Abstract
Purpose
The combination of the oral alkylating agent temozolomide and the oral multi-kinase inhibitor sorafenib was evaluated in advanced melanoma patients.
Patients and Methods
Patients with metastatic melanoma (N=167) were treated on four arms. All patients received sorafenib at 400 mg orally twice daily without interruption. Patients without brain metastases or prior temozolomide were randomized between Arm A: extended dosing of temozolomide (EDT; 75 mg/m2 temozolomide daily for 6/8 weeks) and Arm B: standard dosing (SDT; 150 mg/m2 temozolomide daily for 5/28 days). Patients previously treated with temozolomide were enrolled on Arm C: EDT. Patients with brain metastases and no prior temozolomide were assigned to Arm D: SDT. The primary endpoint was 6-month progression-free survival (PFS) rate. Secondary endpoints included response rate, toxicity rates, and the rates of BRAF or NRAS mutations.
Results
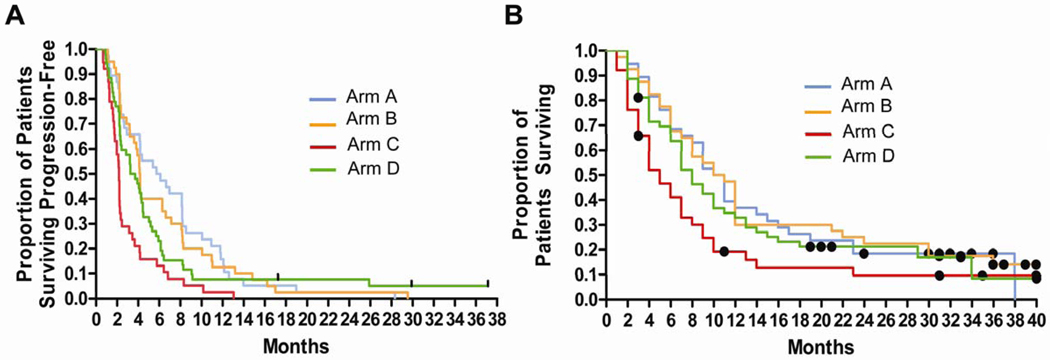
The 6-month PFS rate for arms A, B, C, and D were 50%, 40%, 11%, and 23%. The median PFS for patients on arm A, B, C, and D was 5.9, 4.2, 2.2, and 3.5 months, respectively. No significant differences were observed between Arms A and B in 6-month PFS rate, median PFS, or response rates. Treatment was well tolerated in all arms. No significant differences in toxicity were observed between arms A and B except for more grade 3–4 lymphopenia in arm A.
Conclusion
Temozolomide plus sorafenib was well tolerated and demonstrated activity in melanoma patients without prior history of temozolomide. The activity of this combination regimen warrants further investigation.
Introduction
In the United States an estimated 59,940 new diagnoses of melanoma were made in 2007 (1). Once metastatic disease develops the median survival is less than one year (2). The development of brain metastases in approximately 50% (3, 4) of melanoma patients contributes significantly to the rapid morbidity and mortality in patients with metastatic disease. Various combinations of chemotherapy (5–8), or immunotherapy (9–12) have failed to improve survival compared to single agent dacarbazine. (13) Temozolomide is a related oral alkylating agent that has been reported to produce response rates of 4–13% and median PFS of 1.2 months(14, 15) in patients with brain metastases, and 1.9 months (16) in patients without brain metastases. Despite this limited activity temozolomide is commonly used for the metastatic melanoma, because of its CNS penetration, tolerability, and ease of delivery. A number of therapies have been safely combined with temozolomide in patients with metastatic melanoma. Preclinical studies have identified tumor angiogenesis as a target in melanoma(17). The combination of temozolomide with the antiangiogenic agent thalidomide yielded encouraging response rates initially(18), but concerns for bleeding, thrombosis and infection, limited further development of this regimen.
By 2002, activating mutations in the serine/threonine kinases BRAF, and NRAS were identified in 66% (19) and 15% (20) of melanoma cell lines respectively, establishing MAPK signaling as a new therapeutic target in melanoma. Sorafenib, a kinase inhibitor which inhibits BRAF and vascular endothelial growth factor receptors (VEGFR2 and VEGFR3), in addition to other kinases, demonstrated antitumor activity in preclinical investigations.(21) In a single agent phase II trial sorafenib produced no tumor responses and resulted in a median PFS of 2.8 months. (22) A phase II trial combining sorafenib with carboplatin and paclitaxel appeared promising in metastatic melanoma patients (N=105), with a response rate of 27% and median PFS of 8.8 months, but significant toxicity(23).
This clinical trial was designed to assess the safety and preliminary activity of the combination of temozolomide and sorafenib, in patients with metastatic melanoma. This four-arm trial was designed with the following aims: 1) to determine if there was a significant difference in toxicity or efficacy in patients receiving extended versus standard dosing of temozolomide in combination with sorafenib 2) To determine the activity of temozolomide with sorafenib in patients with previous exposure to temozolomide and 3) to determine the safety and activity of temozolomide and sorafenib in patients with brain metastases.
Patients and Methods
Patients
Patients ≥18 years, with histologically confirmed metastatic or unresectable melanoma, measurable disease, an ECOG performance status of <2, adequate hematologic (White Blood Count [WBC] ≥ 3,000/mm3, absolute neutrophil count [ANC] ≥ 1,500/mm3, platelets ≥ 100,000/mm3), renal (serum creatinine ≤ 2.0 × upper limit of normal [ULN]), hepatic (bilirubin ≤ 1.5 × ULN AST/ALT ≤ 2.5 × ULN or ≤ 5.0 ULN in the presence of liver metastases), and coagulopathic (INR ≤ 1.5 and PTT <ULN; ) function were eligible. There was no limit on the number of prior therapies. Prior sorafenib was allowed. Patients had discontinued prior systemic therapy 4 weeks before entering the study. Prior brain radiotherapy was allowed for patients enrolled on arms C and D. Patients with stable or clinically asymptomatic progressive brain lesions were enrolled on arms C and D. Prior brain radiation therapy was allowed if patients had completed radiation therapy and discontinued steroids prior to enrollment. The study protocol was approved by the institutional review boards at the University of Pennsylvania and Dana Farber/Harvard Cancer Center. All patients provided informed consent before enrollment.
Treatment Plan
The treatment plan is outlined in Table 1. All patients were treated with single-agent sorafenib 400 mg orally twice daily seven days before starting temozolomide and continued without interruption. Patients without brain metastases or prior temozolomide were randomized between: arm A, extended daily dosing (EDT) of temozolomide 75 mg/m2 daily for 6/ 8 weeks (one cycle); or arm B, standard dosing (SDT) of temozolomide 150 mg/m2 daily for 5 /28 days (one hemi-cycle). Patients with prior temozolomide exposure were assigned to arm C, and received EDT with sorafenib. Patients with brain metastases and no prior temozolomide were assigned to arm D, and received SDT with sorafenib.
Table 1.
Four-arm Trial Design
| Arm | Brain Metastases | Prior Temozolomide |
Randomized | Temozolomide Dosing |
|---|---|---|---|---|
| A | No | No | Yes | Extended* |
| B | No | No | Yes | Standard** |
| C | Allowed | Required | No | Extended |
| D | Required | No | No | Standard |
Extended Dosing: Temozolomide 75 mg/m2 po qd for 6 out of every 8 weeks
Standard Dosing: Temozolomide 150 mg/m2 po qd for 5 out of every 28 weeks
Therapy was continued until disease progression or intolerable toxicity. Two dose reductions for sorafenib and one for temozolomide were allowed. For ≤ grade 3 toxicities attributable to sorafenib (e.g. hand-foot syndrome, rash, diarrhea) sorafenib was held (<21 days)_until resolution of the toxicity to ≤ grade 1 and sorafenib was restarted at the same dose. During dose interruption of sorafenib, patients continued on temozolomide. If the toxicity returned at the same severity, sorafenib was held and restarted at a reduced dose of 200 mg b.i.d. or 200 mg q.d. (second dose reduction). Dose re-escalation of sorafenib was allowed for dose-reduced patients with sorafenib-associated toxicities that resolved to ≤ grade 1. For patients that experienced grade 3 granulocytopenia or thrombocytopenia, temozolomide was held (< 28 days)_until resolution of the toxicity to ≤ grade 1 toxicity and was restarted a reduced dose: 100 mg/m2 (arms B, D) or 50 mg/m2 (arms A,C). Prophylactic granulocyte colony stimulating factors were not permitted. All other supportive care measures were permitted.
Assessment of Response and Toxicity
Response assessments consisted of history and physical exam every 4 weeks and CT scans of the chest, abdomen, and pelvis every 8 weeks (one cycle). Responses were investigator-assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines. Responses were confirmed by followup radiographic evaluation ≥ 4 weeks after the initial response criteria were met. MRI scans of the brain were obtained in follow-up only if clinically indicated. Toxicities were graded using the National Cancer Institute Common Toxicity Criteria Version 3.
Mutations in BRAF and NRAS
Blocks or sections were received from 88 (50%) patients for potential evaluation for mutational status. Tumor cell fraction was enriched and DNA isolated using our previously published techniques(23) DNA was extracted using standard protocols. Screening for mutations by PCR sequencing was done in BRAF (exons 11, 15) and NRAS (exons 1, 2) as previously reported (24). The PCR products were sequenced using BigDye Terminator v1.1 Cycle on an ABI PRISM 3130×l Genetic Analyzer (Applied Biosistem). Sequences were analyzed using Mutation Surveyor, DNA Variant Analysis version 3.1 (SoftGenetics LLC). An algorithm for genetic testing was used, in that patients were first screened for mutations in BRAF exon 15 and NRAS exon 2, and if that was negative, then BRAF exon 11 and NRAS exon 1 were evaluated.
Statistical Analysis
The primary endpoint was the rate of 6-month progression-free survival (PFS). Secondary endpoints included median PFS, rate of 1-year overall survival (OS), median OS, overall response rate (ORR), toxicity rates, and BRAF, and NRAS mutational status and PFS. Progression-free survival was defined as the interval of time since receiving first study drug to time of clinical or radiographic progression, or death due to any cause. Patients discontinuing therapy due to toxicity or personal choice, or were actively on trial at the time of analysis were censored from analysis of progression-free survival. For arms A, B, and C a sample size of 38 in each arm provided a power of 0.8 with a two-sided significance level of 0.05 to detect a 25% 6-month PFS rate compared to a null hypothesis of 12% 6-month PFS rate(16). For patients on arm D a sample size of 53 patients provided a power of 0.8 with a two-sided significance level of 0.05 to detect a 6-month PFS of 15% compared to a null hypothesis of 7% 6-month PFS rate(14). Blocked randomization with variable block size was used to randomize patients between arms A and B, stratified by prior sorafenib use. Although not identified as an endpoint in the study protocol, phase II benchmark analysis including the calculation of predicted 6-month PFS rate (Figure 1) and 1-year survival rate (Figure 2) was done according to recommendations of Korn et al.(25) Briefly the predicted 1-year survival rate for each patient was calculated using Table 3 and Appendix table A2(25) and the average predicted OS (π1) or 6-month PFS rate (π2) was determined. The treatment was deemed worthy of further study if the null hypothesis of observed OS rate ≤ π1 or the observed 6-month PFS rate ≤ (π2) was rejected with p-value <0.1. The 38 subjects enrolled to the arms without brain metastasis or prior temozolomide provided 95% power to detect any toxicity occurring at a rate of at least 8%. Rates of interest were estimated along with 95% confidence intervals. Arms A and B were compared using chi-squared tests for 6-month PFS and response rates and the logrank test for PFS curves. Differences between groups for continuous outcomes and categorical outcomes were assessed by t-tests and Fisher exact tests, using StatXact software. The association between continuous outcomes and categorical outcomes were assessed using Pearson or Spearman correlation coefficients, and chi-square or Fisher exact tests. Kaplan-Meier estimates of progression-free survival and 95% confidence intervals were calculated using Graphpad Prism software.
Fig 1.
Progression-free survival (PFS) and overall survival (OS) in melanoma patients treated with temozolomide and sorafenib. (A–B): Kaplan-Meier curves for (A) PFS and (B) OS; Arm A (Blue):patients without brain metastases or prior temozolomide, treated with extended dosing temozolomide (EDT) Arm B (orange); patients without brain metastases or prior temozolomide treated with standard dosing temozolomide (STD), Arm C (red) Patients with prior temozolomide, EDT Arm D (green) patients with brain metastases,,without prior temozolomide, SDT: Dots: censored patients.
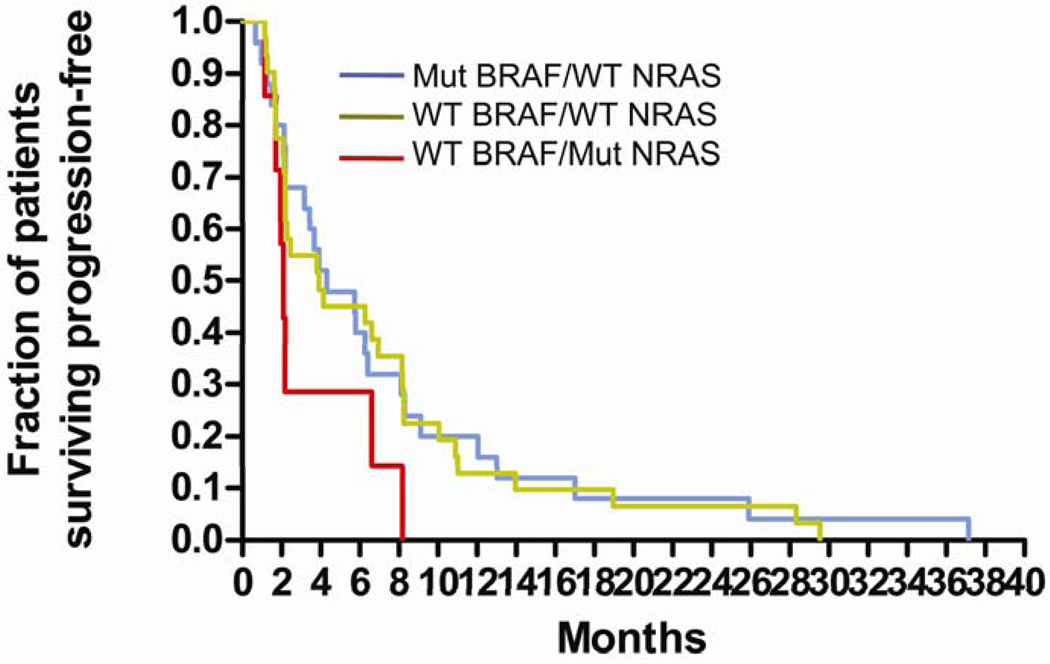
Fig 2.
Kaplan-Meier curves for progression-free survival (PFS) for patients with tumor genotypes wild-type BRAF/mutant NRAS (WT/MuNRAS; red), mutant BRAF/wild-type NRAS (MuBRAF/WT; blue), wild-type BRAF/wild-type NRAS (WT/WT; green)
Table 3.
Benchmark Analysis of Progression Free survival (PES) and Overall Survival (OS)
| Arm | A | p | B | p | C | p | D | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median PFS, months | 5.9 | 4.2 | 2.2 | 3.5 | ||||||||
| Predicated 6-month PFS rate*, % | 16 | 0.002 | 17 | 0.03 | 15 | NS | 16 | NS | ||||
| 6-month PFS rate, % | 50 | 40 | 11 | 23 | ||||||||
| 95% | 33–66 | 25–57 | 4–26 | 14–40 | ||||||||
| Median OS months | 10.5 | 10.5 | 5 | 8 | ||||||||
| Predicted 1 yr OS rate*, % | 34 | NS | 35 | NS | 18 | NS | 21 | <0.1 | ||||
| Observed 1 year OS rate, % | 34 | 48 | 14 | 33 | ||||||||
| 95%Cl | 21–47 | 30–60 | 6–30 | 22–47 | ||||||||
| Number of patients evaluated | 35 | 38 | 35 | 51 |
Calculated from Korm et.al.25
Results
Patient Accrual and Characteristics
Between June 2005 and May 2008, 180 patients were enrolled. Three patients were ineligible due to decline in performance status from screening to enrollment, and 10 patients were considered inevaluable for response or toxicity because they were removed from study before the first evaluation (one patient) or never received combination therapy (nine patients). A total of 78 patients were randomized between arms A and B. Due to block randomization, 2 additional patients were accrued to arm B in order to meet the target accrual for arm A. Characteristics of patients which are known to be prognostic in Stage IV melanoma patients (age, sex, stage, and LDH) were well balanced (Table 2) in patients randomized between arms A and B. The median number of prior chemotherapy regimens in patients on arms A, B and D was 0 (range: A:0–5, B:0–3, D:0–3). Six patients had prior sorafenib and were not excluded from further analyses. At the time of design there was no evidence that progression on non-temozolomide chemotherapy plus sorafenib regimen predicts inactivity of temozolomide with sorafenib so patients with prior sorafenib or prior sorefnib with non-temozolomide chemotherapy were permitted. A significantly higher percentage of patients on arm C had a baseline ECOG performance status of 1 compared to patients enrolled on arms A, B and D. The average number of prior therapies in patients on arm C was 1.67±1.17.
Table 2.
Patient Characteristics
| Arm | |||||
|---|---|---|---|---|---|
| Characteristic | A | B | C | D | Total |
| Number of patients | 38 | 40 | 38 | 53 | 167 |
| Age, years | |||||
| Median | 62 | 61 | 61 | 58 | 61 |
| Range | (26–83) | (44–84) | (35–78) | (37–83) | (26–84) |
| Sex, % | |||||
| Male | 58 | 75 | 74 | 66 | 69 |
| Female | 42 | 25 | 26 | 34 | 31 |
| AJCC Stage, % | |||||
| M1a | 2 | 5 | 0 | 0 | 2 |
| M1b | 8 | 18 | 3 | 0 | 6 |
| M1c | 90 | 77 | 97 | 100 | 92 |
| EECOG PS, % | |||||
| PS 0 | 71 | 90 | 50 | 72 | 75 |
| PS1 | 29 | 10 | 50* | 28 | 25 |
| No. prior therapies, median | 0 | 0 | 1 | 0 | 0 |
| Prior chemotherapy, % | 11 | 10 | 100 | 19 | 37 |
| LDH, % | |||||
| <ULN | 61 | 58 | 36 | 47 | 51 |
| ULN < LDH <2XULN | 23 | 23 | 42 | 36 | 31 |
| >2XULN | 16 | 19 | 22 | 17 | 18 |
p <0.05 compared to Arms A, B, D. Abbreviations: AJCC, American Joint Committee on Cancer; ECOG, Eastern Cooperative Group; PS Performance Status; ULN, Upper Limit of Normal
Survival and Response
Kaplan-Meier analysis of PFS was conducted (Figure 1A). There was no significant difference in PFS between arms A and B (log-rank test p=0.692). The 6-month PFS rate for arms A, B, C, and D were 50%, 40%, 11%, 23% respectively (Table 3) . The median PFS for arms A, B, C, and D were 5.9, 4.2, 2.2, and 3.5 months, respectively. Using a recently reported benchmark analysis(25) the predicted 6- month PFS rate was compared to the observed 6-month PFS rate for arms A (16% v. 50%; p=0.002), B (17% v. 40%; p=0.03, C (15% v. 11%), and D (16%v. 23%; p=N.S.).
By September 2008, survival data was available for 157 out of 167 patients with 10 patients lost to follow-up and whose survival duration was censored at the time of last contact. One hundred forty-one deaths had occurred. Kaplan-Meier analysis of OS was conducted for all 4 arms (Figure 1B). The median survival of patients on arms A, B, C, and D were 10.5, 10.5, 5, and 8 months, respectively. Using benchmark analysis (Table 3), the predicted 1-year survival rate was compared to the observed 1-year survival rate for arms A (34% v. 33%; p= N.S.), B (35% v. 48%; p= N.S.), C (18% v. 14%), and D (21%v. 33%; p=0.08).
The combination of temozolomide and sorafenib yielded one complete response in a patient on Arm B (Table 4). All other responses were partial. There was no significant difference in overall response rate (ORR) between arms A (24%, [95% CI 11–40%]) and B (15%, [95% CI 6–30%]). Patients with prior temozolomide had no responses. In patients with brain metastases (arm D), a 15% ORR in peripheral tumors and 48% SD (best response) rate was observed from temozolomide and sorafenib. CNS tumors were not included as target lesions since the majority of patients were treated with prior radiation therapy. Of the 6 patients with prior sorafenib 4 had SD and 2 had PD. The 15–24% response rate observed in patients without prior temozolomide treatment is similar to previous reported response rates for single agent temozolomide. Nevertheless, responses were durable; the median PFS from time of enrollment for patients with tumor response by RECIST criteria was 11 months.
Table 4.
Response Rates
| Randomized | |||||
|---|---|---|---|---|---|
| Arms | A | B | A+B | C | D |
| Temozolomide dosing | Extended | Standard | Extended | Standard | |
| Brain Metastases | − | − | − | +/− | + |
| Proir Temozolomide | − | − | − | + | − |
| Overall Response Rate*,% | 24 | 15 | 19 | 0 | 15 |
| 95% Cl | [11–40] | [6–30] | [11–30] | − | [7–29] |
| Stable Disease Rate, % | 42 | 58 | 50 | 26 | 48 |
| 95% Cl | [29–59] | [41–73] | [39–62] | [14–44] | [33–62] |
| Progressive Disease Rate, % | 34 | 27 | 31 | 74 | 37 |
| Totel Evaluable(No.) | 38 | 40 | 78 | 38 | 52 |
Overall Response Rate: There was one complete response in Arm A. All other response were partial responses.
Safety
The only significant difference in grade 3–4 toxicities (Table 5) in patients randomized between arms A and B was lymphopenia (53% v,15%, p<0.05). Grade 3–4 lymphopenia as also observed in 32% of patients on arm C who had significantly less exposure to extended dosing of temozolomide compared to patients enrolled on arm A. Despite this high rate of lymphopenia there were no documented opportunistic infections, and no prophylactic antibiotics were given.
Table 5.
Toxicity
| Arm A | Arm B | Arm C | Arm D | |||||
|---|---|---|---|---|---|---|---|---|
| Toxicity, % | Gr 1–2 | Gr 3–4 | Gr 1–2 | Gr 3–4 | Gr 1–2 | Gr 3–4 | Gr 1–2 | Gr 3–4 |
| Hematolgocial Toxicity | ||||||||
| Anemia | 34 | - | 45 | 5 | 24 | - | 30 | - |
| Leukopenia | 55 | 16 | 30 | 5 | 21 | 8 | 25 | 9 |
| Lymphopenia | 79 | 53* | 68 | 15* | 74 | 32 | 64 | 13 |
| Noutroponia | 34 | - | 15 | 3 | 5 | 5 | 9 | 9 |
| Thrombocytopenia | 37 | 5 | 23 | 3 | 24 | 3 | 32 | - |
| Non-Hematological Toxicity | ||||||||
| Abdominal pain | 18 | - | 13 | - | 5 | 3 | 11 | - |
| Alopecia | 61 | - | 40 | - | 13 | 30 | - | |
| Anorexia | 66 | 3 | 55 | - | 47 | 5 | 53 | 15 |
| Constipation | 55 | - | 38 | - | 24 | 5 | 30 | 2 |
| Dehydration | 11 | - | 8 | 3 | 5 | - | 13 | 6 |
| Diarrhea | 61 | 11 | 48 | 3 | 18 | 3 | 55 | 6 |
| Fatigue | 79 | 16 | 73 | 13 | 58 | 5 | 74 | 6 |
| Hand/foot syndrome | 71 | 26 | 73 | 13 | 45 | 11 | 64 | 8 |
| Headache | 18 | - | 15 | 8 | 11 | - | 4 | - |
| Hoarseness | 16 | - | 18 | 16 | - | 17 | 2 | |
| Hypertension | 29 | 3 | 13 | 13 | 16 | 5 | 26 | 2 |
| Myalgia | 34 | 3 | 25 | 3 | 26 | 23 | 2 | |
| Nausea | 71 | 11 | 75 | 3 | 55 | 5 | 58 | 13 |
| Rash | 87 | 13 | 85 | 3 | 50 | 8 | 62 | 11 |
| Stomatitis | 47 | 3 | 38 | 0 | 39 | 3 | 47 | 4 |
| Vomiting | 32 | 8 | 35 | - | 42 | 3 | 47 | 9 |
| Weight loss | 63 | 16 | 55 | 5 | 37 | 5 | 55 | 6 |
P<0.05
Other common (>10%) grade 3–4 toxicities were fatigue, hand/foot skin reaction, rash, hypertension, and diarrhea. A high proportion of patients experienced chronic grade 1–2 toxicity, with the most common being nausea, vomiting, anorexia, and weight loss. One patient had significant delay in wound healing at the site of previous radiation. Three patients had deep venous thrombosis and one patient had arterial thrombosis. There were no episodes of symptomatic hemorrhage into an existing CNS lesion.
BRAF and NRAS mutational analysis
Eighteen of 88 (20%) tumor blocks obtained could not be used for DNA extraction for one of several reasons: volume of tumor was too small, admixture of stroma and tumor did not allow us to obtain over 70% tumor, and no tumor was present in the blocks provided. Genotyping results for both BRAF exons 11, 15 and NRAS exons 1, 2 from high-quality DNA was available from tumor blocks for 62/167 patients (37%) evaluable for response (Table 6). BRAF V600E and V600K mutations were identified in 26/62 (42%) patients. NRAS mutations (G13D, G13R, Q61R, Q61K, or Q61L) were identified in 8/62 (13%) patients. The remaining patients, 28/62 (45%) had no detectable mutations at either locus (WT/WT). Kaplan-Meier analysis of PFS ( Figure 2) did not identify a significant difference between patients with mutant BRAF /WT NRAS versus WT BRAF/WT NRAS (HR=1.0, 95% CI 0.56–1.8; p>0.05) or WT BRAF/Mutant NRAS versus WT BRAF/WT NRAS (HR 2.6, 95%CI 0.86–7.7; p>0.05).
Table 6.
BRAF, NRAS mutations and PFS
| Mu BRAF/WT | WT/WT | Wt/Mu NRAS | Total No. | |
|---|---|---|---|---|
| Total No Sequenced (%) |
26(42) | 28(45) | 8(13) | 62 |
| Arm A, No. (%) | 3(19) | 10(63) | 3(18) | 16 |
| Arm B, No. (%) | 4(25) | 10(63) | 2(12) | 16 |
| Arm C, No. (%) | 11(61) | 2(11) | 5(28) | 15 |
| Arm D, No. (%) | 8(53) | 6(40) | 1(7) | 15 |
| Median PFS, mo | 4.3 | 3.9 | 2.1 | |
| HR [95% Cl] | ||||
Abbreviations: Mu BRAS Mutant BRAF includes BRAF V600K mutations; WT Wild-Type; Mu NRAS includes NRAS G13D, G13R, Q61R, Q61K, Q61L mutations; PFS, Progression-free survival.
Discussion
This study evaluated the activity and safety of combining temozolomide and sorafenib in patients with Stage IV melanoma. The primary endpoint (rate of 6 month PFS) was significantly different from the null hypothesis in patients without prior temozolimide, indicating that temozolomide and sorafenib is an active regimen in metastatic melanoma. While response rates were not significantly different than those observed with other temozolomide-based regimens, a high rate of stable disease contributed to prolongation of PFS compared to reported PFS in patients with or without brain metastases receiving temozolimide. BRAF or NRAS mutational status of the patient’s tumor was not predictive of outcome. The safety profile of this completely oral regimen appears to be excellent. While profound and persistent lymphopenia was observed, there were no opportunistic infections (26). Most grade 3–4 toxicities were reversible with brief drug holidays. Chronic administration of this combination did result in a high percentage of burdensome grade 1–2 toxicities including fatigue, nausea, anorexia, diarrhea, weight loss and rash.
One of the goals of this study was to determine if extended versus standard dosing of temozolomide was more active or safe in combination with sorafenib. There was no significant difference in efficacy outcomes between extended versus standard dosing of temozolomide in combination with sorafenib. There was a trend towards increased response rate and 6-month PFS with extended dosing of temozolomide and sorafenib compared to standard dosing of temozolomide with sorafenib, without a significant increase in toxicity. While the sample size for each arm was sufficient to compare the rates of 6 month PFS of each schedule with historical benchmarks (see below), the sample size may not have been adequate to completely rule out a significant difference in activity between the two temozolomide dosing schedule. It should also be noted that there are other schedules of temozolimide which were not tested in combination with sorafenib. Some consider standard dosing schedule for temozolomide as 200 mg/m2 p.o. q.d. for 5 /28 days instead of 150 mg/m2 that was used in this study. Recently a dose intense bi-weekly schedule of temozolomide 150 mg/m2 po for 7 out of every 14 days was compared with DTIC in a randomized phase III trial for patients with stage IV melanoma (27). While this regimen provides the most temozolomide exposure of any temozolomide dosing schedule commonly used, there was no difference in overall survival in this phase III trial and increased toxicity in patients treated with dose intense temozolomide compared to patients treated with standard temozolomide in a randomized phase III trial versus DTIC was observed. These results suggest that exploring additional schedules of temozolomide in combination with sorafenib is not warranted.
Patients with prior exposure to temozolomide (arm C) had a 0% response rate and short PFS. Few patients on arms A (11%), B (10%), and D (22%) had received prior chemotherapy for metastatic melanoma. The 23% 6 month progression free survival rate, median PFS of 3.5 months and overall survival of 8 months for patients on arm D are superior to any previous single agent temozolomide trial in this patient population. Increasing use of stereotactic radiosurgery alone or in combination with whole brain radiation therapy could have contributed independently to prolonged CNS-progression free survival and overall survival in patients on arm D. While concurrent steroid use was not permitted upon entry to the trial for safety concerns, once treatment was deemed well tolerated in individual patients, steroids for treatment of brain edema was frequently employed for symptomatic brain edema in patients on arm D.
The addition of sorafenib failed to improve the response rate or progression-free survival compared to carboplatin/paclitaxel alone among patients who previously received dacarbazine or temozolomide (28). The efficacy of carboplatin/paclitaxel with sorafenib versus carboplatin/paclitaxel/placebo in the first-line setting in patients with metastatic melanoma was tested in a randomized phase III (ECOG 2603). Recently, this study was stopped early after an interim analysis found that further study conduct was futile with regard to achieving the primary endpoint of significantly improved overall survival.
While randomized trials have determined that sorafenib does not improve progression-free or overall survival for melanoma when it is added to carboplatin and paclitaxel there is evidence that sorafenib may augment the efficacy of other chemotherapy backbones. A randomized phase II trial (N=101) found no overall survival benefit, but a significant difference in time to progression between treatment with dacarbazine with sorafenib compared to dacarbazine and placebo in patients with chemotherapy-naive metastatic melanoma (29). Dacarbazine and sorafenib yielded a 24% ORR which is identical to the ORR for Arm A in this trial. Patients treated with dacarbazine and sorafenib had a 41% 6-month PFS rate, and the similar population of patients on Arms A and B in this trial collectively had a 44% 6-month PFS rate. These results suggest that the type of chemotherapy that is paired with sorafenib may be a key determinant of efficacy.
Recently, Korn et al. reported a meta-analysis that established benchmarks to assess the activity of phase II regimens for metastatic melanoma. Benchmark analysis found a significant difference between observed and predicted 6-month PFS rate for patients without prior temozolomide or brain metastases (arms A and B), but not for patients with brain metastases without prior temozolimide (arm D). In contrast, observed rates of 1-year survival were significantly different from predicted rates only in patients with brain metastases and no prior temozolomide (arm D). These results underscore some of the prevailing controversies that accompany the interpretation of phase II trials in metastatic melanoma. Based on benchmark analysis, it is reasonable to recommend a randomized phase III trial of temozolomide and sorafenib in patients without brain metastases, since the discrepancy between the 6-month PFS and the 1-year survival results points out that the large variance in the estimates for median overall survival suggests that a larger sample size is necessary to detect a survival advantage compared to historical benchmarks. Unlike the method to calculate the predicted 1-year survival rate, the calculation of predicted PFS rate does not take into account the inclusion of patients with brain metastases. Most trials including patients with brain metastases do not report 6-month PFS and therefore the true benchmark rates of 6-month PFS for this patient population may not be captured by the methodology of Korn et al. Based on these results it is reasonable to propose a randomized phase III trial of temozolomide and sorafenib versus temozolomide in patients with brain metastases.
Mechanistically, sorafenib is a small molecule multi-kinase inhibitor. Although sorafenib is a potent BRAF inhibitor in vitro, it is not clear that this is the case in patients at clinically achievable doses sorafenib. The BRAF V600E mutation rate (41%) in these patients was at the low end of the range observed in previous reports. While initial studies conducted in melanoma cell lines described the BRAF mutation rate at 60–70% (30), this genotyping effort identified a lower rate in one of the larger (n=62) prospectively conducted studies of an unselected population of human melanoma. There was no significant association between BRAF mutational status and response rate or PFS in patients treated with temozolomide and sorafenib. Previous studies with sorafenib (22, 23) did not identify a significant association between BRAF mutational status and outcome. Based on the cumulative data available, BRAF mutation status can not be recommended as a selection criteria for future clinical trials involving sorafenib and chemotherapy for advanced melanoma. BRAF and NRAS mutational status could be a predictive maker in clinical trials of more specific BRAF inhibitors, and this approach is currently being investigated(31). These results raise the possibility that the activity observed when sorafenib is combined with chemotherapy may be related to inhibition of angiogenesis. This hypothesis is further supported by analysis of pretreatment tumors tissue from patients on a phase I/II clinical trial of sorafenib, carboplatin and paclitaxel(23), which found a significant correlation between high VEGFR2 expression and response rate(32).
The current results indicate that temozolomide and sorafenib may be an active regimen in temozolomide-naïve metastatic melanoma patients without and with brain metastases. Especially since there is no well-defined treatment algorithm for the treatment of patients without targetable oncogenic mutations, and patients with mutated tumors who have progressed on selective kinase inhibitors, these results deserve further investigation. The major limitation of this phase II study is the lack of a control group. The negative results of two prior randomized trials testing the efficacy of sorafenib, carboplatin and paclitaxel highlights the need to conduct large randomized controlled trials to determine the true efficacy of sorafenib and temozolomide.
Statement of Translational Relevance
This four arm phase II trial characterizes the safety and activity of combining the Raf /VEGFR inhibitor sorafenib with temozolomide,in patients with metastatic melanoma. Preclinical evidence indicates activating BRAF mutations and angiogenesis are important molecular targets in melanoma. The results suggest this regimen is active in melanoma patients including patients with brain metastases, who have a poor prognois. The identification of an active treatment regimens for melanoma patients with brain metastases is an unmet need in oncology practice. This manuscript reports the BRAF and NRAS genotyping of one of the largest prospectively collected unselected population of human melanoma tumors and determines that there is no correlation between mutation status and outcome in patients treated with sorafenib and temozolomide. The results of this study will guide the design of future clinical trials combining multikinase inhibitors such as sorafenib with chemotherapy for metastatic melanoma.
Acknowledgements
The authors would like to thank Elaine Almendras, Yan Wang, David Elder, and Pat Van Belle for receiving, preparing and analyzing paraffin-embedded tumor tissue for the genotyping studies.
This research was supported by NCI SPORE Career Development Award 5 P50 CA 93372-04, 1K23CA120862-01A2 (R.K.A.), 5K23CA104884-02 (K.T.F.) Partially supported by Bayer/Onyx Pharmaceuticals Partially supported by Schering-Plough Pharmaceuticals
Footnotes
Presented in part at the 42nd annual meeting of the American Society for Clinical Oncology Chicago, IL June1–5 2006; the 43rd annual meeting of the American Society for Clinical Oncology Chicago, IL June1–5 2007; and the Chemotherapy Foundation Symposium XXV, New York, NY Nov 6–10 2007.
Author Contributions
Conception and Design: Ravi Amaravadi, Keith Flaherty, Lynn Schuchter, Peter O’Dwyer, Kate Nathanson, Andrea Troxel,
Provision of study materials or patients: Ravi Amaravadi, Keith Flaherty, Lynn Schuchter, David McDermott, Michael Atkins
Collection and Assembly of data: Ravi Amaravadi, Keith Flaherty, Lynn Schuchter, Kate Nathanson, Lydia Giles, Kristi Gramlich, Mary Carberry, Richard Letrero
Data Analysis and Interpretation: Ravi Amaravadi, Keith Flaherty, Lynn Schuchter, Peter O’Dwyer, Kate Nathanson, Andrea Troxel
Manuscript Writing: Ravi Amaravadi, Keith Flaherty, David McDermott, Michael Atkins, Lynn Schuchter, Peter O’Dwyer, Kate Nathanson, Andrea Troxel
References
- 1.American Cancer Society. Atlanta: American Cancer Society, 2007. Atlanta: American Cancer Society; 2007. Cancer Facts and Figures 2007. [Google Scholar]
- 2.Gimotty PA, Botbyl J, Soong SJ, Guerry D. A population-based validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2005;23:8065–8075. doi: 10.1200/JCO.2005.02.4976. [DOI] [PubMed] [Google Scholar]
- 3.Amer MH, Al-Sarraf M, Baker LH, Vaitkevicius VK. Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer. 1978;42:660–668. doi: 10.1002/1097-0142(197808)42:2<660::aid-cncr2820420237>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807–810. doi: 10.1016/0002-9610(78)90171-x. [DOI] [PubMed] [Google Scholar]
- 5.Aamdal S, Wolff I, Kaplan S, et al. Docetaxel (Taxotere) in advanced malignant melanoma: a phase II study of the EORTC Early Clinical Trials Group. Eur J Cancer. 1994;30A:1061–1065. doi: 10.1016/0959-8049(94)90456-1. [DOI] [PubMed] [Google Scholar]
- 6.Bedikian AY, Plager C, Papadopoulos N, Eton O, Ellerhorst J, Smith T. Phase II evaluation of paclitaxel by short intravenous infusion in metastatic melanoma. Melanoma Res. 2004;14:63–66. doi: 10.1097/00008390-200402000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol. 2004;22:1118–1125. doi: 10.1200/JCO.2004.04.165. [DOI] [PubMed] [Google Scholar]
- 8.Jimeno A, Hitt R, Quintela-Fandino M, Cortes-Funes H. Phase II trial of vinorelbine tartrate in patients with treatment-naive metastatic melanoma. Anticancer Drugs. 2005;16:53–57. doi: 10.1097/00001813-200501000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Legha SS, Ring S, Eton O, et al. Development of a biochemotherapy regimen with concurrent administration of cisplatin, vinblastine, dacarbazine, interferon alfa, and interleukin-2 for patients with metastatic melanoma. J Clin Oncol. 1998;16:1752–1759. doi: 10.1200/JCO.1998.16.5.1752. [DOI] [PubMed] [Google Scholar]
- 10.Ridolfi R, Chiarion-Sileni V, Guida M, et al. Cisplatin, dacarbazine with or without subcutaneous interleukin-2, and interferon alpha-2b in advanced melanoma outpatients: results from an Italian multicenter phase III randomized clinical trial. J Clin Oncol. 2002;20:1600–1607. doi: 10.1200/JCO.2002.20.6.1600. [DOI] [PubMed] [Google Scholar]
- 11.Keilholz U, Punt CJ, Gore M, et al. Dacarbazine, cisplatin, and interferon-alfa-2b with or without interleukin-2 in metastatic melanoma: a randomized phase III trial (18951) of the European Organisation for Research and Treatment of Cancer Melanoma Group. J Clin Oncol. 2005;23:6747–6755. doi: 10.1200/JCO.2005.03.202. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann R, Spieth K, Leiter U, et al. Temozolomide in combination with interferon-alfa versus temozolomide alone in patients with advanced metastatic melanoma: a randomized, phase III, multicenter study from the Dermatologic Cooperative Oncology Group. J Clin Oncol. 2005;23:9001–9007. doi: 10.1200/JCO.2005.01.1551. [DOI] [PubMed] [Google Scholar]
- 13.Gogas HJ, Kirkwood JM, Sondak VK. Chemotherapy for metastatic melanoma: time for a change? Cancer. 2007;109:455–464. doi: 10.1002/cncr.22427. [DOI] [PubMed] [Google Scholar]
- 14.Agarwala SS, Kirkwood JM, Gore M, et al. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol. 2004;22:2101–2107. doi: 10.1200/JCO.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 15.Schadendorf D, Hauschild A, Ugurel S, et al. Dose-intensified bi-weekly temozolomide in patients with asymptomatic brain metastases from malignant melanoma: a phase II DeCOG/ADO study. Ann Oncol. 2006;17:1592–1597. doi: 10.1093/annonc/mdl148. [DOI] [PubMed] [Google Scholar]
- 16.Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 17.Streit M, Detmar M. Angiogenesis, lymphangiogenesis, and melanoma metastasis. Oncogene. 2003;22:3172–3179. doi: 10.1038/sj.onc.1206457. [DOI] [PubMed] [Google Scholar]
- 18.Hwu WJ, Krown SE, Menell JH, et al. Phase II study of temozolomide plus thalidomide for the treatment of metastatic melanoma. J Clin Oncol. 2003;21:3351–3356. doi: 10.1200/JCO.2003.02.061. [DOI] [PubMed] [Google Scholar]
- 19.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 20.Tsao H, Zhang X, Fowlkes K, Haluska FG. Relative reciprocity of NRAS and PTEN/MMAC1 alterations in cutaneous melanoma cell lines. Cancer Res. 2000;60:1800–1804. [PubMed] [Google Scholar]
- 21.Wilhelm S, Chien DS. BAY 43-9006: preclinical data. Curr Pharm Des. 2002;8:2255–2257. doi: 10.2174/1381612023393026. [DOI] [PubMed] [Google Scholar]
- 22.Eisen T, Ahmad T, Flaherty KT, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95:581–586. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flaherty KT, Schiller J, Schuchter LM, et al. A phase I trial of the oral, multikinase inhibitor sorafenib in combination with carboplatin and paclitaxel. Clin Cancer Res. 2008;14:4836–4842. doi: 10.1158/1078-0432.CCR-07-4123. [DOI] [PubMed] [Google Scholar]
- 24.Smalley KS, Contractor R, Nguyen TK, et al. Identification of a novel subgroup of melanomas with KIT/cyclin-dependent kinase-4 overexpression. Cancer Res. 2008;68:5743–5752. doi: 10.1158/0008-5472.CAN-08-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 26.Su YB, Sohn S, Krown SE, et al. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22:610–616. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 27.Patel Pea. Extended schedule, escalated dose temozolomide versus dacarbazine in Stage IV malignant melanoma: final results of the randomized phase III study: EORTC 180322; presented at 33rd congress of the European Society for Medical Oncology; Stockholm. 2008. #LBA8. [DOI] [PubMed] [Google Scholar]
- 28.Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–2930. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 29.McDermott DF, Sosman JA, Gonzalez R, et al. Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 Study Group. J Clin Oncol. 2008;26:2178–2185. doi: 10.1200/JCO.2007.14.8288. [DOI] [PubMed] [Google Scholar]
- 30.Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
- 31.Fecher LA, Amaravadi RK, Flaherty KT. The MAPK pathway in melanoma. Curr Opin Oncol. 2008;20:183–189. doi: 10.1097/CCO.0b013e3282f5271c. [DOI] [PubMed] [Google Scholar]
- 32.Jilaveanu L, Zito C, Lee SJ, et al. Expression of sorafenib targets in melanoma patients treated with carboplatin, paclitaxel and sorafenib. Clin Cancer Res. 2009;15:1076–1085. doi: 10.1158/1078-0432.CCR-08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]