Abstract
Despite research and clinical significance, limited information is available on the relations between skeletal muscle (SM) and age in adults, specifically among Hispanics, African Americans (AA), and Asians. The aim was to investigate possible sex and ethnic SM differences in adults over an age range of 60 years. Subjects were 468 male and 1280 female adults (≥18 years). SM was estimated based on DXA-measured appendicular lean-soft tissue using a previously reported prediction equation. Locally weighted regression smoothing lines were fit to examine SM trends and to localize age cutoffs; piecewise multiple linear regression models were then applied, controlling for weight and height, to identify age cutoffs for sex-specific changes in SM among the ethnic groups. The age of 27 years was identified for women and men as the cut-off after which SM starts to show a negative association with age. Both sexes had a similar ethnic pattern for expected mean SM at the age cutoff, with AA presenting the highest SM values, followed by Whites, Hispanics, and Asians. After the age cutoffs, the lowering of SM differed by ethnicity and sex: AA women showed the greatest SM lowering whereas Hispanic women had the least. Hispanic men tended to show a higher negative association of SM with age followed by AA and Whites. To conclude, significant sex and ethnic differences exist in the magnitude of negative associations of SM with age >27 years. Further studies using a longitudinal design are needed to explore the associations of ethnicity-related decline of SM with health risks.
Skeletal muscle (SM), the largest component of adipose tissue-free body mass in humans, is central to the study of nutritional, physiologic, and metabolic processes (Janssen et al., 2000; Lukaski, 2005; Malina, 1996). Total-body and regional SM mass can now be accurately quantified with imaging methods, including computed axial tomography (CT) and magnetic resonance imaging (MRI) (Heymsfield et al., 1997; Lee et al., 2001). However, CT and MRI are costly methods and instrument access is limited.
An alternative approach for measuring total-body SM is dual energy X-ray absorptiometry (DXA), because DXA instruments are widely available and are relatively inexpensive; and radiation exposure is also minimal (Lukaski, 2005; Pietrobelli et al., 1996; Wang et al., 1999). DXA systems provide a measure of appendicular lean soft tissue (ALST), a fat- and bone mineral-free component that includes muscle and other components such as skin, tendons, and connective tissues (Fuller et al., 1992; Levine et al., 2000; Shih et al., 2000; Visser et al., 1999). A large proportion of total-body SM is found in the extremities, and a large proportion of ALST is SM. Hence, DXA potentially affords a practical and available means for quantifying total-body SM mass.
Previous investigators proposed several models for predicting SM with DXA (Fuller et al., 1992; Heymsfield et al., 1990; Wang et al., 1996, 1999); however some of these models (Fuller et al., 1992; Heymsfield et al., 1990) are now recognized as being either inaccurate or of limited applicability because of model imprecision or because of the complexity of the required measurements and calculations. Recently, Kim et al. (2004) developed SM DXA-based models for adults using MRI as the reference method which provided reliable and accurate estimates of total-body SM mass in adults.
Despite research and clinical significance, SM assessment remains difficult or impractical on a large scale basis. Although several studies have assessed the influence of ageing and gender on SM (Cohn et al., 1980; Forbes, 1987; Gallagher and Heymsfield 1998; Gallagher et al., 1997; Kehayias et al., 1997; Tzankoff and Norris, 1977), limited information is available on how the SM tissue compartment develops across the lifespan, especially in an ethnically diverse sample. Other than MRI-measured SM evaluated in the relatively large sample of adults (n = 488) studied by Janssen et al. (2000), previous studies are generally characterized by relatively small sample sizes. Given the importance of SM in both clinical and applied medicine (Evans, 1996 1997), understanding the independent influence of age and ethnicity on SM mass may be useful to improve functional capacity, and decrease health risks, particularly in elderly of different ethnic groups.
The aims of the present study were to (1) provide a cross-sectional report of SM mass from age 18 years onward using SM estimates derived by DXA in a large multi-ethnic sample and (2) identify age cutoffs after which SM values are negatively associated with age in a cohort of African American, Asian, Hispanic, and Whites women and men.
SUBJECTS AND METHODS
Protocol and subjects
Subjects were a convenience sample of adult men (n = 468) and women (n = 1280) participating in other unrelated investigations (He et al., 2003). Ethnicity was determined by self-report. Subjects were asked to choose from four categories Asian, Black (African American), and Whites. All parents and grandparents of the African American and White subjects were required to be non-Hispanic African American and non-Hispanic White, respectively. Four ethnic groups were, hence, identified: Whites, African American, Hispanics, and Asians.
The subjects varied in age (18–80 years) and body mass index (18.5–39.9 kg/m2). In addition, all subjects were ambulatory, without orthopedic problems, and completed a medical examination that included screening blood tests after fasting overnight. Only subjects who denied any major current health problems were enrolled in the study. Each subject performed all of the body composition measurements on the same day, after fasting overnight, at the Body Composition Unit of St. Luke’s-Roosevelt Hospital in New York City. The Institutional Review Board of St Luke’s-Roosevelt Hospital Center approved the study, and all subjects gave written consent before participation.
Body composition measurements
Anthropometric measurements
Body weight was measured to the nearest 0.1 kg (Weight Tronix, New York, NY) and height to the nearest 0.5 cm using a stadiometer (Holtain, Crosswell, Wales).
Dual-energy X-ray absorptiometry
Whole-body and regional body composition were estimated with a Lunar DPX scanner (GE Medical, Madison, WI) with software version 3.6. ALST was considered equivalent to the sum of lean soft tissue in both the right and left arms and legs. Appendages were isolated from the trunk and head by using regional computer-generated default lines, with manual adjustment, on the anterior view planogram. Specific anatomical landmarks were used to define the legs (i.e., soft tissue extending from a line drawn through and perpendicular to the axis of the femoral neck and angled with the pelvic brim to the phalange tips) and arms (i.e., soft tissue extending from the center of the arm socket to the phalange tips) (Kim et al., 2002). The system software provided the total mass, fat, lean soft tissue, and bone mineral mass for each of the selected regions. Repeated daily measurements over 5 d in 4 adult subjects showed a CV of 1.5% for leg lean soft tissue and 2.2% for arm lean soft tissue (Song et al., 2002).
Skeletal muscle
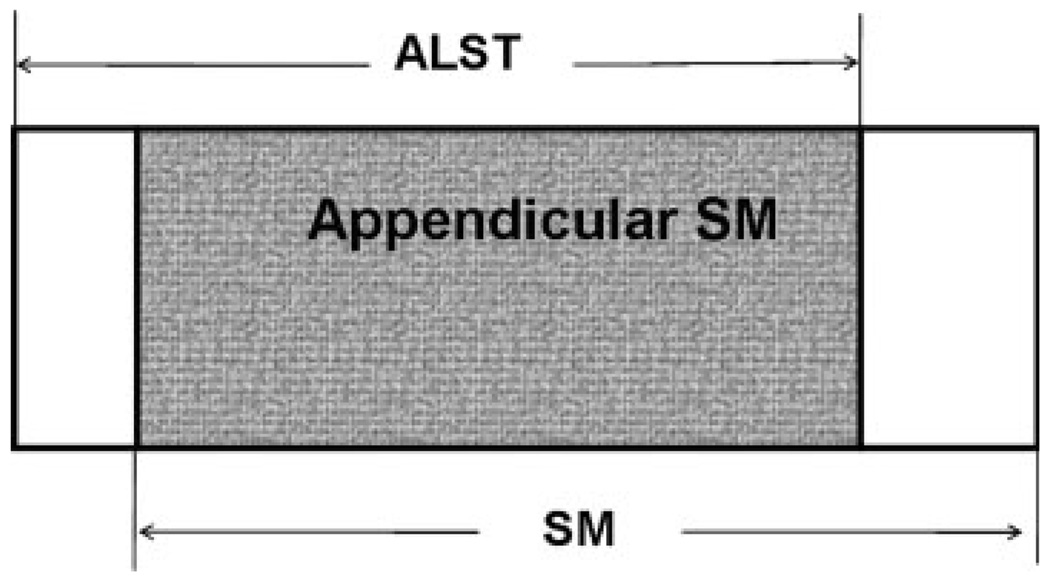
A model developed for adults that used magnetic resonance imaging (MRI) as the reference was used (Kim et al., 2004) to assess SM based on DXA-ALST. The developed model was based on the observation that: 1) a relatively large fraction of total body SM is present in the appendages; and 2) a high percentage of appendicular lean soft tissue (ALST) is SM as illustrated in Figure 1. ALST alone was highly correlated with whole body inter-muscular adipose tissue-free SM, and the model we used had an R2 of 0.96 and a standard error of 1.46 kg. The equation is as follows: SM = 1.19 × ALST − 1.65.
Fig. 1.
Relationship between appendicular lean soft tissue, appendicular skeletal muscle, and total-body skeletal muscle.
Statistical methods
Group data are presented as the mean ± SD. Independent sample t-tests were used to compare values between genders while among ethnic groups by sex, a one-way analysis of variance with Bonferroni correction was used.
SM was plotted against age for men and women separately and a locally weighted regression smoothing line was fitted to examine lifespan trends of SM and to localize age cutoffs that differentiate SM growth and decline. SM was plotted for each sex against age for Whites, African Americans, Hispanics, and also Asians (only in the female group). To estimate peak SM associated with age cutoffs, we applied multiple piecewise linear regression modeling controlling for weight and height that allows for different intercepts and slopes before and after a range of age cutoffs (Naumova et al., 2001). Candidate age cutoffs between 20 and 40 years were tested in 1-year increments. An optimal age cutoff was determined as the age that satisfied the following two conditions: (1) most significant difference in slope before compared with after the specified age and (2) reversed directions of slope after the specified age. We tested the age by ethnicity interaction to examine whether the slopes were significantly different across the four ethnic groups.
All statistical analyses were carried out using SPSS (SPSS for Windows, 14.0, SPSS, Inc., Chicago). Two-tailed (α = 0.05) tests of significance were used.
RESULTS
The subject demographic and body composition data are presented in Table 1. The multiethnic group included 1748 adults (i.e., age ≥ 18 years) (468 males and 1280 females) ranging in age from 18 to 80 years, with a mean body mass index (BMI) approximating the observed in the general US population (Hedley et al., 2004).
TABLE 1.
Subject characteristics
| Males | Females | |
|---|---|---|
| N (total sample = 1748) | 468 | 1280 |
| Ethnicity | ||
| Whites | 235 | 597 |
| AA | 85 | 384 |
| Hispanic | 148 | 207 |
| Asiana | – | 92 |
| Age (years) | 40.5 ± 14.3b | 44.5 ± 15.9 |
| Weight (kg) | 81.7 ± 14.2b | 73.7 ± 16.0 |
| Height (m) | 1.75 ± 0.08b | 1.62 ± 0.07 |
| BMI (kg/m2) | 26.6 ± 4.2b | 28.0 ± 5.6 |
| AALST (kg) | 7.5 ± 1.4b | 4.6 ± 1.0 |
| ALLST (kg) | 21.1 ± 3.3b | 14.6 ± 2.5 |
| SM (kg) | 32.4 ± 5.4b | 21.1 ± 4.0 |
Results are expressed as mean ± SD.
The Asian sample is a multi-generation mixture of Chinese, Indian, Korean, and Japanese (an insufficient number of males subjects participated in this study and were not considered in the data analysis of this study). Abbreviations: AA, African American; AALST, ALLST, and SM are appendicular arms lean soft tissue, appendicular legs lean soft tissue, and skeletal mass.
Males significantly differed from females, P < 0.001.
Males were younger than females (P < 0.001) and had a lower mean BMI (P < 0.001). As observed in Table 1, appendicular arm lean soft tissue (AALST), leg appendicular lean soft tissue (ALLST), and skeletal muscle were significantly smaller in females compared to males (P < 0.001).
Age and gender effects
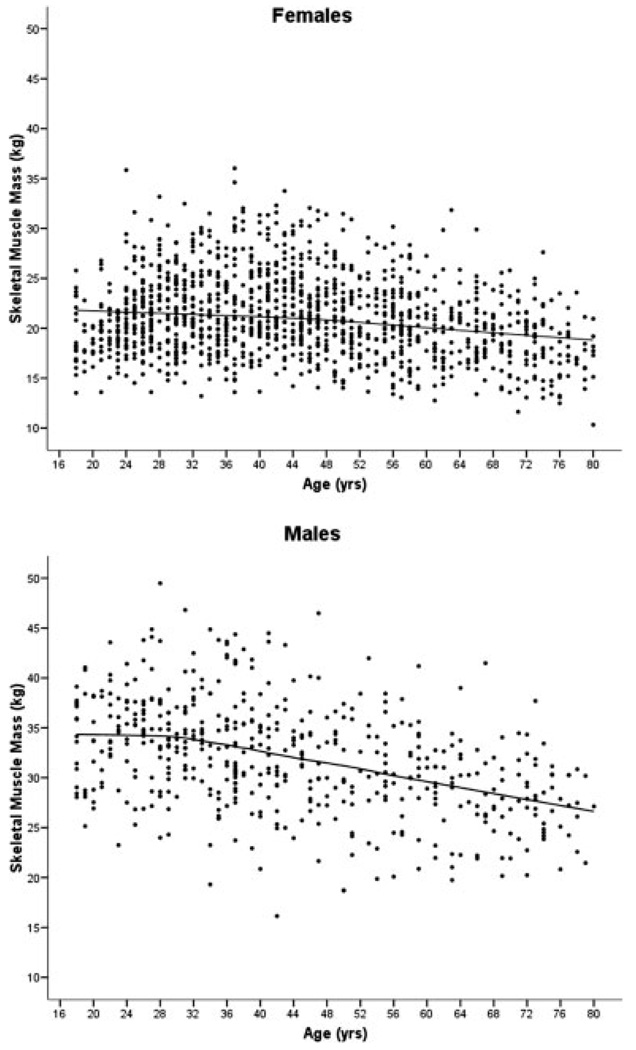
The smoothed scatter-plots for SM versus age are shown in Figure 2. From the observation of the cross-sectional values in Figure 2, SM tends to remain relatively stable in males during the age period of 20–30 years while for females stability in SM appeared until about the age of 40 years. Men also appear to have a greater negative association of SM with age than women, though a higher absolute SM in men is observed throughout the age range (Fig. 2).
Fig. 2.
Locally weighted regression smoothing line for skeletal muscle (SM) versus age for males (lower panel) and females (upper panel).
We further examined the trends observed, specifically the negative association of SM with age from the locally weighted regression lines shown in the Figure 2. For women and men, the age of 27 years was identified as the cutoff after which the SM regression line showed a negative association with age, adjusting for body weight and height. Hence, separate regression models were developed for women and men before and after the age cutoff, and the regression coefficients are presented in Table 2. In general, the men had higher expected mean SM than did the women. When the ethnicity-by-slope interactions were excluded from the models to estimate overall slopes after the age cutoffs, the SM rates of decline were 0.81 kg/decade and 1.58 kg/decade (P < 0.0001) for women and men, respectively.
TABLE 2.
Expected mean skeletal muscle (SM) values and slopes estimated from the regression models for women and men in 4 ethnic groupsa
| Value | |
|---|---|
| Men (n = 469) | |
| Expected mean SM at age 27 years (kg)b | |
| African American | 35.5 |
| Whites | 33.3 |
| Hispanic | 34.0 |
| Common slope before age 27 years (kg/y) | 0.865 |
| Slope after age 27 years (kg/y)c | |
| African American | −0.181 |
| Whites | −0.126 |
| Hispanic | −0.203 |
| Women (n = 1280) | |
| Expected mean SM at age 27 years (kg)b | |
| African American | 21.5A |
| Asian | 19.5B |
| Whites | 20.3B |
| Hispanic | 19.9B |
| Common slope before age 27 years (kg/y) | 0.208 |
| Slope after age 27 years (kg/y)c | |
| African American | −0.111 |
| Asian | −0.069 |
| Whites | −0.065 |
| Hispanic | −0.048 |
Means among the ethnic groups by sex with different superscript uppercase alphabets are significantly different, two-tailed P < 0.05.
All expected mean SM values were evaluated after adjustment for baseline weight and height.
Slopes across the 4 ethnic groups differ as the test of the age by ethnicity interaction was found significant P < 0.05.
Ethnicity effects
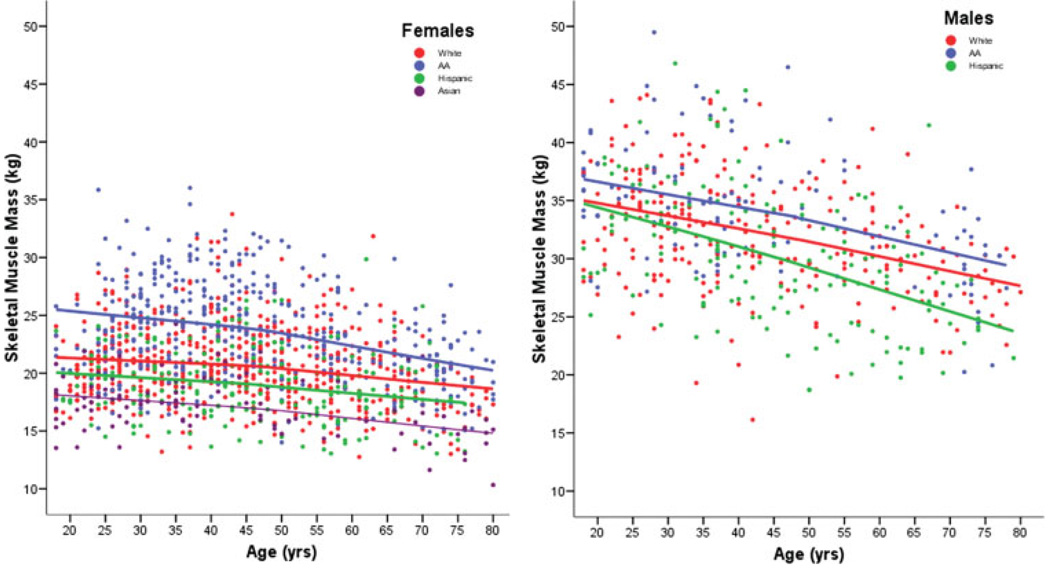
African American (AA) males and females tended to have higher values of SM mass across the lifespan, while Asian females and Hispanic males had the smaller absolute SM mass compared to the other groups (Fig. 3). We further explored the negative association between SM and age after the age cutoff across the ethnic groups, as indicated by the locally weighted regression lines shown in Figure 3. Among the women, African Americans had the largest expected mean SM values, followed by Whites, Hispanics, and Asians. A similar pattern is present in men, in whom African American men had the largest SM and Whites the smallest SM estimates.
Fig. 3.
Locally weighted regression smoothing line for skeletal muscle (SM) for ethnicity effects with age in females (left panel) and males (right panel). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com].
Additional analyses by ethnicity with a common age cutoff of 27 years showed that before the age of 27 years the positive association of SM with age did not significantly differed between men and women within Hispanics, Whites, and AA (all P values <0.01). After the age cut-off the negative associations observed between SM and age were significantly different between African Americans and Whites (P values <0.01) but not for Hispanics (P 5 0.072). Overall, after the age cutoff, the slope estimate of decline in SM in men was twice as large as that in women for African Americans and Whites. The slope estimate of decline in SM in male Hispanics was 4 times that of female Hispanics.
In women, SM started to have a negative association with age at the age of 27 years. Asian women had the lowest SM values while African American showed the highest values of SM throughout the age range studied (P < 0.05). Also, African American women displayed the greatest negative association of SM with age throughout the age range studied. The negative association of SM with age in women was greatest in African Americans (1.11 kg/decade) followed by Asians (0.69 kg/decade), Whites (0.65 kg/decade), and Hispanics (0.48 kg/decade).
In men, SM began to show a negative association with age after the age of 27 y, with Hispanics showing SM decline per decade of 2.03 kg/decade, followed by African Americans (1.81 kg/decade), and Whites (1.26 kg/decade).
DISCUSSION
Our study is the first that depicts SM distribution across most of the adult life span in a large and diverse cross-sectional sample. Our principal finding is that SM is relatively stable within individuals during adulthood up to about age 30 years, after which SM mass begins to decline. Adjusting for body weight and height, the rate of decrease is greater in men than in women, and in AA females and Hispanic males compared to their counterparts.
Sex and age’s difference in skeletal muscle
Our data show a sexual dimorphism in SM mass, with males having a greater positive association of SM with age before age 27, after which females have a negative association of SM with age, controlling for the effect of body weight and height. The findings of this cross-sectional study extend and strengthen the results of previous studies that report that men have more appendicular muscle than women, as estimated by DXA (Gallagher and Heymsfield, 1998; Gallagher et al., 1997), a single CT image (Miller et al., 1993), and MRI (Janssen et al., 2000). According to Janssen et al. (2000) there are sex differences for regional and whole body SM mass. These authors published cross-sectional data for changes with age in SM, as measured using whole-body MRI, in men and women aged 18 to 88 years. The data indicate that SM mass is relatively stable, on average to 45 years, after which there are accelerating rates of loss in both sexes. The findings of Janssen et al. (2000) also indicate that SM mass in men was 36% greater than in women remaining even after adjusting for sex differences in body weight and height. These sex differences may have a hormonal basis. Gonadal steroids are major mediators of adult sexual dimorphism in body composition, including fat-free soft tissues (Rosenbaum and Leibel, 1999). Considering absolute age-related SM mass, our data extend these results by showing larger values for males across the evaluated age range.
The age-associated decrease in SM using DXA based models confirms previous observations wherein SM was measured by MRI (Janssen et al., 2000) elemental analysis (total body potassium and/or nitrogen) (Cohn et al., 1980), urinary creatinine excretion (Tzankoff and Norris, 1977), DXA (Gallagher and Heymsfield, 1998; Gallagher et al., 1997), muscle biopsy (Lexell et al., 1986), and CT (Borkan et al., 1983; Rice et al., 1989). As reported previously with appendicular muscle (Gallagher and Heymsfield, 1998), the loss in whole body SM mass was independent of changes in body weight and stature and was greater in men than in women. The observed lower SM mass values after age 27 years (weight and height adjusted) differs with others who report that muscle fiber cross sectional area (i.e., contractile muscle) (Lexell et al., 1986), body cell mass (Forbes, 1987; Kehayias et al., 1997), and isometric (Bemben et al., 1991; Clement, 1974; Hurley, 1995) and isokinetic (Bemben et al., 1991; Clement, 1974; Hurley, 1995; Tseng et al., 1995) strength do not change substantially until ~45 years of age. On the contrary, a single study reported a lowering of absolute DXA-measured appendicular lean soft tissue beginning in the third decade (Gallagher et al., 1997). Most total-body potassium (TBK) exists in SM, and Gallagher et al. (1997) indicated that the cutoff ages for TBK decline (weight and height adjusted) were 30 and 31 years for women and men, similar to our findings of 27, assuming the life changes of SM should be comparable with those observed for TBK.
The age-related loss of SM, strength and function in old age, known as sarcopenia, has garnered interest in the last decade because it is related with low SM mass and reduced strength. Low muscle mass is associated with physical inactivity and declining levels of testosterone in elderly men, and possibly, growth hormone in both sexes (Baumgartner et al., 1998, 1999). The prevalence of sarcopenia increases rapidly with age greater than 60 years, as shown in studies of several data sets, including the large, nationally representative NHANES III (Baumgartner et al., 1998; Janssen et al., 2002; Tanko et al., 2002). Given the strong influence that SM has on bone mineral density (Bevier et al., 1989; Snow-Harter et al., 1990), the increased prevalence of osteoporosis in women may be explained, in part, by their lower SM mass. The reduced values of SM over the whole age range in the females in our study are not sufficient to affirm that women of this study are at a higher risk of bone fractures at older ages.
Ethnicity and skeletal muscle
In agreement with previous findings (Flynn et al., 1989; He et al., 2003; Pierson et al., 1974), the current study showed that SM appears to be more negatively associated with age in men compared to that in women. However, this sex difference in the association of SM with age varied by ethnicity. Overall, after the age 27 years, the magnitude of negative association of SM with age in men was twice that in women for African Americans and Whites. The magnitude of negative association of SM with age in male Hispanics was 4 times that of female Hispanics whereas the magnitude of the negative association in Asian females was similar to that observed in Hispanic and White women. Ethnic differences in SM loss were previously estimated in AA and White women in cross-sectional studies (Aloia et al., 2000; Gallagher et al., 1997). An investigation conducted by Gallagher et al. (1997) indicated a greater loss in TBK (weight and height adjusted) in African Americans than in White women, which is in accordance with the current findings. On the other hand, a cross-sectional study of 20–69-year-old women reported that the lifetime decline in TBK was 8% for AA women compared with 22% for White women (Aloia et al., 2000). According to He et al. (He et al., 2003; Kim et al., 2006) differences in the age range studied and in the statistical adjustments made may have accounted for these inconsistent findings. He et al. (He et al., 2003; Kim et al., 2006) reported that both sexes had similar ethnic patterns for expected mean TBK at the age cutoffs: African Americans had the highest value, followed by whites, Hispanics, and Asians. After the age cutoffs, the decline in TBK differed by ethnicity and sex. In women, African Americans showed the most rapid decline, almost the double of the other ethnic groups. In men, Hispanics had the most rapid decline in TBK, followed by African Americans, and Whites. Generally, the reported findings are consistent with our results.
Baumgartner et al. (1998) reported a greater prevalence of sarcopenia in elderly Hispanics than in non-Hispanic whites. In our study we observed in Hispanic males a trend for a pronounced negative association of SM with age after age 27. However, we also found an age-related negative association in other ethnic groups. Whether greater risk is associated with a more rapid loss of SM in healthy adults is unknown. The recognition of ethnic differences in SM loss may be of clinical importance because body composition varies by ethnicity. Asians and Hispanics are among the most rapidly growing ethnic groups according to the United States Census Bureau(US), with increases of 20% for non-Hispanic Asians and Pacific Islanders and 21% for persons of Hispanic origin between 1995 and 2000 (compared with 2% for non-Hispanic whites and 6% for non- Hispanic blacks). It was previously reported that Asian or Hispanic heritage is one variable associated with a significantly increased likelihood of osteoporosis in postmenopausal women (Siris et al., 2001). The identification of ethnic differences in the rate of SM loss needs to be followed up in metabolic studies to identify or clarify associations with health risk.
Study limitations
There are several limitations of this study. First, our results are based on a cross-sectional analysis and we cannot make within-subject temporal inferences about muscle mass and distribution for a given increment in age. The effects of environmental conditions on the growth and aging periods of the older subjects compared to younger subjects may also have an influence on the present study findings.
Second, we cannot exclude the possibility of the existence of a subject selection bias since this study is based on a convenience sample from the New York area. Nevertheless, the relatively large sample size could minimize the potential selection bias.
Third, this study used DXA to estimate SM while CT and MRI are the reference methods for assessing this tissue-level component. However, the availability of DXA has provided a technique that allows for the indirect assessment of total and regional lean soft tissue mass in adults, using appendicular skeletal muscle to calculate SM. Fourth, the observed ethnic differences in SM may reflect in part differences in dietary intake, acculturation, body size, or physical activity, detailed information not available for our study population. Moreover, ethnic differences in body composition (Ellis, 1990; Gallagher et al., 1997; Song et al., 2002) are likely reflected in part in the SM differences observed.
Last, and most important, we did not control for physical activity, which is related to muscle mass development. However, data from a multiethnic sample of adult, including residents of New York City area revealed a high density of availability of resources for physical activity practice which was an important factor that influence individuals’ physical activity behaviors (Diez Roux et al., 2007). Whether a higher engaged in a physical active behavior was present in our sample, mainly recruited from the New York city area, it is unknown. Moreover, in comparison to longitudinal studies, it is reported that cross-sectional studies underestimate the age-associated loss in muscular strength (Bassey and Harries, 1993; Clement, 1974; Hurley, 1995). When combined with the observation that the decrease in muscular strength with aging is predominantly due to a corresponding decrease in muscle size (Evans, 1997; Frontera et al., 1988), it is possible that we have underestimated the true effect of aging on muscle. Studies wherein muscle mass is longitudinally studied are required to confirm the findings reported here. Also this study did not control for menopausal status and for hormonal therapy replacement which may affect SM.
CONCLUSION
The results support and extend limited earlier studies demonstrating a clear sexual dimorphism in the relationship between age and SM mass compartment. Based on the observation of this cross-sectional sample, the available data set beginning at age 18 years through age 80 indicate that males and African American had more SM than females and the other ethnic groups across the entire age range, even adjusting for weight and height. Although an identical age cutoff for peak SM was found in women and men, at age 27 years, men attained higher peak values and had steeper negative association between SM and age than did women throughout the age range studied. Additionally, Hispanic males and African American females displayed the steepest negative relationship between SM and age within each gender. These findings confirm that body composition should be interpreted according to gender and ethnicity and, in particular, that different standards for skeletal muscle should be applicable for multi-ethnic populations.
Acknowledgments
Contract grant sponsor: National Institutes of Health; Contract grant number: NIDDK-42618.
LITERATURE CITED
- Aloia JF, Vaswani A, Feuerman M, Mikhail M, Ma R. Differences in skeletal and muscle mass with aging in black and white women. Am J Physiol Endocrinol Metab. 2000;278:E1153–E1157. doi: 10.1152/ajpendo.2000.278.6.E1153. [DOI] [PubMed] [Google Scholar]
- Bassey EJ, Harries UJ. Normal values for handgrip strength in 920 men and women aged over 65 years, and longitudinal changes over 4 years in 620 survivors. Clin Sci (Lond) 1993;84:331–337. doi: 10.1042/cs0840331. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107:123–136. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- Bemben MG, Massey BH, Bemben DA, Misner JE, Boileau RA. Isometric muscle force production as a function of age in healthy 20- to 74- yr-old men. Med Sci Sports Exerc. 1991;23:1302–1310. [PubMed] [Google Scholar]
- Bevier WC, Wiswell RA, Pyka G, Kozak KC, Newhall KM, Marcus R. Relationship of body composition, muscle strength, and aerobic capacity to bone mineral density in older men and women. J Bone Miner Res. 1989;4:421–432. doi: 10.1002/jbmr.5650040318. [DOI] [PubMed] [Google Scholar]
- Borkan GA, Hults DE, Gerzof SG, Robbins AH, Silbert CK. Age changes in body composition revealed by computed tomography. J Gerontol. 1983;38:673–677. doi: 10.1093/geronj/38.6.673. [DOI] [PubMed] [Google Scholar]
- Clement FJ. Longitudinal and cross-sectional assessments of age changes in physical strength as related to sex, social class, and mental ability. J Gerontol. 1974;29:423–429. doi: 10.1093/geronj/29.4.423. [DOI] [PubMed] [Google Scholar]
- Cohn SH, Vartsky D, Yasumura S, Sawitsky A, Zanzi I, Vaswani A, Ellis KJ. Compartmental body composition based on total-body nitrogen, potassium, and calcium. Am J Physiol. 1980;239:E524–E530. doi: 10.1152/ajpendo.1980.239.6.E524. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV, Evenson KR, McGinn AP, Brown DG, Moore L, Brines S, Jacobs DR., Jr Availability of recreational resources and physical activity in adults. Am J Public Health. 2007;97:493–499. doi: 10.2105/AJPH.2006.087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis KJ. Reference man and woman more fully characterized. Variations on the basis of body size, age, sex, and race. Biol Trace Elem Res. 1990;26–27:385–400. doi: 10.1007/BF02992693. [DOI] [PubMed] [Google Scholar]
- Evans WJ. Reversing sarcopenia: how weight training can build strength and vitality. Geriatrics. 1996;51:46–47. 51–53. quiz, 54. [PubMed] [Google Scholar]
- Evans WJ. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127(5 Suppl):998S–1003S. doi: 10.1093/jn/127.5.998S. [DOI] [PubMed] [Google Scholar]
- Flynn MA, Nolph GB, Baker AS, Martin WM, Krause G. Total body potassium in aging humans: a longitudinal study. Am J Clin Nutr. 1989;50:713–717. doi: 10.1093/ajcn/50.4.713. [DOI] [PubMed] [Google Scholar]
- Forbes GB. Human body composition: growth, aging, nutrition, and activity. New York: Springer-Verlag; 1987. [Google Scholar]
- Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64:1038–1044. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- Fuller NJ, Laskey MA, Elia M. Assessment of the composition of major body regions by dual-energy X-ray absorptiometry (DEXA), with special reference to limb muscle mass. Clin Physiol. 1992;12:253–266. doi: 10.1111/j.1475-097x.1992.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Gallagher D, Heymsfield SB. Muscle distribution: variations with body weight, gender, and age. Appl Radiat Isot. 1998;49:733–734. doi: 10.1016/s0969-8043(97)00096-1. [DOI] [PubMed] [Google Scholar]
- Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, Harris T, Heymsfield SB. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83:229–239. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- He q, Heo M, Heshka S, Wang J, Pierson RN, Jr, Albu J, Wang Z, Heymsfield SB, Gallagher D. Total body potassium differs by sex and race across the adult age span. Am J Clin Nutr. 2003;78:72–77. doi: 10.1093/ajcn/78.1.72. [DOI] [PubMed] [Google Scholar]
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Ross R, Wang Z, Frager D. Imaging techniques of body composition: advantages of measurement and new uses. In: Carlson-Newberry SJ, Costello RB, editors. Emerging technologies for nutrition research. Washington, DC: National Academy of Science Press; 1997. pp. 127–150. [Google Scholar]
- Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, Pierson RN., Jr Appendicular skeletal muscle mass: measurement by dualphoton absorptiometry. Am J Clin Nutr. 1990;52:214–218. doi: 10.1093/ajcn/52.2.214. [DOI] [PubMed] [Google Scholar]
- Hurley BF. Age, gender, and muscular strength. J Gerontol A Biol Sci Med Sci. 1995;50:41–44. doi: 10.1093/gerona/50a.special_issue.41. spec no. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- Kehayias JJ, Fiatarone MA, Zhuang H, Roubenoff R. Total body potassium and body fat: relevance to aging. Am J Clin Nutr. 1997;66:904–910. doi: 10.1093/ajcn/66.4.904. [DOI] [PubMed] [Google Scholar]
- Kim J, Heshka S, Gallagher D, Kotler DP, Mayer L, Albu J, Shen W, Freda PU, Heymsfield SB. Intermuscular adipose tissue-free skeletal muscle mass: estimation by dual-energy X-ray absorptiometry in adults. J Appl Physiol. 2004;97:655–660. doi: 10.1152/japplphysiol.00260.2004. [DOI] [PubMed] [Google Scholar]
- Kim J, Shen W, Gallagher D, Jones A, Jr, Wang Z, Wang J, Heshka S, Heymsfield SB. Total-body skeletal muscle mass: estimation by dual-energy X-ray absorptiometry in children and adolescents. Am J Clin Nutr. 2006;84:1014–1020. doi: 10.1093/ajcn/84.5.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76:378–383. doi: 10.1093/ajcn/76.2.378. [DOI] [PubMed] [Google Scholar]
- Lee RC, Wang ZM, Heymsfield SB. Skeletal muscle mass and aging: regional and whole-body measurement methods. Can J Appl Physiol. 2001;26:102–122. doi: 10.1139/h01-008. [DOI] [PubMed] [Google Scholar]
- Levine JA, Abboud L, Barry M, Reed JE, Sheedy PF, Jensen MD. Measuring leg muscle and fat mass in humans: comparison of CT and dual-energy X-ray absorptiometry. J Appl Physiol. 2000;88:452–456. doi: 10.1152/jappl.2000.88.2.452. [DOI] [PubMed] [Google Scholar]
- Lexell J, Downham D, Sjostrom M. Distribution of different fibre types in human skeletal muscles. Fibre type arrangement in m. vastus lateralis from three groups of healthy men between 15 and 83 years. J Neurol Sci. 1986;72:211–222. doi: 10.1016/0022-510x(86)90009-2. [DOI] [PubMed] [Google Scholar]
- Lukaski HC. Measurement of muscle mass. In: Heymsfield SB, Lohman TG, Wang Z, Going SB, editors. Human body composition. Champaign, IL: Human Kinetics; 2005. pp. 203–218. [Google Scholar]
- Malina RM. Regional body composition: age, sex, and ethnic variation. In: Roche AF, Heymsfield SB, Lohman TG, editors. Human body composition. Champaign, IL: Human Kinetics; 1996. pp. 217–256. [Google Scholar]
- Miller AE, MacDougall JD, Tarnopolsky MA, Sale DG. Gender differences in strength and muscle fiber characteristics. Eur J Appl Physiol Occup Physiol. 1993;66:254–262. doi: 10.1007/BF00235103. [DOI] [PubMed] [Google Scholar]
- Naumova EN, Must A, Laird NM. Tutorial in biostatistics: evaluating the impact of “critical periods” in longitudinal studies of growth using piecewise mixed effects models. Int J Epidemiol. 2001;30:1332–1341. doi: 10.1093/ije/30.6.1332. [DOI] [PubMed] [Google Scholar]
- Pierson RN, Jr, Lin DH, Phillips RA. Total-body potassium in health: effects of age, sex, height, and fat. Am J Physiol. 1974;226:206–212. doi: 10.1152/ajplegacy.1974.226.1.206. [DOI] [PubMed] [Google Scholar]
- Pietrobelli A, Formica C, Wang Z, Heymsfield SB. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol. 1996;271(6 Part 1):E941–E951. doi: 10.1152/ajpendo.1996.271.6.E941. [DOI] [PubMed] [Google Scholar]
- Rice CL, Cunningham DA, Paterson DH, Lefcoe MS. Arm and leg composition determined by computed tomography in young and elderly men. Clin Physiol. 1989;9:207–220. doi: 10.1111/j.1475-097x.1989.tb00973.x. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Leibel RL. Clinical review 107: role of gonadal steroids in the sexual dimorphisms in body composition and circulating concentrations of leptin. J Clin Endocrinol Metab. 1999;84:1784–1789. doi: 10.1210/jcem.84.6.5787. [DOI] [PubMed] [Google Scholar]
- Shih R, Wang Z, Heo M, Wang W, Heymsfield SB. Lower limb skeletal muscle mass: development of dual-energy X-ray absorptiometry prediction model. J Appl Physiol. 2000;89:1380–1386. doi: 10.1152/jappl.2000.89.4.1380. [DOI] [PubMed] [Google Scholar]
- Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- Snow-Harter C, Bouxsein M, Lewis B, Charette S, Weinstein P, Marcus R. Muscle strength as a predictor of bone mineral density in young women. J Bone Miner Res. 1990;5:589–595. doi: 10.1002/jbmr.5650050608. [DOI] [PubMed] [Google Scholar]
- Song MY, Kim J, Horlick M, Wang J, Pierson RN, Jr, Heo M, Gallagher D. Prepubertal Asians have less limb skeletal muscle. J Appl Physiol. 2002;92:2285–2291. doi: 10.1152/japplphysiol.01066.2001. [DOI] [PubMed] [Google Scholar]
- Tanko LB, Movsesyan L, Mouritzen U, Christiansen C, Svendsen OL. Appendicular lean tissue mass and the prevalence of sarcopenia among healthy women. Metabolism. 2002;51:69–74. doi: 10.1053/meta.2002.28960. [DOI] [PubMed] [Google Scholar]
- Tseng BS, Marsh DR, Hamilton MT, Booth FW. Strength and aerobic training attenuate muscle wasting and improve resistance to the development of disability with aging. J Gerontol A Biol Sci Med Sci. 1995;50:113–119. doi: 10.1093/gerona/50a.special_issue.113. spec no. [DOI] [PubMed] [Google Scholar]
- Tzankoff SP, Norris AH. Effect of muscle mass decrease on age-related BMR changes. J Appl Physiol. 1977;43:1001–1006. doi: 10.1152/jappl.1977.43.6.1001. [DOI] [PubMed] [Google Scholar]
- US Census Bureau. [accessed 16 August 2002];Population estimates program. 2000 December; http://eire.census.gov/popest/archives/national/nation3/intfile3-1.txt.
- Health, Aging, and Body Composition Study–Dual-Energy X-ray Absorptiometry and Body Composition Working Group. Visser M, Fuerst T, Lang T, Salamone L, Harris TB. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. J Appl Physiol. 1999;87:1513–1520. doi: 10.1152/jappl.1999.87.4.1513. [DOI] [PubMed] [Google Scholar]
- Wang ZM, Visser M, Ma R, Baumgartner RN, Kotler D, Gallagher D, Heymsfield SB. Skeletal muscle mass: evaluation of neutron activation and dual-energy X-ray absorptiometry methods. J Appl Physiol. 1996;80:824–831. doi: 10.1152/jappl.1996.80.3.824. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang Z, Faith MS, Kotler D, Shih R, Heymsfield SB. Regional skeletal muscle measurement: evaluation of new dual-energy X-ray absorptiometry model. J Appl Physiol. 1999;87:1163–1171. doi: 10.1152/jappl.1999.87.3.1163. [DOI] [PubMed] [Google Scholar]