Abstract
We recently showed that culture-derived metacyclic trypomastigotes (CMT), but not epimastigotes (Epi), of the Miranda 88 strain of Trypanosoma cruzi evade lysis by the human alternative complement pathway because of inefficient binding of factor B to complement component C3b on the parasite surface. These results suggested that CMT and tissue-culture-derived trypomastigotes (TCT), which also activate the alternative pathway poorly, might produce a molecule capable of interfering with factor B binding to C3b. We now demonstrate that CMT and TCT lysates, as well as molecules spontaneously shed from CMT and TCT but not Epi, accelerate decay of 125I-labeled factor Bb from the alternative-pathway C3 convertase (C3bBb) assembled on zymosan or Epi and also accelerate decay of the classical-pathway C3 convertase (C4b2a) on sheep erythrocytes. Parasites metabolically labeled with [35S]methionine spontaneously shed a limited number of radioactive components ranging in molecular mass from 86 to 155 kDa for trypomastigotes and 25 to 80 kDa for Epi. Decay-accelerating activity within supernatants is inactivated by papain and is coeluted with 35S-containing polypeptides on FPLC anion-exchange chromatography, suggesting that the active constituents are protein molecules. Molecules with decay-accelerating activity may explain the developmentally regulated resistance to complement-mediated lysis in infective and vertebrate stages of the T. cruzi life cycle.
Full text
PDF

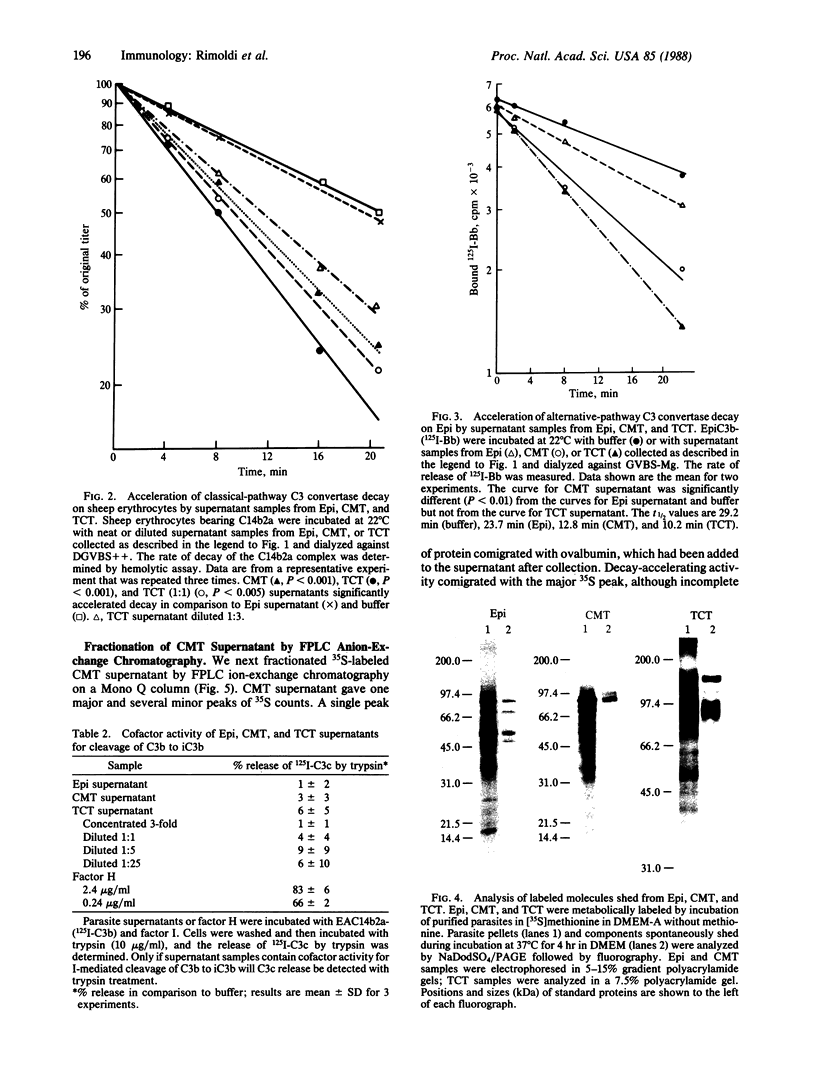
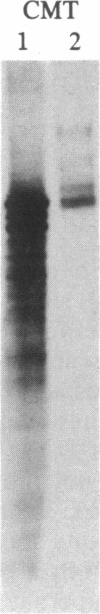
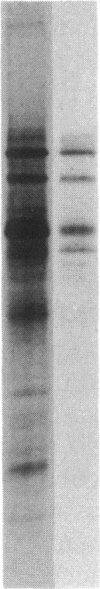
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dvorak J. A., Hyde T. P. Trypanosoma cruzi: interaction with vertebrate cells in vitro. 1. Individual interactions at the cellular and subcellular levels. Exp Parasitol. 1973 Oct;34(2):268–283. doi: 10.1016/0014-4894(73)90087-8. [DOI] [PubMed] [Google Scholar]
- Gaither T. A., Hammer C. H., Frank M. M. Studies of the molecular mechanisms of C3b inactivation and a simplified assay of beta 1H and the C3b inactivator (C3bINA). J Immunol. 1979 Sep;123(3):1195–1204. [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Joiner K., Hieny S., Kirchhoff L. V., Sher A. gp72, the 72 kilodalton glycoprotein, is the membrane acceptor site for C3 on Trypanosoma cruzi epimastigotes. J Exp Med. 1985 May 1;161(5):1196–1212. doi: 10.1084/jem.161.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K., Sher A., Gaither T., Hammer C. Evasion of alternative complement pathway by Trypanosoma cruzi results from inefficient binding of factor B. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6593–6597. doi: 10.1073/pnas.83.17.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis T. L., Tambourgi D. V., Sucupira M., Dias-da-Silva W. Effect of Trypanosoma cruzi membrane components on the formation of the classical pathway C3 convertase. Braz J Med Biol Res. 1986;19(2):271–278. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Rutgers J. L., Knowles D. M., Nussenzweig V. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med. 1987 Mar 1;165(3):848–864. doi: 10.1084/jem.165.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- O'Shea J. J., Brown E. J., Seligmann B. E., Metcalf J. A., Frank M. M., Gallin J. I. Evidence for distinct intracellular pools of receptors for C3b and C3bi in human neutrophils. J Immunol. 1985 Apr;134(4):2580–2587. [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5430–5434. doi: 10.1073/pnas.80.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977 Jul 1;146(1):257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Hieny S., Joiner K. Evasion of the alternative complement pathway by metacyclic trypomastigotes of Trypanosoma cruzi: dependence on the developmentally regulated synthesis of surface protein and N-linked carbohydrate. J Immunol. 1986 Nov 1;137(9):2961–2967. [PubMed] [Google Scholar]