Abstract
The postnatal maternal environment is known to increase susceptibility to a number of autoimmune diseases. Here we asked whether the postnatal maternal environment could influence autoimmune disease development to day 3 thymectomy (d3tx)-induced autoimmune ovarian disease (AOD) and experimental allergic encephalomyelitis (EAE) in cross-fostered A/J and B6 mice. A/J pups foster-nursed by B6 mothers exhibit an increase in autoimmune disease development while cross-fostering B6 pups on A/J mothers did not alter their susceptibility. The increase in AOD incidence seen in foster-nursed d3tx A/J mice correlated with a decrease in the total number of CD4+ T cells in the lymph nodes of these animals. Analysis of the cellular composition in the milk revealed that B6 mice shed significantly more maternally derived lymphocytes into their milk compared to A/J mothers. These data suggest that there are maternally derived postnatal factors that influence the development of autoimmune disease in A/J mice.
Keywords: Autoimmunity, CD4+ T cells, maternal environment, cross-fostered, A/J mice, microchimerism
1. Introduction
Self-tolerance encompasses a critical regulatory component of the immune system that prevents the activation and proliferation of autoreactive cells [1]. This is achieved at two principle levels, including central and peripheral tolerance. Central tolerance is dependent on lymphocytes encountering self-antigens during maturation in the primary lymphoid organs, including the thymus and bone marrow. If the lymphocytes prove to be autoreactive, they will either be deleted or edited to alter their antigen affinity [2]. Peripheral tolerance is applied when autoreactive cells escape detection by central tolerance mechanisms and enter into the periphery. However, when these self-tolerance mechanisms fail to render autoreactive cells inactive, autoimmune disorders may ensue [3]. It is not known what causes the breakdown of central and/or peripheral tolerance to trigger autoreactive lymphocytes, but both environmental and genetic factors are thought to be crucial.
Autoimmune disorders can be induced in inbred mouse strains by thymectomizing the animals at 3 days after birth (d3tx) [4,5]. D3tx induces numerous autoimmune disorders in mice depending on the background of the inbred strain [5]. One such disease is autoimmune ovarian disease (AOD), which leads to ovarian atrophy [6,7]. The emergence of d3tx-induced AOD is attributed to the presence of autoreactive CD4+ T cells in the periphery of susceptible mice that arise by escaping central tolerance in the neonatal thymus [8]. Since autoreactive CD4+ T cells expand in the periphery of d3tx mice, inhibition of these cells is presumed to occur as a result of peripheral tolerance in resistant mouse strains.
Another autoimmune disease linked to autoreactive CD4+ T cell responses is experimental allergic encephalomyelitis (EAE), the mouse model for multiple sclerosis (MS). EAE can be induced in susceptible mouse strains by immunizing the animals with components of self-myelin, such as myelin oligodendrocyte glycoprotein (MOG), combined with an adjuvant [9]. This results in the activation of myelin specific CD4+ T cells that have escaped central tolerance and reside in the periphery [10]. These cells enter into the central nervous system and initiate a cascade of events leading to the development of ascending progressive paralysis beginning with a flaccid tail and progressing to the hind and forelimbs in susceptible mice [11].
It has been well documented that different genetic backgrounds show varying degrees of susceptibility to d3tx-induced AOD [5] and EAE [12]. For instance, A/J female mice are highly susceptible to d3tx-induced AOD and develop anti-ovarian autoantibodies, oophoritis and atrophy while d3tx C57BL/6J (B6) female mice are resistant [13]. In contrast, A/J mice immunized with MOG are relatively resistant to the induction of EAE compared to B6 mice. Genetic analysis has revealed a complex genetic mechanism linked to 5 quantitative trait loci (QTL) that control susceptibility to AOD [13-16] and over 30 QTLs that control susceptibility to EAE [17-19]. While genetics plays a significant role in disease susceptibility, autoimmune disease development arises in genetically susceptible individuals through complex interactions between genes and environmental factors. This is exemplified in the EAE model, where non-genetic factors, including age and season at immunization, contribute to gene-by-environment interactions and susceptibility to disease [20].
The postnatal maternal environment provides a highly influential source of environmental variability between mice from different strains and presents developing newborns with a barrage of factors that can influence their propensity for the development of certain diseases. In addition to the influence of maternal behavior on offspring development, the quality of maternal milk is known to sculpt phenotypic differences. Studies have shown that breast milk containing antigen can lead to oral tolerance and the prevention of allergic asthma in nursing offspring [21, 22]. It has also been documented that mice genetically predisposed to develop diabesity, a disease defined as obese diabetics, can be influenced by the quality of milk being ingested. When nursed by obese mothers, these mice weighed substantially more than when they were nursed by lean mothers, suggesting that the milk from obese mothers contains diabesity-promoting factors [23]. Additionally, it has been documented that nonobese diabetic (NOD) mice can be protected from type 1 diabetes depending on the strain of the foster mother [24].
Here we addressed whether the postnatal maternal environment significantly influenced the disease course of d3tx-induced AOD and EAE in A/J and B6 mice by conducting foster nursing experiments. These experiments revealed that A/J mice become more susceptible to d3tx-induced AOD and to EAE when nursed by B6 mothers. Conversely, foster nursing B6 mice by A/J mothers did not alter the natural disease course in these animals. Foster-nursed d3tx A/J mice exhibit a reduced number of CD4+ T cells in their lymph nodes compared to naturally nursed A/J mice. In addition, A/J mice naturally possess significantly fewer CD4+ T cells in their spleens compared to B6 mice. The analysis of milk obtained from B6 and A/J mothers revealed a significant difference in the percentage of lymphocytes shed into the milk. B6 mice pass a higher percentage of lymphocytes to nursing pups at 3 days after birth, which has been previously shown to be the critical time point for maternal cell uptake [25]. The milk also contained detectable levels of IL-9, IL-13 and CXCL1 cytokines and chemokines, however the quantity of these cytokines did not significantly differ between A/J and B6 milk. These data suggest that there are maternally derived postnatal factors that influence the development of AOD and EAE in A/J mice.
2. Materials and Methods
2.1. Animals and Day Three Thymectomy
Adult naturally and cross-fostered litters of d3tx B6 and A/J mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Cross-fostering was accomplished within 24 hours of birth using matched B6 and A/J litters. D3tx was conducted under hypothermic anesthesia using a suction pipette technique [26]. At 120 days of age the mice were euthanized, tissues collected and processed for histopathology and immune profiling. Animals with a residual thymus were excluded from the study. Age matched A/J and B6 female mice immunized for EAE were purchased from The Jackson Laboratory. The experimental procedures performed in this study were approved by the Institutional Animal Care and Use Committee of the University of Vermont.
2.2. Analysis of AOD phenotypes
Ovaries were fixed in Bouin's fixative and embedded in paraffin, and 5-μm sections were stained with H&E. Multiple-step section were evaluated in a double-blind manner and the ovaries scored for oophoritis and atrophy as previously described [4, 5, 27, 28].
2.3. Induction and Evaluation of EAE
Mice were immunized for the induction of EAE using the double-inoculation (2×) and single-inoculation (1×) protocols [29]. Briefly, for the 2× protocol mice were injected subcutaneously with a sonicated emulsion of 100 μg of MOG35-55 for B6 mice or MOG97-114 for A/J mice in combination with an equal volume of CFA containing 200 μg of Mycobacterium tuberculosis H37RA (Difco Laboratories, Detroit, MI) in the posterior right and left flank; one week later all mice were similarly injected at two sites on the right and left flank anterior of the initial injection sites. The 1× immunized mice received 200 μg of encephalitogenic peptide and an equal volume of CFA containing 400 μg of Mycobacterium tuberculosis H37RA (Difco Laboratories, Detroit, MI) in the right and left flank followed by the i.v. injection of 200 ng pertussis toxin (PTX) (List Biological Laboratories, Campbell, CA). Mice were scored daily starting at day 10 post-injection and clinical quantitative trait variables including disease incidence, mean day of onset, cumulative disease score, number of days affected, overall severity index, and peak score were generated.
Brains and spinal cords were dissected from calvaria and vertebral columns, respectively, and fixed by immersion in 10% phosphate-buffered formalin (pH 7.2). Following adequate fixation, the tissues were trimmed and representative transverse section embedded in paraffin, sectioned at 5 μm, and mounted on glass slides. Sections were stained with H&E for routine evaluation and Luxol fast blue-periodic acid Schiff for demyelination. Sections from representative areas of the brain and spinal cord were scored in a semiquantitative fashion for the various histopathological parameters as previously described [18, 19, 30, 31]. Briefly, EAE pathology was scored for the overall severity of the lesions observed, the extent and degree of demyelination and tissue injury (swollen axon sheathes, swollen axons, and reactive gliosis), severity of the acute inflammatory response such as neutrophil infiltration, and the severity of the chronic inflammatory response (lymphocytes/monocytes).
2.4. Lymphocyte isolation and FACs analysis
The spleens, inguinal, axillary and brachial lymph nodes were excised and single cell suspensions generated by gently teasing the tissues through nylon mesh (Small Parts, Inc). Cells numbers were obtained using ADVIA® 120 Hematology System (Siemens Healthcare Diagnostics, Deerfield, IL). Fc receptors were neutralized using 2.4G2 mAb (BD Pharmingen, Franklin Lakes, NJ) and cells were stained with anti-mouse CD4-TexasRed (Caltag, Bulingame, CA), anti-mouse CD8-Alexa647 (BD Pharmingen, Franklin Lakes, NJ) and anti-mouse TCRβ̃FITC (eBioscience, San Diego, CA). Maternal WBCs from milk samples were stained with anti-mouse CD45.2-PerCP and anti-mouse CD45.1-FITC (eBioscience, San Diego, CA). Flow cytometric analysis was performed on LSR II (BD Bioscience, San Jose, CA) and analyzed with FlowJo 8.8.4 software (TreeStar, Ashland, OR).
2.5. Enumeration of maternally derived cells in milk
Milk filled stomachs were extracted from A/J and B6 pups 3 days after birth and cells were isolated as previously described [32] with modifications. The isolated stomachs were weighed and combined so that the milk samples collected from each mother contained approximately 200 mgs of milk. The milk was extracted from the stomach lining and placed in a 60 cm dish containing 1 ml PBS and homogenized using a 3 ml syringe plunger. The milk was transferred to a 15 ml tube and the 60 cm dish was rinsed with an additional 1 ml of PBS and transferred to the tube. Samples were spun down at 834 g for 10 minutes at 4°C and the skim milk layer was removed and frozen at -80°C and subsequently used to determine the cytokines present in the milk. The pellet was washed with 10 mls of PBS and spun down at 834 g for 10 minutes at 4°C up to 5 times to eliminate as much fat as possible. The pellet was then resuspended in 10 mls of 37% Percoll and layered over 5 mls of 70% Percoll (GE Healthcare, Piscataway, NJ). The gradient was spun down at 390 g for 30 minutes at room temperature. The middle layer containing cells was removed, transferred to a new tube and washed in 10 ml of PBS twice. The pellet was resuspended in 1 ml of PBS and analyzed using the ADVIA® 120 Hematology System (Siemens Healthcare Diagnostics, Deerfield, IL) to determine WBC differentials.
2.6. Cytokine detection in milk
The skim milk isolated from the stomachs as described above was thawed and placed on ice. Undiluted samples were run on a Bio-plex containing 23 analytes including IL-1β, IL-2, IL-4, IL-5, IL-10, GM-CSF, IFN-γ, TNF-α, IL-3, IL-6, IL-12(p40), IL-12(p70), IL-17, G-CSF, KC, MIP-1α, RANTES, IL-1α, IL-9, IL-13, Eotaxin, MCP-1 and MIP-1β as described in the manufacturers protocol (Bio-Rad, Hercules, CA).
3. Results
3.1. AOD disease incidence is increased in d3tx A/J mice cross-fostered on B6 females
Inbred strains of mice exhibit differential susceptibility to d3tx-induced AOD. The inbred mouse strain A/J is highly susceptible to d3tx-induced AOD and exhibit the hallmarks of this disease phenotype including the presence of anti-ovarian autoantibodies, oophoritis and atrophy [13]. In contrast, B6 female mice are known to be resistant to d3tx-induced AOD [13]. We hypothesized that the postnatal maternal environment may contribute to the induction or protection of d3tx-induced AOD in A/J and B6 mice, respectively. Therefore, we cross-fostered susceptible d3tx A/J pups on resistant B6 mothers and resistant d3tx B6 pups on susceptible A/J mothers by 24 hours after birth to determine whether cross-fostering would alter their susceptibility to d3tx-induced AOD development. Surprisingly, we found that the incidence of AOD in A/J pups cross-fostered by B6 mothers was significantly greater (16/18) than that seen in naturally nursed A/J mice (7/13) (p < 0.0001) (Table I). However, while the mean AOD pathology score for A/J mice cross-fostered on B6 mice was greater (14.53 ± 5.37) than that of naturally fostered pups (9.15 ± 8.39), the difference was not statistically significant (p = 0.32). Lastly, foster nursing d3tx B6 pups on A/J mothers did not trigger AOD development in these mice (0/18) (Table I). Therefore, the maternal environment supplied by the resistant B6 mouse strain is capable of amplifying the number of d3tx A/J mice that develop AOD while susceptible A/J mothers do not influence the d3tx resistant phenotype seen in B6 mice.
Table I.
AOD in naturally nursed and cross-fostered adult d3tx A/J and B6 mice.
| B6 Pups | A/J Pups | |||
|---|---|---|---|---|
| Disease | B6 Mothers | A/J Mothers | B6 Mothers | A/J Mothers |
| AOD1 | 0/20 (0%) | 0/18 (0%) | 16/18 (89%) | 7/13 (54%) |
The number of mice developing AOD out of the total mice tested is shown with the percentage in parentheses.
3.2 EAE disease incidence and pathology is amplified in A/J mice cross-fostered on B6 females
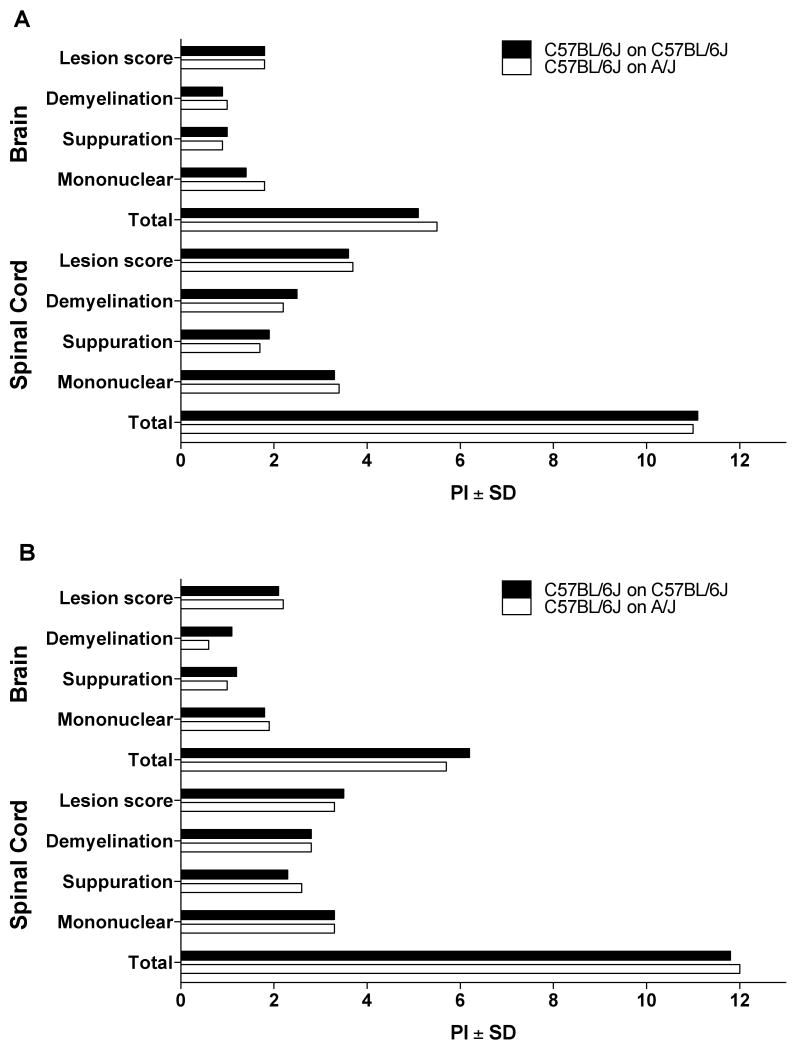
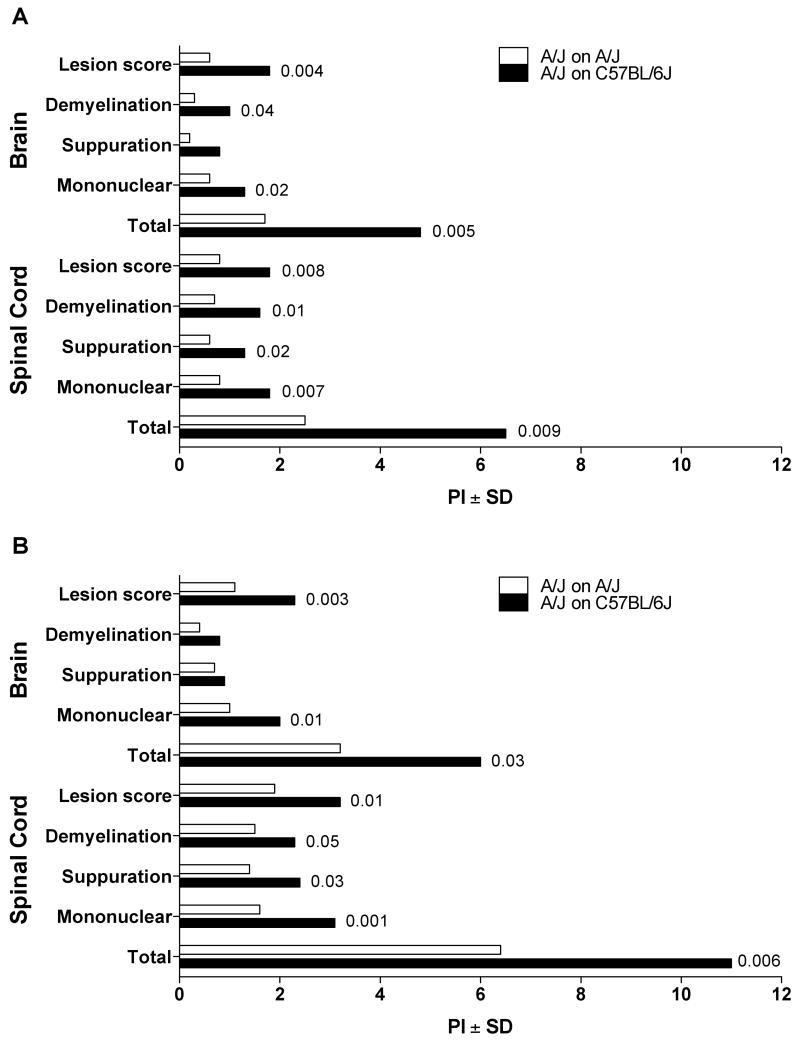
It was of interest to determine whether the increase in susceptibility to AOD in cross-fostered d3tx A/J mice was specific to d3tx-induced disease or if it was a more general phenomenon seen in response to other autoimmune diseases. Similar to the autoimmune disease mounted in susceptible d3tx mice, EAE is an induced autoimmune disease mediated by CD4+ T cells that have escaped central tolerance mechanisms and circulate in the periphery of adult mice [10]. However, animals induced for EAE are not thymectomized but rather immunized with self-myelin in CFA. In addition, unlike the susceptible phenotype displayed by d3tx A/J mice to AOD, A/J mice are relatively resistant to EAE compared to B6 mice. Therefore, to determine the affect of cross-fostering on the induction of EAE in A/J mice, we immunized either B6 mice or A/J mice that were either cross-fostered or nursed naturally using both the single injection (1×) and the double injection (2×) protocol. There were no significant differences in any of the clinical quantitative trait variables including incidence, day of onset, peak score, cumulative disease score, days affected and overall severity index between natural and cross fostered B6 mice elicited with either the 2× or 1× protocols (Table II). Similarly, lesion severity, degree of demyelination, suppuration, and monocyte/lymphocyte infiltration, and total EAE pathology score for both the brain and spinal cord were not significantly different between natural and cross fostered B6 mice (Fig. 1A, B). In contrast, compared to naturally fostered A/J mice the incidence of disease, peak score, cumulative disease score, days affected and overall severity index were significantly greater in A/J mice cross fostered on B6 mice (Table III). A/J mice cross fostered on B6 mice also exhibited significantly more sever EAE pathology in both the brain and spinal cord than did naturally fostered A/J mice (Fig. 2A, B). Therefore, increased susceptibility to the development of autoimmunity in cross-fostered A/J mice is applicable to two different autoimmune disease models.
Table II.
EAE in natural and cross-fostered B6 mice.
| Induction Protocol | Foster Mother | Incidence | Day of Onset1 | Peak Score | Cumulative Disease Score | Days Affected | Severity Index |
|---|---|---|---|---|---|---|---|
| 2× | C57BL/6J | 10/10 | 17.4±2.1 | 3.2±1.1 | 31.4±12.1 | 13.1±3.5 | 2.3±0.6 |
| A/J | 10/10 | 17.2±3.7 | 3.2±0.9 | 35.5±16.3 | 13.6±3.9 | 2.5±0.6 | |
| 1× | C57BL/6J | 11/11 | 13.0±1.2 | 3.9±0.3 | 52.5±10.8 | 18.0±1.3 | 2.9±0.5 |
| A/J | 11/11 | 14.1±2.6 | 3.9±0.3 | 53.5±13.0 | 16.7±2.6 | 3.2±0.6 |
Significance of differences between clinical quantitative trait variables was determined using the Mann Whitney test with a significance threshold of p=0.05.
Figure 1.
Quantification of EAE pathology in natural and cross-fostered B6 female mice. EAE pathology in the brains and spinal cords of natural and cross-fostered mice elicited by immunization with (A) 1× MOG35–55+CFA+PTX and (B) 2× MOG35–55+CFA was evaluated in a semiquantitative manner. Lesions in the brains and spinal cords of natural and cross-fostered mice elicited with both protocols were not significantly different. The significance of differences was determined using the Mann Whitney test with a significance threshold of p = 0.05.
Table III.
EAE in natural and cross-fostered A/J mice.
| Induction Protocol | Foster Mother | Incidence1 | Day of Onset2 | Peak Score | Cumulative Disease Score | Days Affected | Severity Index |
|---|---|---|---|---|---|---|---|
| 2× | A/J | 6/12 | 17.8±0.7 | 1.1±0.4 | 12.8±4.4 | 6.3±2.0 | 1.1±0.3 |
| C57BL/6J | 11/11 | 17.3±0.9 | 2.8±0.1 | 30.7±2.6 | 13.7±0.9 | 2.2±0.1 | |
| p=0.01 | 0.3 | 0.002 | 0.02 | 0.006 | 0.009 | ||
| 1× | A/J | 8/11 | 12.8±0.4 | 2.4±0.5 | 27.9±5.8 | 13.1±2.6 | 1.5±0.3 |
| C57BL/6J | 12/12 | 14.6±0.9 | 3.5±0.2 | 45.1±2.5 | 16.4±0.9 | 2.8±0.1 | |
| p=0.05 | 0.1 | 0.03 | 0.007 | 0.8 | 0.0003 |
Significance of differences in disease incidence was defined using the Chi-square test.
Significance of differences between clinical quantitative trait variables was determined using the Mann Whitney test with a significance threshold of p=0.05.
Figure 2.
Quantification of EAE pathology in natural and cross fostered A/J female mice. EAE pathology in the brains and spinal cords of natural and cross fostered mice elicited by immunization with (A) 1× MOG97-114+CFA+PTX and (B) 2× MOG97-114+CFA was evaluated in a semiquantitative manner. EAE pathology in the brains and spinal cords of A/J mice cross fostered on C57BL/6J females was significantly more severe than that observed in naturally fostered A/J mice. The significance of differences was determined using the Mann Whitney test with a significance threshold of p = 0.05.
3.3 The percentage of maternally derived lymphocytes varied between A/J and B6 derived milk
Maternally derived cells that are shed into the milk and ingested by nursing newborns can cross the GI tract and colonize various organs in the juvenile mouse, a phenomenon known as maternal cell microchimerism [25, 32]. The presence of a specific maternal effect on the induction of AOD and EAE in the A/J mouse strain foster-nursed by B6 mothers prompted us to investigate the maternal cells that are excreted into the milk. Therefore, milk was isolated from the stomachs of pups nursed by either A/J or B6 mothers 3 days after birth. We collected 200 mgs of milk from 7 A/J and 7 B6 mothers by extracting the stomachs from 2 to 3 pups that had recently been fed. Although this method of milk collection has been successfully used to extract maternal cells from milk [32], we wanted to confirm that the cells we were recovering in the milk fractions were of maternal origin. Consequently, we cross-fostered B6 pups expressing the CD45.1 allele on B6 mothers expressing the CD45.2 allele and the milk-derived cells were stained with antibodies against both allelic variants and analyzed by flow cytometry. 90.8% of the cells present in the milk stained positive for CD45.2 and represented the cells in the milk that are of maternal origin. Only 3.32% of the cells were positive for CD45.1 indicating the cells in the milk derived from the nursing pup (Fig. 3A). Therefore, the majority of bone marrow derived cells present in the milk extracted from the stomachs of nursing pups are of maternal origin.
Figure 3.
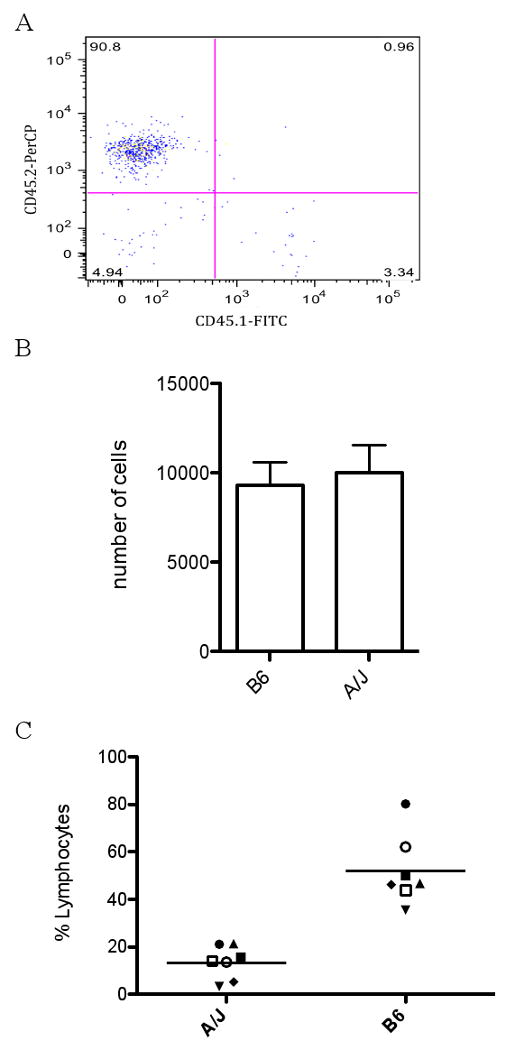
Analysis of maternal cells shed into milk by B6 and A/J mice. (A) Leukocytes present in the milk are of maternal origin. CD45.2+ B6 mothers foster-nursed CD45.1+ B6 pups and WBCs were obtained from the milk extracted from the stomachs of the nursing pups. The cells were stained with anti-CD45.1 FITC and anti-CD45.2 PerCyp and analyzed by Flow cytometry. (B) A/J and B6 shed similar numbers of WBCs in milk. Milk was extracted from the stomachs of pups 3 days after birth from 7 individual lactating B6 or A/J mothers and assessed for the total number of WBCs shed into the milk using ADVIA. Results are expressed as the mean ± SEM. No significant difference was detected. (C) B6 derived milk contains significantly more lymphocytes compared to milk from A/J. The same milk samples used in (B) were analyzed for the total percentage of lymphocytes using ADVIA. Each point represents the percentage of milk-derived lymphocytes obtained from an individual lactating mother. B6 milk contained significantly more lymphocytes compared to A/J milk. The significance of differences was determined using the unpaired Student's t-test (p=0.0001).
To determined the type of maternal white blood cells (WBCs) present in the milk, we utilized the ADVIA® 120 Hematology System to obtain WBC differentials. The total number of cells shed into the milk by both B6 and A/J mice did not differ significantly, with B6 milk containing an average of 9,286 ± 1,304 (n=7) and A/J milk containing 10,530 ± 1,543 (n=7) maternally derived cells (Fig. 3B). Of the cell populations assessed, including lymphocytes, neutrophils, monocytes, eosinophils and basophils, only the lymphocyte population was consistently represented between the individual milk samples obtained within the same mouse strain. The remaining cell populations were generally undetectable or fluctuated between individuals within the same strain and, therefore, only the lymphocyte population has been reported. The milk obtained from B6 mice contained an average of 52 ± 5.6% of maternal lymphocytes (n=7) (Fig. 3C). Interestingly, maternal cells in the milk from individual A/J mothers contained a significantly reduced population of lymphocytes compared to B6, having on average 14 ± 2.6% lymphocytes (n=7) (p=0.0001) (Fig. 3C). As a result, the maternally transmitted environmental factor inherited by B6 mice that leads to increased autoimmune disease development in A/J mice may be arising from the lymphocytes shed into the milk.
3.4. IL-9, IL-13 and CXCL1 are detected in the milk derived from both A/J and B6 mice
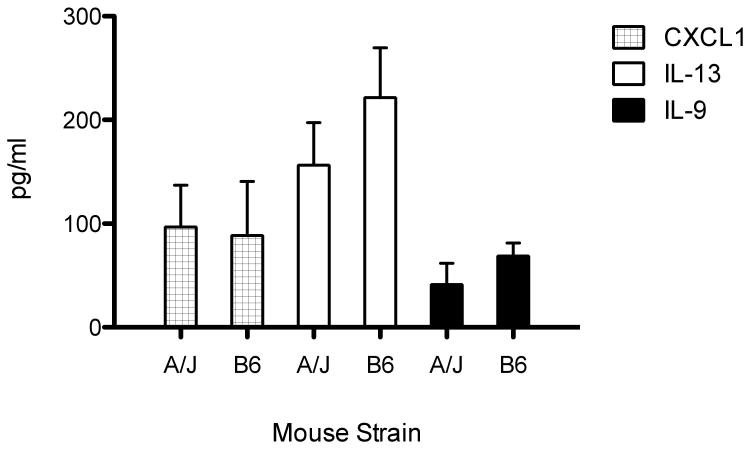
In addition to maternal cell infiltrates, breast milk contains other immunological agents, like cytokines, that have been implicated as regulators of the neonatal immune system [33]. Lymphocytes are notorious for their ability to secrete and respond to numerous types of cytokines and, therefore, we were prompted to investigate whether the differences observed in lymphocyte number also included differences in cytokine levels between A/J and B6 milk. To test this, we isolated the skim milk fraction from the stomachs of nursing mice and analyzed the cytokine, chemokine and growth factor content using a Bio-plex containing 23 analytes. Of the 23 analytes tested, we observed a significant increase above background in the cytokines IL-9, IL-13 and in the chemokine CXCL1. However, we did not detect a significant difference between the levels of these cytokines in the milk derived from A/J versus B6 mice (Fig. 4). Therefore, despite the significant difference between the percentage of maternal lymphocytes secreted into the milk, variability in cytokine quantities does not appear to be contributing to the increase in autoimmune susceptibility in foster-nursed d3tx A/J mice.
Figure 4.
Cytokine analysis in the milk from both B6 and A/J mice. Cytokine levels were measured in the skim milk from the stomachs of nursing pups 3 days after birth using a BioRad Bioplex containing 23 analytes. The majority of the cytokines were below the limits of detection except for IL-9, IL-13 and CXCL1. The levels of these cytokines did not significantly differ between B6 and A/J milk.
3.5. The total number of CD4+ T cells is decreased in the lymph nodes of cross-fostered A/J mice
In our work described thus far, we have shown that susceptibility to two different autoimmune disease models is increased in A/J mice when cross-fostered by B6 mothers and this phenotype may be due to the increased percentage of maternal lymphocytes passed in the milk of B6 mothers. The ability of maternally derived lymphocytes to take up residency in nursing pups suggests that these cells may influence the development of the immune system in these mice [25, 32]. Therefore, we asked whether there was an effect of foster nursing on the percentage of CD4+ T cells in the spleens and lymph nodes of adult d3tx B6 and A/J mice.
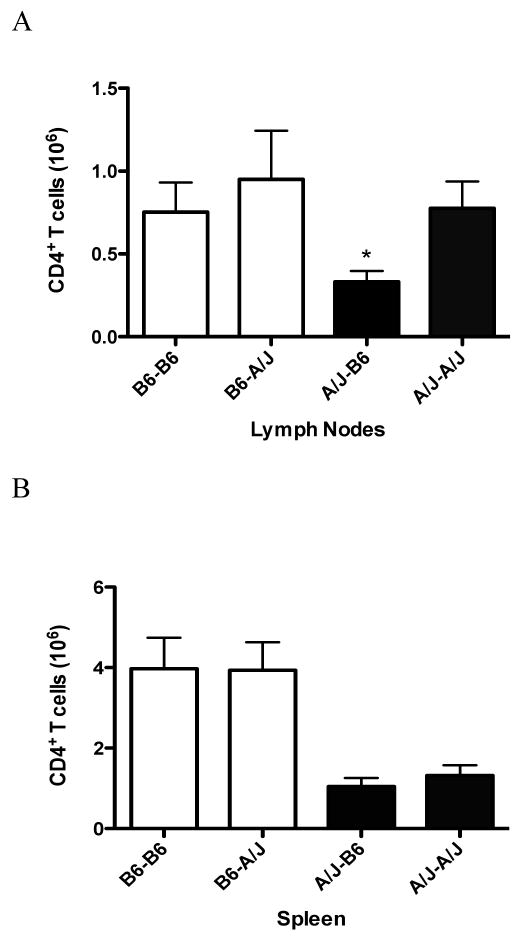
Cross-fostered d3tx resistant B6 mice exhibit approximately equal numbers of CD4+ T cells in their spleen and lymph nodes when compared to foster nursed mice (Fig. 5A). Additionally, naturally nursed d3tx A/J mice possess similar numbers of CD4+ T cells in their lymph nodes compared to B6 mice (Fig. 5A). However, the number of CD4+ T cells is drastically reduced in the lymph nodes of cross-fostered A/J mice compared to naturally nursed animals (p < 0.02) (Fig. 5A). Interestingly, the overall number of CD4+ T cells is significantly lower in the spleens of A/J mice compared to B6, regardless of maternal influence, suggesting a genetic propensity for fewer CD4+ T cells in this organ (p = 0.0002) (Fig. 5B). This data indicates that there are postnatal maternal factors impacting the overall number of CD4+ T cells in the lymph nodes but not the spleen of cross-fostered A/J mice.
Figure 5.
Absolute numbers of CD4+ T cells in the lymph nodes and spleen of adult d3tx A/J and B6 mice. The total number of CD4+ T cells isolated from the (A) Lymph nodes and (B) spleen of natural and cross-fostered B6 and A/J mice were analyzed by FACS. CD4+ T cells from the lymph nodes of cross-fostered A/J mice are significantly lower than naturally nursed A/J mice. The significance of differences was determined using the unpaired Student's t-test (p < 0.02). The spleen from A/J mice naturally exhibit reduced numbers of CD4+ T cells compared to B6 that is independent of maternal influence. The significance of differences was determined using one way ANOVA followed by Bonferroni's multiple comparison test (p = 0.0002). B6-B6, B6 mother and B6 pup; B6-A/J, B6 mother and A/J pup; A/J-B6, A/J mother and B6 pup; A/J-A/J, A/J mother and A/J pup.
4. Discussion
The influence of the postnatal maternal environment on the development and severity of several mouse models of human disease has been well established. However, the ability of the postnatal maternal environment to influence the degree of susceptibility to d3tx-induced AOD or EAE was left largely unexplored. Here we looked at the impact of foster nursing on B6 and A/J mice and examined their susceptibility to disease. If a maternally derived factor influenced susceptibility and resistance, it seemed logical to predict that foster nursing d3tx susceptible A/J pups on resistant B6 mothers would yield adult A/J mice that showed increased resistance to d3tx-induced AOD. Similarly, it seemed plausible that foster nursing EAE susceptible B6 pups on resistant A/J mothers would yield adult B6 mice that showed increased resistance to EAE. Therefore, it was surprising to discover that our data indicates that there is instead an increase in disease susceptibility in A/J mice cross-fostered by B6 mothers in both autoimmune models. Analysis of the cellular composition in the breast milk revealed that there are significantly more maternally derived lymphocytes in B6 milk compared to A/J. In addition, we have shown that d3tx A/J mice possess drastically reduced numbers of CD4+ T cells in their lymph nodes when cross-fostered by B6 mothers and naturally exhibit reduced numbers of CD4+ T cells in their spleens compared to B6 mice. Since the degree of susceptibility to autoimmune diseases increased in A/J mice when nursed by B6 mothers, our data suggests that there are maternally derived environmental factors, perhaps generated by the maternal lymphocytes shed into the milk, that results in an alteration of the immune system leading to an increase in autoimmune disease development in A/J mice. A maternally derived contribution to disease susceptibility is further supported in reciprocal F1 hybrid mice, where susceptibility to AOD is significantly greater when the maternal strain is also the susceptible strain [34].
Maternal cell microchimerism has become a well documented phenomenon where maternally derived cells are passed from mother to offspring, either through the placenta or during breast feeding, which then take up residency in the offspring [25]. When maternally derived WBCs are passed to newborns through breast milk between 3 to 8 days after birth, the cells infiltrate through the wall of the digestive tract and localize in the livers of nursing newborns [25]. This observation is quite interesting when taken into consideration that neonatal thymectomy must be conducted within the first 3 to 5 days after birth to induce autoimmune diseases [16]. The lack of a thymus may result in the inability of the maternal cells to be tolerized in the recipient offspring during this time window when the maximal number of cells are being transferred to nursing pups.
A recent study has shown that the majority of maternal cells taking up residency in the nursing offspring are T lymphocytes [32]. This group also demonstrated that the sensitization of lactating mice with Candida, which elicits a T cell-mediated delayed-type-hypersensitivity (DTH) response, results in an amplified DTH response in nursing females yet suppressed a DTH response in nursing males [32]. The female specific effect of the DTH response correlates with the gender bias seen in MS, where the female to male ratio of MS patients has been significantly increasing over the past 50 years [35]. In studies of half siblings, it has been reported that a significant maternal parent-of-origin (POO) effect contributes to the etiology of MS [36]. Although the mechanism underlying the POO effect is unknown, it may be arising through the gestational and/or the neonatal environment thought to influence the risk of MS later in life [37]. In fact, the POO effect may be associated with microchimerism since higher rates of microchimerism have been observed in women with MS compared to unaffected siblings [38]. Taken together, these studies support the hypothesis that microchimerism can lead to an increase in autoimmune disease susceptibility in cross-fostered A/J mice. A/J mice fostered by B6 mothers may incorporate more maternally derived lymphocytes into their immune system compared to naturally nursed A/J mice. These maternally derived cells may be capable of activating resident lymphocytes in the neonatal immune system by expressing antigens that these cells are reactive for and will ultimately lead to the increased susceptibility we see in fostered A/J mice to d3tx-induced AOD and EAE.
In addition to the transfer of maternal cells to nursing pups, we also detected the presence of IL-9, IL-13 and CXCL1 in the milk. IL-9 is a T helper cell type 2 (TH2) cytokine that when overexpressed in the intestine will promote intestinal mastocytosis, which leads to the permeability of the intestinal wall, known as a leaky gut [48]. Similarly, IL-13 is also a TH2 cytokine that can induce mastocytosis and a leaky gut phenotype in the intestine [49]. Lastly, the receptor for CXCL1, CXCR2, is expressed on mast cell progenitors and is required for the homing of mast cells to the intestine [51]. It is in this way that CXCL1 expression may also contribute to mastocytosis and a leaky gut phenotype. Therefore, IL-9, IL-13 and CXCL1 secretion in the milk from both A/J and B6 mice may be produced in order to facilitate the uptake of maternal cells through the intestinal wall in nursing pups.
Along with the quality of milk being ingested, the postnatal maternal environment can also shape the development of offspring through epigenetic programming influenced by maternal behavior. It has long been recognized that the maternal environment during the postnatal period can influence the behavior of adult animals regardless of their genetic background [52]. More recently, this maternal behavior has been linked to epistatic changes in the genes of the offspring. One such example of this phenomenon was witnessed in rats, where there is a clear distinction between two types of maternal care among individuals, including low frequency of licking and grooming (LG) and arched-back nursing (ABN) versus a high frequency of these traits [53]. Offspring from low frequency LG and ABN mothers are more fearful and susceptible to stress compared to rats from high frequency mothers [54-56]. This phenotype could be reversed after cross-fostering pups from low frequency mothers on high frequency mothers, or vice versa, and this acquired characteristic persisted throughout the life of the animal [56]. Remarkably, this disparity in maternal care directly affected the methylation status of the promoter region of the glucocorticoid receptor gene, which is linked to LG and ABN, during the first week of life [57]. This provides a clear example of altering DNA methylation, and hence the expression of a gene, solely through the postnatal maternal environment in the absence of germ line transmission.
It has been established that CD4+ T cell development and differentiation is aided by the epigenetic modification of genes involved in this process [58-61]. The control of peripheral tolerance in CD4+ T cells has also recently been shown to be under the control of epigenetic mechanisms [62]. T cell anergy is one such method of peripheral tolerance that evokes from the loss of proinflammatory cytokine production by previously activated T cells. Using a Vβ8.1+ TCR transgenic mouse line, it was shown that the methylation status of the IL-2 promoter and IFN-γ enhancer in CD4+ T cells differed depending on the activation status of the CD4+ T cells. When the Vβ8.1+ T cells were activated in vitro, there was substancial histone acetylation and demethylation within the IL-2 promoter and IFN-γ enhancer. In contrast, when the Vβ8.1+ T cells were tolerized in vivo due to endogenous mtv-7 sag expression, histones remained unaceytlated and the DNA remained methlyated at these regulatory loci [62]. It is in this way that epigenetic mechanisms can regulate the production of proinflammatory cytokines and ultimately the activation status of CD4+ T cells.
Both AOD and EAE are immune diseases thought to arise in susceptible mice due to the inadequacy of central and/or peripheral tolerance mechanisms to silence autoreactive T cells. The actions of Tregs constitute one component of the immune system governing peripheral tolerance [63, 64] and the involvement of Tregs during self-tolerance has been intensely investigated using the d3tx model of autoimmune disease [65]. Indeed, recent evidence has emerged indicating that susceptible d3tx mice possess functional Tregs in their lymph nodes despite the removal of the thymus [66]. Tregs are defined by the expression of the transcription factor FoxP3 and numerous signals have been identified as important mediators of FoxP3 expression, including T cell receptor activation, CD28 co-stimulation and cytokine mediated signals, especially IL-2 and TGFβ. Importantly, the gene for IL-2 has been identified as a candidate for a shared autoimmune susceptibility gene in both AOD and EAE, which resides on Chromosome 3 and positioned within the Aod2 and eae3/20 QTLs [67-69]. The fact that the regulatory elements of Il2 are under the control of epigenetic mechanisms suggests that epistatic changes in this locus may also contribute to the enhanced susceptibility to AOD and EAE seen in cross-fostered A/J mice.
We have suggested that the increased susceptibility to AOD and EAE seen in A/J mice foster-nursed by B6 mothers may result from differences in the maternal lymphocytes shed into the milk. However, the possibility remains that the effects of maternal behavior influence disease development through epigenetic programming. Therefore, it is as likely that the changes we observed in the fostered A/J immune system arises through differences in maternal behavior resulting in epistatic changes in immune gene networks. Accordingly, these two components of the postnatal maternal environment may be acting together to cause the increase in disease seen in foster-nursed A/J mice. Future studies need to be conducted to discriminate between these equally convincing scenarios.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health Grants NS060901, NS036526 and AI041747
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parijs LK, Abbas Abul K. Homeostasis and self-tolerance in the immune system: Turning lymphocytes off. Science. 1998;280:243–248. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 2.Nemazze D. Receptor editing in lymphocyte development and central tolerance. Nature. 2006;6:728–740. doi: 10.1038/nri1939. [DOI] [PubMed] [Google Scholar]
- 3.Sinha AA, Lopez MT, McDevitt HO. Autoimmune diseases: the failure of self tolerance. Science. 1990;248:1380–1388. doi: 10.1126/science.1972595. [DOI] [PubMed] [Google Scholar]
- 4.Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesis of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–755. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- 5.Kojima A, Prehn RT. Genetic susceptibility to post-thymectomy autoimmune diseases in mice. Immunogenetics. 1981;14:15–27. doi: 10.1007/BF00344296. [DOI] [PubMed] [Google Scholar]
- 6.Shevach Ethan M. Regulatory T cells in autoimmunity. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 7.Kojima A, Prehn RT. Genetic susceptibility to post-thymectomy autoimmune disease in mice. Immunogenetics. 1981;14:15–27. doi: 10.1007/BF00344296. [DOI] [PubMed] [Google Scholar]
- 8.Smith H, Chen IM, Kubo R, Tung KSK. Neonatal thymectomy results in a repertoire enriched in T cells deleted in adult thymus. Science. 1989;245:749–752. doi: 10.1126/science.2788921. [DOI] [PubMed] [Google Scholar]
- 9.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nature Protocols. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 10.Seamons A, Perchellet A, Goverman J. Immune tolerance to myelin proteins. Immunol Res. 2003;28:201–221. doi: 10.1385/IR:28:3:201. [DOI] [PubMed] [Google Scholar]
- 11.Levine S, Sowinski R. Experimental allergic encephalomyelitis in inbred and outbred mice. J Immunol. 1973;110:139–143. [PubMed] [Google Scholar]
- 12.Lando Z, Teitelbaum D, Arnon R. Induction of experimental allergic encephalomyelitis in genetically resistant strains of mice. Nature. 1980;287:551–552. doi: 10.1038/287551a0. [DOI] [PubMed] [Google Scholar]
- 13.Wardell BB, Michael SD, Tung KSK, Todd JA, Blankenhorn EP, McEntee K, Sudweeks JD, Hansen WK, Meeker ND, Griffith JS, Livingstone KD, Teuscher C. Aod1, the immunoregulatory locus controlling abrogation of tolerance in neonatal thymectomy-induced autoimmune ovarian dysgenesis, maps to chromosome 16. Proc Natl Acad Sci USA. 1995;92:4758–4762. doi: 10.1073/pnas.92.11.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teuscher C, Wardell BB, Lunceford JK, Michael SD, Tung KS. Aod2, the locus controlling development of atrophy inneonatal thymectomy-induced autoimmune ovarian dysgenesis, co-localizes with Il2, Fgfb, and Idd3. J Exp Med. 1996;183:631–637. doi: 10.1084/jem.183.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roper RJ, Ma RZ, Biggis JE, Butterfield RJ, Michael SD, Tung KS, Doerge RW, Teuscher C. Interacting quantitative trait loci control loss of peripheral tolerance and susceptibility to autoimmune ovarian dysgenesis after day 3 thymectomy in mice. J Immunol. 2002;169:1640–1646. doi: 10.4049/jimmunol.169.3.1640. [DOI] [PubMed] [Google Scholar]
- 16.Tung KSK, Setiady YY, Samy ET, Lewis J, Teuscher C. Autoimmune Ovarian Disease in Day 3-Thymectomized mice: The neonatal time window, antigen specificity of disease suppression, and genetic control. Curr Top Microbiol Immunol. 2005;293:207–244. doi: 10.1007/3-540-27702-1_10. [DOI] [PubMed] [Google Scholar]
- 17.Encinas JA, Lees MB, Sobel RA, Symonowisz C, Greer JM, Shovlin CL, Seidman HL, Seidman JG, Kuchroo VK. Genetic analysis of susceptibility to experimental autoimmune encephalomyelitis in a cross between SJL/J and B10.S mice. J Immunol. 1996;157:2186–2192. [PubMed] [Google Scholar]
- 18.Butterfield RJ, Sudweeks JD, Blankenhorn EP, Korngold R, Marini JC, Todd JA, Roper RJ, Teuscher C. New genetic loci that controls susceptibility and symptoms of experimental allergic encephalomyelitis in inbred mice. J Immunol. 1998;161:1860–1867. [PubMed] [Google Scholar]
- 19.Butterfield RJ, Blankenhorn EP, Roper RJ, Zachary JF, Doerge RW, Teuscher C. Identification of genetic loci controlling the characteristics and severity of brain and spinal cord lesions in experimental allergic encephalomyelitis. Am J Pathol. 2000;157:637–645. doi: 10.1016/S0002-9440(10)64574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teuscher C, Doerge RW, Fillmore PD, Blankenhorn EP. Eae36, a locus on mouse chromosome 4, controls susceptibility to experimental allergic encephalomyelitis in older mice and mice immunized in winter. Genetics. 2006;172:1147–1153. doi: 10.1534/genetics.105.049049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leme AS, Hubeau C, Xiang Y, Goldman A, Hamada K, Suzaki Y, Kobzik L. Role of breast milk in a mouse model of maternal transmission of asthma susceptibility. J Immunol. 2006;176:762–769. doi: 10.4049/jimmunol.176.2.762. [DOI] [PubMed] [Google Scholar]
- 22.Verhasselt V, Milcent V, Cazareth J, Kanda A, Fleury S, Dombrowicz D, Glaichenhaus N, Julia V. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat Med. 2008;14:170–175. doi: 10.1038/nm1718. [DOI] [PubMed] [Google Scholar]
- 23.Reifsnyder PC, Churchill G, Leiter EH. Maternal environment and genotype interact to establish diabesity in mice. Gen Res. 2000;10:1568–1578. doi: 10.1101/gr.147000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Washburn LR, Dand H, Tian J, Kaufman DL. The postnatal maternal environment influences diabetes development in nonobese diabetic mice. J Autoimmun. 2007;28:19–23. doi: 10.1016/j.jaut.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou L, Yoshimura Y, Huang YY, Suzuki R, Yokoyama M, Okabe M, Shimamura M. Two independent pathways of maternal cell transmission to offspring: through placenta during pregnancy and by breast-feeding after birth. Immun. 2000;101:570–581. doi: 10.1046/j.1365-2567.2000.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishizuka Y, Sakakura T. Ovarian dysgenesis induced by neonatal thymectomy in the mouse. Endocrinology. 1971;89:886–893. doi: 10.1210/endo-89-3-886. [DOI] [PubMed] [Google Scholar]
- 27.Tung KSK, Smith S, Teuscher C, Cook C, Anderson RE. Murine autoimmune oophritis, epididymoorchitis and gastritis induced by day 3 thymectomy: immunopathology. Am J Pathol. 1987;126:293–302. [PMC free article] [PubMed] [Google Scholar]
- 28.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spach KM, Noubade R, McElvany B, Hickey WF, Blankenhorn EP, Teuscher C. A single nucleotide polymorphism in Tyk2 controls susceptibility to experimental allergic encephalomyelitis. J Immunol. 2009;182:7776–7783. doi: 10.4049/jimmunol.0900142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teuscher C, Noubade R, Spach K, McElvany B, Bunn JY, Fillmore PD, Zachary JF, Blankenhorn EP. Evidence that the Y chromosome influences autoimmune disease in male and female mice. Proc Natl Acad Sci USA. 2006;103:8024–8029. doi: 10.1073/pnas.0600536103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butterfield RJ, Blankenhorn EP, Roper RJ, Zachary JF, Doerge RW, Sudweeks J, Rose J, Teuscher C. Genetic analysis of disease subtypes and sexual dimorphisms in mouse experimental allergic encephalomyelitis (EAE): relapsing/remitting and monophasic remitting/nonrelapsing EAE are immunogenetically distinct. J Immunol. 1999;162:3096–3102. [PubMed] [Google Scholar]
- 32.Ma LJ, Walter B, DeGuzman A, Muller HK, Walker AM. Trans-epithelial immune cell transfer during suckling modulates delayed-type hypersensitivity in recipients as a function of gender. PLoS One. 2008;3:e3562. doi: 10.1371/journal.pone.0003562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellis LA, Mastro AM, Picciano MF. Do milk-borne cytokines and hormones influence neonatal immune cell function? J Nutr. 1997;127:985S–988S. doi: 10.1093/jn/127.5.985S. [DOI] [PubMed] [Google Scholar]
- 34.Kojima A, Prehn RT. Genetic susceptibility to post-thymectomy autoimmune disease in mice. Immunogen. 1981;14:15–27. doi: 10.1007/BF00344296. [DOI] [PubMed] [Google Scholar]
- 35.Orton SM, Herrera BM, Yee IM, Valdar W, Ramagopalan SV, Sadovnick AD, Ebers GC. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol. 2006;5:932–936. doi: 10.1016/S1474-4422(06)70581-6. [DOI] [PubMed] [Google Scholar]
- 36.Ebers GC, Sadovnick AD, Dyment DA, Yee IM, Willer CJ, Risch N. Parent-of-origin effect in multiple sclerosis: observations in half-siblings. Lancet. 2004;363:1773–1774. doi: 10.1016/S0140-6736(04)16304-6. [DOI] [PubMed] [Google Scholar]
- 37.Willer CJ, Dyment DA, Sadovnick AD, Rothwell PM, Murray TJ, Ebers GC. Timing of birth and risk of multiple sclerosis: population based study. Bmj. 2005;330:120. doi: 10.1136/bmj.38301.686030.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willer CJ, Herrera BM, Morrison KM, Sadovnick AD, Ebers GC. Association between microchimerism and multiple sclerosis in Canadian twins. J Neuroimmunol. 2006;179:145–151. doi: 10.1016/j.jneuroim.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Kotzin BL, Leung DYM, Kappler J, Marrack P. Superantigens and their potential role in human disease. Adv Immunol. 1993;54:99–166. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- 40.Renno T, Acha-Orbea H. Superantigens in autoimmune disease: still more shades of gray. Immunol Rev. 1996;154:175–191. doi: 10.1111/j.1600-065x.1996.tb00934.x. [DOI] [PubMed] [Google Scholar]
- 41.Li H, Llera A, Malchiodi EL, Mariuzza RA. The structural basis of T cell activation by superantigens. Annu Rev Immunol. 1999;17:435–466. doi: 10.1146/annurev.immunol.17.1.435. [DOI] [PubMed] [Google Scholar]
- 42.Cohen JC, Varmus HE. Endogenous mammary tumor virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979;278:418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- 43.Acha-Orbea H, MacDonald HR. Superantigens of mouse mammary tumor virus. Annu Rev Immunol. 1995;13:459–486. doi: 10.1146/annurev.iy.13.040195.002331. [DOI] [PubMed] [Google Scholar]
- 44.Kozak C, Peters G, Pauley R, Morris V, Michalides R, Dudley J, Green M, Davisson M, Prakash O, Vaidya A, Hilgers J, Verstraeten A, Hynes N, Diggelmann H, Peterson D, Cohen JC, Dickson C, Sarkar N, Nusse R, Varmus H, Callahan R. A standardized nomenclature for endogenous mouse mammary tumor viruses. J Virol. 1987;61:1651–1654. doi: 10.1128/jvi.61.5.1651-1654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Acha-Orbea H, MacDonald HR. Superantigens of mouse mammary tumor virus. Annu Rev Immunol. 1995;13:459–486. doi: 10.1146/annurev.iy.13.040195.002331. [DOI] [PubMed] [Google Scholar]
- 46.Ben-Nun A, Soffer D. Minor lymphocyte stimulating (Mls) gene products in mice influence their genetic resistance or susceptibility to induction of autoimmune encephalomyelitis. Eur J Immunol. 1990;20:195–200. doi: 10.1002/eji.1830200128. [DOI] [PubMed] [Google Scholar]
- 47.Hauber HP, Bergeron C, Hamid Q. IL-9 in allergic inflammation. Int Arch Allergy Immunol. 2004;134:79–87. doi: 10.1159/000078384. [DOI] [PubMed] [Google Scholar]
- 48.Forbes FE, Groschwitz K, Abonia JP, Brandt RB, Cohen E, Blanchard C, Ahrens R, Seidu L, McKenzie A, Strait R, Finkelman FD, Foster PS, Matthaei KI, Rothenberg ME, Hogan SP. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knight PA, Brown JK, Pemberton AD. Innate immune response mechanisms in the intestinal epithelium: potential roles for mast cells and goblet cells in the expulsion of adult Trichinella spiralis. Parasitology. 2008;135:655–670. doi: 10.1017/S0031182008004319. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi Y. Neutrophil infiltration and chemokines. Crit Revs Immunol. 2006;26:307–315. doi: 10.1615/critrevimmunol.v26.i4.20. [DOI] [PubMed] [Google Scholar]
- 51.Hallgren J, Gurish MF. Pathways of murine mast cell development and trafficking: tracking the roots and routes of the mast cell. Immunol Rev. 2007;217:8–18. doi: 10.1111/j.1600-065X.2007.00502.x. [DOI] [PubMed] [Google Scholar]
- 52.Zhang TY, Bagot R, Parent C, Nesbitt C, Bredy TW, Caldji C, Fish E, Anisman H, Szyf M, Meaney MJ. Maternal programming of defensive responses through sustained effects on gene expression. Biol Psych. 2006;73:72–89. doi: 10.1016/j.biopsycho.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 53.Champagne FA, Francis DD, Mar A, Meaney MJ. Naturally-occurring variations in maternal care in the rat as a mediating influence for the effects of environment on the development of individual differences in stress reactivity. Physiol Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 54.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 55.Liu D, Diorio J, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 57.Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuro. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 58.Hozumi K, Abe N, Chiba S, Hirai H, Habu S. Active form of Notch members can enforce T lymphopoiesis on lymphoid progenitors in the monolayer culture specific for B cell development. J Immunol. 2003;170:4973–4979. doi: 10.4049/jimmunol.170.10.4973. [DOI] [PubMed] [Google Scholar]
- 59.Taniuchi I, Ellmeier W, Littman DR. The CD4/CD8 lineage choice: new insights into epigenetic regulation during T cell development. Adv Immunol. 2004;83:55–89. doi: 10.1016/S0065-2776(04)83002-5. [DOI] [PubMed] [Google Scholar]
- 60.Kioussis D, Ellmeier W. Chromatin and CD4, CD8A and CD8B gene expression during thymic differentiation. Nat Rev Immunol. 2002;2:909–919. doi: 10.1038/nri952. [DOI] [PubMed] [Google Scholar]
- 61.Ansel KM, Lee DU, Rao A. An epigenetic view of helper T cell differentiation. Nat Immunol. 2003;4:616–623. doi: 10.1038/ni0703-616. [DOI] [PubMed] [Google Scholar]
- 62.Thomas RM, Saouaf SJ, Wells AD. Superantigen-induced CD4+ T cell tolerance is associated with DNA methylation and histone hypo-acetylation at cytokine gene loci. Genes and Immunity. 2007;8:613–618. doi: 10.1038/sj.gene.6364415. [DOI] [PubMed] [Google Scholar]
- 63.Sakaguchi S. Naturally arising CD4+ Regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 64.Vignali D, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shevach Ethan M. Regulatory T cells in autoimmunity. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 66.Samy ET, Wheeler KM, Roper RJ, Teuscher C, Tung KSK. Cutting edge: autoimmune disease in day 3 thymectomized mice is actively controlled by endogenous disease-specific regulatory T cells. J Immunol. 2008;180:4366–4370. doi: 10.4049/jimmunol.180.7.4366. [DOI] [PubMed] [Google Scholar]
- 67.Teuscher C, Wardell BB, Lunceford JK, Michael SD, Tung KS. Aod2, the locus controlling development of atrophy in neonatal thymectomy-induced autoimmune ovarian dysgenesis, co-localizes with Il2, Fgfb, and Idd3. J Exp Med. 1996;183:631–637. doi: 10.1084/jem.183.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Encinas JA, Wicker LS, Peterson LB, Mukasa A, Teuscher C, Sobel R, Weiner HL, Seidman CE, Seidman JG, Kuchroo VK. QTL influencing autoimmune diabetes and encephalomyelitis map to a 0.15-cM region containing Il2. Nat Genet. 1999;21:158–160. doi: 10.1038/5941. [DOI] [PubMed] [Google Scholar]
- 69.del Rio R, Noubade R, Subramanian M, Saligrama N, Diehl S, Rincon M, Teuscher C. SNPs upstream of the minimal promoter control IL-2 expression and are candidates for the autoimmune disease-susceptibility locus Aod2/Idd3/Eae3. Genes Immun. 2008;9:115–121. doi: 10.1038/sj.gene.6364455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.