Abstract
Purpose
Histologic findings in 51 pancreata resected from patients with a strong family history of pancreatic cancer were compared to the findings in 40 pancreata resected from patients with sporadic pancreatic cancer. None of the patients in the familial group had a known inherited syndrome, other than familial pancreatic cancer.
Experimental Design
Precursor lesions, including pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasm (IPMN) and incipient IPMN, were quantified. Invasive cancers were classified using established histologic criteria.
Results
The individual precursor lesions identified in both groups were histologically similar. Precursor lesions were more common in the familial cases than in the sporadic cases. The Relative Rate of PanINs per cm2 was 2.75 fold higher (95% CI 2.05-3.70, adjusted for age) in familial compared to sporadic cases. PanIN-3 lesions were more common in familial versus sporadic pancreatic cancer patients (Relative Rate 4.20; 95%CI 2.22-7.93 adjusted for age). High-grade incipient IPMNs were only observed in the familial cases. Nine (18%) of the 51 familial pancreatic cancers and 4 (10%) of the 40 sporadic cancers arose in association with an IPMN. No significant differences were found in the types of invasive cancers.
Conclusions
Non-invasive precursor lesions are more common in patients with a strong family history of pancreatic cancer than in patients with sporadic disease, and precursor lesions are of a higher grade in patients with a strong family history of pancreatic cancer. These findings can form a basis for the design of screening tests for the early detection of pancreatic neoplasia.
Introduction
Up to 10% of patients with pancreatic cancer have a family history of the disease(1, 2). Individuals with a family history of pancreatic cancer have an increased risk of developing pancreatic cancer themselves(3). It has been estimated that individuals with one first-degree relative with a pancreatic cancer have a 2- fold increased risk of developing pancreatic cancer, and the risk increases significantly with greater numbers of affected first-degree relatives(3, 4). Several genes have been identified which predispose to the familial aggregation of pancreatic cancer, and these include BRCA2, CDKN2A/p16, STK11/LKB1, PALB2 and PRSS1(5-11). These known genes account for only a minority of the cases of familial pancreatic cancer. The genetic basis for the majority of the familial aggregation of pancreatic cancer remains unknown.
One approach to understand the biological properties of a familial cancer gene is to carefully examine the precursor lesions that arise in the patients with the gene defect. For example, understanding of precursor lesions can help classify familial cancer genes as either “gatekeeper” or “caretaker” genes. Gatekeepers are genes that directly regulate the growth of neoplasms by inhibiting their growth or by promoting their death(12). The functions of these genes are rate-limiting for the growth of the neoplasm(12). Germline mutations in gatekeeper genes produce a dramatic increase in the number of precursor lesions, as, for example, is observed in familial adenomatous polyposis (FAP), a syndrome in which affected patients develop hundreds or in some cases even thousands of adenomas of the colon(13, 14). By contrast, caretaker genes do not directly promote the growth of neoplasms. Instead, the inactivation of a caretaker gene leads to a genetic instability that in turn indirectly promotes neoplastic growth by increasing the mutation rate(12). Germline mutations in caretaker genes, as seen for example in hereditary nonpolyposis colorectal syndrome (HNPCC), are not associated with increased numbers of precursor lesions. The neoplasms that arise in patients with germline mutations in a caretaker gene rapidly progress to invasive cancer (13, 15). A careful examination of the precursor lesions could, therefore, help define the biological properties of the gene(s) responsible for familial pancreatic cancer.
An understanding of the precursor lesions of familial and sporadic pancreatic cancer can also form the basis of the development of rationale strategies for the early detection of pancreatic neoplasia. Early detection has been shown to save many lives that would otherwise be lost to breast, cervical and colon cancer, and early detection is likely to improve survival of patients with pancreatic neoplasms(16). For example, Furukawa et al. reported a 4 year postoperative survival rate of 78% in patients with stage I infiltrating ductal adenocarcinomas of the pancreas(17), and Canto et al. and Brentnall et al. have reported that curable precursor lesions, including pancreatic intraepithelial neoplasia (PanIN) and intraductal papillary mucinous neoplasm (IPMN), can be detected when asymptomatic patients with a strong family history of pancreatic cancer are screened by endoscopic ultrasound (EUS)(18-20).
Screening of patients with a strong family history of pancreatic cancer for early disease requires an understanding of the precursor lesions in these patients. For example, Brune et al. have shown that multifocal neoplastic precursor lesions are associated with lobulocentric atrophy of the pancreas in patients with a strong family history of pancreatic cancer and that this multifocal lobulocentric atrophy can be detected by EUS(21, 22). Furthermore, an understanding of the precursor lesions in patients with a strong family history of pancreatic cancer is needed for the development of clinical approaches to the treatment of the precursor lesions identified in these patients(23).
The purpose of this study was to define the histologic features of the non-invasive and invasive lesions in patients with familial pancreatic cancer by histologic review of a large series of pancreata resected from these patients.
Materials and Methods
Patients
The National Familial Pancreas Tumor Registry (NFPTR) was established at The Johns Hopkins Medical Institutes in 1994(24). All procedures related to the NFPTR have been approved by The Johns Hopkins Medical Institutional Review Board. As of August 10, 2009, 3,367 families have enrolled in the NFPTR. Among them, 1,114 families meet the established definition of familial pancreatic cancer (defined as a kindred in which at least a pair of first-degree relatives has been diagnosed with pancreatic cancer). Fifty-one familial pancreatic cancer patients and 40 sporadic pancreatic cancer patients (defined as a kindred without a pair of affected first-degree relatives) enrolled in the NFPTR who underwent surgical resection for their pancreatic cancer at The Johns Hopkins Hospital were included in these analysis. Patients were limited to those who underwent surgery at The Johns Hopkins Hospital to ensure uniform sampling and processing of the resected specimens(25). Family history of pancreatic cancer was obtained by questionnaire and when possible confirmed by pathology report, review of histologic slides, medical record and/or death certificate, the details of which have been described elsewhere (3, 26). All patients in the familial group did not have a known genetic syndrome (BRCA2, HNPCC, FAMMM) other than familial pancreatic cancer.
Microscopic examination
All available histological slides from the surgically resected pancreata were reviewed for non-invasive precursor lesions including PanINs, incipient IPMNs and IPMNs, as well as for other histologic changes (27). The number of duct profiles containing a precursor lesion was counted, and the area with no invasive carcinoma was measured. The densities of precursor lesion were calculated (#lesion per cm2). The histologic type of each invasive cancer was documented, as were the parenchymal changes associated with the precursor lesions(21).
PanIN
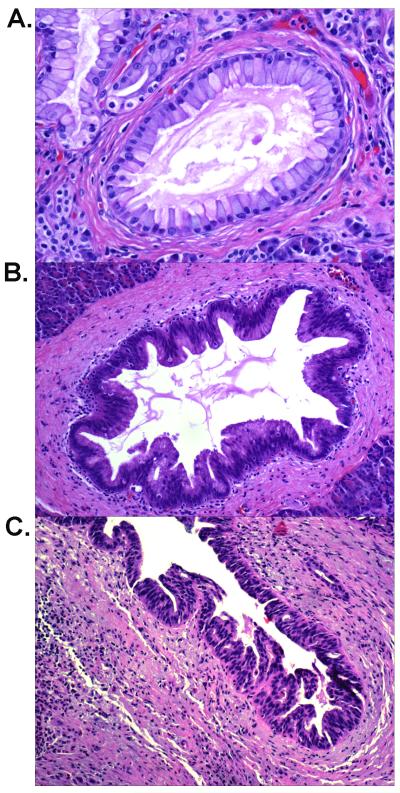
Pancreatic intraepithelial neoplasia (PanIN) is a microscopic flat or papillary, noninvasive epithelial neoplasm arising in a smaller (<0.5 cm) pancreatic duct. PanIN lesions are further classified into three grades based on their architectural and cytologic atypia(28). PanIN-1 is defined as flat or papillary lesions composed of uniform columnar mucinous cells with little if any nuclear atypia (Figure 1A)(28). PanIN-2 lesions have some nuclear abnormalities including loss of polarity, nuclear crowding, enlarged nuclei, pseudostratification and hyperchromasia (Figure 1B). PanIN-3 lesions are characterized by the presence of significant architectural and/or cytologic atypia (Figure 1C)(28).
Figure 1.
Representative hematoxylin-eosin stained PanIN lesions from case 51 of the familial group. A: A PanIN-1 lesion showing mucinous columnar epithelial proliferation with little nuclear atypia (200X); B: A PanIN-2 lesion showing proliferated ductal epithelium with some nuclear atypia and pseudostratification (100X). C: A PanIN-3 lesion showing marked architectural and nuclear atypia (100X).
IPMN
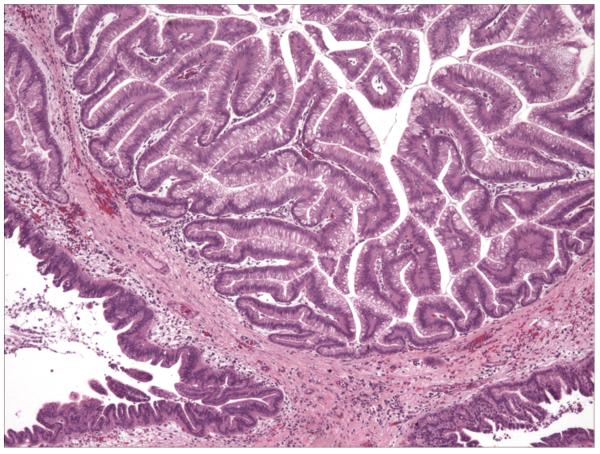
Intraductal papillary mucinous neoplasms (IPMNs) are grossly visible mucin-producing epithelial neoplasms (≥1 cm), which predominantly arise within the main pancreatic duct or one of its branches(28). IPMNs cause varying degrees of duct dilatation, have a prominent papillary architecture, and produce abundant intracellular and extracellular mucin (Figure 2). IPMNs can be further classified into IPMN with low-grade dysplasia (IPMN-adenoma), IPMN with moderate dysplasia (IPMN-borderline) and IPMN with high-grade dyspasia (in situ carcinoma) based on the degree of architectural and cytologic changes(28).
Figure 2.
Representative hematoxylin-eosin section (40X) containing an IPMN lesion from case 21 of the familial group. The lesion had a prominent papillary structure associated with moderate to marked nuclear atypia.
Incipient IPMN
“Incipient” IPMNs are histologically similar to IPMNs, but they are <1cm and fall short of size criteria for an IPMN. In this study, a precursor lesion with a size of 0.5 cm -1 cm was defined as an incipient IPMN.
Invasive Carcinoma
Histologic types of invasive pancreatic carcinoma were reviewed and classified using standard nomenclature(27).
Statistics
The number of PanIN profiles and the number of incipient IPMNs in areas with no invasive carcinoma observed in the familial pancreatic cancer patients and the sporadic pancreatic cancer patients were compared using negative binomial regression, with an offset for area in cm2. The histologic types of the invasive pancreatic cancer and number of IPMNs were compared using Fisher’s exact tests and/or Kruskal-Wallis Rank Test. Data analysis was conducted using STATA v10.0.
Results
Histologic types of invasive pancreatic cancer
The majority of the invasive carcinomas in the 51 patients with familial pancreatic cancer were classic infiltrating ductal adenocarcinomas (34 of 51, 67%, Table 1). Other types of invasive cancer identified in the familial group included 6 adenosquamous carcinomas (12%), 9 IPMNs with an associated invasive adenocarcinoma (IPMN + CA, 18%), 1 undifferentiated carcinoma (2%), and 1 signet ring cell carcinoma (2%). Classic infiltrating ductal adenocarcinoma also accounted for the majority of the infiltrating cancers in the sporadic group (34 of the 40 cases, 85%). The other histologic types of invasive cancer identified in the sporadic pancreatic cancer group included 1 adenosquamous carcinoma (2%), 4 IPMN+CA (10%) and 1 undifferentiated carcinoma (2%). These observed differences in the histological types of invasive carcinoma between the patients with familial and sporadic pancreatic cancer were not statistically significant (p=0.214). There was a trend towards more adenosquamous carcinomas in the familial group (12% vs. 2%, p=0.12).
Table 1.
Demographics and histologic findings.
| Familial Cases (n=51) |
Sporadic Cases (n=40) |
||
|---|---|---|---|
| Age | 66.98 ± 11.47 | 65.25 ± 10.62 (p=0.43) | |
| Race | |||
| White | 48 | 37 | |
| Black | 0 | 1 | |
| Other | 3 | 2 (p=0.80) | |
| Gender | |||
| Male | 29 | 21 | |
| Female | 22 | 19 (p=0.85) | |
| Stage | |||
| T1N0 | 1 | 3 | |
| T2N0 | 5 | 2 | |
| T2N1 | 2 | 4 | |
| T3N0 | 8 | 6 | |
| T3N1 | 35 | 25 (p=0.274) | |
| Histologic types |
Ductal CA | 34 | 34 |
| IPMN+CA | 9 | 4 | |
| Adenosquamous CA | 6 | 1 | |
| Signet ring cell CA | 1 | 0 | |
| Undifferentiated CA | 1 | 1 (p=1.0) | |
| Tumor location | |||
| Head | 43 | 33 | |
| Body | 3 | 2 | |
| Tail | 2 | 2 | |
| others | 3 | 3 (p=1.0) | |
| Margin | |||
| Positive | 8 | 10 | |
| Negative | 43 | 30 (p=0.299) |
Precursor lesions
Pancreatic parenchyma not involved by the patient’s invasive carcinoma was available for review from 49 of the 51 familial pancreatic cancer cases and all 40 of the sporadic cases. The number of non-invasive precursor lesions, including PanINs and incipient IPMNs, were counted in these tissues (Table 2). Precursor lesions were seen in 48 of 49 familial cases, and multiple precursor lesions were present in 46 (94%) of the 49 (Figure 1) in contrast only 35 of the 40 sporadic case had precursor lesions and 34 of the 40 (85%) had multiple lesions. The Relative Rate of PanIN lesions per cm2 was 2.75 fold higher (95% CI 2.05-3.70, adjusted for age) in familial compared to sporadic cases. This corresponds to a rate of 1.51 PanIN lesions per cm2 for a familial case and 0.55 for a sporadic case at the observed mean age of 66 years. In the familial cases, the number of the PanIN lesions per cm2 ranged from 0 to up to 3.8. Of the 5 sporadic cases without precursor lesions all had a classic infiltrating ductal adenocarcinoma . The number of PanIN lesions in the sporadic cases ranged from 0.0 to 2.01 per cm2.
Table 2.
Precursor lesions in the familial (n=49) and sporadic cases (n=40)
| Precursor | Familial (per cm2) | Sporadic (per cm2) |
|---|---|---|
| Total PanIN | 1.51 | 0.55* |
| PanIN-1 | 0.84 | 0.35 |
| PanIN-2 | 0.51 | 0.14 |
| PanIN-3 | 0.19 | 0.04 |
| Total incipient IPMN | 0.04 | 0.01* |
| HG Incipient IPMN | 0.03 | 0 |
| Total Precursor | 1.55 | 0.56* |
| Total HG precursor | 0.22 | 0.04* |
Note: HG=High grade , IPMN= intraductal papillary mucinous neoplasm, PanIN=pancreatic intraepithelial neoplasia,
=p<0.05
Total precursor=PanIN-1 + PanIN-2 + PanIN-3 + Incipient IPMN.
Total high-grade precursor=PanIN3 + High-grade incipient IPMN.
In the pancreata from the patients with familial pancreatic cancer, the PanIN lesions identified were mostly PanIN-1 (0.84/cm2) and PanIN-2 (0.51/cm2). However, 32 (65%) of the 49 pancreata also harbored at least one PanIN-3 lesion. Similar to the familial cases, the PanIN lesions in the sporadic cases were mostly PanIN-1 (0.35/cm2) lesions. PanIN-2 (0.14/cm2) and PanIN-3 lesions were significantly less common. Only 14 (35%) of the 40 sporadic cases had one or more PanIN-3 lesions. Overall, the rate of PanIN-3 lesions per cm2 was greater (Relative Rate 4.20;95%CI 2.23-7.93) for familial compared to sporadic pancreatic cancer patients. This corresponds to a rate of PanIN-3 lesions per cm2 in familial cases of 0.19 and in sporadic cases a rate of 0.04 at the observed mean age of 66 years. Cigarette smoking was not significantly associated with the number of PanIN lesions.
Incipient IPMNs were identified in 16 (33%) of the 49 familial cases and 3 (6%) of the 40 sporadic cases. Ten of these 16 familial patients (63%) had incipient IPMNs with high-grade dysplasia. Two of the 3 sporadic cases had only low-grade dysplasia, one had both low-grade and moderate dysplasia. No incipient IPMN with high-grade dysplasia was seen in the sporadic cases. The rate of incipient IMPNs per cm2 was 11.82 (95% CI 1.88-74.08) fold higher in familial compared to sporadic pancreatic cancer patients after controlling for age. Cigarette smoking was not significantly associated with the rate of incipient IPMN lesions.
Nine (18%) of the 51 familial pancreatic cancers arose associated with an IPMN (Table 1). These invasive carcinomas included 8 classic ductal adenocarcinomas and 1 colloid (mucinous non-cystic) adenocarcinoma. Pancreatic parenchyma with no invasive carcinoma was available for review from 7 of the 9 IPMN + CA cases. Each harbored a single IPMN lesion with high-grade dysplasia (in situ carcinoma). IPMNs were observed in 4 of the 40 sporadic cases (10%) (Table 1), all were associated with an invasive carcinoma. This difference in prevalence was not statistically significant (p=0.28). The invasive carcinomas arising in association with these IPMNs included 3 classic infiltrating ductal carcinomas and 1 colloid adenocarcinoma. One of the 4 sporadic cases with IPMN harbored two IPMNs: one IPMN with moderate dysplasia, and the other with high-grade dysplasia associated with invasive carcinoma. The other 3 sporadic IPMN cases had a single IPMN with high-grade dysplasia.
Histologic subtypes of IPMNs in both familial and sporadic group were also analyzed. Three of 4 IPMNs in the sporadic group were pancreatobiliary subtype, the remaining one was intestinal subtype. Histologic subtypes of IPMNs in the familial group included pancreatobiliary subtypes in 5 cases, intestinal subtype in 2 cases, gastric subtype in 1 case, and oncocytic subtype in 1 case.
High-grade Precursor Lesions
Thirty-five of the 49 familial cases (71%) harbored high-grade precursor lesions (including PanIN-3 and incipient IPMNs). Twenty-two of these 35 had multiple high-grade lesions. By contrast, 14 (35%) of the 40 sporadic cases had high-grade lesions and 10 of these 14 contained more than one high-grade lesion. The total number of PanIN and Incipient IPMN lesions per cm2 was 2.78 fold higher (95% CI 2.07 – 3.74) in familial compared to sporadic pancreatic cancer patients.
Parenchymal changes associated with the precursors
With the exception of a few cases with marked chronic pancreatitis in the background pancreatic parenchyma, the pancreatic parenchyma not associated with non-invasive precursor lesions or with invasive cancer was histologically unremarkable. As expected, the pancreatic parenchyma adjacent to the infiltrating carcinomas in both the familial and sporadic cases uniformly showed marked fibrosis, parenchymal atrophy and a mixed inflammatory response.
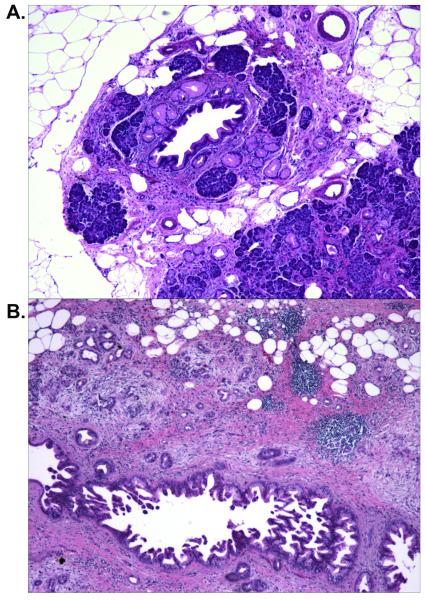
The pancreatic parenchyma adjacent to the non-invasive precursor lesions often showed various degrees of lobulocentric atrophy and/or fibrosis (21, 29). This lobulocentric atrophy was characterized by loss of acinar parenchyma in the lobule surrounding the precursor lesion, fibrosis, acinar to ductal metaplasia and aggregation of the islets of Langerhans. The degree of the parenchymal changes associated with the precursor lesions was variable, ranging from partial acinar atrophy with mainly focal acinar to ductal metaplasia (Figure 3A) to a complete loss of acinar cells and replacement of the lobular unit by acinar to ductal metaplasia, fibrotic stroma and aggregates of islets of Langerhans (Figure 3B). Lobulocentric atrophy of varying degrees was seen in 42 of the 49 pancreata (86%) from the patients with familial pancreatic cancer. Similar changes were also seen in the pancreata from the patients with sporadic pancreatic cancer, but at a lower frequency (21/40, 53%) (p=0.001). This can be explained by the smaller numbers of precursor lesions in the pancreata of the sporadic group.
Figure 3.
PanIN lesions associated parenchymal abnormalities (hematoxylin-eosin, 40X) . A: A PanIN-1B lesion with associated lobular parenchyma showing partial acinar atrophy, acinar to ductal metaplasia. Note: residual normal acinar cells. B: A PanIN-3 lesion with associated parenchymal showing lobulocentric atrophy with loss of acinar parenchyma in a lobular pattern, fibrosis, and acinar-ductal metaplasia, and clusters of islet of Langerhans.
Discussion
Familial pancreatic cancers appear to arise from both PanINs and IPMNs, the same types of precursor lesions as do sporadic pancreatic cancers. We found that PanINs and incipient IPMNs are more numerous and of a higher grade in the pancreata from the patients with familial pancreatic cancer than they are in the pancreata from patients with sporadic pancreatic cancer. In both the familial and sporadic cases, the precursor lesions were associated with lobulocentric atrophy with fibrosis as well as acinar to ductal metaplasia. Since the precursor lesions were more common in the familial cases, these foci of lobulocentric atrophy were also more common in the familial cases. No significant differences were found in the histologic types of infiltrating carcinoma seen in the two groups, although there was a trend towards more adenosquamous carcinoma in the familial group. These findings have a number of significant implications.
First, since familial and sporadic pancreatic cancers appear to arise from the same types of precursors (PanINs and IPMNs), it is likely that tests designed to screen for sporadic precursor lesions should be able to detect familial precursor lesions and vise a versa. In particular, approximately 10 to 18% of sporadic and familial cancers arise in association with an IPMN, and imaging modalities shown to be sensitive and specific for precursor lesions such as IPMNs will be effective in both populations.
Second, the multifocalitiy of the non-invasive precursor lesions seen in the patients with familial pancreatic cancer suggests that some familial pancreatic cancers are caused by a mutation in a “gatekeeper” gene rather than a “caretaker gene”. Indeed, Brune et al. reported that in some patients as many as 20% of the pancreatic ducts in patients with a strong family history of pancreatic cancer harbor a precursor lesion(21). The classification of the familial pancreatic cancer gene as a gatekeeper gene in turn suggests that examining multiple precursor lesions for shared loci of loss of heterozygosity should help localize the genetic locus of the familial pancreatic cancer gene(30). The multifocality of the precursor lesions in patients with a family history of pancreatic cancer also suggests that these patients remain at-risk for multifocal synchronous or metachronous disease following partial pancreatectomy. This risk of synchronous and metachronous disease has already been observed in patients with apparently sporadic IPMNs(31, 32).
Third, the association of multifocal precursor lesions with multifocal lobulocentric atrophy in patients with a strong family history of pancreatic cancer has significant implications for screening for early disease. Several screening tactics are already being evaluated using technologies such as EUS, computed tomography (CT) and magnetic resonance cholangiopancreatography (MRCP)(18-22). In these studies, EUS imaging has demonstrated chronic pancreatitis-like abnormalities in the pancreata that harbored multifocal precursor lesions, a pattern produced by the multiple foci of lobulocentric atrophy caused by multifocal PanINs(18, 19, 21, 22, 33). Thus, an individual PanIN lesion may not be detectable using currently available imaging modalities, but the parenchymal changes induced by multifocal PanINs can be appreciated using EUS(18, 19, 21, 22).
Finally, some inherited cancer syndromes are associated with neoplasms with a specific histology. For example, a medullary phenotype is seen in patients with a familial history of HNPCC(34-36), and individuals with Peutz-Jeghers syndrome may be predisposed to develop IPMNs(37-39). Similarly, Koorstra JB and colleagues have recently reported an undifferentiated carcinoma with osteoclast-like giant cells (UCOCGC) of the pancreas in a patient with the familial atypical multiple mole melanoma syndrome (FAMMM)(40). Here we were able to characterize the histologic subtypes of invasive pancreatic cancer in familial pancreatic cancer patients without a known genetic defect. We found no significant differences in the type of infiltrating carcinomas observed in patients with familial and sporadic pancreatic cancer, however, there was a trend towards more adenosquamous carcinomas in the familial group (12% vs. 2%). Of interest, Whelan et al. reported a patient with the FAMMM syndrome who developed a squamous (presumably an adenosquamous) carcinoma of the pancreas(41). Should follow-up studies confirm a greater proportion of adenosquamous carcinomas in patients with familial pancreatic cancer, this would be clinically significant because adenosquamous carcinomas are very aggressive neoplasms with a median survival of only six months after resection(27).
A limitation of the current study is that we looked at precursor lesions at a single point in time (the moment of surgical resection). We are therefore not able to define the frequency and speed at which non-invasive precursor lesions progress to infiltrating cancer. These two characteristics of precursor lesions are critical because they will define the optimal frequency at which at-risk patients should be screened, and they will guide the clinical management of precursor lesions once they are identified.
In summary, we demonstrated that precursor lesions are more common in patients with familial pancreatic cancer than they are in patients with sporadic disease. These precursor lesions in patients with familial pancreatic cancer also tend to be of a higher grade. The multifocality of the precursor lesions suggests that the gene responsible for the some cases of familial pancreatic cancer has “gatekeeper” properties. The lobulocentric atrophy and resultant heterogeneity of the pancreatic parenchyma produced by these multifocal precursor lesions provides a basis for the design of screening tests for the early detection of pancreatic neoplasia(21).
Statement of Translational Relevance.
Individuals with a family history of pancreatic cancer have an increased risk of developing pancreatic cancer themselves. Significant effort has been placed in screening these at-risk individuals for early disease, but little is known of the precursor lesions associated with familial pancreatic cancer. In this study, we demonstrate that precursor lesions are more numerous and are higher grade in patients with familial pancreatic cancer than they are in patients with sporadic disease. These findings have significant implications for the design of screening tests for the early detection of pancreatic cancer.
Acknowledgments
Supported by the NCI SPORE grant in Gastrointestinal Cancer CA62924 and the Michael Rolfe Pancreatic Cancer Foundation
References
- 1.Lynch HT. Genetics and pancreatic cancer. Arch Surg. 1994;129:266–8. doi: 10.1001/archsurg.1994.01420270042009. [DOI] [PubMed] [Google Scholar]
- 2.Petersen GM, de Andrade M, Goggins M, et al. Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2006;15:704–10. doi: 10.1158/1055-9965.EPI-05-0734. [DOI] [PubMed] [Google Scholar]
- 3.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer research. 2004;64:2634–8. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 4.Amundadottir LT, Thorvaldsson S, Gudbjartsson DF, et al. Cancer as a complex phenotype: pattern of cancer distribution within and beyond the nuclear family. PLoS Med. 2004;1:e65. doi: 10.1371/journal.pmed.0010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–7. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein AM, Fraser MC, Struewing JP, et al. Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N Engl J Med. 1995;333:970–4. doi: 10.1056/NEJM199510123331504. [DOI] [PubMed] [Google Scholar]
- 7.Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–5. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 8.Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–53. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- 9.Murphy KM, Brune KA, Griffin C, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17% Cancer research. 2002;62:3789–93. [PubMed] [Google Scholar]
- 10.Couch FJ, Johnson MR, Rabe KG, et al. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:342–6. doi: 10.1158/1055-9965.EPI-06-0783. [DOI] [PubMed] [Google Scholar]
- 11.Jones S, Hruban RH, Kamiyama M, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science (New York, NY. 2009;324:217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 13.Kinzler KW, Vogelstein B. Cancer-susceptibility genes. Gatekeepers and caretakers. Nature. 1997;386:761, 3. doi: 10.1038/386761a0. [DOI] [PubMed] [Google Scholar]
- 14.Kinzler KW, Nilbert MC, Su LK, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–5. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 15.Leach FS, Nicolaides NC, Papadopoulos N, et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993;75:1215–25. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- 16.Hruban RH, Takaori K, Canto M, et al. Clinical importance of precursor lesions in the pancreas. J Hepatobiliary Pancreat Surg. 2007;14:255–63. doi: 10.1007/s00534-006-1170-9. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa H, Okada S, Saisho H, et al. Clinicopathologic features of small pancreatic adenocarcinoma. A collective study. Cancer. 1996;78:986–90. doi: 10.1002/(SICI)1097-0142(19960901)78:5<986::AID-CNCR7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 18.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–81. doi: 10.1016/j.cgh.2006.02.005. quiz 665. [DOI] [PubMed] [Google Scholar]
- 19.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606–21. doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 20.Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999;131:247–55. doi: 10.7326/0003-4819-131-4-199908170-00003. [DOI] [PubMed] [Google Scholar]
- 21.Brune K, Abe T, Canto M, et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol. 2006;30:1067–76. [PMC free article] [PubMed] [Google Scholar]
- 22.Takaori K, Matsusue S, Fujikawa T, et al. Carcinoma in situ of the pancreas associated with localized fibrosis: a clue to early detection of neoplastic lesions arising from pancreatic ducts. Pancreas. 1998;17:102–5. doi: 10.1097/00006676-199807000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Hruban RH, Schulick RD. Is surgery required for patients with intraductal papillary mucinous neoplasms without mural nodules? Nat Clin Pract Gastroenterol Hepatol. 2008 doi: 10.1038/ncpgasthep1261. [DOI] [PubMed] [Google Scholar]
- 24.Hruban RH, Canto MI, Griffin C, et al. Treatment of familial pancreatic cancer and its precursors. Curr Treat Options Gastroenterol. 2005;8:365–75. doi: 10.1007/s11938-005-0039-3. [DOI] [PubMed] [Google Scholar]
- 25.Hruban RH, Pitman MB, Klimstra DS. The tumor of the Pancreas. The American Registry of Pathology; Washington, DC: 2007. [Google Scholar]
- 26.Klein AP, Beaty TH, Bailey-Wilson JE, Brune KA, Hruban RH, Petersen GM. Evidence for a major gene influencing risk of pancreatic cancer. Genetic epidemiology. 2002;23:133–49. doi: 10.1002/gepi.1102. [DOI] [PubMed] [Google Scholar]
- 27.Hruban RHPM, Klimstra DS, editors. Tumors of the Pancreas. American Registry of Pathology and Armed Forces Institute of Pathology; Washington, DC: 2007. [Google Scholar]
- 28.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–87. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 29.Detlefsen S, Sipos B, Feyerabend B, Kloppel G. Pancreatic fibrosis associated with age and ductal papillary hyperplasia. Virchows Arch. 2005;447:800–5. doi: 10.1007/s00428-005-0032-1. [DOI] [PubMed] [Google Scholar]
- 30.Abe T, Fukushima N, Brune K, et al. Genome-wide allelotypes of familial pancreatic adenocarcinomas and familial and sporadic intraductal papillary mucinous neoplasms. Clin Cancer Res. 2007;13:6019–25. doi: 10.1158/1078-0432.CCR-07-0471. [DOI] [PubMed] [Google Scholar]
- 31.Chari ST, Yadav D, Smyrk TC, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–7. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- 32.Tajima Y, Kuroki T, Tsuneoka N, et al. Multifocal branch-duct pancreatic intraductal papillary mucinous neoplasms. American journal of surgery. 2008;196:e50–2. doi: 10.1016/j.amjsurg.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Aimoto T, Uchida E, Nakamura Y, et al. Multicentric pancreatic intraepithelial neoplasias (PanINs) presenting with the clinical features of chronic pancreatitis. J Hepatobiliary Pancreat Surg. 2008;15:549–53. doi: 10.1007/s00534-007-1269-7. [DOI] [PubMed] [Google Scholar]
- 34.Goggins M, Offerhaus GJ, Hilgers W, et al. Pancreatic adenocarcinomas with DNA replication errors (RER+) are associated with wild-type K-ras and characteristic histopathology. Poor differentiation, a syncytial growth pattern, and pushing borders suggest RER+ Am J Pathol. 1998;152:1501–7. [PMC free article] [PubMed] [Google Scholar]
- 35.Wilentz RE, Goggins M, Redston M, et al. Genetic, immunohistochemical, and clinical features of medullary carcinoma of the pancreas: A newly described and characterized entity. Am J Pathol. 2000;156:1641–51. doi: 10.1016/S0002-9440(10)65035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banville N, Geraghty R, Fox E, et al. Medullary carcinoma of the pancreas in a man with hereditary nonpolyposis colorectal cancer due to a mutation of the MSH2 mismatch repair gene. Hum Pathol. 2006;37:1498–502. doi: 10.1016/j.humpath.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 37.Su GH, Hruban RH, Bansal RK, et al. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am J Pathol. 1999;154:1835–40. doi: 10.1016/S0002-9440(10)65440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato N, Rosty C, Jansen M, et al. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 2001;159:2017–22. doi: 10.1016/S0002-9440(10)63053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furukawa T. Molecular genetics of intraductal papillary-mucinous neoplasms of the pancreas. J Hepatobiliary Pancreat Surg. 2007;14:233–7. doi: 10.1007/s00534-006-1167-4. [DOI] [PubMed] [Google Scholar]
- 40.Koorstra JB, Maitra A, Morsink FH, et al. Undifferentiated Carcinoma With Osteoclastic Giant Cells (UCOCGC) of the Pancreas Associated With the Familial Atypical Multiple Mole Melanoma Syndrome (FAMMM) Am J Surg Pathol. 2008 doi: 10.1097/PAS.0b013e31818371cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whelan AJ, Bartsch D, Goodfellow PJ. Brief report: a familial syndrome of pancreatic cancer and melanoma with a mutation in the CDKN2 tumor-suppressor gene. N Engl J Med. 1995;333:975–7. doi: 10.1056/NEJM199510123331505. [DOI] [PubMed] [Google Scholar]