Abstract
Postpartum depression (PPD) is a debilitating illness, yet little is known about its causes. The purpose of this study was to examine a major symptom of depression during the postpartum period, anhedonia, by comparing sucrose preference in female rats that had undergone actual pregnancy or hormone-simulated pregnancy (HSP) to their respective controls. Whereas HSP rats showed significantly less preference than vehicle-control rats for 1% sucrose solution during the first three weeks of the “postpartum” period, previously pregnant females showed only slightly depressed sucrose preference for the first 1–2 days postpartum, compared to non-pregnant controls. Habituation to 1% sucrose during the pregnancy period, which increased preference upon later testing in previously pregnant rats tested on postpartum day 2, did not significantly increase preference in HSP rats, suggesting that depressed preference in the latter group was not due to neophobia. Pre-treatment with desipramine did not prevent suppressed sucrose preference in HSP rats, and preference was even further suppressed following chronic sertraline treatment. These results suggest that estradiol withdrawal following HSP may cause anhedonia during the early “postpartum” period. In contrast, females that have undergone actual pregnancy are less likely to show this effect, suggesting that postpartum hormonal changes other than the dramatic decline in estradiol may buffer its negative mood effects.
Keywords: Estradiol, Postpartum depression, Pregnancy, Hormone-simulated pregnancy, Sucrose preference, Sertraline, Desipramine
1. Introduction
Characterized by symptoms mimicking major depression, postpartum depression (PPD) is a debilitating illness that affects 10–15% of new mothers [34]. Symptoms typically arise within the first 5–6 weeks following birth, and may persist for as long as a year [13]. The devastating consequences of PPD have been described in a number of studies. For example, children of depressed mothers tend to show abnormal cognitive, motor and social development [60], and are more likely to experience depression or anxiety later in life [46]. Untreated PPD also has been linked to negative infant feeding outcomes [17], as well as a number of child abuse and infanticide cases [12]. A better understanding of the etiology of PPD is important for developing better preventative care and treatment options.
Although the physiological mechanism underlying PPD onset is debated, rapid changes in reproductive hormones immediately following childbirth are believed to contribute [49]. Circulating estradiol and progesterone reach very high levels during pregnancy (increasing 50- and 10-fold, respectively, compared to maximum menstrual cycle levels), and decline to early follicular levels by postpartum day 3–7 [8]. Given such dramatic physiological changes, it is not surprising that several studies have suggested a link between ovarian hormone withdrawal and the onset of PPD. For example, Bloch and colleagues [8] demonstrated that pharmacological withdrawal from estradiol and progesterone induced depressive symptoms in women with a history of PPD. In addition, postpartum estradiol treatment reduced depressive symptoms in women with PPD [2,3,31,58]. Studies examining the use of progesterone for preventing PPD have been less successful [15]. For example, women who were administered a long-acting progesterone contraceptive after delivery showed increased risk for developing PPD compared to placebo-treated women [38]. Such studies support the hypothesis that estradiol but not progesterone withdrawal contributes significantly to the development of PPD. In general, human studies addressing hormonal mechanisms of PPD have failed to rule out psychosocial factors such as a history of depression, low social support, stressful life events, complicated pregnancy and low socioeconomic status, which also have been linked to the development of PPD [16,27,29]. Therefore, the degree to which estradiol withdrawal alone contributes to the onset of this mood disorder remains unclear.
Animal models that utilize hormone manipulations to simulate pregnancy are useful for investigating mood effects that may be associated with hormone withdrawal. The hormone-simulated pregnancy (HSP) regimen [9] has been used previously as a model for examining depression-like behavior in “postpartum” female rats [26,61]. In this model, increases in estradiol and progesterone that occur during normal pregnancy are simulated by administering daily hormone injections; after terminating hormone treatment, females are tested for depression-like behavior in the forced swim test. These studies have demonstrated that females undergoing estradiol withdrawal after HSP show increased immobility in the forced swim test compared to controls [26,61]. Additionally, gonadally intact, previously pregnant female rats tested during the early postpartum period showed greater immobility than rats tested during mid-pregnancy in the forced swim test [41]. Although these results suggest a link between postpartum ovarian hormone withdrawal and PPD, additional tests of depression-like behaviors would provide more convincing evidence for this relationship.
Anhedonia, or the decreased ability to experience pleasure, represents one of the core symptoms of depression (including PPD: [20]) that can be examined in rats. The sucrose preference test, which essentially measures a rat’s appetite for a highly palatable, rewarding substance, has been used extensively to assess anhedonia in chronic mild stress models of depression (e.g., [48,67]). In the current study, we administered a sucrose preference test to rats during the “postpartum” period following an HSP, to test the hypothesis that postpartum estradiol withdrawal decreases reward-seeking behavior (i.e., produces anhedonia).
The second aim of this study was to determine whether actual pregnant rats tested during the postpartum period also show an increase in depression-like behavior, as previously suggested in a forced swim test study [41]. Sucrose preference testing was again utilized for this comparison because preference results can be readily compared between actual pregnant and HSP rats: whereas the weight gain associated with actual pregnancy may itself increase immobility in the forced swim test, greater body weight is less likely to affect sucrose preference (although it may affect absolute consumption). Studies in women suggest that PPD occurs in only 10–15% of all pregnancies; thus we predicted that a majority of rats that had undergone actual pregnancy would not show suppressed sucrose preference during the postpartum period.
Evidence from a number of studies suggests that the correlation between postpartum estradiol withdrawal and depressive symptoms likely involves disruption of serotonergic and/or noradrenergic neurotransmission. Estradiol is known to downregulate the serotonin (5-HT) autoreceptor and upregulate tryptophan hydroxylase, the enzyme responsible for converting tryptophan into 5-HT [51,52,56]. Regulation of brain serotonergic activity is also the basis for successful treatment of PPD with selective serotonin reuptake inhibitors such as fluoxetine and sertraline [23,28,68]. Because estradiol may modulate 5-HT neurotransmission, we investigated the effects of sertraline (a selective serotonin reuptake inhibitor) and desipramine (a tricyclic antidepressant) on “postpartum” sucrose preference in HSP rats. Sertraline was chosen based on previous research demonstrating an interaction between estradiol and this SSRI (e.g., [57]), among others. Prevention of suppressed sucrose preference by two widely used antidepressant medications would provide further evidence that estradiol withdrawal after HSP leads to a depression-like state.
2. Materials and Methods
2.1. Subjects
Female Sprague-Dawley rats served as subjects (N=426, 70–100 days old, bred in-house; breeders obtained from Taconic Farms, Germantown, NY). Rats were housed in the Psychology Department vivarium (12:12 h light:dark cycle; lights on at 0600 h). Ovariectomized females were housed in pairs with a cage mate from the same treatment group (i.e., a vehicle-treated female was always housed with another vehicle-treated female). Gonadally intact rats were housed in pairs during the mating period (female/male pairs for rats assigned to the “pregnant” group, female/female pairs for rats assigned to the non-pregnant control group), then housed individually for the remainder of the experiment. Adult male Sprague-Dawley rats (N=32, 90–300 days old) were used only for mating. Access to rat chow and water was ad libitum except during surgery and sucrose preference testing. Rats were cared for according to the guidelines of the Institutional Animal Care and Use Committee at Washington State University, and the NIH “Guide for the Care and Use of Laboratory Animals” [32].
2.2. Surgical procedure and the hormone-simulated pregnancy (HSP) regimen
Ovariectomy and the HSP regimen were conducted according to the methods outlined by Stoffel and Craft [61], with one exception: rats in the current study were allowed to recover for 24 h, rather than 1 week, before beginning daily hormone injections (see Table 1).
Table 1.
Hormone-Simulated Pregnancy (HSP) and Timed Pregnancy Regimens
| Treatment | Procedure (Day 0) | Pregnancy Period | Postpartum Period | ||
|---|---|---|---|---|---|
| Days 1–16 | Days 17–22 | Day 0 | Day 1, 2, 4, 7, 14 or 21 | ||
| Vehicle | ovariectomy | 2 vehicle injections | 1 vehicle injection | injections stop | sucrose preference test |
| HSP | ovariectomy | estradiol + progester. injections* | estradiol injection only# | injections stop | sucrose preference test |
| Non-pregnant | female/female paired | (daily handling) | sucrose preference test | ||
| Pregnant | male/female paired | gestation (daily handling) | pups born | sucrose preference test | |
estradiol benzoate 2.5 μg/rat and progesterone 4 mg/rat
estradiol benzoate 50 μg/rat
2.3. Timed pregnancy regimen
Female rats were assigned to either a pregnancy (mated) or control (unmated) group. Daily vaginal smears were performed so that sexual receptivity could be determined (indicated by a large number of nucleated cells characteristic of the proestrous phase of the estrous cycle) [24]. Females determined to be in proestrus were paired with a male rat until sperm were observed in the vaginal smear. The first day that sperm were observed was designated as the first day of pregnancy. The non-pregnant control group consisted of unmated females (paired with another female) and a few females that were paired with a male but did not get pregnant within approximately 2 weeks. As much as possible, the number of days of pair-housing and subsequent single-housing was matched between the control and pregnant groups.
All females were picked up by the base of the tail daily between 1100 – 1300 h to simulate the handling that occurred when HSP females received daily injections (and to accustom females to handling). Once nesting behavior was observed, pregnant females were checked three times daily so that the date and time of pup birth could be accurately determined. The discovery of pups marked the beginning of the postpartum period, or postpartum day 0 (see Table 1). Pups remained with the female until just prior to sucrose preference testing.
2.4. Sucrose preference test
Sucrose preference testing was conducted at 1800 h on postpartum day 1, 2, 4, 7, 14 or 21. For pregnant females, the postpartum time point in hours or days after parturition was defined as: postpartum day 1 = 20–28 h; postpartum day 2 = 40–56 h; postpartum day 4 = 3.5–4.5 days; postpartum day 7 = 6.5–7.5 days; postpartum day 14 = 13.5–14.5 days; postpartum day 21 = 20.5–21.5 days. Rats were weighed and placed into separate cages. Two pre-weighed bottles, one containing 0% (tap water) and the other containing either 0.5%, 1% or 2% sucrose solution, were placed on each cage. Left-right placement of water vs. sucrose bottles was counterbalanced among rats in each group. After 1 h, bottles were removed and re-weighed to determine consumption in g. Separate rats were tested at each time point or sucrose concentration in a given experiment.
2.5. Habituation procedure
Some rats were habituated to sucrose before the postpartum test. Rats undergoing habituation were pre-exposed to 1% sucrose solution on day 17 of the HSP or timed pregnancy regimen. The regular water bottle was removed from each rat’s home cage, and a pre-weighed bottle containing 1% sucrose solution was placed on each cage for 24 h. The bottle was then removed and re-weighed so that consumption (in g) during the habituation period could be determined. Seven or eight days later (on day 2 of the postpartum period), preference for 1% sucrose solution was measured in both habituated and non-habituated rats.
2.6. Antidepressant treatment regimen
The effects of two widely used antidepressants were examined in HSP rats only. Sertraline (10 mg/kg i.p.), desipramine (10 mg/kg i.p.) or vehicle (2.5% Tween80 or saline) were administered chronically (one injection/day for 16 days) or sub-chronically (3 injections over 1 day) before sucrose preference testing. Chronic and sub-chronic treatment with antidepressants has been used previously to demonstrate antidepressant effects in the forced swim test, with chronic treatment typically being more effective than sub-chronic treatment (e.g., [19]). We are not aware of studies in which sub-chronic antidepressant treatment has been examined in the sucrose preference test, but chronic antidepressant treatment of at least two weeks has been shown to prevent or reverse suppressed sucrose preference in rodents exposed to chronic mild stress [42,43,67]. Thus, in the present study rats in the chronic treatment groups received a daily injection of drug or vehicle at 1700 h on “pregnancy” day 9 through “postpartum” day 2 (16 days). Preference for 1% sucrose solution was measured at 1800 h on “postpartum” day 2, so the last antidepressant or vehicle injection was 1 h before testing.
To ensure that all rats received the same amount of handling, rats in the sub-chronic treatment groups received a vehicle injection on “pregnancy” days 9–22. On “postpartum” day 1, rats were injected with sertraline, desipramine or vehicle at 1800 h. The same treatment was administered again on “postpartum” day 2, at 1300 and 1700 h. Preference for 1% sucrose solution was measured at 1800 h on “postpartum” day 2. Thus, rats in the sub-chronic treatment groups received drug injections at 24, 5 and 1 h before sucrose preference testing.
2.7. Hormones and drugs
Estradiol benzoate and progesterone (Steraloids Inc., Newport, RI) were dissolved in safflower oil. Both concentrations of estradiol benzoate were administered in a volume of 0.1 ml/rat. Progesterone was administered in a volume of 0.3 ml/rat. Vehicle control rats received safflower oil in the same volumes. All hormone and oil injections were administered s.c.
Sertraline hydrochloride (generously donated by Pfizer Inc., Groton, CT) was dissolved in 2.5% Tween80. Desipramine hydrochloride (Sigma-Aldrich Inc., St. Louis, MO) was dissolved in physiological saline. Rats in the vehicle control group received vehicle injections of either 2.5% Tween80 or physiological saline. Drug and vehicle injections were i.p., in a volume of 1.0 ml/kg.
2.8. Uterine weights
Immediately after testing in the 1% sucrose time course experiment, rats were euthanized. The uterus was harvested from a subset of rats from each treatment group and time point, to provide an index of circulating ovarian hormone levels [39]. Each uterus was stored in 10% formalin for a minimum of two weeks before it was trimmed, blotted dry and weighed.
2.9. Data analyses
Data from rats in which the water and/or sucrose bottles leaked during testing (i.e., large wet spot in bedding observed directly under one or both bottles immediately after testing) were not included in analyses (N=12 rats). Sucrose and water consumption in g were converted into % sucrose preference using the following formula: [sucrose consumption (g)/total fluid consumption (g)] × 100. In Experiments 1–4, sucrose preference data were analyzed by two-way, between-subjects ANOVA with the following variables: Time course experiment, treatment group (2 levels) and time (4–6 levels); Sucrose concentration experiment, treatment group (2 levels) and sucrose concentration (3 levels); Habituation experiment, treatment group (2 levels) and habituation condition (2 levels); Antidepressant experiment, drug regimen (2 levels: sub-chronic vs. chronic) and drug (3 levels). Uterine weights (g/kg body weight) were analyzed separately for HSP vs. vehicle control and previously pregnant vs. non-pregnant rats by two-way, between-subjects ANOVA: treatment group (2 levels) and time (4–6 levels). In the habituation experiment, body weight-adjusted sucrose intake (sucrose intake (g)/body weight (kg)) was also calculated for the 24-h sucrose habituation period. Body weight-adjusted intake data were analyzed by one-way, between-subjects ANOVA: treatment group (4). Independent sample t-tests with a Bonferroni correction were performed in the event of a main effect of treatment group or interaction, in order to identify the time point, sucrose concentration, habituation condition, drug regimen or drug at which the significant effect occurred. Significance level was set at p ≤ 0.05.
3. Results
3.1. Time course of 1% sucrose preference during the postpartum period
Figure 1 shows preference for a 1% sucrose solution in HSP vs. vehicle control rats, and previously pregnant vs. non-pregnant control rats during the postpartum period. Over the 3-week postpartum period, HSP rats showed significantly less preference than vehicle control rats for 1% sucrose solution (top left panel; F(1,104)=7.26, p=0.008). Although there was no significant interaction between treatment group and postpartum day (F(5,104)=0.17, n.s.), the group difference appears to be waning by day 21.
Figure 1.
Top panels: Percent preference (mean ± 1 S.E.M) for a 1% sucrose solution during the postpartum period, in HSP vs. vehicle control rats (left; N=5–11 rats/treatment group/time point), and in previously pregnant vs. non-pregnant control rats (right; N=6–14 rats/group/time point). The dotted line at 50% preference denotes equal consumption of sucrose and water (i.e. no preference for sucrose); data points above this line reflect a preference for sucrose solution over water. Bottom panels: Body-weight adjusted uterine weight in HSP vs. vehicle control rats (left; N=4–10 rats/group/time point), and in previously pregnant vs. non-pregnant control rats (right; N=6–7 rats/group/time point). *Significantly greater than control group, p≤0.05.
In gonadally intact rats (Fig. 1, top right panel), sucrose preference was somewhat suppressed in previously pregnant compared to non-pregnant controls on postpartum days 1–2; however, the treatment group by day interaction was not significant (F(3,78)=0.26, n.s.).
Figure 1 (lower panels) also shows uterine weights for rats in all groups at the same postpartum time points. HSP rats had larger uteruses than vehicle control rats over the entire 3-week postpartum period, with uterine weights in HSP rats gradually declining from postpartum day 2 to 21 (bottom left panel; group × day: F(5,57)=11.86, p<0.001). In contrast, uterine weights in previously pregnant rats were very high on postpartum day 1 but declined rapidly so that they were no different from those in non-pregnant controls by postpartum day 2 (bottom right panel; group × day: F(3,43)=16.49, p<0.001).
3.2. [Sucrose]-Effect Function
Figure 2 shows results from the next experiment, conducted to determine whether group differences in sucrose preference varied by concentration of the sucrose solution. Sucrose preference testing was conducted on postpartum day 2, with sucrose concentrations of 0.5%, 1% or 2%. Figure 2 (left panel) shows that in both HSP and vehicle control groups, sucrose preference increased as the concentration of sucrose increased (F(2,55)=7.97, p=0.001), and overall, HSP rats showed significantly less preference than vehicle control rats for these sucrose concentrations (F(1,55)=6.21, p=0.02), with no interaction between group and sucrose concentration (F(2,55)=0.33, n.s.).
Figure 2.

Percent preference (mean ± 1 S.E.M.) for 0.5%, 1% or 2% sucrose solutions on postpartum day 2, in HSP vs. vehicle control rats (left panel; N=8–12 rats/treatment group/[sucrose]), and in previously pregnant vs. non-pregnant control rats (right panel; N=7–10 rats/treatment group/[sucrose]). Other details as in Fig. 1.
Figure 2 (right panel) shows that in previously pregnant and non-pregnant control rats, sucrose preference also increased as the concentration of sucrose increased (F(3,75)=3.36, p=0.02). However, there was no significant group difference in preference at any of the sucrose concentrations tested.
3.3. Effect of pre-test habituation to sucrose solution on sucrose preference
Figure 3 shows results of the next experiment, conducted to determine whether suppressed sucrose preference (demonstrated by HSP rats in Experiments 1 and 2) could reflect heightened neophobia (fear of an unfamiliar substance) rather than anhedonia. Preference for a 1% sucrose solution was measured on postpartum day 2 in rats pre-exposed to 1% sucrose approximately 1 week prior to testing (Habituation), compared to rats given no prior exposure to sucrose solution (No Habituation, similar to previous experiments). Overall, HSP rats showed significantly less preference than vehicle control rats for 1% sucrose solution (Fig. 3, left panel; F(1,46)=13.23, p=0.001), and preference was not significantly affected by habituation to the sucrose solution (F(1,46)= 0.07, n.s.). However, post-hoc analysis revealed that sucrose preference was significantly reduced only in HSP rats in the non-habituated condition.
Figure 3.
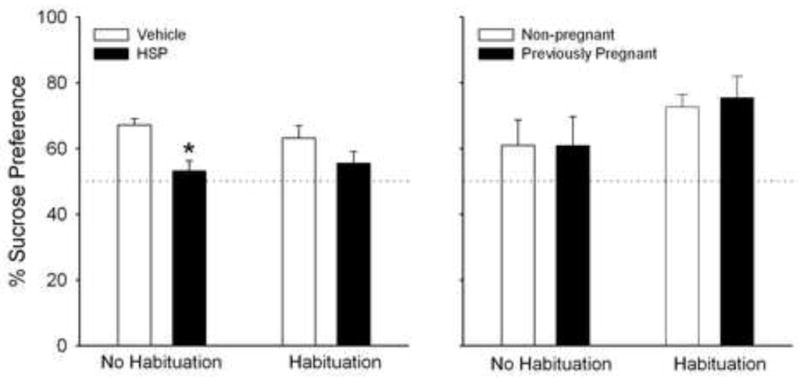
Percent preference (mean + 1 S.E.M) for a 1% sucrose solution on postpartum day 2 in rats previously exposed to 1% sucrose (Habituation) vs. those not pre-exposed to sucrose (No Habituation). Left panel: HSP vs. vehicle control rats; N=11–12 rats/treatment group/habituation condition. Right panel: Previously pregnant vs. non-pregnant control rats; N=6–9 rats/treatment group/habituation condition. *Significantly different than non-habituated, vehicle control rats, P< 0.05. Other details as in Fig. 1.
In contrast, previously pregnant and non-pregnant control rats that were exposed to the sucrose solution 1 week before testing showed significantly greater preference than non-habituated rats for 1% sucrose (Fig. 3, right panel; F(1,27)=4.56, p=0.04). Similar to previous experiments, however, previously pregnant and non-pregnant control rats did not differ in sucrose preference under either habituation condition (F(1,27)=0.05, n.s.). During the 24-h sucrose pre-exposure period, body weight-adjusted 1% sucrose consumption did not differ significantly between habituated rats in the HSP, previously pregnant or control groups (F(3,22)=2.10, n.s.), with a mean consumption of 246 g sucrose solution/kg body weight during the 24-h pre-exposure period.
3.4. Effect of sub-chronic vs. chronic antidepressant treatment on sucrose preference (HSP rats only)
Figure 4 shows results of the final experiment, conducted to determine whether pre-treatment with antidepressant medications would prevent suppressed sucrose preference in HSP rats. Preference for 1% sucrose on postpartum day 2 was examined in rats that were pre-treated either sub-chronically (3 injections over 1 day before testing) or chronically (daily for 16 days before testing) with vehicle or the antidepressants sertraline (10 mg/kg) or desipramine (10 mg/kg). In rats treated with the sub-chronic antidepressant regimen, there was a main effect of drug (F(2,23)=5.31, p=0.014) – however, the drug effect was opposite to the expected outcome: rats that received sertraline showed significantly less preference for 1% sucrose than rats that received vehicle. In rats treated with antidepressants under the chronic regimen, a similar decrease in sucrose preference was observed with desipramine, although overall there was no significant effect of drug condition (F(2,14)=1.09, n.s.).
Figure 4.
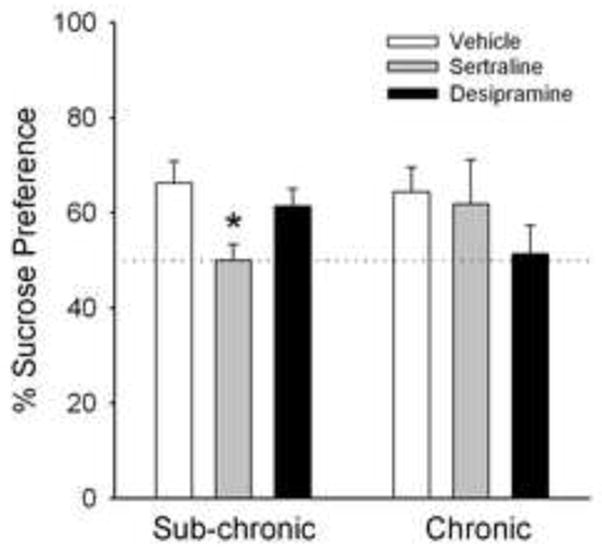
Effect of sub-chronic or chronic pretreatment with antidepressant medications on preference for a 1% sucrose solution (mean + 1 S.E.M) in HSP rats on postpartum day 2. Rats were injected sub-chronically (3 injections over 1 day pre-test) or chronically (once-daily for 16 days) with vehicle, sertraline (10 mg/kg) or desipramine (10 mg/kg); N=4–8 rats/drug/regimen. *Significantly different than sub-chronic, vehicle control rats, P< 0.05. Other details as in Fig. 1.
4. Discussion
4.1. Estradiol withdrawal-induced anhedonia
In the time course experiment, HSP rats showed significantly less preference for 1% sucrose than vehicle control rats over the 3-week postpartum period. Given that the “postpartum” period of the HSP regimen mainly simulates estradiol withdrawal (only estradiol is administered for the last 6 days of “pregnancy”), this result suggests that withdrawal from pregnancy levels of estradiol leads to the development of anhedonia in the “postpartum” period. Uterine weights gradually decreased in HSP rats over the same 3-week postpartum period during which suppressed sucrose preference was observed. Previous studies have shown a strong positive correlation between uterine weight and circulating estradiol levels in female rats (e.g., [64]) — therefore it appears that sucrose preference was suppressed in HSP rats during the period of declining plasma estradiol. The present results demonstrating that estradiol withdrawal suppresses sucrose consumption agree with previous findings of increased immobility in the forced swim test after “parturition” in HSP rats [26,61], and after ovariectomy in non-pregnant rodents [6,47], although immobility appeared to be declining by one week postpartum in the Stoffel and Craft study [61]. The present results also agree with those of a recent study in which sucrose preference in HSP rats decreased significantly from the “pregnancy” period to “postpartum” days 2–3 [30].
A correlation between postpartum estradiol levels and negative mood effects associated with PPD has been reported in several studies in women [1,8,37]. In addition, treatment with estradiol has been shown to reverse the negative mood symptoms experienced by women with PPD [3,31,58], and to prevent depression-like behavior in HSP rats tested during the “postpartum” period [26,30], as well as in ovariectomized rodents [47]. Thus, the present results using the sucrose preference test provide additional evidence that estradiol withdrawal during the postpartum period contributes to negative mood changes such as those observed in PPD.
The mechanism for this relationship likely involves estradiol regulation of central serotonergic neurotransmission. Disturbance in serotonergic activity has been linked to depression, and is the basis for successful treatment of PPD with selective serotonin reuptake inhibitors (SSRI’s) such as fluoxetine and sertraline [23,28,68]. Studies examining the interaction between estradiol and serotonergic neurotransmission reveal that estradiol downregulates the serotonin (5HT) autoreceptor and upregulates tryptophan hydroxylase [51,52,56]; each of these effects would tend to enhance serotonergic activity. Therefore, it likely that the period of estradiol withdrawal following parturition (or HSP) is accompanied by decreased serotonergic activity. This may increase vulnerability to PPD symptoms such as anhedonia.
In accordance with this hypothesis, a number of studies in rodents have reported that chronic administration (i.e., 2–4 weeks) of a variety of antidepressants prevents anhedonia precipitated by exposure to chronic mild stress (for review see [66]). This prompted our prediction that chronic, and perhaps sub-chronic (3 injections over 24 h) treatment with the antidepressants sertraline (a selective serotonin reuptake inhibitor) or desipramine (a tricyclic antidepressant) would prevent suppressed sucrose preference precipitated by estradiol withdrawal. Although the sub-chronic antidepressant treatment regimen has not been examined in the sucrose preference model of anhedonia, it has been shown – at the doses we used – to decrease immobility in the forced swim test [18,54], a paradigm which tends to parallel chronic mild stress in terms of antidepressant effects [7]. Contrary to our prediction, the present results show that neither sub-chronic nor chronic pre-treatment with antidepressants prevented suppressed sucrose preference on postpartum day 2. In fact, preference for 1% sucrose solution was essentially abolished in rats treated sub-chronically with sertraline or chronically with desipramine. Such results suggest that variables specific to the HSP regimen (i.e., ovariectomy or hormone withdrawal) decrease sensitivity to antidepressant treatment. A number of molecular and behavioral studies in women and ovariectomized rodents suggest that estradiol modulates the efficacy of antidepressants that target serotonin and norepinephrine function. Specifically, antidepressants are less effective in women and rodents with low or declining estradiol levels [22,53,57,60,62]. Given that rats in the present study experienced withdrawal from pregnancy levels of estradiol—a more dramatic hormone withdrawal than after ovariectomy — it is perhaps not surprising that neither sertraline nor desipramine treatment prevented decreases in sucrose preference. Although the abolishment of sucrose preference following sub-chronic sertraline treatment was unexpected, it is worth noting that our results agree with those of a recent study in which chronic treatment with imipramine not only failed to prevent suppressed sucrose preference in HSP rats tested during the “postpartum” period, but actually further suppressed preference [30].
The lack of efficacy of antidepressant treatment in HSP rats was surprising given that a majority of studies in women report a modest reversal or delayed recurrence of PPD symptoms following chronic antidepressant treatment [14,50,69,70]. The discrepant findings between “postpartum” rodents and postpartum women might result from suboptimal timing of treatment in the rodent models. In the present study, antidepressant treatment occurred primarily during the high-estradiol phase of the HSP (hormone injection day 9 to “postpartum” day 2) and its effects on sucrose preference evaluated during estradiol withdrawal (“postpartum” day 2). Some studies in women show that the metabolism of antidepressants increases during pregnancy and that plasma levels are significantly reduced in some women during the third trimester of pregnancy [25,35], the period during which plasma estradiol reaches its highest levels. At least two studies have shown that higher doses of antidepressants are required to treat depressive symptoms in women during late pregnancy [36,59], although another study demonstrates that maternal paroxetine concentrations (and antidepressant effect) depend entirely on the cytochrome P450 (specifically, CYP2D6) genotype of the individual woman – with plasma levels of paroxetine decreasing in some women but increasing in others during pregnancy [63]. Although it is unclear whether rising plasma estradiol alone increases antidepressant drug metabolism, there is some evidence from molecular studies to support this speculation [65]. At present the literature is quite mixed regarding the potential interactions of estrogens and antidepressants via cytochrome P450 modulation; conclusions appear to be highly dependent on the specific estrogen and antidepressant drug (i.e., the specific P450 isozyme affected by each and needed to metabolize each), the chronicity of estrogen and antidepressant treatment, the species, and how the cytochrome P450 activity is measured (e.g., in vitro or in vivo) [4,11,33,55]. Whatever the mechanism underlying hormone-antidepressant interaction, studies in both women and rodents indicate that antidepressants are less effective during periods of declining estradiol. This hypothesis can be tested in future PPD studies by combining the antidepressants with a low dose of estradiol, or treating with higher doses of antidepressants.
4.2. Neophobia does not account for suppressed sucrose preference in HSP rats
We predicted that neophobia might also influence sucrose preference, perhaps differentially in HSP vs. vehicle control rats. That is, if HSP rats were more neophobic to sucrose solution than vehicle control rats, one might expect the HSP rats to show less sucrose preference than vehicle control rats. The habituation experiment showed that previous exposure to sucrose (habituation) failed to significantly increase sucrose preference in HSP and vehicle control rats (Fig. 3, left panel). This result suggests that either neophobia was not limiting rats’ consumption of sucrose, or that the habituation procedure we used was inadequate for familiarizing rats to the sucrose solution. In regard to the latter possibility, non-pregnant and previously pregnant rats did increase sucrose consumption after undergoing the same pre-exposure procedure, suggesting that it was adequate for habituating rats to the sucrose solution. In addition, sucrose intake on the habituation day (“pregnancy” day 17) did not differ significantly among the four treatment groups (HSP, vehicle control, pregnant and non-pregnant control) suggesting that pre-exposure to sucrose was same in all groups. Thus, although habituation slightly decreased the size of the group difference between HSP and vehicle control rats, the fact that there was no significant difference in preference between habituated and non-habituated rats in each group suggests that the group difference in sucrose preference is not due to greater neophobia to sucrose in the HSP group.
4.3. Hormone-simulated pregnancy vs. actual pregnancy
Another important question addressed in this study was how closely HSP modeled actual pregnancy in terms of the employed measure of depression-like behavior. Sucrose preference results from HSP and previously pregnant rats across three experiments indicates that the two groups of rats are not comparable. First, whereas HSP rats showed suppressed preference for a 1% sucrose solution for approximately 2 weeks postpartum, previously pregnant rats showed only a trend towards suppressed sucrose preference on postpartum days 1–2. Moreover, previously pregnant rats showed no suppression of sucrose preference at any of three sucrose concentrations tested. Finally, pre-exposure to the sucrose solution significantly increased postpartum sucrose preference in previously pregnant rats but not in HSP rats. Thus, neophobia appears to have limited sucrose consumption in previously pregnant but not HSP rats.
The lack of comparable sucrose preference profiles between HSP and previously pregnant rats may result from differences in the rate of decline of estradiol during the postpartum period. Uterine weights differed substantially between HSP and vehicle control rats for two weeks into the postpartum period, but differed only on postpartum day 1 between previously pregnant and non-pregnant rats. The period during which rats in both groups experienced the most dramatic decline in uterine weight (the period of declining estradiol) correlated with suppressed sucrose preference. This result suggests that estradiol withdrawal may also suppress sucrose preference, albeit to a much lesser extent, in previously pregnant rats.
In addition to the more rapid decline in estradiol that occurs in actual pregnant rats during the early postpartum period, the lack of a significant difference in sucrose preference between previously pregnant vs. non-pregnant rats may be due to the “buffering” effects of other hormones that were not investigated in the present study. Of particular interest are prolactin and oxytocin, which rise dramatically during pregnancy and the early postpartum period but are not modeled per se in the HSP regimen. Recent studies suggest that breastfeeding – an event accompanied by marked increases in prolactin and oxytocin – may decrease the risk of developing PPD [10,40]. Prolactin and oxytocin also have been shown to possess anxiolytic/anti-stress and antidepressant effects in rats [5,21,44,45] Thus, increases in these hormones during late pregnancy/early postpartum may buffer the negative mood symptoms caused by estradiol withdrawal. Future studies aimed at determining the effects of additional hormones such as prolactin and oxytocin on depression-like behaviors in HSP rats will be necessary to test this possibility.
4.4. Conclusions
The present study provides valuable insight into the potential role of estradiol withdrawal in the development of PPD. Estradiol withdrawal after HSP suppresses sucrose preference, suggesting the development of anhedonia, one of the core symptoms of PPD. Taken together with previous studies in HSP rats demonstrating increased immobility in the forced swim test [26,61], and suppressed sucrose preference [30] when rats were tested during the “postpartum” period, the present results suggest that estradiol withdrawal occurring in the early postpartum period contributes to the development of PPD.
Acknowledgments
The authors thank Jessica Rogers, Jaime Ellis, Latisha Sternod and Kimberly Tsutsui for excellent technical assistance. This research was funded by a grant from the National Institute of Mental Health (MH070381) to R.M.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abou-Saleh M, Ghubash R, Karim L, Krymski M, Bhai I. Hormonal aspects of postpartum depression. Psychoneuroendocrinology. 1998;23:465–75. doi: 10.1016/s0306-4530(98)00022-5. [DOI] [PubMed] [Google Scholar]
- 2.Ahokas A, Kaukoranta J, Aito M. Effect of oestadiol on postpartum depression. Psychopharmacology. 1999;146:108–10. doi: 10.1007/s002130051095. [DOI] [PubMed] [Google Scholar]
- 3.Ahokas A, Kaukoranta J, Wahlbeck K, Aito M. Estrogen deficiency in severe postpartum depression: successful treatment with sublingual physiologic 17beta-estradiol: a preliminary study. J Clin Psychiatry. 2001;62:332–6. doi: 10.4088/jcp.v62n0504. [DOI] [PubMed] [Google Scholar]
- 4.Anakk S, Ku CY, Vore M, Strobel HW. Insights into gender bias: Rat cytochrome P450 3A9. J Pharmacol Exp Ther. 2003;305:703–9. doi: 10.1124/jpet.102.048090. [DOI] [PubMed] [Google Scholar]
- 5.Arletti R, Bertolini A. Oxytocin acts as an antidepressant in two animal models of depression. Life Sci. 1987;41:1725–30. doi: 10.1016/0024-3205(87)90600-x. [DOI] [PubMed] [Google Scholar]
- 6.Bekku N, Yoshimura H, Araki H. Factors producing a menopausal depressive-like state in mice following ovariectomy. Psychopharmacology. 2006;187:170–80. doi: 10.1007/s00213-006-0395-2. [DOI] [PubMed] [Google Scholar]
- 7.Bessa JM, Mesquita AR, Oliveira M, Pego JM, Cerqueira JJ, Palha JA, Almeida OFX, Sousa N. A trans-dimensional approach to the behavioral aspects of depression. Front Beh Neurosci. 2009;3:1–7. doi: 10.3389/neuro.08.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloch M, Daly R, Rubinow D. Endocrine factors in the etiology of postpartum depression. Comp Psychiatry. 2003;44:234–46. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- 9.Bridges RS. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology. 1984;114:930–40. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- 10.Carter CS, Altemus M, Chrousos GP. Neuroendocrine and emotional changes in the post-partum period. Prog Brain Res. 2001;133:241–9. doi: 10.1016/s0079-6123(01)33018-2. [DOI] [PubMed] [Google Scholar]
- 11.Chang S-Y, Chen C, Yang Z, Rodrigues AD. Further assessment of 17α-ethinyl estradiol as an inhibitor of different human cytochrome P450 forms in vitro. Drug Metab Disp. 2009;37:1667–75. doi: 10.1124/dmd.109.026997. [DOI] [PubMed] [Google Scholar]
- 12.Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med. 1998;129:229–40. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- 13.Cox JL, Murray D, Chapman G. A controlled study of the onset, duration and prevalence of postnatal depression. Br J Psychiatry. 1993;163:27–31. doi: 10.1192/bjp.163.1.27. [DOI] [PubMed] [Google Scholar]
- 14.Cohen LS, Viguera AC, Bouffard SM, Nonac RM, Morabito C, Collins MH, Ablon JS. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62:592–6. doi: 10.4088/jcp.v62n0803. [DOI] [PubMed] [Google Scholar]
- 15.Dennis CLE. Preventing postpartum depression part 1: A review of biological interventions. Can J Psychiatry. 2004;49:467–74. doi: 10.1177/070674370404900708. [DOI] [PubMed] [Google Scholar]
- 16.Dennis CLE, Janssen PA, Singer J. Identifying women at-risk for postpartum depression in the immediate postpartum period. Acta Psychiatr Scand. 2004;110:338–46. doi: 10.1111/j.1600-0447.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- 17.Dennis CL, McQueen K. The relationship between infant-feeding outcomes and postpartum depression: A qualitative systematic review. Pediatrics. 2009;123:e736–51. doi: 10.1542/peds.2008-1629. [DOI] [PubMed] [Google Scholar]
- 18.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test diferentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 19.Detke MJ, Johnson J, Lucki I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol. 1997;5:107–12. doi: 10.1037//1064-1297.5.2.107. [DOI] [PubMed] [Google Scholar]
- 20.Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, D.C: American Psychiatric Association; 1994. p. 327.p. 386. (DSM-IV) [Google Scholar]
- 21.Donner N, Bredewold R, Maloumby R, Neumann ID. Chronic intracerebral prolactin attenuates neuronal stress circuitries in virgin rats. Eur J Neurosci. 2007;25:1804–14. doi: 10.1111/j.1460-9568.2007.05416.x. [DOI] [PubMed] [Google Scholar]
- 22.Estrada-Camarena E, Fernandez-Guasti A, Lopez-Rubalcava C. Interaction between estrogens and antidepressants in the forced swimming test in rats. Psychopharmacology. 2004;173:139–45. doi: 10.1007/s00213-003-1707-4. [DOI] [PubMed] [Google Scholar]
- 23.Flores D, Hendrick V. Etiology and treatment of postpartum depression. Curr Psychiatry Rep. 2002;4:461–6. doi: 10.1007/s11920-002-0074-x. [DOI] [PubMed] [Google Scholar]
- 24.Freeman ME. The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E, Neill J, editors. The Physiology of Reproduction. New York: Raven Press; 1988. pp. 1893–1928. [Google Scholar]
- 25.Freeman MP, Nolan PE, Jr, Davis MR, Anthony M, Fried K, Fankhauser M, Woosley RL, Moreno F. Pharmacokinetics of sertraline across pregnancy and postpartum. J Clin Psychopharmacology. 2008;28:646–53. doi: 10.1097/JCP.0b013e31818d2048. [DOI] [PubMed] [Google Scholar]
- 26.Galea LA, Wide JD, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- 27.Gentile S. The role of estrogen therapy in postpartum psychiatric disorders: an update. CNS Spectrums. 2005;10:944–52. doi: 10.1017/s1092852900010518. [DOI] [PubMed] [Google Scholar]
- 28.Gjerdingen D. The effectiveness of various postpartum depression treatments and the impact of antidepressant drugs on nursing infants. J Am Board Fam Pract. 2003;16:372–82. doi: 10.3122/jabfm.16.5.372. [DOI] [PubMed] [Google Scholar]
- 29.Gotlib JH, Whiffen VE, Mount JH, Milne K, Cordy NI. Prevalence and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57:269–74. doi: 10.1037//0022-006x.57.2.269. [DOI] [PubMed] [Google Scholar]
- 30.Green AD, Barr AM, Galea LAM. Role of estradiol withdrawal in ‘anhedonic’ sucrose consumption: a model of postpartum depression. Physiol Behav. 2009;97:259–65. doi: 10.1016/j.physbeh.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 31.Gregoire A, Kumar R, Everitt B, Henderson A, Studd J. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347:930–3. doi: 10.1016/s0140-6736(96)91414-2. [DOI] [PubMed] [Google Scholar]
- 32.Guide for the care and use of laboratory animals. Washington (DC): National Academy Press; 1996. [Google Scholar]
- 33.Haduch A, Wojcikowski J, Daniel WA. The effect of tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs) and new antidepressant drug on the activity and level of rat CYP3A. Eur Neuropsychopharm. 2006;16:178–86. doi: 10.1016/j.euroneuro.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Halbreich U. Postpartum disorders: Multiple interacting underlying mechanisms and risk factors. J Affect Dis. 2005;88:1–7. doi: 10.1016/j.jad.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Heikkinen T, Ekblad U, Palo P, Laine K. Pharmacokinetics of fluoxetine and norfluoxetine in pregnancy and lactation. Clin Pharmacol Ther. 2003;73:330–7. doi: 10.1016/s0009-9236(02)17634-x. [DOI] [PubMed] [Google Scholar]
- 36.Hostetter A, Stowe ZN, Strader JR, Jr, McLaughlin E, Llewellyn A. Dose of selective serotonin uptake inhibitors across pregnancy: clinical implications. Depress Anxiety. 2000;11:51–7. [PubMed] [Google Scholar]
- 37.Josefsson A, Angelsioo L, Berg G, Ekstrom CM, Gunnervik C, Nordin C, Sydsjo G. Obstetric, somatic and demographic risk factors for postpartum depressive symptoms. Obstet Gyn. 2002;99:223–8. doi: 10.1016/s0029-7844(01)01722-7. [DOI] [PubMed] [Google Scholar]
- 38.Lawrie TA, Hofmeyr GJ, De Jager M, Berk M, Paiker J, Viljoen E. A double-blind randomized placebo controlled trial of postnatal norethisterone enanthate: the effect on postnatal depression and serum hormones. Br J Obstet Gynaecol. 1998;105:1089–90. doi: 10.1111/j.1471-0528.1998.tb09940.x. [DOI] [PubMed] [Google Scholar]
- 39.Medlock KL, Lyttle CR, Kelepouris N, Newman ED, Sheehan DM. Estradiol down-regulation of the rat uterine estrogen receptor. Proc Soc Exp Biol Med. 1991;196:293–300. doi: 10.3181/00379727-196-43191. [DOI] [PubMed] [Google Scholar]
- 40.Mezzacappa ES, Katkin ES. Breast-feeding is associated with reductions in perceived stress and negative mood in mothers. Health Psychol. 2002;21:187–93. [PubMed] [Google Scholar]
- 41.Molina-Hernandez M, Tellez-Alcantara NP. Antidepressant-like actions of pregnancy, and progesterone in Wistar rats forced to swim. Psychoneuroendocrinology. 2001;26:479–91. doi: 10.1016/s0306-4530(01)00007-5. [DOI] [PubMed] [Google Scholar]
- 42.Monleon S, D’Aquila P, Parra A, Simon VM, Brain PF, Willner P. Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology. 1995;117:453–57. doi: 10.1007/BF02246218. [DOI] [PubMed] [Google Scholar]
- 43.Muscat R, Papp M, Willner P. Reversal of stress-induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline. Psychopharmacology. 1992;109:433–8. doi: 10.1007/BF02247719. [DOI] [PubMed] [Google Scholar]
- 44.Neumann ID. Brain mechanisms underlying emotional alterations in the peripartum period in rats. Depress Anxiety. 2003;17:111–21. doi: 10.1002/da.10070. [DOI] [PubMed] [Google Scholar]
- 45.Neumann ID. Stimuli and consequences of dendritic release of oxytocin within the brain. Biochem Soc Trans. 2007;35:1252–7. doi: 10.1042/BST0351252. [DOI] [PubMed] [Google Scholar]
- 46.Nomura Y, Wickramaratne PJ, Warner V, Mufson L, Weissman MM. Family discord, parental depression, and psychopathology in offspring; ten-year follow-up. J Am Acad Child Adolesc Psychiatry. 2002;41:402–9. doi: 10.1097/00004583-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Okada M, Hayashi N, Kometani M, Nakao K, Inukai T. Influences of ovariectomy and continuous replacement of 17β-estradiol on the tail skin temperature and behavior in the forced swimming test in rats. Jpn J Pharmacol. 1997;73:93–6. doi: 10.1254/jjp.73.93. [DOI] [PubMed] [Google Scholar]
- 48.Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology. 1991;104:255–9. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- 49.Parry BL, Sorenson DL, Meliska CJ, Basavaraj N, Zirpoli GG, Gamst A, Hauger R. Hormonal basis of mood and postpartum disorders. Curr Women’s Health Rep. 2003;3:230–5. [PubMed] [Google Scholar]
- 50.Pearlstein T. Perinatal depression: treatment options and dilemmas. J Psychiatry Neurosci. 2008;33:302–18. [PMC free article] [PubMed] [Google Scholar]
- 51.Pecins-Thompson M, Bethea C. Ovarian steroid regulation of serotonin-1A autoreceptor messenger RNA expression in the dorsal raphe of rhesus macaques. Neuroscience. 1999;89:267–77. doi: 10.1016/s0306-4522(98)00326-1. [DOI] [PubMed] [Google Scholar]
- 52.Pecins-Thompson M, Brown N, Kohama S, Bethea C. Ovarian steroid regulation of tryptophan hydroxylase mRNA expression in rhesus macaques. J Neuroscience. 1996;16:7021–9. doi: 10.1523/JNEUROSCI.16-21-07021.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pinto-Meza A, Usall J, Serrano-Blanco A, Suarez D, Haro JM. Gender differences in response to antidepressant treatment prescribed in primary care. J Affect Disord. 2006;93:53–60. doi: 10.1016/j.jad.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 54.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 55.Preskorn SH, Greenblatt DJ, Flockhart D, Luo Y, Perloff ES, Harmatz JS, Baker B, Klick-Davis A, Desta Z, Burt T. Comparison of duloxetine, escitalopram, and sertraline effects on cytochrome P450 2D6 function in healthy volunteers. J Clin Psychopharm. 2007;27:28–34. doi: 10.1097/00004714-200702000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Rubinow D, Schmidt P, Roca C. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44:839–50. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- 57.Sell SL, Craft RM, Seitz PK, Stutz SJ, Cunningham KA, Thomas ML. Estradiol-sertraline synergy in ovariectomized rats. Psychoneuroendocrinology. 2008;33:1051–60. doi: 10.1016/j.psyneuen.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Sichel DA, Cohen LS, Robertson LM, Ruttenberg A, Rosenbaum JF. Prophylactic estrogen in recurrent postpartum affective disorder. Biol Psychiatry. 1995;38:814–18. doi: 10.1016/0006-3223(95)00063-1. [DOI] [PubMed] [Google Scholar]
- 59.Sit DK, Perel JM, Helsel JC, Wisner KL. Changes in antidepressant metabolism and dosing across pregnancy and early postpartum. J Clin Psychiatry. 2008;69:652–8. doi: 10.4088/jcp.v69n0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spinelli MG. Neuroendocrine effects on mood. Rev Endocr Metab Disord. 2005;6:109–15. doi: 10.1007/s11154-005-6723-8. [DOI] [PubMed] [Google Scholar]
- 61.Stoffel EC, Craft RM. Ovarian hormone withdrawal-induced “depression” in female rats. Physiol Behav. 2004;83:505–13. doi: 10.1016/j.physbeh.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 62.Thase ME, Entsuah R, Cantillon M, Kornstein SG. Relative antidepressant efficacy of venlafaxine and SSRIs: sex-age interactions. J Womens Health (Larchmt) 2005;14:609–16. doi: 10.1089/jwh.2005.14.609. [DOI] [PubMed] [Google Scholar]
- 63.Ververs FF, Voorbij HA, Zwarts P, Belitser SV, Egberts TC, Visser GH, Schobben AF. Effects of cytochrome P450 2D6 genotype on maternal paroxetine plasma concentrations during pregnancy. Clin Pharmacokinet. 2009;48:677–83. doi: 10.2165/11318050-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 64.Walf AA, Frye CA. Effects of two estradiol regimens on anxiety and depressive behaviors and trophic effects in peripheral tissues in a rodent model. Gender Med. 2009;6:300–11. doi: 10.1016/j.genm.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang H, Strobel HW. Regulation of CYP3A9 gene expression by estrogen and catalytic studies using cytochrome P450 3A9 expressed in Escherichia coli. Arch Biochem Biophysics. 1997;344:365–372. doi: 10.1006/abbi.1997.0230. [DOI] [PubMed] [Google Scholar]
- 66.Willner P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 67.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–64. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 68.Wisner K, Parry B, Piontek C. Postpartum Depression. New Engl J Med. 2002;347:194–9. doi: 10.1056/NEJMcp011542. [DOI] [PubMed] [Google Scholar]
- 69.Wisner K, Perel JM, Peindl KS, Hanusa BH, Piontek CM, Findling RL. Prevention of postpartum depression: a pilot randomized clinical trial. Am J Psychiatry. 2004;161:1290–2. doi: 10.1176/appi.ajp.161.7.1290. [DOI] [PubMed] [Google Scholar]
- 70.Yonkers KA, Lin H, Howell HB, Heath AC, Cohen LS. Pharmacologic treatment of postpartum women with new-onset major depressive disorder: a randomized controlled trial with paroxetine. J Clin Psychiatry. 2008;69:659–65. doi: 10.4088/jcp.v69n0420. [DOI] [PMC free article] [PubMed] [Google Scholar]



