 years (31 males with a mean age of 45.4 yrs, and 22 females with a mean age of 47.4 yrs.). The effect of age on [18F]altanserin binding is controlled in the present study.
Despite a prominent role in mood disorders, impulsive-aggression, eating disorders and suicide, the behavioral significance of 5HT2A receptor function in normal subjects has not been well studied. In a large sample study of 83 healthy volunteers, Frokjaer et.al., (2008) found neuroticism (on the Neo-PI-R personality questionnaire) was related to increased [18F]altanserin binding in frontolimbic cortex, especially the entorhinal (parahippocampal) cortex, superior and inferior frontal cortex and posterior cingulate. The personality trait of vulnerability (e.g. coping with stress) had the strongest association with [18F]altanserin binding. Anxiety, depression and self-consciousness had an intermediate relationship, and impulsiveness and angry hostility little or no association.
Moresco et.al., (2002) found an inverse relationship between the temperamental trait of Harm Avoidance and binding of 3-(2’ [18F]flouroethyl) spiperone ([18F]FESP), a 5HT2 and D2 receptor ligand, in a small sample of healthy subjects (3 female, 8 male). They examined correlations between [18F]FESP binding and Harm Avoidance on a pixel by pixel basis using Statistical Parametric Mapping (SPM) and found significant inverse correlations in inferior frontal gyrus bilaterally, left precentral gyrus, left inferior parietal lobule, middle and inferior temporal gyrus, left post central gyrus, fusiform gyrus, anterior cingulate and hypothalamus. Taken together, these two studies suggest that increased receptor number or affinity, consistent with a hypothesis of diminished serotonergic agonism, may be associated with low scores on Harm Avoidance and increased neuroticism. Behavioral effects of diminished serotonergic agonism may differ depending upon the affected brain region.
We assessed gender differences in the binding of [18F]altanserin in a sample of healthy male and female subjects (with an age range of 18 – 46 years), and asked whether 5HT2A receptor binding in specific cortical areas was related to standardized measures of mood, impulsivity, aggression and temperament.
2. METHODS
This study was approved by the Institutional Review Board of the University of Pittsburgh. All subjects were recruited by advertisement from the community and gave written informed consent. To be included in this study, all subjects had to be free of current and lifetime Axis I and II disorders, determined by trained raters using the Structured Clinical Interview for DSM III-R (Spitzer et.al., 1988) and the International Personality Disorders Examination (IPDE) (Loranger et.al., 1987). (DSM III-R was used to allow comparison with ongoing longitudinal studies). All subjects had a normal physical examination, and were free of all medications, including oral contraceptives. Female subjects had normal menstrual cycles, though it was not possible to control for day of menstrual cycle in scheduling the PET scans. No subject had a current or past diagnosis of a substance use disorder. All had abstained completely from social use of alcohol for at least seven days prior to the PET scan (range 7 – 288 days, mean (sd): 48.5 (72.5) days), 7 non-drinkers). None were casual drug users. All subjects had a negative urinalysis for drugs of abuse at the time of the scan, and women had a negative pregnancy test. Subjects were requested to eat a low tryptophan breakfast with no caffeine-containing beverages on the day of the scan. Psychological measures obtained in the week before the scan included the Hamilton Rating Scale for Depression, 24 item format (HamD) (Guy W., 1976), the Barratt Impulsiveness Scale-Version 11 (BIS) (Barratt and Slaughter, 1998), the Brown-Goodwin Lifetime History of Aggression (LHA) (Brown and Goodwin, 1986), the Buss-Durkee Hostility Inventory (Buss and Durkee, 1957), and the Temperament and Character Inventory (Cloninger et.al., 1994.)
A magnetic resonance imaging (MRI) scan was obtained prior to the PET study for region-of-interest definition and partial volume correction. The MRI scans were acquired with a 1.5 Tesla Signa scanner (GE Healthcare, Milwaukee, WI). A T1-weighted sagittal scout image was obtained for graphic prescription of the coronal and axial images. 3D gradient echo imaging (Spoiled Gradient Recalled Acquisition, SPGR) was performed in the coronal plane (TR = 25 ms, TE = 5 ms, nutation angle = 40°, field of view = 24 cm, section thickness =1.5 mm, intersection gap = 0 mm, NEX = 1, matrix size = 256×192 pixels) to obtain 124 images covering the entire brain. Additionally, a double echo-spin echo sequence was used to obtain T2 and proton weighted density images in the axial plane to screen for neuroradiological abnormalities.
[18F]altanserin was synthesized using routine methods based on Lemaire et al. (1991). [18O]Water (>95 % enriched, Rotem Inc., Port Chester, NY) was irradiated at 18 mA for 60 min using an RDS 112 (CTI/Siemens, Inc., Knoxville, TN) and then transferred to a 5 mL reaction V-vial (Pierce, St. Louis, MO) containing Kryptofix® 222 (22.3 mg, 60 mmol,) and K2CO3 (7.1 mg, 52 mmol) in Milli-Q water (57 mL). The solution was repeatedly evaporated to dryness using acetonitrile (3 × 1 mL) at 100°C under a stream of argon. To the dried Kryptofix® 222/[18F]fluoride complex in a V-vial was added nitroaltanserin (ABX, Inc. Germany) (4.0 ± 0.5 mg, 9.1 mmol ± 1.1 mmol dissolved in 500 mL DMF). The V-vial was sealed and irradiated in a commercial microwave (Sears Kenmore™, 1000W) at 60% power for 2 min. After heating, the reaction solution was cooled in an ice-water bath followed by purification by semi-preparative HPLC utilizing two Phenomenex Prodigy™ ODS-Prep (10 × 250 mm) columns in series equipped for peak enrichment between the two columns. The semi-preparative eluent consisted of 30% acetonitrile /70 % sodium acetate (80 mM)/ascorbic acid (25 mM) buffer at a flow of 5 mL/min for 10 min and 8 mL/min for the remainder. The desired product, [18F]altanserin eluted at ~35min. The fraction containing [18F]altanserin was diluted with 100 mL of the sodium acetate/ascorbic acid buffer and the desired product was isolated using a Waters C18 Sep-Pak® Plus cartridge. The final product was formulated in 1 mL of ethanol (Dehydrated Alcohol Injection, USP) and 14 mL 0.9% Sodium Chloride Injection, USP and 20 mL Ascorbic Acid Injection, USP (CENOLATE ®, 500 mg/mL) and was sterile-filtered (Millipore, USA Millex®-FG filter (0.2 micron). The average radiochemical yield of [18F]altanserin was 18 ± 10 % at end of synthesis (EOS), based on [18F]fluoride yielding between 1.5 – 7.2 GBq (40 – 194 mCi) of [18F]altanserin at the end of the approximately 70 min synthesis (n=110). The average specific activity of [18F]altanserin (EOS) was determined to be 600 GBq/mmol (16.1 Ci/mmol}. The radiochemical identity of [18F]altanserin was assessed by radio-HPLC using analytical HPLC (Phenomenex Prodigy™ ODS-3, 5m, 4.6 × 250 mm) and 30% acetonitrile and 70% 80 mM acetate buffer (pH 5.2) at a flow rate of 2.0 mL/min, providing ~14 min retention time for [18F]altanserin. The radiochemical and chemical purity of [18F]altanserin was > 90%, using the same analytical conditions used for radiochemical identity.
The PET study was conducted in the University of Pittsburgh PET Facility. All subjects were scanned on an ECAT HR+ PET scanner (Siemens/CTI) in two-dimensional (2D) imaging mode, septa extended, with 63 image planes acquired over a 152-mm axial field-of-view. Subjects were placed in a recumbent position and a short 21-gauge plastic catheter inserted into either the left or right (non-dominant hand) radial artery under local anesthesia. An intravenous line was placed in the antecubital vein on the other side and infused with normal saline to keep open. Subjects were positioned in the scanner with the head oriented approximately parallel to the canthomeatal line to include imaging from vertex through cerebellum. A softened thermoplastic facemask with generous holes for eyes, nose, mouth and ears was fitted closely around the head and attached to the head holder to minimize subject motion. A 10-minute transmission scan was performed for attenuation correction using rotating 68Ge/ 68Ga rods. The [18F]altanserin was administered as a slow bolus (20 sec bolus, 10 mCi, high specific activity (≥ 1.04 Ci/umol.)). A 90-minute dynamic PET acquisition began at injection along with arterial blood sampling for the determination of the arterial input function (Bailer et al., 2004). The PET emission data were corrected for deadtime, attenuation, radioactive decay, and scatter. PET data were reconstructed using filtered back-projection. The final reconstructed PET image resolution was about 6 mm (transverse and axial).
For each subject, the PET data were co-registered to the MRI data set using an automated algorithm for image alignment and reslicing (Woods et al., 1993). Data were examined for subject motion and inter-frame motion was corrected using a more extensive registration procedure on a frame-by-frame basis. Region-of-interest (ROI) sampling of the PET data was performed based upon the co-registered SPGR MR image data. Eight ROIs were selected based on prior reports of involvement in mood and impulse regulation. In the prefrontal cortex, 3 ROIs were sampled, including: the medial orbital frontal cortex inferiorly (MOF: BA 11), the medial frontal cortex superiorly (MFC: BA 9/10), and the lateral orbital frontal cortex (LOF: BA 45/47). The MOF includes the cortex within the gyrus rectus, while the MFC samples the superior frontal gyrus and medial portion of the medial frontal gyrus at the level of the superior-most section through the body of the lateral ventricles. The anterior cingulate (ANC: BA 32) and its subdivisions were sampled: the pregenual cingulate (PRG: BA 24/32, anterior to the anterior-most part of the corpus callosum), and the subgenual cingulate (SUG: BA 25, inferior to the genu of the corpus callosum). The hippocampus (HIP) was sampled as a discrete ROI, and as part of a larger area in medial temporal cortex (MTC), which included the hippocampal-amygdala complex. Two control regions were sampled: the occipital cortex (OCC: BA 18) and the thalamus (THL), representing cortical and limbic regions not previously associated with impulsive or suicidal behavior. The cerebellum (CER) was defined as the reference region, emphasizing cerebellar hemisphere gray matter at the level of the inferior portion of the fourth ventricle. ROIs were hand drawn (multiple contiguous planes) on the MR images according to anatomic landmarks and transferred to the co-registered summed PET images for regional sampling. The ROIs were applied to the dynamic PET images to generate regional time-activity curves. Drawing of ROIs was done blind to diagnosis.
The [18F]altanserin PET data were analyzed using the linear Logan graphical method that was applied across the 12 to 90 min integration intervals (Logan et al., 1990; Meltzer et. al. 1998; Bailer 2004) and regional volume of distribution (VT) values were obtained. It is important to clarify that the graphical [18F]altanserin VT is an overestimate of the true [18F]altanserin VT because radiolabeled metabolites (radiometabolites) of [18F]altanserin pass the blood-brain barrier (BBB) and contribute nonspecifically to the overall PET signal (Price 2001a). We previously performed PET studies in baboons after the bolus injection of [18F]altanserin or one of its two major radiometabolites, at baseline and after pharmacologic receptor blockade (blocking data) (Price 2001a). The cerebellar and regional blocking data were analyzed using either single (parent radiotracer) or dual (parent radiotracer and radiometabolites) input function methods. The study showed that the major radiometabolites of [18F]altanserin crossed the BBB and contributed to a fairly uniform background of nonspecific radioactivity. Subsequent human studies were performed with detailed consideration of the impact of these BBB-permeable metabolites on the specific binding parameters (Price 2001b). The human studies showed that the single-input Logan graphical results (used in the present study) were highly correlated with the more comprehensive dual-input results and its bias was fairly constant across regions and subjects.
We have analyzed dynamic [18F]altanserin PET data using the Logan method because it provided a good compromise between validity, sensitivity, and reliability of implementation (Price et al., 2001b; Bailer et al., 2004). The VT measure used in the current work, therefore, reflects radioactivity concentration in brain that arises from [18F]altanserin that is free, nonspecifically bound, and specifically bound in brain, as well as nonspecific uptake that arises from radiometabolites and radioactivity in the vasculature. The cerebellum was used as the reference region as it was assumed to reflect minimal levels of specific binding and to provide a reasonable estimate of the nondisplaceable volume of distribution (or VND), despite overestimation of the nonspecific component as a result of the BBB-permeable radiometabolites. Accordingly, the cerebellar VT values were used to correct the regional [18F]altanserin VT values to obtain binding potential measures of specific binding, i.e., BPP and BPND, according to traditional relationships: BPP = VT - VND and BPND = VT/VND (Innis et al., 2007). Both BPP and BPND were examined in this study to allow for the assessment of binding measures relative to nonspecific uptake in plasma and brain, respectively. The BP measures were corrected for partial volume effects due to cerebral spinal fluid dilution using MR based correction factors that varied from 0 to 1 (no dilution) using a routinely applied method (Meltzer et.al. 1999). For each ROI, BP values were determined for left and right hemispheres and results determined for the left and right ROI data and also for the average of the left and right ROI data. Both BPP and BPND were examined as both measures have been reported in previous [18F]altanserin publications.
We further acknowledge the importance of evaluating group differences in plasma metabolism and cerebellar VT (Meltzer et.al. 1998, Bailer et al., 2004) for [18F]altanserin or any other PET radiotracer. In addition to concerns regarding radiometabolite brain uptake, [18F]altanserin binding is not be entirely absent in cerebellum (Dwivedi and Pandey 1998; Staley et.al., 2001). To address these concerns gender differences were examined in the fractional level of radiometabolites in plasma (over the study duration) and in the cerebellar VT measures. Other study concerns include potential limitations with regard to quantification of lateralized binding potential measures in small structures and the vulnerability of such regions to scatter and motion artifacts. In this study, PET imaging was performed in 2D mode and this minimizes scatter-induced variation (relative to 3D imaging) which is advantageous with respect to test-retest variation (Smith et al., 1998). Study motion was corrected for during image co-registration as described above.
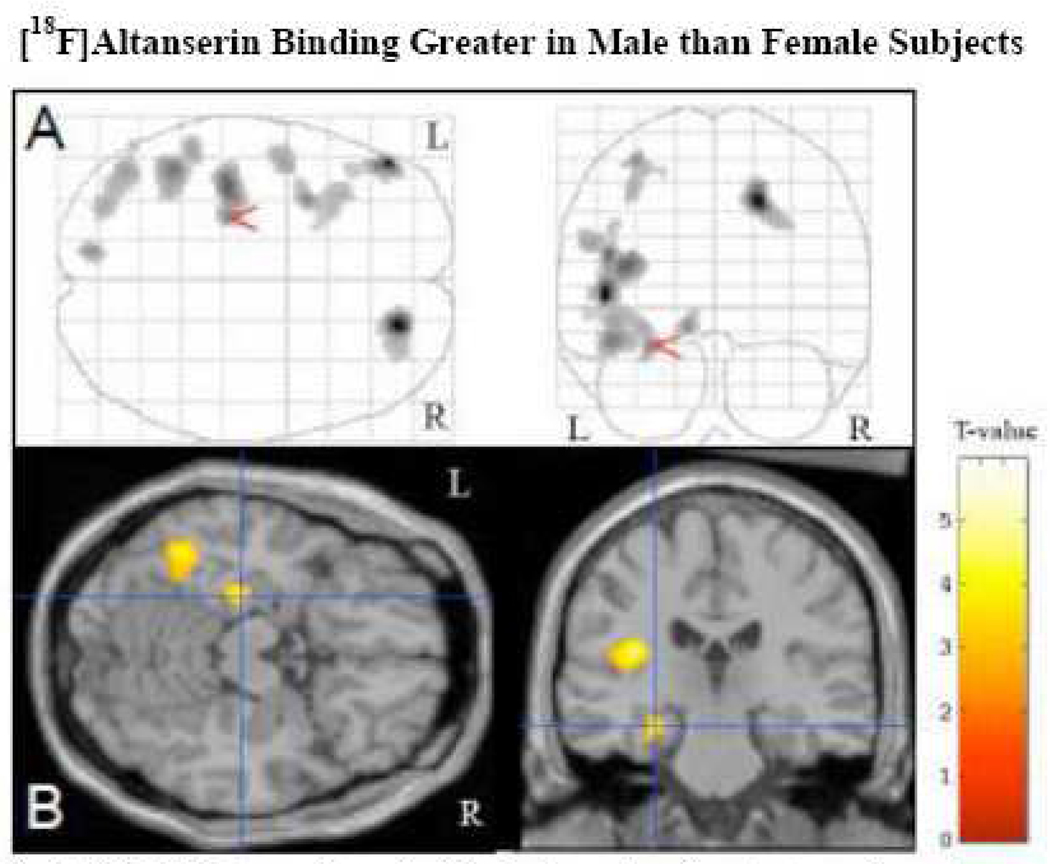
The data were further examined using Statistical Parametric Mapping (SPM5: http://www.fil.ion.ucl.ac.uk/spm/software/spm5/) as a supplemental analysis. The small sample size restricts the SPM analysis to a qualitative confirmation of the regional analysis, specifically illustrating a voxel-wise consistency of the lateralized differences. The supplemental analysis was limited to the BPP binding measure. Parametric [18F]altanserin VT images were created by applying the Logan graphical method on a voxel basis. Each individual’s Logan VT image was sampled using the individual’s cerebellar ROI in order to generate parametric BPP images. The BPP images were normalized to the MNI (ICBM 152 template) space within SPM5 using the unified method. This process began with the coregistration of each subject’s MR and BPP images with subsequent segmentation and normalization of the MR to the MNI template, and normalization of the coregistered PET using the MR transformation parameters obtained in the previous step. The normalized images were written out using the template bounding box and an isotropic voxel size of 2 mm. Gender difference images were explored using a two-sample t-test with age as a covariate. The results of this qualitative confirmation are shown in Figure 1 (uncorrected p=0.008 (T=2.658), voxel extent of 50).
Fig. 1.
SPM Maximum Intensity Projections showing clusters of voxels where there is evidence of greater [18F]altanserin binding in male compared to female subjects. The glass brain images (A) illustrate the overall left hemisphere dominance of the binding difference across all planes. The transaxial and coronal planes of the MR template (B) depict voxel differences at the left hippocampus (cursor) and the left fusiform gyrus. The images were created with a threshould of p=0.008 (T threshould of 2.658) and extent thershould of 50 voxels.
SPSS version 16.0 was used for statistical analyses (SPSS, Inc., Chicago,Ill.) Normality and homogeneity of variance were tested using the Kolmogorov-Smirnov (K-S) one sample test, and Levine’s test, respectively. BP and BPp values were also examined for outliers based on standard deviation of each value from the mean for the region. BPp values were compared to BP using Pearson correlations. Demographic and psychological variables were compared between groups by t-tests. BP and BPp values for male and female subjects were first compared by ANOVA for each ROI, co-varying for age, then by lateralized left and right samples. (Results are presented in the text in terms of BPp. Both data sets are shown in Table 2). To minimize Type I error, analyses between psychological variables and BPp values were limited to ROIs with significant differences between groups (p.<.05). Relationships between gender, age, BPp values and psychological variables were examined for each ROI using linear regression (with psychological variables as dependents). Partial correlation coefficients are reported with gender and/or age co-varied as required. Only the 4 Temperament scales of the TCI were used (e.g. Novelty Seeking, Harm Avoidance, Reward Dependence and Persistence). Data from the female control sample was previously included in a study of female subjects with borderline personality disorder (Soloff et.al., 2007).
Table 2.
Non-specific binding, metabolite, and Altanserin BPp values
| N | Female | Male | |||
|---|---|---|---|---|---|
| (Mean, S.D.) | (Mean, S.D.) | t-value | df | P-value | |
| 11 | 10 | ||||
| A. Non-specific binding | |||||
| CER_DV | 1.13 (0.25) | 1.15 (0.08) | 0.23 | 12.1 | .ns |
| B. Metabolites | |||||
| 2 min. | 0.98 (0.01) | 0.97 (0.02) | 1.16 | 19 | .ns |
| 10 min. | 0.87 (0.03) | 0.88 (0.04) | 0.30 | 19 | .ns |
| 30 min. | 0.71 (0.08) | 0.71 (0.06) | 0.074 | 19 | .ns |
| 60 min. | 0.56 (0.08) | 0.58 (0.07) | 0.56 | 19 | .ns |
| 90 min. | 0.50 (0.08) | 0.51 (0.06) | 0.35 | 19 | .ns |
| C. BP values* (Logan) | |||||
| HIP | 0.31 (0.12) | 0.43 (0.09) | 2.63 | 19 | 0.02 |
| Lt. | 0.29 (0.11) | 0.44 (0.10) | 3.25 | 19 | 0.004 |
| Rt. | 0.32 (0.18) | 0.42 (0.10) | 1.57 | 19 | .ns |
| LOF | 1.48 (0.32) | 1.73 (0.25) | 1.93 | 19 | 0.07 |
| Lt. | 1.46 (0.31) | 1.79 (0.27) | 2.60 | 19 | 0.02 |
| Rt. | 1.50 (0.35) | 1.70 (0.26) | 1.12 | 19 | .ns |
| MTC | 0.38 (0.10) | 0.45 (0.92) | 1.48 | 19 | .ns |
| Lt. | 0.34 (0.09) | 0.47 (0.08) | 3.31 | 19 | 0.004 |
| Rt. | 0.42 (0.16) | 0.42 (0.12) | 0.09 | 19 | .ns |
| THL | 0.24 (0.08) | 0.31 (0.08) | 1.98 | 19 | 0.06 |
| Ft. | 0.22 (0.09) | 0.31 (0.08) | 2.36 | 19 | 0.03 |
| Rt. | 0.26 (0.10) | 0.31 (0.08) | 1.18 | 19 | .ns |
| D. BPp values* | |||||
| HIP | 0.34 (0.12) | 0.50 (0.10) | 3.31 | 19 | 0.004 |
| Lt. | 0.32 (0.11) | 0.50 (0.11) | 3.77 | 19 | 0.001 |
| Rt. | 0.34 (0.18) | 0.48 (0.11) | 2.10 | 19 | 0.05 |
| LOF | 1.63 (0.24) | 1.98 (0.35) | 2.75 | 19 | 0.01 |
| Lt. LOF | 1.61 (0.27) | 2.05 (0.35) | 3.33 | 19 | 0.003 |
| MFC | 2.14 (0.31) | 2.54 (0.48) | 2.30 | 19 | 0.03 |
| Lt. MFC | 2.17 (0.30) | 2.67 (0.56) | 2.61 | 19 | 0.02 |
| MOF | 1.94 (0.34) | 2.23 (0.27) | 2.11 | 19 | 0.05 |
| Lt. MOF | 1.94 (0.32) | 2.23 (0.30) | 2.13 | 19 | 0.05 |
| MTC | 0.41 (0.08) | 0.51 (0.10) | 2.36 | 19 | 0.03 |
| Lt. MTC | 0.37 (0.08) | 0.53 (0.09) | 4.15 | 19 | 0.001 |
| OCC | 1.44 (0.26) | 1.68 (0.20) | 2.39 | 19 | 0.03 |
| Lt. OCC | 1.41 (0.27) | 1.69 (0.25) | 2.46 | 19 | 0.03 |
| Lt. SUG | 1.76 (0.33) | 2.08 (0.35) | 2.13 | 19 | 0.05 |
| Lt. THL | 0.26 (0.10) | 0.36 (0.09) | 2.20 | 19 | 0.04 |
BP, BPp values not corrected for age
3. RESULTS
3.1. Sample characteristics
We compared 10 healthy male and 11 healthy female subjects, with an age range of 18–46 years, and a mean age (s.d.) of 25.4 (8.6) years (Table 1). The groups did not differ significantly in age or BMI.
Table 1.
Clinical characteristics of healthy female and male subjects
| N | Female | Male | |||
|---|---|---|---|---|---|
| (Mean, S.D.) | (Mean, S.D.) | t-value | df | P-value | |
| 11 | 10 | ||||
| Age (yrs.) | 27.3 (10.6) | 23.4 (5.7) | 1.10 | 15.6 | .ns |
| BMI | 23.9 (2.5) | 24.8 (3.8) | 0.61 | 19 | .ns |
| HamD | 0.45 (0.69) | 1.00 (1.7) | 0.98 | 19 | .ns |
| BIS | 57.0 (8.6) | 57.5 (7.5) | 0.14 | 18 | .ns |
| BDHI-total | 20.5 (8.4) | 19.6 (9.3) | 0.25 | 19 | .ns |
| BDHI-Neg. | 1.45 (1.03) | 1.80 (0.79) | 0.85 | 19 | .ns |
| LHA | 11.8 (3.6) | 17.7 (2.8) | 4.13 | 19 | .001 |
| Novelty Seeking | 14.5 (5.2) | 18.1 (4.9) | 1.60 | 18 | .ns |
| Harm Avoidance | 11.6 (4.2) | 16.9 (3.2) | 3.18 | 18 | .005 |
| Reward Dependence | 10.5 (3.1) | 11.4 (2.2) | 0.74 | 18 | .ns |
| Persistence | 2.6 (1.6) | 3.5 (1.0) | 1.54 | 18 | .ns |
All female subjects reported normal menstrual cycles with a mean (s.d) of 17.4 (14.1) days between onset of flow and PET study. In this healthy sample, levels of depressed mood (Ham-D-24) were negligible, with no significant differences between groups (range 0 – 5, mean 0.71 (s.d.1.3)). Similarly, there were no significant group differences in trait impulsivity (BIS), or anger-hostility (BDHI). Male subjects had higher scores on lifetime aggression (LHA) compared to females (t = 4.13, df =19, p = 0.001). There were no significant differences between groups on the TCI temperament scales for Novelty Seeking, Reward Dependence or Persistence; however, males had significantly higher scores than female subjects on Harm Avoidance (t = 3.18, df = 18, p = 0.005) (Table 1). TCI temperament scales were inter-correlated: Novelty Seeking with Harm Avoidance (r = 0.54, p = 0.014); Harm Avoidance with Reward Dependence (r = 0.57, p = 0.009) and Persistence (r = 0.59, p = 0.006).
3.2. Altanserin binding
There were no significant gender differences in non-specific binding (measured by cerebellar distribution volumes, CER VND), or metabolite levels sampled at 2, 10, 30, 60 and 90 minutes post injection (Table 2). MR-determined partial volume effects for the ROIs, separated by gender, ranged from 0.74 – 0.98. Atrophy correction factors were significantly greater in female subjects than in males in 2 of 10 ROIs: LOF (t = 2.78.df = 19, p = 0.012) and OCC (t = 2.45, df = 19, p = 0.02). Altanserin binding potentials were corrected for partial volume effects.
BP and BPp values for all ROIs in the two groups were normally distributed (K-S one sample test). Homogeneity of variance was demonstrated for BPp values for all pooled, left and right ROIs, and for BP values in all ROIs except OCC and Rt. OCC. In the total sample (N=21), BP values were highly correlated with BPp in each ROI. e.g. HIP: r = 0.94, p < .001; LOF: r = 0.70, p <.001; MFC: r = 0.69, p = .001; MOF: r = 0.66, p = .001; MTC: r = 0.83, p <.001; OCC: r = 0.59, p = .005; SUG: r = 0.68, p = .001; THL: r = 0.92, p <.001. Comparisons by gender are presented in terms of BPp (below). Both data sets are reported in Table 2.
Healthy male subjects had significantly greater mean BPp values than healthy females in 6 pooled and 9 lateralized ROIs (t-tests, Table 2.). After co-varying for age, gender differences in the pooled samples remained significant for 3 ROIs: HIP (F = 9.11, df = 1,20, p = 0.007), LOF (F = 5.85, df =1,20, p = 0.03), and THL (F = 4.49, df = 1,20, p = 0.05). In the lateralized samples, significant gender differences remained in 6 ROIs, all in the left hemisphere: Lt.-HIP (F = 12.11, df = 1,20, p = 0.003), Lt.-LOF (F = 9.17, df = 1,20, p = 0.007), Lt.-MFC (F = 5.22, df = 1,20, p = 0.04), Lt. MTC (F = 14.97, df = 1,20, p = 0.001), Lt. OCC(F = 4.67, df = 1,20, p = 0.04), and Lt. THL(F = 6.78, df = 1,20, p = 0.02). (With Bonferroni correction for multiple comparisons (p = 0.004), robust results remain in Lt. HIP and Lt. MTL, with a near significant trend in Lt.LOF.) These results were not attributable to outliers. Further analyses were limited to these 9 ROIs. (Similar results were found using BP values. With age covaried, male subjects had significantly greater altanserin binding than females in HIP and Lt. HIP, Lt. LOF, Lt. MTC, and Lt.THL).
3.3 SPM analysis
Altanserin binding was compared between male and female subjects on a voxel basis using SPM with age as a covariate. Male subjects demonstrated trends for greater altanserin binding than females in the left hemisphere, though results for clusters were too weak to withstand correction for multiple comparisons. Trends (p uncorr < .1) were identified in the areas of the left inferior frontal gyrus (BA 46), left insula and left temporal lobe (BA 37).(Fig.1.)
3.4. Psychological variables and BPp values
In the combined gender sample, regression analyses demonstrated no significant relationships between BPp values with age and gender as independent variables, and depressed mood (HamD) or impulsivity (BIS) as dependent variables. Gender was significantly related to aggression (LHA) in each ROI. With age and gender as covariates, aggression was inversely related to BPp values in Lt. MTC (r = − 0.71, df = 17, p = 0.001), Lt. LOF (r = −0.47, df = 17, p = 0.04) and Lt. MFC (r = −0.46, df =17, p = 0.05).
Negative correlations between aggression and BPp (with age covaried) were attributable to male, but not female subjects in Lt.LOF and Lt. MFC (e.g. Lt. LOF (r = −0.84, df = 7, p = 0.004), Lt. MFC (r = −0.82, df = 7, p = 0.007). Aggression was inversely related to BPp values in Lt.MTC in both male and female subjects, though more robust in the male (r = −0.85, df = 7, p = 0.004) than in the female subjects (r = −0.64, df = 8, p = 0.05).
Suspiciousness, a subscale of the BDHI, was positively correlated with BPp values in LOF (r = 0.54, df = 17, p = 0.02), Lt. LOF (r = 0.49, df = 17, p =0.04), and Lt. MFC (r = 0.46, df =17, p = 0.05). These correlations were attributable to male, but not female subjects. (e.g. LOF (r = 0.85, df = 7, p = 0.004), Lt.LOF (r = 0.75, df = 7, p = 0.02), and Lt. MFC (r = 0.67, df = 7, p = 0.05). Female, but not male subjects, had positive correlations between the Negativism subscale of the BDHI and BPp values in HIP (r = 0.70, df = 8, p = 0.025), and between the Verbal Hostility subscale and BPp values in Lt.HIP (r = 0.68, df = 8, p = 0.03).
The relationship between temperament (TCI) and altanserin binding was investigated in regression models, with age, gender and BPp values in each ROI as independent variables, Novelty Seeking, Harm Avoidance, Reward Dependence and Persistance examined individually as dependent variables. Persistance was positively related to BPp values in Lt. MTC (r = 0.51, df = 16, p = 0.03) with no significant gender effect. BPp values were not significantly related to Novelty Seeking, Harm Avoidance or Reward Dependence in any ROI.
4. DISCUSSION
Among healthy subjects, significant gender differences were found in altanserin binding in areas of the frontal lobes (LOF, MFC), temporal lobes (HIP, MTC), thalamus, and occipital cortex, with greater BPp values among male subjects relative to females in each ROI. Increased BPp values suggest increased 5HT2A receptor number or affinity in these areas. Our results are comparable, though not identical, to the findings of Biver et. al.,(1996), who found increased altanserin binding in healthy male compared to female subjects in frontal, parietal, and temporal lobes, bilaterally, and in median cingulate cortex. Differences in outcome between our studies and three negative reports (Baeken et.al., 1998, Meltzer et.al., 1998, Frokjaer et.al.2008), may be due to different methods, age range of subjects, or inter-subject variability, amplified by small sample sizes.
Gender differences in central serotonergic function are widely reported in pre-clinical and clinical studies, involving serotonin neurobiology at many levels of function. In animal models, region-specific gender differences are noted in the expression of serotonin-1A and -2A receptor messenger RNA and 5-HT2A receptor binding (Zhang et.al., 1999). Manipulation of sex steroids in male rats (e.g. gonadectomy, exogenous testosterone) modulates region-specific transcriptional regulation of 5-HT1A and 5-HT2A receptors. (e.g. Gonadectomy increases serotonin-1A m-RNA, decreases serotonin-2A m-RNA in discrete regions (Zhang et.al. 1999)). In clinical studies, hormone replacement therapy (estradiol, progesterone) in post menopausal women is associated with upregulation and increased 5HT2A binding in areas of PFC, pregenual cortex and dorsal anterior cingulate (Moses et.al., 2000).
Gender-related differences are reported in the neuroendocrine response to serotonergic challenge with d,l, fenfluramine (FEN) in healthy non-patient subjects (McBride et.al., 1990), and in cortical metabolic responses to FEN- activation in PET neuroimaging studies, in both healthy control and impulsive BPD subjects (Soloff et.al., 2005). Serotonin synthesis, assessed using alpha [11C]-methyl-L-tryptophan and PET neuroimaging is reported to be 52% greater in healthy male subjects compared to females (Nishizawa et.al., 1997). Some, but not all, early studies of the serotonin transporter site using [3H]imipramine or [3H]paroxetine in the platelet model, or in postmortem brain samples, reported increased affinity (lower Kd) in healthy female subjects compared to males (Klompenhouwer et al., 1990; Halbreich et al., 1991; Marazziti et al., 1998; Arato et.al., 1991). An age × gender interaction has been reported for [3H]paroxetine binding in the platelet model, especially noteworthy in female subjects, where affinity is greater in younger females compared to younger males, and reversed with age. i.e. Older females have decreased affinity (high Kd) compared to older males (Marazziti et al., 1998).
Female subjects demonstrate greater binding of [11C-carbonyl]WAY-100635 to the 5HT1A receptor compared to healthy male subjects (Parsey et.al., 2002). In this study, sex differences were significant in dorsal raphe, amygdala, anterior cingulate, cingulate body, medial and orbital prefrontal cortex. Binding potential was inversely related to aggression in several regions-of-interest.
Gender specific aging effects have also been noted in studies of 5HT1A receptor function. In a PET study using [11C-carbonyl]WAY100635, Meltzer et.al., (2001) found an inverse relationship between age and binding to the 5HT1A receptor throughout the cortex and brainstem in healthy men but not in women. Other investigators have also noted a greater loss of 5HT1A receptor density with age in men compared to women (Matsubara et.al., 1991; Dillon et.al., 1991). The current study, (and that of Bivers et.al., (1996)) suggests that female subjects have diminished binding to the post-synaptic 5HT2A receptor, compared to healthy males.
The functional significance of these gender differences in normal subjects remains unclear. Some investigators have suggested that decreased serotonin synthesis in females relative to males, and decreased affinity of the serotonin transporter site among older females, may increase vulnerability to depression in females (Nishizawa et.al., 1997; Marazziti et.al., 1998). Sex differences in serotonergic function are also region-specific, with behavioral consequences related to the function of specific neural networks. e.g. Increased [3H]imipramine binding to the serotonin transporter site in orbital frontal cortex of female subjects compared to males may mediate better impulse regulation in women (Arato et.al., 1991). Similarly, increased 5HT1A binding in prefrontal cortex in healthy female subjects relative to males (Parsey et.al., 2002), and decreased post-synaptic 5HT2A binding in healthy females relative to male subjects in frontal and cingulate cortex (Biver et.al., 1996), may be associated with diminished aggression in female subjects. The increased 5HT2A receptor binding found in our male subjects relative to females, especially in areas of prefrontal cortex (e.g. Lt. LOF), is consistent with a hypothesis of diminished serotonergic agonism, and increased vulnerability to impulsive and aggressive behavior in male compared to female subjects. Alternatively, increased 5HT2A in males (or decreased binding in females) could reflect the effect of sex hormones on receptor expression. The laterality of our findings, with a preponderance of gender differences on the left side, suggests that gender may be a determinant in the expression of behaviors mediated by left-sided neural pathways in these ROIs. e.g. The left frontal cortex, (especially Lt. LOF) may be more involved in regulation of impulsive-aggressive behavior than the right (New et.al., 2002).
We found clear gender differences in the co-variation of psychological traits with BPp values; however, given the small sample sizes, and the possibility of Type I error, results of our regression analyses must be viewed with caution as exploratory. Female, but not male subjects had positive correlations between Negativism and Verbal Hostility on the BDHI, and altanserin binding in areas of the hippocampus. Negativism is defined by “oppositional behavior,” especially toward authority figures (Buss and Durkee, 1957) and may be conceptually related to Beck’s construct of “dysfunctional attitudes.” (Dysfunctional attitudes are formally defined as “negatively biased views of oneself, the world and the future, independent of depressed mood” (Meyer et.al., 2003)). Scores on a Dysfunctional Attitude Scale are positively correlated with 5-HT2 receptor binding, independent of depressed mood, in subjects with acute or remitted depressive disorder (Meyer et.al., 2003; Bhagwagar, et al., 2006). In healthy control subjects, Dysfunctional Attitude Scale scores diminish with FEN challenge and increased serotonergic agonism (Meyer et.al., 2003). Increased BPp values in HIP may be associated with a vulnerability to such dysfunctional attitudes in normal female subjects, which, in turn, may predispose to development of mood disorders.
Few investigators have specifically addressed the relationship between gender, psychological traits and 5HT2A receptor function. Moresco et.al., (2002), found a negative correlation between Harm Avoidance and binding of ([18F] FESP in frontal cortex, bilaterally, and in left parietal cortex, using a manual ROI method. Use of an automated SPM analysis extended the area with significant correlation to include regions of prefrontal, tempoparietal, parietal and occipitotemporal cortex, anterior cingulate and hypothalamus. In this small sample study (3 females vs. 8 males), no gender effects were found.
Frokjaer et.al., (2008) reported a positive correlation between the Neuroticism factor score (a NEO-PI-R subscale) and [18F]altanserin binding in a large area of frontolimbic cortex in a sample of 83 healthy volunteers. With age and gender co-varied, two constituent traits of Neuroticism, vulnerability and anxiety, were significantly correlated with frontolimbic binding (with a trend for depression). They found no gender effects on frontolimbic binding or on the correlation between BP values and Neuroticism. In contrast, we found positive correlations in male, but not female subjects, between Suspiciousness and BPp values in frontal ROIs (LOF, MFC), and in female but not male subjects, between BPp values, Negativism and Verbal Hostility in hippocampus. Differences in definitions of ROIs limit comparisons between our studies. e.g. Frokjaer et. al., (2008) did not report discrete BP values for HIP or LOF.
Among our healthy male, but not female subjects, aggression (LHA) was negatively correlated with BPp values in areas of left frontal and temporal cortex (Lt. LOF, Lt. MFC, Lt. MTC). The orbital frontal cortex and hippocampal-amygdala complex are integral parts of a neural circuit involved with emotion regulation and response inhibition. (Our MTC includes both HIP and amygdala). Impulsivity and impulsive-aggressive behavior in both patient and healthy subjects have been associated with evidence of diminished central serotonergic function, especially in areas of prefrontal cortex (Oquendo and Mann, 2000). Finding an association between heightened aggression and diminished 5-HT2A receptor number or affinity in these areas is contrary to expectation. The positive correlations between Suspiciousness in males, Negativism and Verbal Hostility in females, and altanserin binding in specific ROIs is consistent with the hypothesis of diminished serotonergic agonism. However, since our study was not designed to assess the relationship between endogenous serotonin levels and 5HT2A receptor binding, alternative hypotheses should be considered (e.g. an effect of sex hormones on on receptor expression.) Larger sample studies are needed to test the validity of these findings in healthy subjects.
4.1 Limitations
We did not sample estrogen or progesterone blood levels at the time of the scan; nor was it possible to co-ordinate the PET study with each subject’s menstrual cycle. Fluctuations in ovular hormone levels may play a role in serotonergic receptor binding, i.e. hormone regulation may influence receptor expression rates. The clinical literature is not consistent in regards to specific effects of ovular hormones on the 5HT2A receptor. Moses et.al. (2000) conducted serial PET scans on 5 post-menopausal women (mean age (s.d.) = 52.0 (3.3) yrs.) before hormone replacement, after estradiol replacement (8–14 wks.), and after combined estradiol plus progesterone therapy (2 – 6 wks). Compared to baseline, they found increased 5HT2A binding in widespread areas of the cerebral cortex following the hormone replacement treatments, with greatest effect after combined treatment. Frokjaer et.al. (2008) found no effect of exogenous or replacement hormone therapy on 5HT2A binding in a sample of 31 healthy women, 9 of whom were on hormonal contraception or replacement therapy. They concluded that endogenous or therapeutic levels of estradiol did not significantly affect 5HT2A receptor levels.
By self-report, all of our female subjects had normal menstrual cycles at the time of the PET study; however, it is possible that some could have been “peri-menopausal” (e.g. with low levels of ovular hormones). Future studies should sample ovular hormones at the time of the scan to control for this potentially confounding variable.
The use of modest sample sizes in this study begs the question of whether we had sufficient statistical power to detect differences between groups. Gender differences in BP and BPp values reflect large effect sizes for ROIs with significant differences (e.g. 0.88 – 1.53 for BP values). For an effect size greater than 1.40 (e.g. BP values for Lt. HIP, Lt. MTC), sample sizes of 9 subjects per group would suffice for 80% power for detecting differences at the p = 0.05 level (Cohen J., 1988). While adequately powered to detect these large effects, our sample size is insufficient to detect small or medium effects. Similarly, we were underpowered to detect differences in our exploratory regression analyses of the relationship between altanserin binding and psychological characteristics. For our regression models with three independent variables (age, gender, BP values), 34 subjects are required to detect large effects with power = .80 and alpha = .05. (Cohen J., 1992). Significant findings in our exploratory analyses require replication with larger numbers.
The use of multiple comparisons in our analyses raises the issue of controlling for Type I error. We reported Bonferroni correction in the main contrast of gender with BPp values and noted that significant differences remained between groups for Lt. HIP and Lt. MTL after correction. Our analyses of the relationship between altanserin binding and psychological characteristics is exploratory in nature. Although correction for multiple comparisons is not appropriate in this setting, significant findings support the need for confirmation with larger samples.
4.2 Conclusions
Our results suggest that altanserin binding differs between healthy male and female subjects in areas of the brain involved in regulation of impulse and mood. Region-specific gender differences in serotonergic functioning in healthy subjects may mediate differences in the expression of personality characteristics such as aggression, suspiciousness, negativism and verbal hostility. Regional differences in 5HT2A receptor function may also contribute to the neurobiology of temperament independent of gender, e.g. Persistence in our study, Harm Avoidance in Moresco et.al. (2002) and Neuroticism in Frokjaer et.al. (2008). Future studies of the 5HT2A receptor and its behavioral correlates in both normal and clinical subjects should control for gender.
ACKNOWLEDGEMENT
This work was supported by grants from the American Foundation for Suicide Prevention (PHS) and the National Institute of Mental Health: MH48463 (PHS), KO AG027998 (JCP), MH59945, MH67602, MH64625 (CCM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams K, Pinborg LH, Svarer C, Hasselbalch SG, Holm S, Haugbol S, Madsen K, Frokjaer V, Martiny L, Paulson OB, Knudsen GM. A database of [18F]-altanserin binding to 5HT2a receptors in normal volunteers: normative data and relationship to physiological and demographic variables. Neuroimage. 2004;21:1105–1113. doi: 10.1016/j.neuroimage.2003.10.046. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre-and post synaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Research. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Mann JJ. Postmortem findings in suicide victims. Implications for in vivo studies. Annals of the New York Academy of Science. 1997;836:269–287. doi: 10.1111/j.1749-6632.1997.tb52365.x. [DOI] [PubMed] [Google Scholar]
- Arato M, Frecska E, Tekes K, MacCrimmon DJ. Serotonergic interhemispheric asymmetry: gender difference in the orbital cortex. Acta Psychiatrica Scandinavica. 1991;84:110–111. doi: 10.1111/j.1600-0447.1991.tb01431.x. [DOI] [PubMed] [Google Scholar]
- Audenaert K, Van Laere K, Dumont F, Slegers G, Mertens J, van Heeringen C, Dierckx RA. Decreased frontal serotonin 5HT2a receptor binding index in deliberate self-harm patients. European Journal of Nuclear Medicine. 2001;28(2):175–182. doi: 10.1007/s002590000392. [DOI] [PubMed] [Google Scholar]
- Baeken C, D’haenen H, Flamen P, Mertens J, Terriere D, Chavatte K, Boumon R, Bossuyt A. 123I-5-I- R91150, a new single photon emission tomography ligand for 5-HT2A receptors: influence of age and gender in healthy subjects. European Journal of Nuclear Medicine. 1998;25:1617–1622. doi: 10.1007/s002590050339. [DOI] [PubMed] [Google Scholar]
- Bailer UF, Price JC, Meltzer CC, Mathis CA, Frank GK, Weissfeld L, McConaha CW, Henry SE, Brooks-Achenbach S, Barbarich NC, Kaye WH. Altered 5-HT2A receptor binding after recovery from bulimia-type anorexia nervosa: Relationships to harm avoidance and drive for thinness. Neuropsychopharmacology. 2004;29:1143–1155. doi: 10.1038/sj.npp.1300430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt ES, Slaughter L. Defining, measuring and predicting impulsive-aggression: A heuristic model. Behavioral Sciences and the Law. 1998;16:285–302. doi: 10.1002/(sici)1099-0798(199822)16:3<285::aid-bsl308>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Hinz R, Taylor M, Fancy S, Cowen P, Grasby P. Increased 5-HT2A receptor binding in euthymic, medication-free patients recovered from depression: A positron emission study with [11 C] MDL 100, 907. American Journal of Psychiatry. 2006;163:1580–1587. doi: 10.1176/ajp.2006.163.9.1580. [DOI] [PubMed] [Google Scholar]
- Biver F, Lotstra F, Monclus M, Wikler D, Damhaut P, Mendlewicz J, Goldman S. Sex difference in 5-HT2 receptor in the living human brain. Neuroscience Letters. 1996;204(1–2):25–28. doi: 10.1016/0304-3940(96)12307-7. [DOI] [PubMed] [Google Scholar]
- Biver F, Wikler D, Lotstra F, Damhaut P, Goldman S, Mendlewicz J. Serotonin 5-HT2 receptor imaging in major depression: focal changes in orbito-insular cortex. British Journal of Psychiatry. 1997;171:444–448. doi: 10.1192/bjp.171.5.444. [DOI] [PubMed] [Google Scholar]
- Brown GL, Goodwin FK. Cerebrospinal fluid correlates of suicide attempts and aggression. Annals of the New york Academy of Science. 1986;487:175–188. doi: 10.1111/j.1749-6632.1986.tb27897.x. [DOI] [PubMed] [Google Scholar]
- Buss AH, Durkee A. An inventory for assessing different kinds of hostility. Journal of Consulting and Clinical Psychology. 1959;21:343–349. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD. Center for Psychobiology of Personality. St. Loius, Missouri: Washington University; 1994. The Temperament and Character Inventory (TCI): A guide to its development and use. 1994. [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Dillon K, Gross-Isseroff R, Israeli M, Biegon A. Autoradiographic analysis of serotonin 5-HT1A receptor binding in the human brain post-mortem: effects of age and alcohol. Brain Research. 1991;554:56–64. doi: 10.1016/0006-8993(91)90171-q. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Pandey GN. Quantitation of 5HT2a receptor mRNA in human postmortem brain using competitive RT-PCR. Neuroreport. 1998;9:3761–3765. doi: 10.1097/00001756-199812010-00001. [DOI] [PubMed] [Google Scholar]
- Frank GK, Kaye WH, Meltzer CC, Price JC, Greer P, McConaha C, Skovira K. Reduced 5-HT2A receptor binding after recovery from anorexia nervosa. Biological Psychiatry. 2002;52:896–906. doi: 10.1016/s0006-3223(02)01378-1. [DOI] [PubMed] [Google Scholar]
- Frokjaer VG, Mortensen EL, Nielsen FA, Haugbol S, Pinborg LH, Adams KH, Svarer C, Hasselbalch SG, Holm S, Paulson OB, Knudsen GM. Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biological Psychiatry. 2008;63:569–576. doi: 10.1016/j.biopsych.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual of Psychopharmacology-Revised. Rockville, Md: 1976. DHEW Publ. No. ADM 76–338. [Google Scholar]
- Halbreich U, Rojansky N, Zander KJ, Barkai A. Influence of age, sex, and diurnal variability on imipramine receptor binding and serotonin uptake in platelets of normal subjects. Journal of Psychiatric Research. 1991;25:7–18. doi: 10.1016/0022-3956(91)90012-y. 1991. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gunn RN, Holden J, Houle S, Huang S-C, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun M, Morris ED, Parsey R, Price J, Slifstein M, Sossi V, Suhara T, Votaw J, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow and Metabolism. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Frank GK, Meltzer CC, Price JC, McConaha C, Crossan PJ, Klump KL, Rhodes L. Altered serotonin 2A receptor activity in women who have recovered from bulimia nervosa. American Journal of Psychiatry. 2001;158:1152–1155. doi: 10.1176/appi.ajp.158.7.1152. [DOI] [PubMed] [Google Scholar]
- Klompenhouwer J-L, Fekkes D, van Hulst AM, Moleman P, Pepplinkhuizen L, Mulder PGH. Seasonal variations in binding of 3H-paroxetine to blood platelets in healthy volunteers: indications for a gender difference. Biological Psychiatry. 1990;28:509–517. doi: 10.1016/0006-3223(90)90484-j. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Lemaire C, Cantineau R, Guillaume M, Plenevaux A, Christiaens L. Fluorine-18-altanserine : a radioligand for the study of serotonin receptors with PET : radiolabeling and in vivo biologic behavior in rats. Journal of Nuclear Medicine. 1991;32:2266–2272. [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hitzemann R, Bendriem B, Gatley SJ, Christman DR. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. Journal of Cerebral Blood Flow and Metabolism. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- Loranger AW, Susman VL, Oldham JM, Russakoff LM. The Personality Disorder Examination: A preliminary report. Journal of Personality Disorders. 1987;1:1–3. [Google Scholar]
- Marazziti D, Rossi A, Palego L, Barsanti A, Carrai M, Giannaccini G, Serra P, Lucacchini A, Cassano GB. Effect of aging and sex on the [3H]-paroxetine binding to human platelets. Journal of Affective Disorders. 1998;50:11–15. doi: 10.1016/s0165-0327(97)00074-8. [DOI] [PubMed] [Google Scholar]
- Matsubara S, Arora H, Meltzer H. Serotonergic measures in suicide brain: 5HT1A binding sites in frontal cortex of suicide victims. Journal of Neural Transmission. 1991;85:181–194. doi: 10.1007/BF01244944. [DOI] [PubMed] [Google Scholar]
- McBride PA, Tierney H, DeMeo M, Chen J-S, Mann JJ. Effects of age and gender on CNS serotonergic responsivity in normal adults. Biological Psychiatry. 1990;27:1143–1155. doi: 10.1016/0006-3223(90)90051-3. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Drevets WC, Price JC, Mathis CA, Lopresti B, Villemagne VL, Holt D, Mason NS, Houck PR, Reynolds CF, DeKosky ST. Gender-specific aging effects on the serotonin 1A receptor. Brain Research. 2001;895:9–17. doi: 10.1016/s0006-8993(00)03211-x. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Kinahan PE, Greer PJ, Nichols TE, Comtat C, Cantwell MN, Price JC. Comparative evaluation of MR-based partial volume correction schemes for PET. Journal of Nuclear Medicine. 1999;40:2053–2065. [PubMed] [Google Scholar]
- Meltzer CC, Smith G, Price JC, Reynolds CF, III, Mathis CA, Greer P, Lopresti B, Mintun M, Pollack BG, Ben-Eliezer D, Canwell MN, Kaye W, DeKosky ST. Reduced binding of [18 F] altanserin to serotonin type 2A receptors in aging: persistance of effect after partial volume correction. Brain Research. 1998;813:167–171. doi: 10.1016/s0006-8993(98)00909-3. [DOI] [PubMed] [Google Scholar]
- Messa C, Colombo C, Moresco RM, Gobbo C, Galli L, Lucignanbi G, Gilardi MC, Rizzo G, Simeraldi E, Zanardi R, Artigas F, Fazio F. 5-HT2A receptor binding is reduced in drug-naïve and unchanged in SSRI-responder depressed patients compared to healthy controls: a PET study. Psychopharmacology. 2003;167:72–78. doi: 10.1007/s00213-002-1379-5. [DOI] [PubMed] [Google Scholar]
- Meyer JH, McMain S, Kennedy SH, Korman L, Brown G, DaSilva JN, Wilson AA, Blak T, Eynan-Harvey R, Goulding V, Houle S, Links P. Dysfunctional attitudes and 5HT2 receptors during depression and self-harm. American Journal of Psychiatry. 2003;160:90–99. doi: 10.1176/appi.ajp.160.1.90. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Sheline YI, Moerlein SM, Vlassenko AG, Huang Y, Snyder AZ. Decreased hippocampal 5-HT2A receptor binding in major depressive disorder: In vivo measurement with [18F] altanserin positron emission tomography. Biological Psychiatry. 2004;55:217–224. doi: 10.1016/j.biopsych.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Moresco FM, Dieci M, Vita A, Messa C, Gobbo C, Galli L, Rizzo G, Panzacchi A, De Peri L, Invernizzi G, Fazio F. In vivo serotonin 5HT2A receptor binding and personality traits in healthy subjects: A positron emission tomography study. Neuroimage. 2002;17:1470–1478. doi: 10.1006/nimg.2002.1239. [DOI] [PubMed] [Google Scholar]
- Moses EL, Drevets WC, Smith G, Mathis CA, Kalro BN, Butters MA, Leondires MP, Greer P, Lopresti B, Loucks TL, Berga SL. Effects of estradiol and progesterone administration on human serotonin 2A receptor binding: a PET study. Biological Psychiatry. 2000;48:854–860. doi: 10.1016/s0006-3223(00)00967-7. [DOI] [PubMed] [Google Scholar]
- New AS, Hazlett EA, Buchsbaum MS, Goodman M, Reynolds D, Mitropoulou V, Sprung L, Shaw RB, Jr, Koenigsberg H, Platholi J, Silverman J, Siever LJ. Blunted prefrontal cortical 18Fluorodeoxyglucose positron emission tomography response to meta-chlorophenylpiperazine in impulsive aggression. Archives of General Psychiatry. 2002;59:621–629. doi: 10.1001/archpsyc.59.7.621. [DOI] [PubMed] [Google Scholar]
- Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, Blier P, Diksic M. Differences between males and females in rates of serotonin synthesis in human brain. Proceedings of the National Academy of Sciences, USA. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Mann JJ. The biology of impulsivity and suicidality. Psychiatric Clinics of North America. 2000;23(1):11–27. doi: 10.1016/s0193-953x(05)70140-4. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, Mann JJ. Effects of age, sex, and aggressive traits in man on brain serotonin 5-HT(1A) receptor ligand binding potential measured by PET using [C-11] WAY-100635. Brain Research. 2002;954(2):173–182. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- Price JC, Lopresti BJ, Mason NS, Holt DP, Huang Y, Mathis CA. Analyses of [18 F] altanserin bolus injection PET data. II. Consideration of radiolabeled metabolites in baboons. Synapse. 2001a;41:1–10. doi: 10.1002/syn.1054. [DOI] [PubMed] [Google Scholar]
- Price JC, Lopresti BJ, Meltzer CC, Smith G, Mason NS, Huang Y, Holt DP, Gunn RN, Mathis CA. Analyses of [18 F] altanserin bolus injection PET data. II. Consideration of radiolabeled metabolites in humans. Synapse. 2001b;41:11–21. doi: 10.1002/syn.1055. [DOI] [PubMed] [Google Scholar]
- Rosel P, Arranz B, San L, Vallejo J, Crespo JM, Urretavizcaya M, Navarro MA. Altered 5-HT(2A) binding sites and second messenger inositol triphosphate (IP(3)) levels in hippocampus but not in frontal cortex from depressed suicides. Psychiatry Research. 2000;99:173–181. doi: 10.1016/s0925-4927(00)00076-7. [DOI] [PubMed] [Google Scholar]
- Rosier A, Dupont P, Peuskens J, Bormans G, Vandenberghe R, Maes M, de Groot T, Schiepers C, Verbruggen A, Mortelmans L. Visualization of loss of 5-HT2A receptors with age in healthy volunteers using [18F]altanserin and positron emission tomographic imaging. Psychiatry Research: Neuroimaging Section. 1996;68:11–22. doi: 10.1016/s0925-4927(96)02806-5. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Meltzer CC, Becker C, Greer PJ, Constantine D. Gender differences in a fenfluramine-activated FDG PET study of borderline personality disorder. Psychiatry Research: Neuroimaging. 2005;138:183–195. doi: 10.1016/j.pscychresns.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Price JC, Meltzer CC, Fabio A, Frank GK, Kaye WH. 5HT2A receptor binding is increased in borderline personality disorder. Biological Psychiatry. 2007;62:580–587. doi: 10.1016/j.biopsych.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Instruction Manual for the Structured Clinical Interview for DSMIII-R. 722 West 168th Street, NY, NY: Biometrics Research Department, New York State Psychiatric Inst.; 1988. [Google Scholar]
- Staley JK, Van Dyck CH, Tan PZ, Al Tikriti M, Ramsby Q, Klump H, Ng C, Garg P, Soufer R, Baldwin RM, Innis RB. Comparison of [18 F] altanserin and [18 F] deuteroaltanserin for PET imaging of serotonin 2A receptors in baboon brain: Pharmacologic studies. Nuclear Medicine and Biology. 2001;28:271–279. doi: 10.1016/s0969-8051(00)00212-2. [DOI] [PubMed] [Google Scholar]
- Van Heeringen C, Audenaert K, Van Laere K, Dumont F, Slegers G, Mertens J, Dierckx RA. Prefrontal 5-HT2A receptor binding index, hopelessness and personality characteristics in attempted suicide. Journal of Affective Disorders. 2003;74:149–158. doi: 10.1016/s0165-0327(01)00482-7. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. Journal of Computer Assisted Tomography. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Liddle PF, Shiah I-Shin, Scarrow G, Lam R, Adam MJ, Zis AP, Ruth TJ. Brain serotonin2 receptors in major depression: A positron emission tomography study. Archives of General Psychiatry. 2000;57:850–858. doi: 10.1001/archpsyc.57.9.850. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ma W, Barker JL, Rubinow DR. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: A possible role of testosterone. Neuroscience. 1999;94:251–259. doi: 10.1016/s0306-4522(99)00234-1. [DOI] [PubMed] [Google Scholar]