Abstract
Integrin αvβ3 plays a significant role in tumor angiogenesis, and is a receptor for the extracellular matrix proteins with the exposed arginine-glycine-aspartic (RGD) tripeptide sequence. These include vitronectin, fibronectin, fibrinogen, lamin, collagen, Von Willibrand’s factor, osteoponin, and adenovirus particles. Integrin αvβ3 is expressed at low levels on epithelial cells and mature endothelial cells, but it is overexpressed on the activated endothelial cells of tumor neovasculature and some tumor cells. The restricted expression of integrin αvβ3 during tumor growth, invasion and metastasis present an interesting molecular target for both early detection and treatment of rapidly growing solid tumors. Over the last decade, many radiolabeled linear and cyclic RGD peptide antagonists have been evaluated as the integrin αvβ3-targeted radiotracers. Significant progress has been made on their use for imaging integrin αvβ3-positive tumors by SPECT or PET. Among the radiotracers evaluated in pre-clinical tumor-bearing models, [18F]Galacto-RGD (2-[18F]fluoropropanamide c(RGDfK(SAA); SAA = 7-amino-L-glyero-L-galacto-2,6-anhydro-7-deoxyheptanamide) and [18F]-AH111585 are currently under clinical investigation for visualization of integrin αvβ3 expression in cancer patients. However, their low tumor uptake, high cost and lack of preparative modules for routine radiosynthesis will limit their continued clinical applications. Thus, there is a continuing need for more efficient integrin αvβ3-targeted radiotracers that are readily prepared from a kit formulation without further post-labeling purification. This article will focus on different approaches to maximize the targeting capability of cyclic RGD peptides and to improve the radiotracer excretion kinetics from non-cancerous organs. Improvement of tumor uptake and tumor-to-background ratios is important for early detection of integrin αvβ3-positive tumors and/or noninvasive monitoring of therapeutic efficacy of antiangiogenic therapy.
Keywords: Integrin αvβ3, PET and SPECT radiotracers, tumor imaging
Introduction
Cancer is the second leading cause of death worldwide. Although the exact cause of cancer remains unknown, most cancer patients will survive after surgery, radiation therapy, and chemotherapy or a combination thereof if it can be detected at the early stage. Thus, accurate early detection is highly desirable so that appropriate therapy can be given before the primary tumors become widely spread.
Tumors produce many angiogenic factors, which are able to activate endothelial cells in established blood vessels and induce endothelial proliferation, migration, and new vessel formation (angiogenesis) through a series of sequential but partially overlapping steps. Angiogenesis is a requirement for tumor growth and metastasis (1–7). Without the neovasculature to provide oxygen and nutrients, tumors cannot grow beyond 1–2 mm in size. Once vascularized, the tumors begin to grow rapidly. The angiogenic process depends on vascular endothelial cell migration and invasion, is regulated by cell adhesion receptors. Integrins are such a family of proteins that facilitate the cellular adhesion to and the migration on extracellular matrix proteins in the intercellular spaces and basement membranes, and regulate cellular entry and withdraw from the cell cycle (7–10). Integrin αvβ3 serves as a receptor for extracellular matrix proteins with exposed arginine-glycine-aspartic (RGD) tripeptide sequence (8–13). These include vitronectin, fibronectin, fibrinogen, lamin, collagen, osteoponin and adenovirus particles. Integrin αvβ3 is expressed at low levels on epithelial cells and mature endothelial cells; but it is highly expressed on activated endothelial cells in neovasculature of tumors, including osteosarcomas, neuroblastomas, glioblastomas, melanomas, lung carcinomas and breast cancer (14–16). It has been demonstrated that integrin αvβ3 is overexpressed on both the endothelial cells and tumor cells in human breast cancer xenografts (17). The integrin αvβ3 expression correlates well with tumor progression and invasiveness of melanoma, glioma, ovarian and breast cancers (10–17). The restricted expression of integrin αvβ3 during tumor growth, invasion and metastasis present an interesting molecular target for diagnosis and treatment of the rapidly growing and metastatic tumors (18–32).
Over the last decade, many radiolabeled cyclic RGD peptides have been evaluated as radiotracers for imaging tumors by SPECT or PET (33–68). Several review articles have appeared covering the nuclear medicine applications of radiolabeled cyclic RGD peptide and non-peptide antagonists as radiotracers for diagnosis and radiotherapy of integrin αvβ3–positive tumors (17–29). This article is not intended to be an exhaustive review of the current literature on radiolabeled RGD peptides and nuclear medicine applications. Instead, it will focus on different approaches to maximize their targeting capability and to improve the radiotracer excretion kinetics from non-cancerous organs. Improvement of tumor uptake and tumor-to-background (T/B) ratios is important for both diagnostic and therapeutic radiotracers.
Radiotracer Design
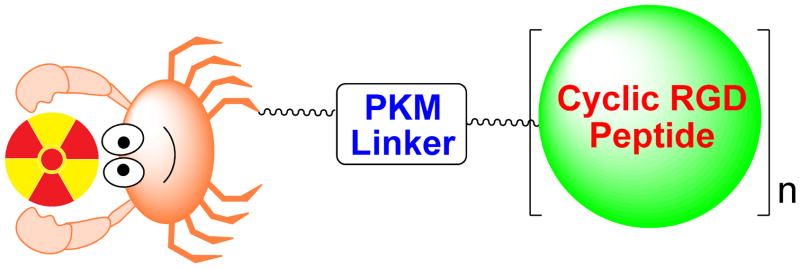
Figure 1 shows a schematic illustration of an integrin αvβ3-targeted radiotracer. Cyclic RGD peptide serves as the targeting biomolecule to carry radionuclide to the integrin αvβ3 overexpressed on tumor cells and activated endothelial cells of tumor neovasculature. A multidentate bifunctional chelator (BFC) is used to attach the metallic radionuclide to the cyclic RGD peptide (69–72), whereas an organic precursor or synthon is often needed for the 18F-labeling (73). The pharmacokinetic modifying linker (PKM) is used to improve the radiotracer excretion kinetics (69–71). For a new integrin αvβ3-targeted radiotracer to be successful, it must show clinical indications for high-incidence tumors (breast, lung, colorectal, prostate and skin cancers). The radiotracer should have high tumor uptake and T/B ratios in a short period of time. To achieve this goal, the radiotracer should have a rapid blood clearance to minimize background radioactivity. Since most high-incidence tumors (breast, lung and colorectal cancers) occur in the torso, renal excretion is necessary to avoid radioactivity accumulation in the gastrointestinal tract. The integrin αvβ3-targeted radiotracer should also be able to distinguish between benign and malignant tumors, to follow tumor growth and metastasis, and to predict therapeutic efficacy in integrin αvβ3-positive cancer patient. In addition, a kit formulation or preparative module is needed for routine preparation of the radiotracer in high yield and radiochemical purity at low cost.
Figure 1.
Schematic presentation of the radiotracer design. The targeting biomolecule is a multimeric cyclic RGD peptide. The PKM linker is used to modify radiotracer pharmacokinetics. For the metal-containing radiotracers, a multidentate BFC is often used to attach the metallic radionuclide to the targeting biomolecule. For 18F-based radiotracers, an organic precursor or synthon is often needed to attach 18F onto the multimeric cyclic RGD peptide.
Choice of Radionuclide
The choice of radionuclide depends largely on the clinical utility of radiotracer. For planar imaging and SPECT, more than 80% of radiotracers used in nuclear medicine departments are 99mTc compounds due to optimal nuclear properties of 99mTc and its easy availability at low cost (69–72). The 6 h half-life is long enough to allow radiopharmacists to carry out radiosynthesis and for physicians to collect clinically useful images. At the same time, it is short enough to permit administration of 20–30 mCi of 99mTc without imposing a significant radiation dose to the patient. The monochromatic 140 KeV photons are readily collimated to give high quality images with high spatial resolution. The clinically most relevant radionuclides for PET include 18F, 62Cu, 64Cu and 68Ga.
18F is a cyclotron-produced isotope suitable for PET. It has a half-life of 110 min. For several years, 18F-FDG (FDG = 2-fluoro-2-deoxyglucose) has been widely used as an imaging tool for diagnosis of cancers, brain and cardiovascular diseases. Despite its short half-life, the availability of preparative modules makes 18F-labeled biomolecules much more accessible to researchers and clinicians in many clinical institutions.
62Cu is a generator-produced radionuclide from the decay of 62Zn. Its 9.7 min half-life allows repeated dosing without imposing a significant radiation burden to the patient. The commercially available 62Zn-62Cu generator has been successfully used in clinical trials (74–77). 64Cu is another PET isotope useful for development of target-specific radiotracers. It has a half-life of 12.7 h and a β+ emission (18%, Emax = 0.655 MeV). Despite poor nuclear properties, its long half-life makes it feasible to prepare, transport, and deliver the 64Cu radiotracer for clinical applications. More importantly, recent breakthroughs in production of 64Cu with high specific activity have made it more available to the small research institutions without on-site cyclotron facilities (78). 64Cu is a viable alternative to 18F for research programs that wish to incorporate high sensitivity and high spatial resolution of PET, but cannot afford to maintain the expensive radionuclide production infrastructure. Copper radionuclides and related radiochemistry have been reviewed by Blower et al (79). Nuclear medicine applications of 64Cu-labeled monoclonal antibodies and peptides have been reviewed by Anderson et al (75, 80).
68Ga is generator-produced PET isotope with a half-life of 68 min. The 68Ge-68Ga generator can be used for more than a year, allowing PET studies without the on-site cyclotron. If the radiotracer is properly designed, 68Ga could become as useful for PET as 99mTc for SPECT (81, 82). In support of this, the 68Ga-labeled somatostatin analogs have been studied extensively for PET imaging of somatostatin-positive tumors in animal models and cancer patients (83–91). Gallium chemistry and related medical applications have been reviewed recently (81, 82, 92).
Bifunctional Chelators
The choice of BFC depends on the radionuclide. 18F can be incorporated into the cyclic RGD peptide via a covalent bond without the need for BFC. In contrast, BFC is an important part of radiotracers containing a metallic radionuclide (69–72, 92). Among various BFCs (Figure 2), 6-hydazinonicotinic acid (HYNIC) is of great interest due to its high 99mTc-labeling efficiency (rapid radiolabeling and high radiolabeling yield), the high solution stability of its 99mTc complexes, and the easy use of different coligands for modification of biodistribution characteristic of 99mTc-labeled small biomolecules (93). DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid), NOTA (1,4,7-tritazacyclononane-1,4,7-triacetic acid) and their derivatives (Figure 2) have been used as BFCs for 68Ga and 64Cu-labeling small biomolecules (84–91). NODAGA is particularly useful for 68Ga- and 64Cu-labeling due to high hydrophilicity and in vivo stability of its 68Ga and 64Cu chelates. It has been reported that NOTA derivatives have much higher 68Ga and 64Cu-labeling efficiency than their DOTA analogs (94–98). The fast and efficient radiolabeling is especially critical for 68Ga and 62Cu due to their short half-life (t1/2 = 68 min for 68Ga and 9.7 min for 62Cu).
Figure 2.
BFCs useful for radiolabeling of multimeric cyclic RGD peptides with 99mTc, 68Ga and 64Cu. DADA (diamidodithiol), MAMA (monoaminemonoamidedithiol), MADT (diaminedithiol), MAG2 (2-mecaptoacetylglycylglycyl) and HYNIC are particularly useful for 99mTc-labeling while DOTA, NOTA and their derivatives are excellent BFCs for chelation of 68Ga and 64Cu.
PKM Linkers
In general, high lipophilicity often leads to more hepatobiliary excretion and/or high protein binding, which will results in longer blood retention of radioactivity. Hepatobiliary excretion is detrimental for improvement of T/B ratio. Thus, an important aspect of radiotracer development is to improve T/B ratios by modifying pharmacokinetics of radiolabeled cyclic RGD peptides. For example, the negatively charged small peptide sequences or amino acids have been proposed as PKM linkers to reduce renal uptake and kidney retention of radiolabeled small biomolecules (70, 71, 92). The di(cysteic acid) linker has successfully been used to improve the blood clearance and minimize the liver and kidney activity of radiolabeled nonpeptide integrin αvβ3 receptor antagonists (99–102). The Asp3 and Ser3 tripeptide sequences were also used to modify excretion kinetics of the 99mTc-labeled cyclic RGD peptide (39). Harris et al reported the use of a PEG4 (15-amino-4,7,10,13-tetraoxapentadecanoic acid) linker to improve the tumor uptake and T/B ratios of the 99mTc-labeled nonpeptide integrin αvβ3 receptor antagonists (99–102). Kessler et al reported the use of HEG (hexaethylene glycolic acid) as the PKM linker for the 18F-labeled cyclic RGDfE dimers and tetramers (40–42). Using the HEG linker also increases the distance between the cyclic RGD motifs. Chen et al also found that the introduction of the PEG (polyethylene glycolic acid) linker can improve the tumor uptake and excretion kinetics of 125I- and 18F-labeled c(RGDyK) and 64Cu-labeled E[c(RGDyK)]2 (48, 49, 54, 103).
Targeting Biomolecules
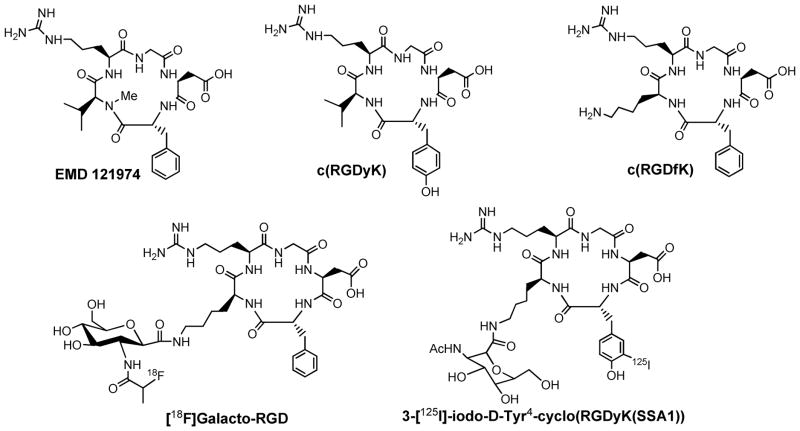
Figure 3 shows several examples of cyclic RGD peptides that have high affinity and selectivity for integrin αvβ3. Among the radiotracers evaluated in pre-clinical tumor-bearing models, [18F]Galacto-RGD (Figure 3: 2-[18F]fluoropropanamide c(RGDfK(SAA); SAA = 7-amino-L-glyero-L-galacto-2,6-anhydro-7-deoxyheptanamide) and [18F]-AH111585, the core sequence of which was originally discovered from a phage display library (as ACDRGDCFCG), are currently under clinical investigation for visualization of integrin αvβ3 expression in cancer patients (104–109). The results from imaging studies in cancer patients show that there is sufficient integrin αvβ3 expression for PET imaging, and the accumulation of radiolabeled RGD peptide radiotracer correlates well with the integrin αvβ3 expression levels in cancer patients (108, 109). However, their relatively low tumor uptake, the high cost and lack of preparative modules for routine radiosynthesis will limit their continued clinical applications. Several steps of manual radiosynthesis and post-labeling purification often cause significant radiation exposure to radiopharmacists. Of course, this problem can be solved by developing preparative modules for routine radiosynthesis, which will definitely lead to added cost for the radiotracer. Thus, this is a continuing need for more efficient integrin αvβ3-specific radiotracers that are readily prepared from a kit formulation without further post-labeling chromatographic purification.
Figure 3.
Chemdraw structures of cyclic RGD peptides useful as targeting biomolecules for the integrin αvβ3-targeted radiotracers. [18F]Galacto-RGD (2-[18F]fluoropropanamide c(RGDfK(SAA); SAA = 7-amino-L-glyero-L-galacto-2,6-anhydro-7-deoxyheptanamide) is currently under clinical investigation for visualization of integrin αvβ3 expression in cancer patients
Maximizing Binding Affinity via Multimerization
Cyclization to Improve Binding Affinity and Selectivity
Many cyclic RGD peptides have been proposed as the integrin αvβ3 antagonists for treatment of cancer. It was found that incorporation of the RGD sequence into a cyclic pentapeptide framework (Figure 3) increases the binding affinity and selectivity for integrin αvβ3 over glycoprotein IIb/IIIa (110–113). After extensive structure-activity evaluations, it was concluded that the amino acid in position 5 has no significant impact on integrin αvβ3 binding affinity. The valine (V) residue in c(RGDfV) can be replaced by lysine (K) or glutamic acid (E) to afford c(RGDfK) and c(RGDfE), respectively, without changing the integrin αvβ3 binding affinity.
Cyclic RGD Dimers
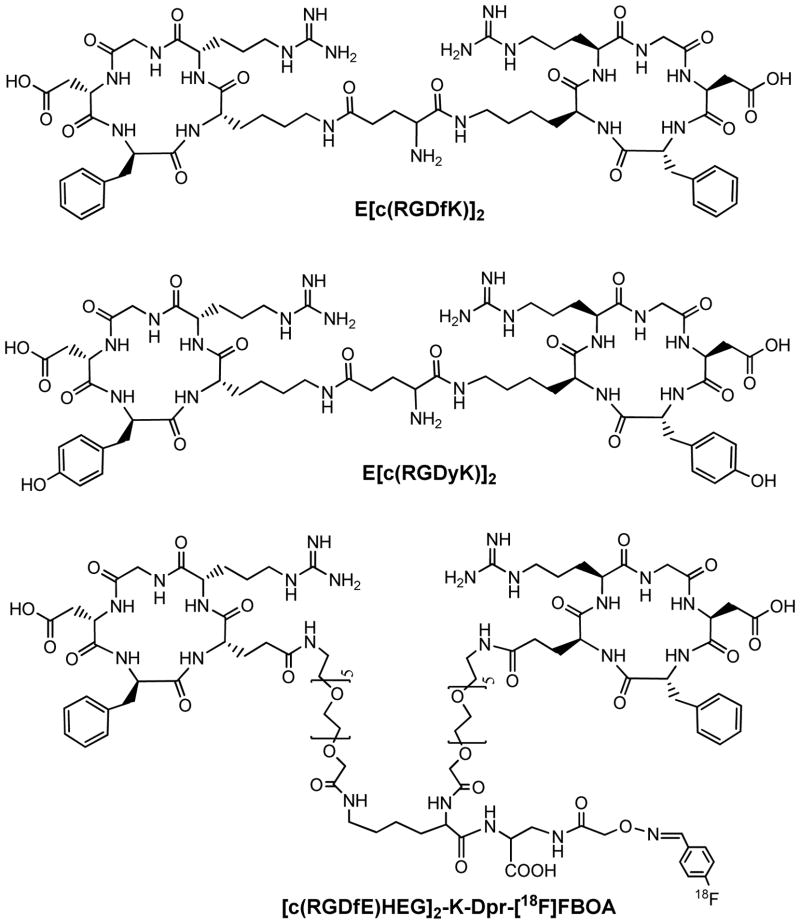
To improve integrin αvβ3 binding affinity, multimeric RGD peptides, such as E[c(RGDfK)]2, have been used to develop integrin αvβ3-targeted radiotracers. For example, Rajopadhye et al were the first to use cyclic RGD dimers, such as E[c(RGDfK)]2 (Figure 4), to develop diagnostic (99mTc) and therapeutic (90Y and 64Cu) radiotracers (56, 57, 114, 115). Recently, Chen et al (50,51) reported the 64Cu and 18F-labeled E[c(RGDyK)]2 (Figure 4) as PET radiotracers. Poethko et al found that the RGDfE dimer [c(RGDfE)HEG]2-K-Dpr-[18F]FBOA (Figure 4) had much better targeting capability as evidenced by its higher integrin αvβ3 binding affinity and tumor uptake as compared to its monomeric analog c(RGDfE)HEG-Dpr-[18F]FBOA (40–42).
Figure 4.
Cyclic RGD peptide dimers (E[c(RGDfK)]2 and E[c(RGDyK)]2) and [c(RGDfE)HEG]2-K-Dpr-[18F]FBOA.
Cyclic RGD Tetramers and Octamers
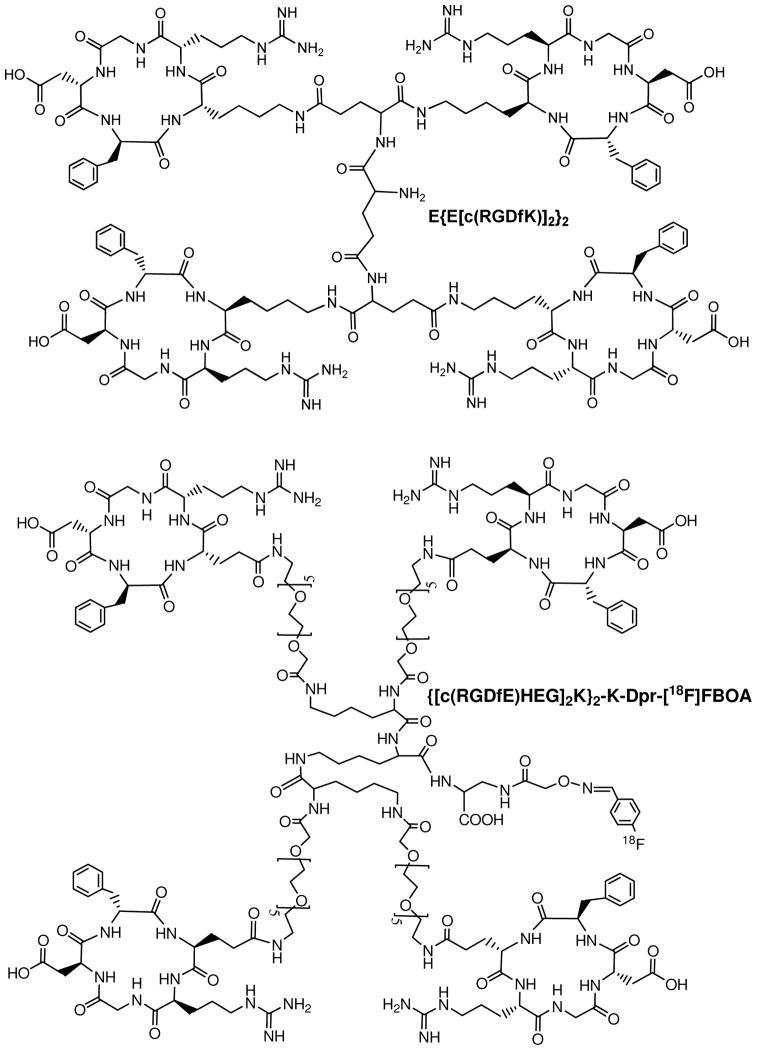
Several groups have used the multimer concept to prepare cyclic RGD tetramers and octamers. For example, Boturyn et al reported a series of cyclic RGDfK tetramers (116), and found that increasing the peptide multiplicity significantly enhanced the integrin αvβ3 binding affinity and internalization. Kessler et al reported a cyclic RGDfE tetramer (Figure 5) that had better integrin αvβ3 binding affinity than its monomeric and dimeric analogs (40–42). Liu et al reported the use a cyclic RGDfK tetramer E[E[c(RGDfK)]2]2 (Figure 5) for development of integrin αvβ3-targeted SPECT and PET radiotracers (52, 64–68). Chen et al recently used 64Cu and 18F-labeled cyclic RGD tetramer E[E[c(RGDxK)]2]2 and octamer E[E[E[c(RGDyK)]2]2]2 for tumor imaging by PET (52, 117). Although the results from both in vitro assays and ex-vivo biodistribution studies have demonstrated that radiolabeled (99mTc, 18F and 64Cu) RGD tetramers and octamer have much better tumor targeting capability (higher integrin αvβ3 binding affinity and better radiotracer tumor uptake) than their dimeric analogs, it remains unclear if the cyclic RGD motifs in E[E[c(RGDxK)]2]2 (x = f and y) and E[E[E[c(RGDyK)]2]2]2 are really multivalent in binding to integrin αvβ3. As the peptide multiplicity increases, the uptake of radiolabeled multimeric RGD peptides in the kidneys, liver, lungs and spleen are also significantly increased. In addition, the cost for E[E[c(RGDfK)]2]2 and E[E[E[c(RGDyK)]2]2]2 is prohibitively high for development of integrin αvβ3-targeted radiotracers in the future. Thus, an alternate approach is needed to improve integrin αvβ3-targeting capability and minimize the radiotracer accumulation in non-cancerous organs.
Figure 5.
A cyclic RGDfK peptide tetramer, E{E[c(RGDfK)]2}2 and a 18F-labeled cyclic RGDfE tetramer, {[c(RGDfE)HEG]2K}2-K-Dpr-[18F]FBOA.
Maximizing Binding Affinity via Bivalency
Factors Influencing Binding Affinity
The success of E[c(RGDfK)]2 as targeting biomolecules is very intriguing. Given the short distance (6 bonds excluding side-arms of K-residues) between two cyclic RGD motifs in E[c(RGDfK)]2, it is unlikely that they would bind to two adjacent integrin αvβ3 sites simultaneously. However, the binding of one RGD motif to integrin αvβ3 will significantly increase the “local concentration” of second RGD motif in the vicinity of integrin αvβ3 sites (Figure 6A). The “locally enhanced RGD concentration” may explain the higher tumor uptake of radiolabeled (99mTc, 111In, 90Y, 18F and 64Cu) cyclic RGD dimers as compared to their monomeric analogs (60–65). To further improve the integrin αvβ3-targeting capability of cyclic RGD dimers, the distance between two cyclic RGD motifs must be increased so that they are to achieve simultaneous integrin αvβ3 binding (Figure 6B). The combination of “bivalency” and the “enriched local RGD concentration” is expected to result in higher integrin αvβ3 targeting capability of cyclic RGD dimers and better tumor uptake with longer tumor retention time for their corresponding radiotracers.
Figure 6.

Schematic illustration of interactions between cyclic RGD peptide dimers and integrin αvβ3. A: The distance between two RGD motifs is not long enough for simultaneous integrin αvβ3 binding. However, the RGD concentration is “locally enriched” in the vicinity of neighboring integrin αvβ3 once the first RGD motif is bound. B: The distance between two RGD motifs is long due to the presence of two linkers (L). As a result, the cyclic RGD dimer is able to bind integrin αvβ3 in a “bivalent” fashion. In both cases, the end-result would be higher integrin αvβ3 binding affinity for the multimeric cyclic RGD peptides.
Improve Integrin αvβ3 Binding Affinity by Increasing Distance between Cyclic RGD Motifs
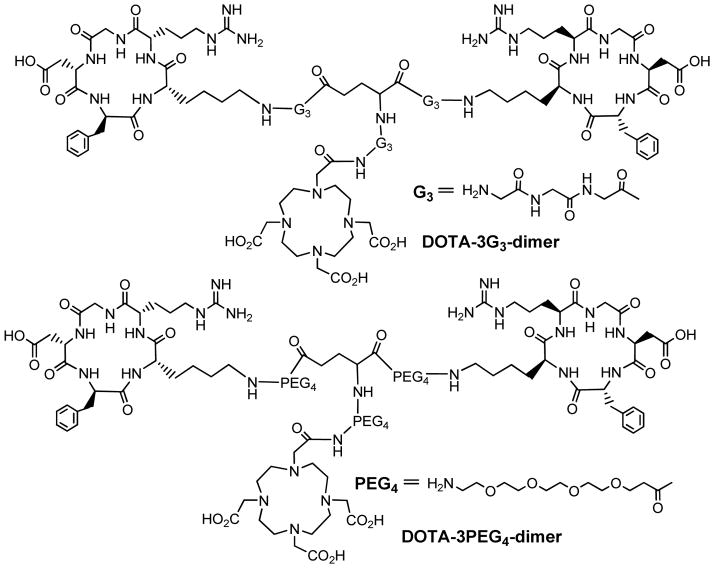
To demonstrate this concept, Shi et al recently reported a series of cyclic RGD peptide dimers (Figure 7) containing triglycine (G3) and PEG4 linkers, which are used to increase the distance between two cyclic RGD motifs from 6 bonds in E[c(RGDfK)]2 to 24 bonds in 3G3-dimer and 38 bonds in 3PEG4-dimer (118, 119). The integrin αvβ3 binding affinities (Table 1) against 125I-echistatin bound to U87MG human glioma cells follow the order of HYNIC-tetramer (IC50 = 7 ± 2 nM) > HYNIC-2PEG4-dimer (IC50 = 52 ± 7 nM) ~ HYNIC-3PEG4-dimer (IC50 = 60 ± 4 nM) ~ HYNIC-3G3-dimer (IC50 = 61 ± 2 nM) > HYNIC-PEG4-dimer (IC50 = 84 ± 7 nM) ~ HYNIC-dimer (IC50 = 112 ± 21 nM) ≫ HYNIC-G3-monomer (IC50 = 358 ± 8 nM) > HYNIC-PEG4-monomer (IC50 = 452 ± 11 nM). These data indicate that the G3 and PEG4 linkers between two RGD motifs are responsible for the improved integrin αvβ3 affinity of HYNIC-3G3-dimer and HYNIC-3PEG4-dimer as compared to that of HYNIC-PEG4-dimer. The higher binding affinity of HYNIC-tetramer is probably due to its two extra cyclic RGD motifs.
Figure 7.
New cyclic RGD peptide dimers useful for the development of integrin αvβ3–targeted radiotracers. The PEG4 and G3 linkers are used to increase the distance between two RGD motifs and to improve radiotracer excretion kinetics from non-cancerous organs.
Table 1.
Integrin αvβ3 binding data for HYNIC-conjugated cyclic RGD peptides against 125I-echistatin bound to the integrin αvβ3–positive U87MG human glioma cells.
| Compound | Radiotracer | IC50 (nM) |
|---|---|---|
| HYNIC-G3-monomer | [99mTc(HYNIC-G3-monomer)(tricine)(TPPTS)] | 358 ± 8 |
| HYNIC-PEG4-monomer | [99mTc(HYNIC-PEG4-monomer)(tricine)(TPPTS)] | 452 ± 11 |
| HYNIC-dimer | [99mTc(HYNIC-dimer)(tricine)(TPPTS)] | 112 ± 21 |
| HYNIC-PEG4-dimer | [99mTc(HYNIC-PEG4-dimer)(tricine)(TPPTS)] | 84 ± 7 |
| HYNIC-2G3-dimer | [99mTc(HYNIC-2G3-dimer)(tricine)(TPPTS)] | 60 ± 4 |
| HYNIC-2PEG4-dimer | [99mTc(HYNIC-2PEG4-dimer)(tricine)(TPPTS)] | 52 ± 7 |
| HYNIC-3G3-dimer | [99mTc(HYNIC-3G3-dimer)(tricine)(TPPTS)] | 61 ± 2 |
| HYNIC-3PEG4-dimer | [99mTc(HYNIC-3PEG4-dimer)(tricine)(TPPTS)] | 62 ± 5 |
| HYNIC-tetramer | [99mTc(HYNIC-dimer)(tricine)(TPPTS)] | 7 ± 2 |
| DOTA-dimer | 64Cu(DOTA-dimer)/111In(DOTA-dimer) | 102 ± 5 |
| DOTA-3G3-dimer | 64Cu(DOTA-3G3-dimer)/111In(DOTA-3G3-dimer) | 74 ± 3 |
| DOTA-3PEG4-dimer | 64Cu(DOTA-3PEG4-dimer)/111In(DOTA-3PEG4-dimer) | 62 ± 6 |
| DOTA-tetramer | 64Cu(DOTA-tetramer)/111In(DOTA-tetramer) | 10 ± 2 |
| NOTA-dimer | 68Ga(NOTA-dimer) | 100 ± 3 |
| NOTA-2G3-dimer | 68Ga(NOTA-2G3-dimer) | 66 ± 4 |
| NOTA-3PEG4-dimer | 68Ga(NOTA-2PEG4-dimer) | 54 ± 2 |
It is very important to note that the IC50 value depends largely on the radioligand (125I-c(RGDyK) vs. 125I-echistatin) and tumor cell lines (U87MG vs. MDA-MB-435) used in the competitive displacement assay. Caution should be taken when comparing the IC50 values of cyclic RGD peptides with those reported in the literature. Whenever possible, a “control compound”, such as c(RGDfK) or c(RGDyK) should be used in each experiment.
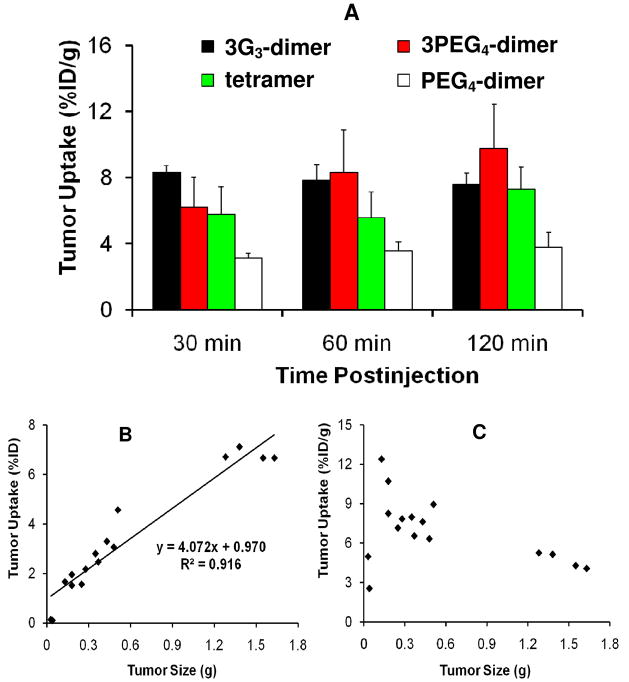
Ternary ligand complexes [99mTc(HYNIC-3PEG4-dimer)(tricine)(TPPTS)] (99mTc-3PEG4-dimer) and [99mTc(HYNIC-3G3-dimer)(tricine)(TPPTS)] (99mTc-3G3-dimer) have been evaluated in the athymic nude mice bearing U87MG glioma and MDA-MB-435 breast tumor xenografts (118, 119). For comparison purposes, [99mTc(HYNIC-PEG4-dimer)(tricine)(TPPTS)] (99mTc-PEG4-dimer) and [99mTc(HYNIC-tetramer)(tricine)(TPPTS)] (99mTc-tetramer) were also evaluated in the same tumor-bearing animal model (118, 119). As expected, the breast tumor uptake of 99mTc-3PEG4-dimer and 99mTc-3G3-dimer was comparable to that of 99mTc-tetramer (Figure 8A), and was >2x higher than that of 99mTc-PEG4-dimer (118). These data suggest that 3PEG4-dimer, 3G3-dimer and tetramer are most likely “bivalent” (Figure 6B) whereas PEG4-dimer is monodentate (Figure 6A). If PEG4-dimer were bivalent, HYNIC-PEG4-dimer would have shared similar integrin αvβ3 binding affinity with HYNIC-3PEG4-dimer and HYNIC-3G3-dimer while 99mTc-PEG4-dimer would have had the tumor uptake comparable to that of 99mTc-3PEG4-dimer and 99mTc-3G3-dimer. In addition, 99mTc-3PEG4-dimer and 99mTc-3G3-dimer had the kidney and liver uptake that was half of that for 99mTc-tetramer, probably because 3PEG4-dimer and 3G3-dimer have only two RGD motifs. Therefore, 99mTc-3PEG4-dimer and 99mTc-3G3-dimer have significant advantages over 99mTc-tetramer with respect to T/B ratios (118, 119).
Figure 8.
Top: direct comparison of the tumor uptake for 99mTc-PEG4-dimer, 99mTc-3G3-dimer, 99mTc-3PEG4-dimer and 99mTc-tetramer in athymic nude mice bearing MDA-MB-435 breast cancer xenografts (A). Bottom: the relationship between the tumor size and tumor uptake (B and C) of 99mTc-3PEG4-dimer at 120 min p.i. in athymic nude mice bearing U87MG glioma xenografts. The linear relationship suggests that 99mTc-3PEG4-dimer is for monitoring the tumor growth or shrinkage during anti-angiogenic therapy.
Multimeric ≠ Multivalent
It is critical to note that multimeric RGD peptides are not necessarily multivalent. There are two factors (bivalency and enhanced local RGD concentration) contributing to high integrin αvβ3 binding affinity of multimeric RGD peptides. The concentration factor exists in all multimeric RGD peptides regardless of the linkers. The key for bivalency is the distance between two cyclic RGD motifs. For example, this distance in 3PEG4-dimer (38 bonds) and 3G3-dimer (26 bonds) is long enough for them to achieve bivalency, which leads to the higher integrin αvβ3 binding affinity of HYNIC-3PEG4-dimer and HYNIC-3G3-dimer than that of HYNIC-PEG4-dimer, and the much higher breast tumor uptake (Figure 8A) for 99mTc-3PEG4-dimer and 99mTc-3G3-dimer as compared to that of 99mTc-PEG4-dimer. In contrast, the concentration factor is responsible for the better binding affinity of HYNIC-tetramer than that of HYNIC-3PEG4-dimer and HYNIC-3G3-dimer. The fact that the breast tumor uptake of 99mTc-3PEG4-dimer and 99mTc-3G3-dimer is comparable to that of 99mTc-tetramer suggests that the contribution from the “concentration factor” might not be as significant as that from the “bivalency factor”. In addition, the ability of a multimeric RGD peptide to achieve bivalency also depends on the integrin αvβ3 density. If the tumor integrin αvβ3 density is high, the distance between two neighboring integrin αvβ3 sites will be short, which makes it easier for the multimeric RGD peptide to achieve bivalency. If the integrin αvβ3 density is very low, the distance between two neighboring integrin αvβ3 sites will be long, and it might be more difficult for the same multimeric RGD peptide to achieve simultaneous integrin αvβ3 binding.
Relationship between Tumor Size and Radiotracer Tumor Uptake
The ability to quantify the integrin αvβ3 in vivo provides opportunities to select the patients more appropriately for anti-angiogenic treatment and to monitor the therapeutic efficacy of integrin αvβ3-positive tumors (120, 121). The %ID tumor uptake reflects the total integrin αvβ3 level while the %ID/g tumor uptake reflects the integrin αvβ3 density. Figure 8B shows the relationship between tumor size and tumor uptake (%ID and %ID/g) of 99mTc-3PEG4-dimer. There was a linear relationship between tumor size and %ID tumor uptake with R2 = 0.9164 (Figure 8B), suggesting that 99mTc-3PEG4-dimer might be useful for monitoring tumor growth during anti-angiogenic therapy (118). If the tumor uptake is expressed as %ID/g (Figure 8C), it seems that 99mTc-3PEG4-dimer has a narrow window to achieve an optimal tumor uptake. When tumor size is small (<0.05 g), there is little angiogenesis with low blood flow, and 99mTc-3PEG4-dimer has low %ID/g tumor uptake. When the tumor size is 0.1 g–0.25 g, the microvessel density and integrin αvβ3 density are high. The %ID/g tumor uptake of 99mTc-3PEG4-dimer is ~10 %ID/g (Figure 8C). As tumors grow, the total integrin αvβ3 level is higher, and the %ID tumor uptake increases (Figure 8B). However, the microvessel density decreases due to maturity of blood vessels. The integrin αvβ3 density also decreases due to larger interstitial space (122). In addition, parts of the tumor may become necrotic, leading to the lower integrin αvβ3 density. As a result, larger tumors have lower %ID/g tumor uptake than smaller ones (Figure 8C).
Radiotracer Tumor Uptake and Tumor Cell Integrin αvβ3 Expression
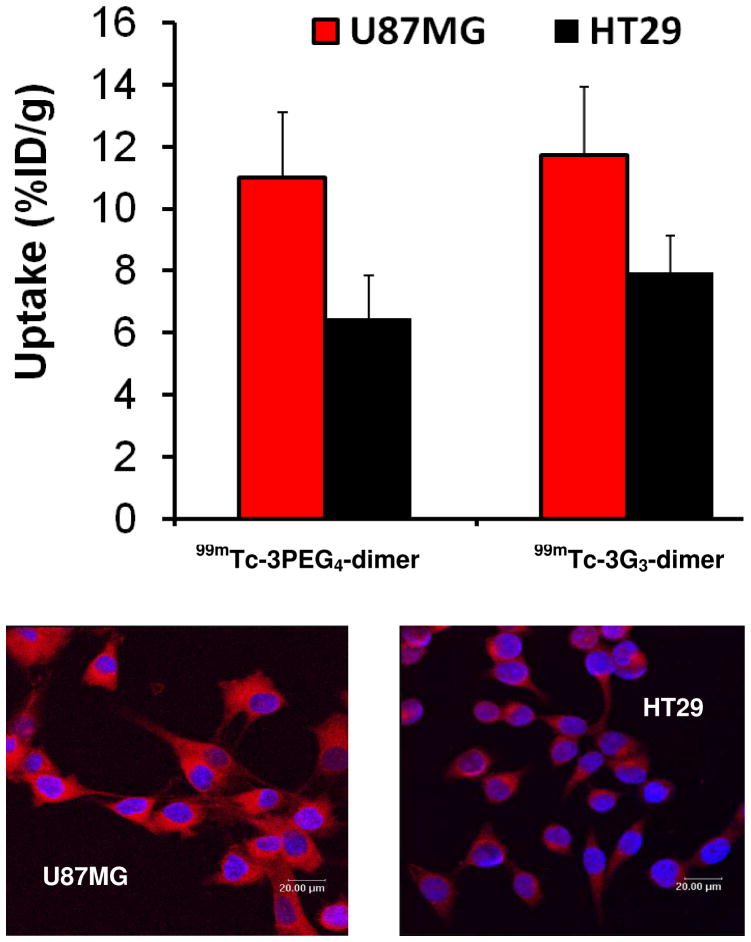
Figure 9 compares the tumor uptake of 99mTc-3PEG4-dimer and 99mTc-3G3-dimer in athymic nude mice bearing U87MG glioma and HT29 colon cancer xenografts, and the integrin αvβ3 expression levels on U87MG glioma and HT29 cells. Both 99mTc-3PEG4-dimer and 99mTc-3G3-dimer have significantly higher uptake (%ID/g) in the glioma than HT29 tumors (Figure 9: top), which is supported by the presence of higher level of integrin αvβ3 expression on U87MG glioma cells than those on HT29 cells (Figure 9: bottom). These data clearly show that the integrin αvβ3 level on tumor cells (U87MG > HT29) plays a significant role in the radiotracer tumor uptake. More fluorescent staining studies are needed to better quantify the contributions from the integrin αvβ3 expressed on the tumor cells and neovasculature in the tumor tissue.
Figure 9.
Top: direct comparison of the tumor uptake for 99mTc-3PEG4-dimer and 99mTc-3G3-dimer in athymic nude mice bearing U87MG glioma and HT29 colon cancer xenografts. Bottom: confocal microscope images of U87MG glioma (left) and HT29 (right) tumor cells. The blue color indicates the presence cell nuclei (the presence of 4′,6′-diamidino-2-phenylindole). The red color indicates the presence of integrin αvβ3 due to the binding of LM609 and TRITC-coupled goat-anti-mouse IgG. These data show that the integrin αvβ3 level on tumor cells (U87MG > HT29) plays a significant role in the radiotracer tumor uptake.
64Cu(DOTA-3PEG4-dimer) and 64Cu(DOTA-3G3-dimer)
Recently, Shi et al (123) reported two cyclic RGD dimer conjugates: DOTA-3PEG4-dimer and DOTA-3G3-dimer (Figure 10). It was found that the integrin αvβ3 binding affinity (Table 1) follow the order of DOTA-tetramer (IC50 = 10 ± 2 nM) > DOTA-3G3-dimer (IC50 = 74 ± 3 nM) ~ DOTA-3PEG4-dimer (IC50 = 62 ± 6, nM) > DOTA-dimer (IC50 = 102 ± 5 nM) against 125I-echistatin bound to U87MG glioma cells. Once again, bivalency is likely responsible for the higher integrin αvβ3 binding affinity of DOTA-3PEG4-dimer and DOTA-3G3-dimer than that of DOTA-dimer. This conclusion is completely consistent with the higher tumor uptake of 64Cu(DOTA-3PEG4-dimer) and 64Cu(DOTA-3G3-dimer) than that of 64Cu(DOTA-dimer) (123). In contrast, the concentration factor is most likely responsible for higher integrin αvβ3 binding affinity of DOTA-tetramer than that of DOTA-3PEG4-dimer and DOTA-3G3-dimer, and the higher initial tumor uptake of 64Cu(DOTA-tetramer) as compared to that of 64Cu(DOTA-3PEG4-dimer) and 64Cu(DOTA-3G3-dimer) (132). However, the uptake of 64Cu(DOTA-3PEG4-dimer) in the liver and kidneys was significantly lower than that reported for 64Cu(DOTA-tetramer) (57), due to the presence of four R-residues in E[E[c(RGDfK)]2]2 as compared to only two R-residues in 64Cu(DOTA-3PEG4-dimer) and 64Cu(DOTA-3G3-dimer) (132).
Figure 10.
Structures of DOTA-3G3-dimer and DOTA-3PEG4-dimer.
111In(DOTA-3PEG4-dimer) and 111In(DOTA-3G3-dimer)
To explore the impact of radiometal chelates, 111In(DOTA-3PEG4-dimer) and 111In(DOTA-3G3-dimer) were evaluated in the same animal model (124). 111In(DOTA-3PEG4-dimer) and 111In(DOTA-3G3-dimer) share the same DOTA chelator, have almost identical lipophilicity (log P = −4.13 ± 0.08 and −4.20 ± 0.21, respectively), and show very similar metabolic stability (124). The tumor uptake of 111In(DOTA-3G3-dimer) is comparable to that of 111In(DOTA-3PEG4-dimer) (Figure 11A). Planar imaging studies showed that they both had very high tumor uptake with a long tumor retention and excellent tumor-to-background contrast (124). The activity accumulation in the chest and abdominal regions almost completely disappeared at 60 min p.i. The combination of the hydrophilic 111In(DOTA) chelate with PEG4/G3 linkers is responsible for the low liver uptake for 111In(DOTA-3PEG4-dimer) and 111In(DOTA-3G3-dimer), leading to high tumor/liver ratios (Figure 11A). Both 111In(DOTA-3PEG4-dimer) and 111In(DOTA-3G3-dimer) also had low kidney and muscle uptake with very high tumor/kidney and tumor/muscle ratios (124). Their integrin αvβ3-specificity has been clearly demonstrated by the blocking experiment, in which 111In(DOTA-3PEG4-dimer) was used as the radiotracer and E[c(RGDfK)]2 as the blocking agent (124). The RGD-specificity of 111In-labeled cyclic RGD dimers was demonstrated by the higher integrin αvβ3 affinity of DOTA-3PEG4-NS (IC50 = 715 ± 45 nM; 3PEG4-NS = PEG4-E[PEG4-(RGKfD)]2, a scrambled nonsense peptide) than that of DOTA-3PEG4-dimer (1.3 ± 0.3 nM), and the much better tumor uptake of 111In(DOTA-3PEG4-dimer) (10.06 ± 3.52 %ID/g) than that of 111In(DOTA-3PEG4-NS) (0.30 ± 0.09 %ID/g) in the same animal model. On the basis of the integrin αvβ3- and RGD-specificity of the 111In-labeled cyclic RGD peptides, it has been suggested that their uptake in several normal organs (e.g. intestine, kidneys, liver, lungs and spleen) might be also integrin αvβ3-mediated (124).
Figure 11.
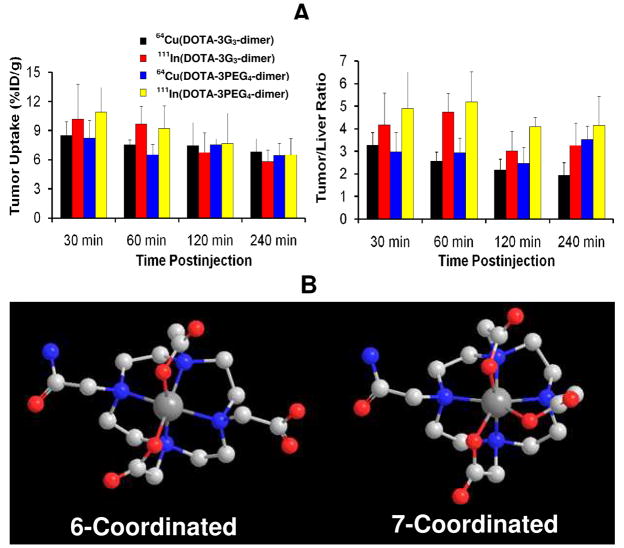
A: comparison of tumor uptake and tumor/liver ratios for 64Cu(DOTA-3PEG4-dimer), 64Cu(DOTA-3G3-dimer), 111In(DOTA-3PEG4-dimer) and 111In(DOTA-3G3-dimer) in athymic nude mice bearing U87MG human glioma xenografts. B: Chem-3D presentation of 64Cu(DOTA-monoamide) (6-coordinated) and 111In(DOTA-monoamide) (7-coordinated).
111In(DOTA-3PEG4-dimer) and 64Cu(DOTA-3PEG4-dimer) share the same DOTA-conjugate. In spite of their difference in radiometal, the tumor uptake 111In(DOTA-3PEG4-dimer) (10.89 ± 2.55 and 7.65 ± 3.17 %ID/g at 30and 240 min p.i., respectively) was close to that of 64Cu(DOTA-3PEG4-dimer) (8.23 ± 1.97 and 6.43 ± 1.22 %ID/g at 30 and 240 min p.i., respectively). They also share similar uptake in normal organs. For example, the kidney uptake of 111In(DOTA-3PEG4-dimer) was 5.80 ± 0.95 and 2.78 ± 0.20 %ID/g at 30 and 240 min p.i., respectively, and was comparable to that of 64Cu(DOTA-3PEG4-dimer) (6.59 ± 0.93 %ID/g at 30 min p.i. and 2.81 ± 0.36 % ID/g at 240 min p.i.). The liver uptake of 111In(DOTA-3PEG4-dimer) is 2.52 ± 0.57 %ID/g at 30 min and 1.61 ± 0.06 %ID/g at 240 min p.i. while 64Cu(DOTA-3PEG4-dimer) has the liver uptake of 2.80 ± 0.35 %ID/g at 30 min p.i. and 1.87 ± 0.51 %ID/g at 240 min p.i. Similar conclusion can be made by comparing biodistribution properties of 64Cu(DOTA-3G3-dimer) and 111In(DOTA-3G3-dimer). These data suggest that the radiometal chelate (Figure 11B) has minimal impact on radiotracer tumor uptake and excretion kinetics.
68Ga(NOTA-2PEG4-dimer) and 68Ga(NOTA-2G3-dimer)
We also prepared conjugates NOTA-2PEG4-dimer and NOTA-2G3-dimer (Figure 12: NOTA = 1,4,7-triaazacyclononane-1,4,7-tetracetic acid) and their 68Ga complexes, 68Ga(NOTA-2PEG4-dimer) and 68Ga(NOTA-2G3-dimer) (125). The integrin αvβ3 binding affinity (Table 1) of NOTA-dimer (IC50 = 100 ± 3 nM), NOTA-2G3-dimer (IC50 = 66 ± 4 nM) and NOTA-2PEG4-dimer (IC50 = 54 ± 2 nM) were very close to those for DOTA-dimer (IC50 = 102 ± 5 nM), DOTA-3G3-dimer (IC50 = 74 ± 3 nM) and DOTA-3PEG4-dimer (IC50 = 62 ± 6 nM), respectively. These data further suggest that the G3 and PEG4 linkers between cyclic RGD motifs in dimeric RGD peptides make it possible for them to bind integrin αvβ3 in a bivalent fashion. The tumor uptake of 68Ga(NOTA-2G3-dimer) and 68Ga(NOTA-2PEG4-dimer) was much higher than that of 68Ga(NOTA-dimer) in the same tumor-bearing animal model (125), suggesting that the addition of G3 and PEG4 linkers between two cyclic RGD motifs increases the radiotracer tumor uptake. In all cases, the tumors can be clearly visualized as early as 30 min p.i. with excellent contrast (Figure 12). It was also found that the tumor uptake of 68Ga(NOTA-2PEG4-dimer) in MDA-MB-435 breast tumor was significantly lower than that in U87MG glioma. Similar results were also obtained for 18F-labeled 3PEG4-dimer (126), which is consistent with that fact that the MDA-MB-435 breast tumors have lower integrin αvβ3 expression than U87MG glioma (52–55).
Figure 12.
Top: structures of NOTA-2G3-dimer and NOTA-2PEG4-dimer. Bottom: microPET images for 68Ga(NOTA-2G3-dimer) and 68Ga(NOTA-2PEG4-dimer). Arrows indicate the presence of tumors.
Conclusions
Over the last several years, many multimeric cyclic RGD peptides have been used to increase the integrin αvβ3–targeting capability. It was found that increasing the peptide multiplicity can significantly enhance their integrin αvβ3 binding affinity, and improve the radiotracers tumor targeting ability. However, as peptide multiplicity increases, the uptake of radiolabeled multimeric RGD peptides is also significantly increased in normal organs. There is no significant advantage in using radiolabeled tetramers E{E[c(RGDxK)]2}2 (x = f and y) over their dimeric analogs E[c(RGDxK)]2 (x = f and y) with respect to T/B ratios. In addition, the cost for E{E[c(RGDxK)]2}2 (x = f and y), E{E[E[c(RGDyK)]2]2}2 is too high for them to be useful for future radiotracer development.
Recent studies on cyclic RGD dimers suggest that two factors (bivalency and enhanced local RGD concentration) contribute to the high integrin αvβ3 binding affinity of multimeric cyclic RGD peptides (118, 119, 123–126). The concentration factor exists in all multimeric RGD peptides regardless of the linker length between two cyclic RGD motifs. To achieve bivalency, the distance between two RGD motifs must be long enough for them to bind the neighboring integrin αvβ3 sites simultaneously. Among the cyclic RGD peptides, 2PEG4-dimer/3PEG4-dimer and 2G3-dimer/3G3-dimer show most promising results with respect to the tumor uptake and T/B ratios of their radiotracers (99mTc, 111In, 64Cu and 68Ga). Thus, 2PEG4-dimer/3PEG4-dimer and 2G3-dimer/3G3-dimer are better integrin αvβ3-targeting biomolecules than the tetramer E{E[c(RGDfK)]2}2 with respect to their cost and the T/B ratios of their 99mTc, 111In, 64Cu and 68Ga radiotracers.
While current research efforts on the integrin αvβ3–targeted radiotracers have been focused on new cyclic RGD peptides, the formulation development for routine preparation of radiotracers is often neglected. It must be emphasized that it is common to use post-labeling chromatographic separation for improvement of radiotracer purity and specific activity for research purposes. In clinical settings, however, post-labeling purification is not practical. Regardless of the beauty of the science involved in the discovery of a new radiotracer, its success relies largely on the availability and capability to improve the quality of cancer patient’s life. As a matter of fact, the main challenge for [18F]Galacto-RGD and [18F]-AH111585 to assume a wide-spread clinical utility is not their biological performance, but their clinical availability at reasonable cost. In this respect, the integrin αvβ3-targeted 99mTc radiotracers will offer significant advantages over their corresponding 18F analogs because of the clinical availability of 99Mo-99mTc generators, and the kit formulation for routine preparation of 99mTc radiotracers at low cost. However, both planar imaging and SPECT suffer a significant drawback with respect to quantification of the radiotracer “absolute” organ uptake, the speed of dynamic imaging, spatial resolution and tissue attenuation.
The successful application of the 68Ga-labeled somatostatin analogs for imaging tumors in cancer patients has clearly demonstrated that 68Ga is an excellent alternative to 18F. 68Ga is available from an in-house commercially available 68Ge/68Ga generator (127–129), and its short half-life is best suited for the fast excretion kinetics of many 68Ga-labeled small peptides. 64Cu is another viable alternative to 18F. The use of NOTA and its derivatives as BFCs allows the development of the kit formulation for routine preparation of 68Ga and 64Cu radiotracers with high specific activity. In addition, 68Ga(NOTA) and 64Cu(NOTA) chelates have very high hydrophilicity, which is extremely important for improving the radiotracer clearance kinetics from non-cancerous organs.
It is very important to note that integrin αvβ3 is also overexpressed on the activated endothelial cells during wound healing and post-infarct remodeling, in rheumatoid arthritis, and atherosclerotic plaque (1–4, 130, 131). The integrin αvβ3–targeted radiotracers have been proposed for imaging myocardial angiogenesis (132), inflammatory diseases (133), and hindlimb ischemia (134). Recent results showed that the 111In-labeled nonpeptide integrin αvβ3 antagonist (RP748) was able to image the angiogenesis in the heart with myocardial infarction (132), and the radiotracer uptake in the infarct region was associated with the integrin αvβ3 expression level. The results reported by Pichler et al suggest that [18F]Galacto-RGD might be a powerful tool to distinguish between acute and chronic phases of T-cell mediated immune responses (133). Studies have also demonstrated the value of a 99mTc-labeled cyclic RGD monomer (NC100692) for imaging the integrin αvβ3 in rodent models of hindlimb ischemia (134). These promising results suggest that the newer and more effective integrin αvβ3–targeted radiotracers under development for tumor imaging might become valuable non-invasive markers of angiogenesis after ischemic injury, myocardial infarction and inflammation. In addition, the combination of high tumor uptake, long tumor retention with favorable pharmacokinetic of 111In(DOTA-3G3-dimer) and 111In(DOTA-3PEG4-dimer) suggests that their corresponding 90Y and 177Lu analogs, M(DOTA-RGD) (M = 90Y and 177Lu; and RGD = 3G3-dimer and 3PEG4-dimer), might be useful for the treatment of integrin αvβ3-positive tumors.
Acknowledgments
The author would like to thank Dr. Xiaoyuan Chen at Stanford Medical School and Dr. Fan Wang at the Beijing University Medical Isotopes Research Center for their collaboration on the radiolabeled multimeric cyclic RGD peptides. This work is supported, in part, by Purdue University and research grants: R01 CA115883 A2 (S.L.) from the National Cancer Institute (NCI) and DE-FG02-08ER64684 from the Department of Energy.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Mousa SA. Mechanism of angiogenesis in vascular disorders: potential therapeutic targets. Drugs of the Future. 1998;23:51–60. [Google Scholar]
- 3.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 4.Meitar D, Crawford SE, Rademaker AW, Cohn SL. Tumor angiogenesis correlates with metastatic disease, N-myc-amplification, and poor outcome in human neuroblastoma. J Clin Oncol. 1996;14:405–414. doi: 10.1200/JCO.1996.14.2.405. [DOI] [PubMed] [Google Scholar]
- 5.Gasparini G, Brooks PC, Biganzoli E, Vermeulen PB, Bonoldi E, Dirix LY, Ranieri G, Miceli R, Cheresh DA. Vascular integrin αvβ3: a new prognostic indicator in breast cancer. Clin Can Res. 1998;4:2625–2634. [PubMed] [Google Scholar]
- 6.Falcioni R, Cimino L, Gentileschi MP, D’Agnano I, Zupi G, Kennel SJ, Sacchi A. Expression of beta 1, beta 3, beta 4, and beta 5 integrins by human lung carcinoma cells of different histotypes. Exp Cell Res. 1994;210:113–122. doi: 10.1006/excr.1994.1017. [DOI] [PubMed] [Google Scholar]
- 7.Sengupta S, Chattopadhyay N, Mitra A, Ray S, Dasgupta S, Chatterjee A. Role of αvβ3 integrin receptors in breast tumor. J Exp Clin Cancer Res. 2001;20:585–590. [PubMed] [Google Scholar]
- 8.Bögler O, Mikkelsen T. Angiogenesis in Glioma: molecular mechanisms and roadblocks to translation. Cancer J. 2003;9:205–213. doi: 10.1097/00130404-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 10.Hwang R, Varner JV. The role of integrins in tumor angiogenesis. Hematol Oncol Clin North Am. 2004;18:991–1006. doi: 10.1016/j.hoc.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Brooks PC, Clark RAF, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 12.Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct αv integrin. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 13.Horton MA. The αvβ3 integrin “vitronectin receptor”. Int J Biochem Cell Biol. 1997;29:721–725. doi: 10.1016/s1357-2725(96)00155-0. [DOI] [PubMed] [Google Scholar]
- 14.Bello L, Francolini M, Marthyn P, Zhang JP, Carroll RS, Nikas DC, Strasser JF, Villani R, Cheresh DA, Black PM. Alpha(v)beta3 and alpha(v)beta5 integrin expression in glioma periphery. Neurosurgery. 2001;49:380–389. doi: 10.1097/00006123-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Albelda SM, Mette SA, Elder DE, Stewart RM, Damjanovich L, Herlyn M, Buck CA. Integrin distribution in maliganant melanoma: association of the beta3 subunit with tumor progression. Cancer Res. 1990;50:6757–6764. [PubMed] [Google Scholar]
- 16.Felding-Habermann B, Mueller BM, Romerdahl CA, Cheresh DA. Involvement of integrin alpha V gene expression in human melanoma tumorigenicity. J Clin Invest. 1992;89:2018–2022. doi: 10.1172/JCI115811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zitzmann S, Ehemann V, Schwab M. Arginine-Glycine-Aspartic acid (RGD)-peptide binds to both tumor and tumor endothelial cells in vivo. Cancer Res. 2002;62:5139–5143. [PubMed] [Google Scholar]
- 18.Weber WA, Haubner R, Vabuliene E, Kuhnast B, Webster HJ, Schwaiger M. Tumor angiogenesis targeting using imaging agents. Q J Nucl Med. 2001;45:179–182. [PubMed] [Google Scholar]
- 19.Costouros NG, Diehn FE, Libutti SK. Molecular imaging of tumor angiogenesis. J Cell Biol Suppl. 2002;39:72–78. doi: 10.1002/jcb.10426. [DOI] [PubMed] [Google Scholar]
- 20.van de Wiele C, Oltenfreiter R, De Winter O, Signore A, Slegers G, Dierckx RA. Tumor angiogenesis pathways: related clinical issues and implications for nuclear medicine imaging. Eur J Nucl Med. 2002;29:699–709. doi: 10.1007/s00259-002-0783-8. [DOI] [PubMed] [Google Scholar]
- 21.Liu S, Edwards DS. Fundamentals of receptor-based diagnostic metalloradiopharmaceuticals. Topics in Current Chem. 2002;222:259–278. [Google Scholar]
- 22.Liu S, Robinson SP, Edwards DS. Integrin αvβ3 directed radiopharmaceuticals for tumor imaging. Drugs of the Future. 2003;28:551–564. [Google Scholar]
- 23.Haubner R, Wester HJ. Radiolabeled tracers for imaging of tumor angiogenesis and evaluation of antiangiogenic therapies. Current Pharmaceutical Design. 2004;10:1439–1455. doi: 10.2174/1381612043384745. [DOI] [PubMed] [Google Scholar]
- 24.D’Andrea LD, Del Gatto A, Pedone C, Benedetti E. Peptide-based molecules in angiogenesis. Chem Biol Drug Des. 2006;67:115–126. doi: 10.1111/j.1747-0285.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen X. Multimodality imaging of tumor integrin αvβ3 expression. Mini-Rev Med Chem. 2006;6:227–234. doi: 10.2174/138955706775475975. [DOI] [PubMed] [Google Scholar]
- 26.Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin αvβ3-targeted radiotracers for tumor imaging. Mol Pharm. 2006;3:472–487. doi: 10.1021/mp060049x. [DOI] [PubMed] [Google Scholar]
- 27.Cai W, Chen X. Multimodality Molecular imaging of tumor angiogenesis. J Nucl Med. 2008;49:113S–128S. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- 28.Cai W, Niu G, Chen X. Imaging of integrins as biomarkers for tumor angiogenesis. Current Pharmaceutical Design. 2008;14:2943–2973. doi: 10.2174/138161208786404308. [DOI] [PubMed] [Google Scholar]
- 29.Chen X. Advances in anatomic, functional, and molecular imaging of angiogenesis. J Nucl Med. 2008;49:511–514. doi: 10.2967/jnumed.107.050179. [DOI] [PubMed] [Google Scholar]
- 30.Jin H, Varner J. Integrins: roles in cancer development and as treatment targets. Br J Cancer. 2004;90:561–565. doi: 10.1038/sj.bjc.6601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar CC. Integrin αvβ3 as a therapeutic target for blocking tumor-induced angiogenesis. Curr Drug Targets. 2003;4:123–131. doi: 10.2174/1389450033346830. [DOI] [PubMed] [Google Scholar]
- 32.Meyer A, Aurenheimer J, Modlinger A, Kessler H. Targeting RGD recognizing integrins: drug development, biomaterial research, tumor imaging and targeting. Current Pharmaceutical Design. 2006;12:2723–2747. doi: 10.2174/138161206777947740. [DOI] [PubMed] [Google Scholar]
- 33.Van Hagen PM, Breeman WAP, Bernard HF, Schaar M, Mooij CM, Srinivasan A, Schmidt MA, Krenning EP, de Jong M. Evaluation of a radiolabeled cyclic DTPA-RGD analog for tumor imaging and radionuclide therapy. Int J Cancer (Radiat Oncol Invest) 2000;90:186–198. [PubMed] [Google Scholar]
- 34.Huabner R, Wester HJ, Senekowitsch-Schmidtke R, Diefenbach B, Kessler H, Stöcklin G, Schwaiger M. RGD-peptides for tumor targeting: biological evaluation of radioiodinated analogs and introduction of a novel glycosylated peptide with improved biokinetics. J Labelled Compounds & Radiopharmaceuticals. 1997;40:383–385. [Google Scholar]
- 35.Sivolapenko GB, Skarlos D, Pectasides D, Stathopoulou E, Milonakis A, Sirmalis G, Stuttle A, Courtenay-Luck NS, Konstantinides K, Epenetos AA. Imaging of metastatic melanoma utilizing a technetium-99m labeled RGD-containing synthetic peptide. Eur J Nucl Med. 1998;25:1383–1389. doi: 10.1007/s002590050312. [DOI] [PubMed] [Google Scholar]
- 36.Haubner R, Wester HJ, Weber WA, Mang C, Ziegler SI, Goodman SL, Senekowitsch-Schmidtke R, Kessler H, Schwaiger M. Noninvasive imaging of αvβ3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. 2001;61:1781–1785. [PubMed] [Google Scholar]
- 37.Haubner R, Wester HJ, Reuning U, Senekowitsch-Schmidtke R, Diefenbach B, Kessler H, Stöcklin G, Schwaiger M. Radiolabeled αvβ3 integrin antagonists: a new class of tracers for tumor imaging. J Nucl Med. 1999;40:1061–1071. [PubMed] [Google Scholar]
- 38.Haubner R, Wester HJ, Burkhart F, Senekowisch-Schmidtke R, Weber W, Goodman SL, Kessler H, Schwaiger M. Glycolated RGD-containing peptides: tracer for tumor targeting and angiogenesis imaging with improved biokinetics. J Nucl Med. 2001;42:326–336. [PubMed] [Google Scholar]
- 39.Haubner R, Bruchertseifer F, Bock M, Kessler H, Schwaiger M, Wester HJ. Synthesis and biological evaluation of 99mTc-labeled cyclic RGD peptide for imaging the αvβ3 expression. Nuklearmedizin. 2004;43:26–32. doi: 10.1267/nukl04010026. [DOI] [PubMed] [Google Scholar]
- 40.Thumshirn G, Hersel U, Goodman SL, Kessler H. Multimeric cyclic RGD peptides as potential tools for tumor targeting: solid-phase peptide synthesis and chemoselective oxime ligation. Chem Eur J. 2003;9:2717–2725. doi: 10.1002/chem.200204304. [DOI] [PubMed] [Google Scholar]
- 41.Poethko T, Schottelius M, Thumshirn G, Herz M, Haubner R, Henriksen G, Kessler H, Schwaiger M, Wester HJ. Chemoselective pre-conjugate radiohalogenation of unprotected mono- and multimeric peptides via oxime formation. Radiochim Acta. 2004;92:317–327. [Google Scholar]
- 42.Poethko T, Schottelius M, Thumshirn G, Hersel U, Herz M, Henriksen G, Kessler H, Schwaiger M, Wester HJ. Two-step methodology for high yield routine radiohalogenation of peptides: 18F-labeled RGD and octreotide analogs. J Nucl Med. 2004;5:892–902. [PubMed] [Google Scholar]
- 43.Alves S, Correia JDG, Gano L, Rold TL, Prasanphanich A, Haubner R, Rupprich M, Alberto R, Decristoforo C, Santos I, Smith CJ. In vitro and in vivo evaluation of a novel 99mTc(CO)3-pyrazolyl conjugate of cyclo-(Arg-Gly-Asp-D-Tyr-Lys) Bioconj Chem. 2007;8:530–537. doi: 10.1021/bc060234t. [DOI] [PubMed] [Google Scholar]
- 44.Fani M, Psimadas D, Zikos C, Xanthopoulos S, Loudos GK, Bouziotis P, Varvarigou AD. Comparative evaluation of linear and cyclic 99mTc-RGD peptides for targeting of integrins in tumor angiogenesis. Anticancer Res. 2006;26:431–434. [PubMed] [Google Scholar]
- 45.Su ZF, Liu G, Gupta S, Zhu Z, Rusckowski M, Hnatowich DJ. In vitro and in vivo evaluation of a technetium-99m-labeled cyclic RGD peptide as specific marker of αvβ3 integrin for tumor imaging. Bioconj Chem. 2002;13:561–570. doi: 10.1021/bc0155566. [DOI] [PubMed] [Google Scholar]
- 46.Decristoforo C, Faintuch-Linkowski B, Rey A, von Guggenberg E, Rupprich M, Hernandez-Gonzales I, Rodrigo T, Haubner R. [99mTc]HYNIC-RGD for imaging integrin αvβ3 expression. Nucl Med Biol. 2006;33:945–952. doi: 10.1016/j.nucmedbio.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Chen X, Park R, Tohme M, Shahinian AH, Bading JR, Conti PS. MicroPET and autoradiographic imaging of breast cancer αv-integrin expression using 18F- and 64Cu-labeled RGD peptide. Bioconj Chem. 2004;15:41–49. doi: 10.1021/bc0300403. [DOI] [PubMed] [Google Scholar]
- 48.Chen X, Park R, Shahinian AH, Tohme M, Khankaldyyan V, Bozorgzadeh MH, Bading JR, Moats R, Laug WE, Conti PS. 18F-labeled RGD peptide: initial evaluation for imaging brain tumor angiogenesis. Nucl Med Biol. 2004;31:179–189. doi: 10.1016/j.nucmedbio.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Chen X, Park R, Shahinian AH, Bading JR, Conti PS. Pharmacokinetics and tumor retention of 125I-labeled RGD peptide are improved by PEGylation. Nucl Med Biol. 2004;31:11–19. doi: 10.1016/j.nucmedbio.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Chen X, Liu S, Hou Y, Tohme M, Park R, Bading JR, Conti PS. MicroPET imaging of breast cancer αv-integrin expression with 64Cu-labeled dimeric RGD peptides. Mol Imag Biol. 2004;6:350–359. doi: 10.1016/j.mibio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Chen X, Tohme M, Park R, Hou Y, Bading JR, Conti PS. MicroPET imaging of breast cancer αv-integrin expression with 18F-labeled dimeric RGD peptide. Mol Imaging. 2004;3:96–104. doi: 10.1162/15353500200404109. [DOI] [PubMed] [Google Scholar]
- 52.Wu Y, Zhang X, Xiong Z, Cheng Z, Fisher DR, Liu S, Gambhir SS, Chen X. MicroPET imaging of glioma integrin αvβ3 expression using 64Cu-labeled tetrameric RGD peptide. J Nucl Med. 2005;46:1707–1718. [PubMed] [Google Scholar]
- 53.Zhang X, Xiong Z, Wu Y, Cai W, Tseng JR, Gambhir SS, Chen X. Quantitative PET imaging of tumor integrin αvβ3 expression with 18F-FRGD2. J Nucl Med. 2006;47:113–121. [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Z, Li Z, Chen K, Cai W, He L, Chin FT, Li F, Chen X. MicroPET of tumor integrin αvβ3 expression using 18F-labeled PEGylated tetrameric RGD peptide (18F-FPRGD4) J Nucl Med. 2007;48:1536–1544. doi: 10.2967/jnumed.107.040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li ZB, Chen K, Chen X. 68Ga-labeled multimeric RGD peptides for microPET imaging of integrin αvβ3 expression. Eur J Nucl Med Mol Imaging. 2008;35:1100–1108. doi: 10.1007/s00259-007-0692-y. [DOI] [PubMed] [Google Scholar]
- 56.Liu S, Cheung E, Rajopadhye M, Ziegler MC, Edwards DS. 90Y- and 177Lu-labeling of a DOTA-conjugated vitronectin receptor antagonist for tumor therapy. Bioconj Chem. 2001;12:559–568. doi: 10.1021/bc000146n. [DOI] [PubMed] [Google Scholar]
- 57.Janssen M, Oyen WJG, Massuger LFAG, Frielink C, Dijkgraaf I, Edwards DS, Rajopadhye M, Corsten FHM, Boerman OC. Comparison of a monomeric and dimeric radiolabeled RGD-peptide for tumor targeting. Cancer Biother Radiopharm. 2002;17:641–646. doi: 10.1089/108497802320970244. [DOI] [PubMed] [Google Scholar]
- 58.Janssen M, Oyen WJG, Dijkgraaf I, Massuger LFAG, Frielink C, Edwards DS, Rajopadyhe M, Boonstra H, Corsten FHM, Boerman OC. Tumor targeting with radiolabeled αvβ3 integrin binding peptides in a nude mice model. Cancer Res. 2002;62:6146–6151. [PubMed] [Google Scholar]
- 59.Janssen ML, Frielink C, Dijkgraaf I, Oyen WJ, Edwards DS, Liu S, Rajopadhye M, Massuger LF, Corstens FHM, Boerman OC. Improved tumor targeting of radiolabeled RGD-peptides using rapid dose fractionation. Cancer Biother Radiopharm. 2004;19:399–404. doi: 10.1089/cbr.2004.19.399. [DOI] [PubMed] [Google Scholar]
- 60.Liu S, Hsieh WY, Kim YS, Mohammed SI. Effect of coligands on biodistribution characteristics of ternary ligand 99mTc complexes of a HYNIC-conjugated cyclic RGDfK dimer. Bioconj Chem. 2005;16:1580–1588. doi: 10.1021/bc0501653. [DOI] [PubMed] [Google Scholar]
- 61.Jia B, Shi J, Yang Z, Xu B, Liu Z, Zhao H, Liu S, Wang F. 99mTc-labeled cyclic RGDfK dimer: initial evaluation for SPECT imaging of glioma integrin αvβ3 expression. Bioconj Chem. 2006;17:1069–1076. doi: 10.1021/bc060055b. [DOI] [PubMed] [Google Scholar]
- 62.Liu S, He ZJ, Hsieh WY, Kim YS, Jiang Y. Impact of PKM linkers on biodistribution characteristics of the 99mTc-labeled cyclic RGDfK dimer. Bioconj Chem. 2006;17:1499–1507. doi: 10.1021/bc060235l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu S, Hsieh WY, Jiang Y, Kim YS, Sreerama SG, Chen X, Jia B, Wang F. Evaluation of a 99mTc-labeled cyclic RGD tetramer for noninvasive imaging integrin αvβ3-positive breast cancer. Bioconj Chem. 2007;18:438–446. doi: 10.1021/bc0603081. [DOI] [PubMed] [Google Scholar]
- 64.Dijkgraaf I, Liu S, Kruijtzer JAW, Soede AC, Oyen WJG, Liskamp RMJ, Corstens FHM, Boerman OC. Effect of linker variation on the in vitro and in vivo characteristics of an 111In-labeled RGD Peptide. Nucl Med Biol. 2007;34:29–35. doi: 10.1016/j.nucmedbio.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 65.Dijkgraaf I, Kruijtzer JAW, Liu S, Soede A, Oyen WJG, Corstens FHM, Liskamp RMJ, Boerman OC. Improved targeting of the αvβ3 integrin by multimerization of RGD peptides. Eur J Nucl Med Mol Imaging. 2007;34:267–273. doi: 10.1007/s00259-006-0180-9. [DOI] [PubMed] [Google Scholar]
- 66.Liu S, Kim YS, Hsieh WY, Sreerama SG. Coligand effects on solution stability, biodistribution and metabolism of 99mTc-labeled cyclic RGDfK tetramer. Nucl Med Biol. 2008;35:111–121. doi: 10.1016/j.nucmedbio.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jia B, Liu Z, Shi J, Yu ZL, Yang Z, Zhao HY, He ZJ, Liu S, Wang F. Linker effects on biological properties of 111In-labeled DTPA conjugates of a cyclic RGDfK dimer. Bioconj Chem. 2008;19:201–210. doi: 10.1021/bc7002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang JJ, Kim YS, He ZJ, Liu S. 99mTc-labeling of HYNIC-conjugated cyclic RGDfK dimer and tetramer using EDDA as coligand. Bioconj Chem. 2008;19:634–642. doi: 10.1021/bc7004208. [DOI] [PubMed] [Google Scholar]
- 69.Liu S, Edwards DS. 99mTc-labeled small peptides as diagnostic radiopharmaceuticals. Chem Rev. 1999;99:2235–2268. doi: 10.1021/cr980436l. [DOI] [PubMed] [Google Scholar]
- 70.Liu S. The role of coordination chemistry in development of target-specific radiopharmaceuticals. Chem Soc Rev. 2004;33:1–18. doi: 10.1039/b309961j. [DOI] [PubMed] [Google Scholar]
- 71.Liu S, Edwards DS. Bifunctional chelators for therapeutic lanthanide radiopharmaceuticals. Bioconj Chem. 2001;12:7–34. doi: 10.1021/bc000070v. [DOI] [PubMed] [Google Scholar]
- 72.Jurisson SS, Lydon JD. Potential technetium small molecule radiopharmaceuticals. Chem Rev. 1999;99:2205–2218. doi: 10.1021/cr980435t. [DOI] [PubMed] [Google Scholar]
- 73.Cai W, Zhang X, Wu Y, Chen X. A thiol-reactive 18F-labeling agent, N-[2-(4-18F-fluorobenzamido)ethyl]maleimide, and synthesis of RGD peptide-based tracer for PET imaging of αvβ3 integrin expression. J Nucl Med. 2006;47:1172–1180. [PMC free article] [PubMed] [Google Scholar]
- 74.Welch MJ, McCarthy TJ. The potential role of generator-produced radiopharmaceuticals in clinical PET. J Nucl Med. 2000;41:315–317. [PubMed] [Google Scholar]
- 75.Anderson CJ, Green MA, Yashi YF. Chemistry of copper radionuclides and radiopharmaceutical products. In: Welch MJ, Redvanly CS, editors. Handbook of Radiopharmaceuticals: Radiochemistry and Applications. John Wiley & Sons; New York: 2003. pp. 402–422. [Google Scholar]
- 76.Bormans G, Janssen A, Adriaens P, Crombez D, Witsenboer A, Degoeij J, Mortelmans L, Verbruggen A. 62Zn/62Cu generator for the routine production of 62Cu-PTSM. Appl Radiat Isot. 1992;43:1437–1441. [Google Scholar]
- 77.Haynes NG, Lacy JL, Nayak N, Martin CS, Dai D, Mathias CJ, Green MA. Performance of a 62Zn/62Cu generator in clinical trials of PET perfusion agent 62Cu-PTSM. J Nucl Med. 2000;41:309–314. [PubMed] [Google Scholar]
- 78.Smith SV. Molecular imaging with copper-64. J Inorg Biochem. 2004;98:1874–1901. doi: 10.1016/j.jinorgbio.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 79.Blower PJ, Lewis JS, Zweit J. Copper radionuclides and radiopharmaceuticals in nuclear medicine. Nucl Med Biol. 1996;23:957–980. doi: 10.1016/s0969-8051(96)00130-8. [DOI] [PubMed] [Google Scholar]
- 80.Reichert DE, Lewis JS, Anderson CJ. Metal complexes as diagnostic tools. Coord Chem Rev. 1999;184:3–66. [Google Scholar]
- 81.Maecke H, Hofmann M, Haberkorn U. 68Ga-labeled peptides in tumor imaging. J Nucl Med. 2005;46:172S–178S. [PubMed] [Google Scholar]
- 82.Heppeler A, Froidevaux S, Eberle AN, Maecke HR. Receptor targeting for tumor localisation and therapy with radiopeptides. Curr Med Chem. 2000;7:971–994. doi: 10.2174/0929867003374516. [DOI] [PubMed] [Google Scholar]
- 83.Heppeler A, Froidevaux S, Mäcke HR, Jermann E, Béhé M, Powell P, Hennig M. Radiometal-labelled macrocyclic chelator-derivatised somatostatin analogue with superb tumour targeting properties and potential for receptor-mediated internal radiotherapy. Chem Eur J. 1999;5:1974–1981. [Google Scholar]
- 84.Henze M, Schuhmacher J, Hipp P, Kowalski J, Becker DW, Doll F, Mäcke HR, Hofmann M, Debus J, Haberkorn U. PET imaging of somatostatin receptors using [68GA]DOTAD- Phe1-Tyr3-octreotide: first results in patients with meningiomas. J Nucl Med. 2001;42:1053–1056. [PubMed] [Google Scholar]
- 85.Froidevaux S, Eberle AN, Christe M, Sumanovski L, Heppeler A, Schmitt JS, Eisenwiener K, Beglinger C, Mäcke HR. Neuroendocrine tumor targeting: study of novel galliumlabeled somatostatin radiopeptides in a rat pancreatic tumor model. Int J Cancer. 2002;98:930–937. doi: 10.1002/ijc.10295. [DOI] [PubMed] [Google Scholar]
- 86.Hofmann M, Maecke H, Borner AR, Weckesser E, Schoffski P, Oei ML, Schumacher J, Henze M, Heppeler A, Meyer GJ, Knapp WH. Biokinetics and imaging with the somatostatin receptor PET radioligand 68Ga-DOTATOC: preliminary data. Eur J Nucl Med. 2001;28:1751–1757. doi: 10.1007/s002590100639. [DOI] [PubMed] [Google Scholar]
- 87.Kowalski J, Henze M, Schuhmacher J, Macke HR, Hofmann M, Haberkorn U. Evaluation of positron emission tomography imaging using 68Ga-DOTA-D Phe(1)-Tyr(3)-octreotide in comparison to 111In-DTPAOC SPECT. First results in patients with neuroendocrine tumors. Mol Imaging Biol. 2003;5:42–48. doi: 10.1016/s1536-1632(03)00038-6. [DOI] [PubMed] [Google Scholar]
- 88.Henze M, Schuhmacher J, Dimitrakopoulou-Strauss A, Strauss LG, Macke HR, Eisenhut M, Haberkorn U. Exceptional increase in somatostatin receptor expression in pancreatic neuroendocrine tumour, visualized with 68Ga-DOTATOC PET. Eur J Nucl Med Mol Imaging. 2004;31:466. doi: 10.1007/s00259-003-1436-2. [DOI] [PubMed] [Google Scholar]
- 89.Koukouraki S, Strauss LG, Georgoulias V, Schuhmacher J, Haberkorn U, Karkavitsas N, Dimitrakopoulou-Strauss A. Eur J Nucl Med Mol Imaging. Vol. 33. 2006. Evaluation of the pharmacokinetics of 68Ga-DOTATOC in patients with metastatic neuroendocrine tumours scheduled for 90Y-DOTATOC therapy; pp. 460–466. [DOI] [PubMed] [Google Scholar]
- 90.Henze M, Dimitrakopoulou-Strauss A, Milker-Zabel S, Schuhmacher J, Strauss LG, Doll J, et al. Characterization of (68Ga)-DOTA-D-Phe1-Tyr3-octreotide (DOTATOC) kinetics in patients with meningiomas. J Nucl Med. 2005;46:763–769. [PubMed] [Google Scholar]
- 91.Koukouraki S, Strauss LG, Georgoulias V, Eisenhut M, Haberkorn U, Dimitrakopoulou-Strauss A. Comparison of the pharmacokinetics of 68Ga-DOTATOC and [18F]FDG in patients with metastatic neuroendocrine tumours scheduled for 90Y-DOTATOC therapy. Eur J Nucl Med Mol Imaging. 2006;33:1115–1122. doi: 10.1007/s00259-006-0110-x. [DOI] [PubMed] [Google Scholar]
- 92.Liu S. Bifunctional coupling agents for target-specific delivery of metallic radionuclides. Advanced Drug Delivery Reviews. 2008;60:1347–1370. doi: 10.1016/j.addr.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu S. HYNIC derivatives as bifunctional coupling agents for 99mTc-labeling of small biomolecules. Topics in Current Chem. 2005;252:193–216. [Google Scholar]
- 94.André J, Maecke H, Zehnder M, Macko L, Akyel K. 1,4,7-triazanonane-1-succinic acid-4,7-diacetic acid (NODASA): a new bifunctional chelator for radio gallium-labeling of biomolecules. Chem Commun. 1998;12:1301–1302. [Google Scholar]
- 95.Eisenwiener KP, Prata MI, Buschmann I, Zhang HW, Santos AC, Wenger S, Reubi JC, Maecke HR. NODAGATOC, a new chelator-coupled somatostatin analogue labeled with [67Ga/68Ga] and [111In] for SPECT, PET, and targeted therapeutic applications of somatostatin receptor (hsst2) expressing tumors. Bioconj Chem. 2002;13:530–541. doi: 10.1021/bc010074f. [DOI] [PubMed] [Google Scholar]
- 96.Eisenwiener KP, Powell P, Maecke HR. A convenient synthesis of novel bifunctional pro-chelators for coupling to bioactive peptides for radiometal labeling. Bioorg Med Chem Lett. 2000;10:2133–2135. doi: 10.1016/s0960-894x(00)00413-3. [DOI] [PubMed] [Google Scholar]
- 97.McQuade P, Miao Y, Yoo J, Quinn TP, Welch MJ, Lewis JS. Imaging of melanoma using 64Cu- and 86Y-DOTA-ReCCMSH(Arg11), a cyclized peptide analogue of α-MSH. J Med Chem. 2005;48:2985–2992. doi: 10.1021/jm0490282. [DOI] [PubMed] [Google Scholar]
- 98.Prasanphanich AF, Nanda PK, Rold TL, Ma L, Lewis MJ, Garrison JC, Hoffman TJ, Sieckman GL, Figueroa SD, Smith CJ. [64Cu-NOTA-8-Aoc-BBN(7-14)NH2] targeting vector for positron-emission tomography imaging of gastrin-releasing peptide receptor-expressing tissues. PNAS. 2007;104:12462–12467. doi: 10.1073/pnas.0705347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harris TD, Kalogeropoulos S, Nguyen T, Liu S, Bartis J, Ellars CE, Edwards DS, Onthank D, Yalamanchili P, Robinson SP, Lazewatsky J, Barrett JA, Bozarth J. Design, synthesis and evaluation of radiolabeled integrin αvβ3 receptor antagonists for tumor imaging and radiotherapy. Cancer Biother Radiopharm. 2003;18:627–641. doi: 10.1089/108497803322287727. [DOI] [PubMed] [Google Scholar]
- 100.Onthank DC, Liu S, Silva PJ, Barrett JA, Harris TD, Robinson SP, Edwards DS. 90Y and 111In complexes of A DOTA-conjugated integrin αvβ3 receptor antagonist: different but biologically equivalent. Bioconj Chem. 2004;15:235–241. doi: 10.1021/bc034108q. [DOI] [PubMed] [Google Scholar]
- 101.Harris TD, Cheesman E, Harris AR, Sachleben R, Edwards DS, Liu S, Bartis J, Ellars C, Onthank D, Yalamanchili P, Heminway S, Silva P, Robinson S, Lazewatsky J, Rajopadhye M, Barrett JA. Radiolabeled divalent peptidomimetic vitronectin receptor antagonists as potential tumor radiotherapeutic and imaging Agents. Bioconj Chem. 2007;18:1266–1279. doi: 10.1021/bc070002+. [DOI] [PubMed] [Google Scholar]
- 102.Harris TD, Kalogeropoulos S, Nguyen T, Dwyer G, Edwards DS, Liu S, Bartis J, Ellars C, Onthank D, Yalamanchili P, Heminway S, Robinson S, Lazewatsky J, Barrett J. Structure-Activity relationships of 111In- and 99mTc-labeled quinolin-4-one peptidomimetics as ligands for the vitronectin receptor: potential tumor imaging agents. Bioconj Chem. 2006;17:1294–1313. doi: 10.1021/bc060063s. [DOI] [PubMed] [Google Scholar]
- 103.Chen X, Sievers E, Hou Y, Park R, Tohme M, Bart R, Bremner R, Bading JR, Conti PS. Integrin αvβ3–targeted imaging of lung cancer. Neoplasia. 2005;7:271–279. doi: 10.1593/neo.04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morrison MS, Ricketts SA, Barnett J, Cuthbertson A, Tessier J, Wedge SR. Use of a novel Arg-Gly-Asp radioligand, 18F-AH111585, to determine changes in tumor vascularity after antitumor therapy. J Nucl Med. 2009;50:116–122. doi: 10.2967/jnumed.108.056077. [DOI] [PubMed] [Google Scholar]
- 105.Kenny LM, Coombes RC, Oulie I, Contractor KB, Miller M, Spinks TJ, McParland B, Cohen PS, Hui A, Palmieri C, Osman S, Glaser M, Turton D, Al-Nahhas A, Aboagye EO. Phase I trial of the positron-emitting Arg-Gly-Asp (RGD) peptide radioligand 18F-AH111585 in breast cancer patients. J Nucl Med. 2008;49:879–886. doi: 10.2967/jnumed.107.049452. [DOI] [PubMed] [Google Scholar]
- 106.Beer AJ, Haubner R, Goebel M, Luderschmidt S, Spilker ME, Wester HJ, Weber WA, Schwaiger M. Biodistribution and pharmacokinetics of the αvβ3-selective tracer 18F-Galacto-RGD in cancer patients. J Nucl Med. 2005;46:1333–1341. [PubMed] [Google Scholar]
- 107.Haubner R, Weber WA, Beer AJ, Vabulience E, Reim D, Sarbia M, Becker KF, Goebel M, Hein R, Wester HJ, Kessler H, Schwaiger M. Noninvasive visualization of the activiated αvβ3 integrin in cancer patients by positron emission tomography and [18F]Galacto-RGD. PLOS Medicine. 2005;2:e70, 244–252. doi: 10.1371/journal.pmed.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beer AJ, Grosu AL, Carlsen J, Kolk A, Sarbia M, Stangier I, Watzlowik P, Wester HJ, Haubner R, Schwaiger M. [18F]Galacto-RGD positron emission tomography for imaging of αvβ3 expression on the neovasculature in patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6610–6616. doi: 10.1158/1078-0432.CCR-07-0528. [DOI] [PubMed] [Google Scholar]
- 109.Beer AJ, Niemeyer M, Carlsen J, Sarbia M, Nahrig J, Watzlowik P, Wester HJ, Harbeck N, Schwaiger M. Patterns of alphavbeta3 expression in primary and metastatic human breast cancer as shown by 18F-Galacto-RGD PET. J Nucl Med. 2008;49:255–259. doi: 10.2967/jnumed.107.045526. [DOI] [PubMed] [Google Scholar]
- 110.Haubner R, Finsinger D, Kessler H. Stereoisomeric peptide libraries and peptidomimetics for designing selective inhibitors of the αvβ3 integrin for a new cancer therapy. Angew Chem Int Ed Engl. 1997;36:1375–1389. [Google Scholar]
- 111.Haubner R, Gratias R, Diefenbach B, Goodman SL, Jonczyk A, Kessler H. Structural and functional aspect of RGD-containing cyclic pentapeptides as highly potent and selective integrin αvβ3 antagonists. J Am Chem Soc. 1996;118:7461–7472. [Google Scholar]
- 112.Aumailley M, Gurrath M, Müller G, Calvete J, Timpl R, Kessler H. Arg-Gly-Asp constrained within cyclic pentapeptides strong and selective inhibitors of cell adhesion to vitronectin and laminin fragment P1. FEBS Lett. 1991;291:50–54. doi: 10.1016/0014-5793(91)81101-d. [DOI] [PubMed] [Google Scholar]
- 113.Gottschalk KE, Kessler H. The structures of integrins and integrin-ligand complexes: Implications for drug design and signal transduction. Angew Chem Int Ed Engl. 2002;41:3767–3774. doi: 10.1002/1521-3773(20021018)41:20<3767::AID-ANIE3767>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 114.Rajopadhye M, Harris AR, Nguyen HM, Overoye KL, Bartis J, Liu S, Edwards DS, Barrett JA. RP593, a 99mTc-labeled αvβ3/αvβ5 antagonist, rapidly detects spontaneous tumors in mice and dogs. 47th Annual Meeting of SNM, St Louis, Missouri. J Nucl Med. 2000;41:34P. [Google Scholar]
- 115.Liu S, Edwards DS, Ziegler MC, Harris AR, Hemingway SJ, Barrett JA. 99mTc-Labeling of a hydrazinonicotinamide-conjugated vitronectin receptor antagonist useful for imaging tumor. Bioconj Chem. 2001;12:624–629. doi: 10.1021/bc010012p. [DOI] [PubMed] [Google Scholar]
- 116.Boturyn D, Coll JL, Garanger E, Favrot MC, Dumy P. Template assembled cyclopeptides as multimeric system for integrin targeting and endocytosis. J Am Chem Soc. 2004;126:5730–5739. doi: 10.1021/ja049926n. [DOI] [PubMed] [Google Scholar]
- 117.Li Z, Cai W, Cao Q, Chen K, Wu Z, Chen X. 64Cu-Labeled tetrameric and octameric RGD peptides for small-animal PET of tumor αvβ3 integrin expression. J Nucl Med. 2007;48:1162–1171. doi: 10.2967/jnumed.107.039859. [DOI] [PubMed] [Google Scholar]
- 118.Wang L, Kim YS, Shi J, Zhai S, Jia B, Liu Z, Zhao H, Wang F, Chen X, Liu S. Improving tumor targeting capability and pharmacokinetics of 99mTc-labeled cyclic RGD dimers with PEG4 linkers. Mol Pharm. 2009;6:231–245. doi: 10.1021/mp800150r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shi J, Wang L, Kim YS, Zhai S, Liu Z, Chen X, Liu S. Improving tumor uptake and excretion kinetics of 99mTc-labeled cyclic Arginine-Glycine-Aspartic (RGD) dimers with triglycine linkers. J Med Chem. 2008;51:7980–7990. doi: 10.1021/jm801134k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cai W, Rao J, Gambhir SS, Chen X. How molecular imaging is speeding up antiangiogenic drug development? Mol Cancer Ther. 2006;5:2624–2633. doi: 10.1158/1535-7163.MCT-06-0395. [DOI] [PubMed] [Google Scholar]
- 121.Niu G, Chen X. Has molecular and cellular imaging enhanced drug discovery and drug development? Drugs in R&D. 2008;9:351–368. doi: 10.2165/0126839-200809060-00002. [DOI] [PubMed] [Google Scholar]
- 122.Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47:3039–3051. [PubMed] [Google Scholar]
- 123.Shi J, Wang L, Kim YS, Zhai S, Liu Z, Chen X, Liu S. Improving tumor uptake and pharmacokinetics of 64Cu-labeled cyclic RGD dimers with triglycine and PEG4 Linkers. Bioconj Chem. 2009;20:750–759. doi: 10.1021/bc800455p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shi J, Kim YS, Chakraborty S, Zhou Y, Wang F, Liu S. Novel 111In-labeled cyclic RGD peptide dimers: improving tumor uptake and pharmacokinetics with G3 and PEG4 Linkers. Dalton Trans. Submitted. [Google Scholar]
- 125.Liu Z, Niu G, Shi J, Liu SL, Wang F, Liu S, Chen X. 68Ga-Labeled cyclic RGD dimers with Gly3 and PEG4 linkers: promising agents for tumor integrin αvβ3 PET imaging. Eur J Nucl Med Mol Imaging. 2009;36:947–957. doi: 10.1007/s00259-008-1045-1. [DOI] [PubMed] [Google Scholar]
- 126.Liu Z, Liu S, Wang F, Liu S, Chen X. Non-invasive imaging of tumor integrin expression using 18F-labeled RGD dimer peptide with PEG4 linkers. Eur J Nucl Med Mol Imaging ASAP. 2009 doi: 10.1007/s00259-009-1112-2. [DOI] [PubMed] [Google Scholar]
- 127.Fani M, Andre JP, Maecke HR. 68Ga-PET: a powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol Imaging. 2008;3:53–63. doi: 10.1002/cmmi.232. [DOI] [PubMed] [Google Scholar]
- 128.Maecke HR, Andre JP. 68Ga-PET radiopharmacy: A generator-based alternative to 18F-radiopharmacy. Ernst Schering Res Found Workshop. 2007;62:215–242. doi: 10.1007/978-3-540-49527-7_8. [DOI] [PubMed] [Google Scholar]
- 129.Al-Nahhas A, Win Z, Szyszko T, Singh A, Khan S, Rubello D. What can gallium-68 PET add to receptor and molecular imaging? Eur J Nucl Med Mol Imaging. 2007;34:1897–1901. doi: 10.1007/s00259-007-0568-1. [DOI] [PubMed] [Google Scholar]
- 130.Creamer D, Allen M, Sousa A, Poston R, Barker J. Altered vascular endothelium integrin expression in psoriasis. Am J Pathol. 1995;147:1661–1667. [PMC free article] [PubMed] [Google Scholar]
- 131.Waldeck J, Häger F, Höltke C, Lanckohr C, von Wallbrunn A, Torsello G, Heindel W, Theilmeier G, Schäfers M, Bremer C. Fluorescent reflectance imaging of macrophage-rich atherosclerostic plaques using αvβ3 integrin-targeted fluorochrome. J Nucl Med. 2008;49:1845–1851. doi: 10.2967/jnumed.108.052514. [DOI] [PubMed] [Google Scholar]
- 132.Meoli DF, Sadeghi MM, Krassilnikova S, Bourke BN, Giordano FJ, Dione DP, Su HL, Edwards DS, Liu S, Harris TD, Madri JA, Zaret BL, Sinusas AJ. Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J Clin Invest. 2004;113:1684–1691. doi: 10.1172/JCI20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pichler BJ, Kneilling M, Haubner R, Braumüller H, Schwaiger M, Röcken M, Weber WA. Imaging of delayed-type hypersensitivity reaction by PET and 18F-Galacto-RGD. J Nucl Med. 2005;46:184–189. [PubMed] [Google Scholar]
- 134.Hua J, Dobrucki LW, Sadeghi MM, Zhang J, Bourke BN, Cavaliere P, Song J, Chow C, Jahanshad N, van Royen N, Buschmann I, Madri JA, Mendizabal M, Sinusas A. Noninvasive imaging of angiogenesis with a 99mTc labeled peptide targeted at αvβ3 integrin after murine hindlimb ischemia. Circulation. 2005;111:3255–3260. doi: 10.1161/CIRCULATIONAHA.104.485029. [DOI] [PubMed] [Google Scholar]