Abstract
Organophosphates are developmental neurotoxicants but recent evidence also points to metabolic dysfunction. We determined whether neonatal parathion exposure in rats has long-term effects on regulation of adipokines and lipid peroxidation. We also assessed the interaction of these effects with increased fat intake. Rats were given parathion on postnatal days 1–4 using doses (0.1 or 0.2 mg/kg/day) that straddle the threshold for barely detectable cholinesterase inhibition and the first signs of systemic toxicity. In adulthood, animals were either maintained on standard chow or switched to a high-fat diet for seven weeks. We assessed serum leptin and adiponectin, tumor necrosis factor-α (TNFα) in adipose tissues, and thiobarbituric acid reactive species (TBARS) in peripheral tissues and brain regions. Neonatal parathion exposure uncoupled serum leptin levels from their dependence on body weight, suppressed adiponectin and elevated TNFα in white adipose tissue. Some of the effects were offset by a high-fat diet. Parathion reduced TBARS in the adipose tissues, skeletal muscle and temporal/occipital cortex but not in heart, liver, kidney or frontal/parietal cortex; it elevated TBARS in the cerebellum; the high-fat diet again reversed many of the effects. Neonatal parathion exposure disrupts the regulation of adipokines that communicate metabolic status between adipose tissues and the brain, while also evoking an inflammatory adipose response. Our results are consistent with impaired fat utilization and prediabetes, as well as exposing a potential relationship between effects on fat metabolism and on synaptic function in the brain.
Keywords: Adipokines, Adiponectin, Developmental toxicity, Diabetes, Dietary fat intake, Leptin, Lipid metabolism, Obesity, Organophosphate insecticides, Oxidative Stress, Parathion, Tumor necrosis factor-α
INTRODUCTION
It is increasingly clear that environmental chemical exposures early in life contribute to the explosive increase in the incidence of obesity and diabetes [28,47]. These likely include exposures to some of the most commonly-used pesticides [25,35]. In that regard, recent attention has turned to the organophosphates, which represent as much as 50% of the worldwide use of insecticides [5]. Childhood exposure to organophosphates is virtually ubiquitous [26] and the same subpopulations that have the greatest exposure also have the highest rates of obesity [8,10,11,29,30,34,49].
Although organophosphates are classified as neurotoxicants, their toxic effects extend beyond the nervous system. Exposure of adult animals to doses high enough to elicit signs of intoxication can lead to subsequent increases in body weight [22] and diabetes-like changes in hepatic energy metabolism [1]. In a recent series of studies with several different organophosphates, we explored whether lower-level exposures early in life can evoke changes in metabolic function that could contribute to obesity and diabetes. Chlorpyrifos given to fetal or neonatal rats led to increased body weight in adulthood, associated with leptin dysregulation, sensitization of hepatic responses to gluconeogenic signals and a serum profile resembling prediabetes [20,23,38]. Subsequently, we demonstrated a specific interaction between neonatal exposure to diazinon or parathion and enhanced weight gain responses to dietary fat intake [21,33]; a detailed analysis of serum metabolites confirmed that the organophosphates were likely targeting lipid metabolism in a complex manner, with distinct sex disparities and a nonmonotonic dose-response relationship [20,21,33,38]; the latter represented a different pattern of effects in comparing exposures below and just above the threshold for initial signs of intoxication.
Alterations in lipid metabolism may even contribute to the neurobehavioral consequences of early-life organophosphate exposure. Adipose tissue communicates with the brain, as well as skeletal muscle, through the release of adipokines such as leptin and adiponectin [2,36]; our earlier work indicated that early exposure to chlorpyrifos leads to leptin dysregulation [20]. Another adipokine, tumor necrosis factor-α (TNFα) is released as a consequence of inflammatory responses and acts as an autocrine/paracrine signal to regulate the function of other adipokines, insulin and other critical metabolic factors [16]; TNFα contributes to insulin resistance and diabetes, and the adipose inflammatory response is thought to play a significant role in obesity [3,45]. In turn, systemic changes in lipid metabolism and adiposity affect the composition of synaptic membranes within the brain, altering the function of the neurotransmitter receptors and signaling molecules that are embedded in the lipid milieu [6,12,14,19,31]. This may explain why the synaptic and behavioral effects of early-life organophosphate exposure do not represent simply the continuing effects of initial damage, but rather evolve over an extended period from juvenile stages through adolescence and adulthood [37,41,43], concurrently with the emergence of metabolic dysfunction and changes in weight and adiposity [20,21,33]. Indeed, we recently showed that consumption of a high-fat diet in adulthood can offset some of the metabolic consequences of neonatal parathion exposure and at the same time, ameliorate adverse effects on indices of cholinergic synaptic function [21,39]. Further, consumption of a high-fat diet alone in control rats elicited synaptic alterations in serotonergic and dopaminergic systems resembling some of the effects evoked by neonatal parathion treatment [43], reinforcing the idea that effects on lipid metabolism can contribute to the neurobehavioral deficits evoked by early-life organophosphate exposure.
In the present study, we evaluated the effects of parathion given to neonatal rats, on adipokines and related metabolic factors in peripheral tissues and the brain, assessed in adulthood. We focused on a well-characterized treatment paradigm spanning the threshold for barely-detectable cholinesterase inhibition and the emergence of signs of systemic intoxication [40,42], known to produce subsequent metabolic dysregulation and to disrupt synaptic and behavioral function [21,37,39,41,43,46]. In adulthood, we switched half the animals to a diet high in saturated fat, thus producing ketogenesis and excessive weight gain [21]. We then evaluated the concentrations of serum adipokines and of TNFα in adipose tissues. Finally, we assessed membrane lipid peroxidation in adipose tissues, brain regions and in peripheral organs, with the latter focused on skeletal muscle, which utilizes fatty acids as a major energy source, as compared to visceral organs (heart, liver, kidney).
MATERIALS AND METHODS
Animal treatments and diet
All experiments were carried out humanely and with regard for alleviation of suffering, with protocols approved by the Duke University Institutional Animal Care and Use Committee and in accordance with all federal and state guidelines. Timed-pregnant Sprague–Dawley rats (Charles River, Raleigh, NC) were housed in breeding cages, with a 12 h light–dark cycle and free access to water and food (LabDiet 5001, PMI Nutrition, St. Louis, MO). On the day after birth, all pups were randomized and redistributed to the dams with a litter size of 10 (5 males, 5 females) to maintain a standard nutritional status. Parathion (99.2% purity; Chem Service, West Chester, PA) was dissolved in dimethylsulfoxide to provide consistent absorption [40,42,48] and was injected subcutaneously in a volume of 1 ml/kg once daily on postnatal days 1–4; control animals received equivalent injections of the dimethylsulfoxide vehicle. Doses of 0.1 and 0.2 mg/kg/day were chosen because they straddle the threshold for barely-detectable cholinesterase inhibition and the first signs of reduced weight gain or impaired viability [40,42]. Brain cholinesterase inhibition 24 hr after the last dose of 0.1 mg/kg parathion is reduced 5–10%, well below the 70% threshold necessary for signs of cholinergic hyperstimulation [7]. Randomization of pup litter assignments within treatment groups was repeated at intervals of several days up until weaning, and in addition, dams were rotated among litters to distribute any maternal caretaking differences randomly across litters and treatment groups. Offspring were weaned on postnatal day 21.
Beginning at 15 weeks of age, half the rats were switched to a high-fat diet (OpenSource D12330, Research Diets Inc., New Brunswick, NJ), providing 58% of total calories as fat; 93% of the fat is hydrogenated coconut oil. The remaining rats continued on the standard LabDiet 5001 diet, which provides 13.5% of total calories as fat; with this diet, only 27% of the fat is saturated. Although the high-fat diet contains 37% more calories per gram, we found that animals on this diet reduce their food intake by approximately the same proportion [21], so that the total dietary intake is isocaloric; nevertheless, animals gain excess weight because of the higher fat content and have more than twice the serum concentration of β-hydroxybutyrate [21]. Serum samples were collected during the 22nd postnatal week. Rats were restrained in a polyfilm restraint cone (Harvard Apparatus, Holliston, MA), and blood was sampled by saphenous venipuncture into serum separator tubes (capiject T-MG; Terumo, Elkton, MD). Samples were allowed to clot for 30 min and were sedimented for collection of serum, which was then stored at −80°C. Food was then removed from the cage overnight, and a second sample was obtained with rats in the fasted state (12–14 hr without food). During the 24th postnatal week, animals were decapitated and tissues were harvested, flash-frozen with liquid nitrogen, and stored at −45°C. We dissected the heart, left kidney, three brain regions (frontal/parietal cortex, temporal/occipital cortex, cerebellum), gastrocnemius muscle, one lobe of the liver and samples of the mesenteric and inguinal white fat pads. Assays were performed on tissues from 6 to 12 rats per treatment group for each sex and with each diet, with no more than one male and one female derived from a given litter in each group.
Assays
Serum leptin and adiponectin were measured using radioimmunoassay kits (Millipore, Billerica, MA). For the TNFα measurements fat samples were prepared using an adaptation of described methods [24]. Individual fat pads were homogenized with a Polytron (Brinkmann Instruments, Westbury, NY), in phosphate buffered saline containing 1% protease inhibitor cocktail (Sigma Chemical Co., St. Louis, MO) plus 0.5% triton X-100. Homogenates were frozen in crushed dry ice, thawed, sonicated (VirTis, Gardiner, NY), sedimented for 30 minutes at 40,000 × g and analyzed using a commercial ELISA kit (Invitrogen, Carlsbad, CA). Lipid peroxidation was assessed through reaction with thiobarbituric acid using a modification [32] of published procedures [13]; the product was then quantitated as thiobarbituric acid reactive species (TBARS).
Data analysis
Data were compiled as means and standard errors. We evaluated several measures that were all related to metabolism, so the initial comparisons were conducted by a global ANOVA (data log-transformed because of heterogeneous variance among tissues and measures) incorporating all the variables and measurements in order to avoid an increased probability of type 1 errors that might otherwise result from multiple tests of the same data set. The variables in the global test were treatment (control, parathion 0.1 mg/kg, parathion 0.2 mg/kg), diet (normal, high-fat), tissue, sex and measure. The latter factor constituted three classes of variables: serum measurements (adiponectin, leptin), TNFα and TBARS; because each class contained multiple measures from the same individual, these were considered to be repeated measures in the ANOVA. Where we identified interactions of treatment with the other variables, data were then subdivided for lower-order ANOVAs to evaluate treatments that differed from the corresponding control. Where permitted by the interaction terms, individual groups that differed from control were identified with Fisher’s Protected Least Significant Difference Test. Significance was assumed at the level of p < 0.05. For interactions at p < 0.1, we also examined whether lower-order main effects were detectable after subdivision of the interactive variables [44]. The criterion for interaction terms was not used to assign significance to the effects but rather to identify interactive variables requiring subdivision for lower-order tests of main effects of parathion, the variable of chief interest [44].
RESULTS
Tissue weights (data not shown)
We reported earlier on the effects of neonatal parathion treatments and dietary manipulations on body weights from the same cohort of animals as used in the present study [21] By itself, parathion caused a small (2–3%) but significant elevation in weight at the low dose in males, and reductions of about 4% at either dose in females. Consumption of a high fat diet increased body weights regardless of whether animals were exposed to parathion as neonates, with an average 10% increase in males and 30% in females. Here, we evaluated changes in tissue weights, except where we sampled only a portion of the tissue (liver, fat pads). Multivariate ANOVA (factors of parathion treatment, diet, sex, tissue) indicated main effects of diet (p < 0.05), sex (p < 0.0001) and tissue (p < 0.0001), as well as interactions of parathion × tissue (p < 0.08), diet × sex (p < 0.06), diet × tissue (p < 0.0001), sex × tissue (p < 0.0001) and diet × sex × tissue (p < 0.05). Accordingly, we subdivided the data into the individual tissues to see which were the most affected. All tissues showed significant sex differences and the peripheral tissues also showed a main effect of diet (p < 0.0007 for gastrocnemius, p < 0.0001 for heart, p < 0.02 for kidney), whereas diet had no effect on brain region weights. Parathion effects were seen in the cerebellum (p < 0.03 for the main effect, p < 0.02 for parathion × diet × sex) and heart (p < 0.05 for parathion × diet × sex); however, after the effects were separated by the interactive variables, the only one that achieved significance was a 10% increase in cerebellar weight caused by the high dose of parathion in females given the high-fat diet (p < 0.004).
Serum leptin and adiponectin
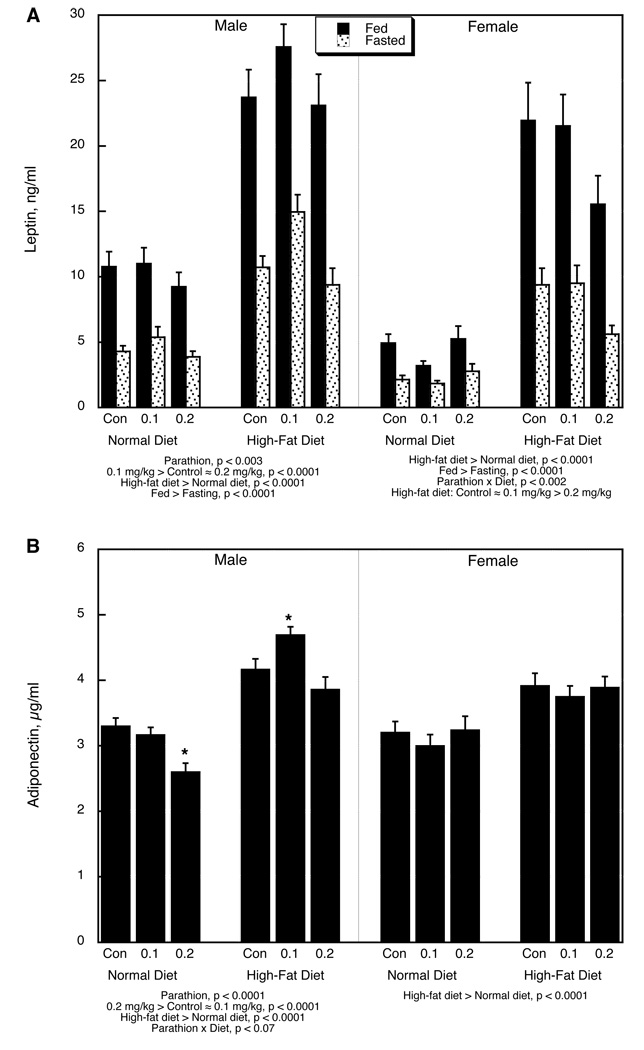
The global ANOVA indicated significant main effects of parathion treatment (p < 0.0001), diet (p < 0.0001), feeding status (p < 0.0001), sex (p < 0.0001), and measure (adiponectin vs. leptin, p < 0.0001), along with interactions of parathion treatment × diet (p < 0.004), parathion × sex (p < 0.002), diet × sex (p < 0.005), diet × measure (p < 0.0001), feeding status × measure (p < 0.0001), feeding status × sex (p < 0.0001, sex × measure (p < 0.0001), parathion × diet × sex (p < 0.03), parathion × diet × measure (p < 0.005), diet × sex × measure (p < 0.0001) and parathion × diet × sex × measure (p < 0.1). Accordingly, we first separated the two measures and performed a lower-order analysis for each. For leptin, the main effects and interactions were maintained (p < 0.04 for parathion treatment, p < 0.0001 for diet, p < 0.0001 for sex, p < 0.0001 for feeding status, p < 0.003 for parathion × diet, p < 0.08 for parathion × sex, p < 0.0001 for diet × sex, p < 0.04 for parathion × diet × sex), whereas for adiponectin, significance was maintained for main effects and interactions of parathion, diet and sex, but not for feeding status (p < 0.02 for parathion, p < 0.0001 for diet, p < 0.0005 for parathion × sex, p < 0.03 for diet × sex). Thus, for adiponectin, the results of fed and fasting values were combined for presentation.
Fasting by itself significantly decreased serum leptin and introduction of a high-fat diet evoked an increase in leptin levels (Figure 1A). In males, neonatal exposure to 0.1 mg/kg parathion produced a significant overall elevation in leptin levels that was lost when the dose was raised to 0.2 mg/kg; the parathion effect was more notable on the high-fat diet. In contrast, females on the normal diet did not show significant parathion effects; when placed on the high-fat diet, females exposed to the high dose of parathion showed a smaller increase in leptin, so that their levels were significantly lower than those in the control or low-dose parathion groups.
Figure 1.
Serum adipokines: (A) leptin, (B) adiponectin. Data represent means and standard errors; values for fed and fasted adiponectin were combined because of the absence of a main effect of feeding status or interaction of status with the other variables. ANOVA for each sex appears below the panels. Asterisks denote individual groups that differ from the corresponding control, evaluated only where permitted by the interaction terms; otherwise, only main effects are shown.
Adiponectin also showed a substantial increase evoked by the high-fat diet alone (Figure 1B). Males exposed to 0.2 mg/kg parathion and maintained on a normal diet evinced a significant decrease in circulating adiponectin; however, they showed a greater increase in response to the high-fat diet, so that the 0.1 mg/kg parathion group then displayed adiponectin values higher than in controls, whereas the 0.2 mg/kg group became indistinguishable from the control. Females showed only the main dietary effect on adiponectin, without any interaction with parathion treatment.
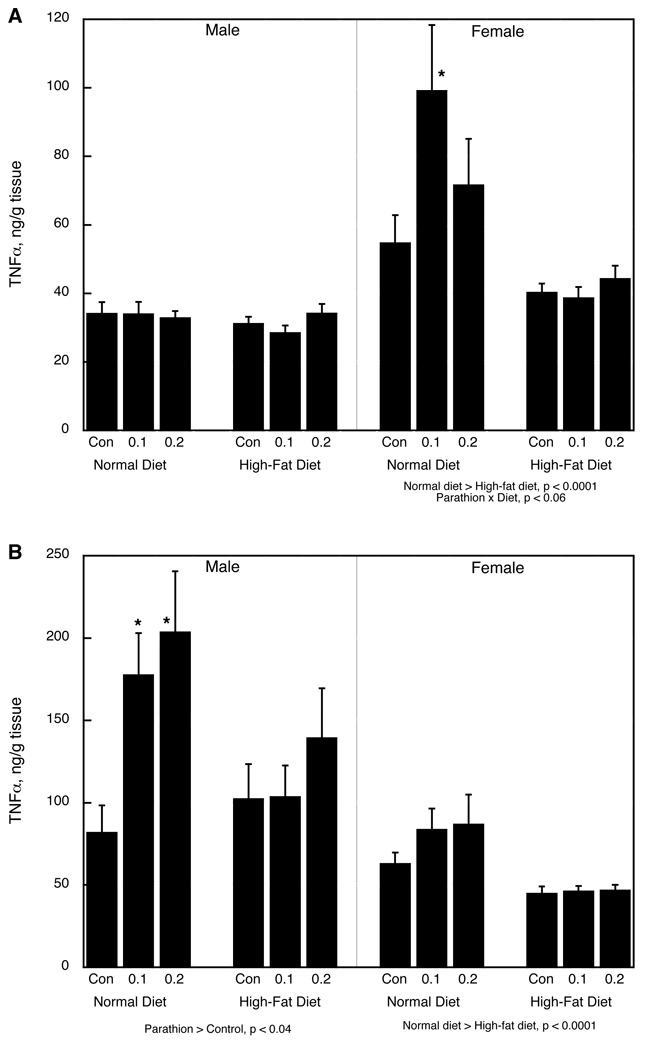
TNFα
Multivariate ANOVA (parathion treatment, diet, sex, tissue) showed significant main effects of parathion (p < 0.004), diet (p < 0.0001) and tissue (mesenteric vs. inguinal fat pad), as well as interactions of parathion × diet (p < 0.03), diet × sex (p < 0.02), sex × tissue (p < 0.0001) and parathion × sex × tissue (p < 0.08). In the mesenteric fat pad in males, neither parathion treatment nor the high-fat diet had any effect on TNFα (Figure 2A). In females maintained on a normal diet, neonatal parathion treatment evoked an increase in TNFα; the high-fat diet by itself reduced the levels and eliminated the effect of parathion exposure. In the inguinal fat pad, males exposed to neonatal parathion and given a normal diet showed significant increases in TNFα, effects that were reduced substantially by the high-fat diet (Figure 2B). Although the effect of parathion was not significant in females in the normal diet group, they showed the same trend as the males and the effect did not actually differ significantly from that in males (no interaction of parathion × sex), so the sex difference needs to be viewed with caution. The high-fat diet lowered TNFα and obtunded the effect of parathion; given the lack of a treatment interaction with sex, we confirmed this by separating the data according to diet and evaluating both sexes; in the normal diet group, there was a main effect of parathion that was highly significant (p < 0.006 overall; p < 0.008 for 0.1 mg/kg vs. control, p < 0.005 for 0.2 mg/kg vs. control), whereas there was no significant effect in the group given the high-fat diet (p > 0.6).
Figure 2.
TNFα in (A) mesenteric white fad pad, (B) inguinal white fat pad. Data represent means and standard errors. ANOVA for each sex appears below the panels. Asterisks denote individual groups that differ from the corresponding control, evaluated only where permitted by the interaction terms; otherwise, only main effects are shown.
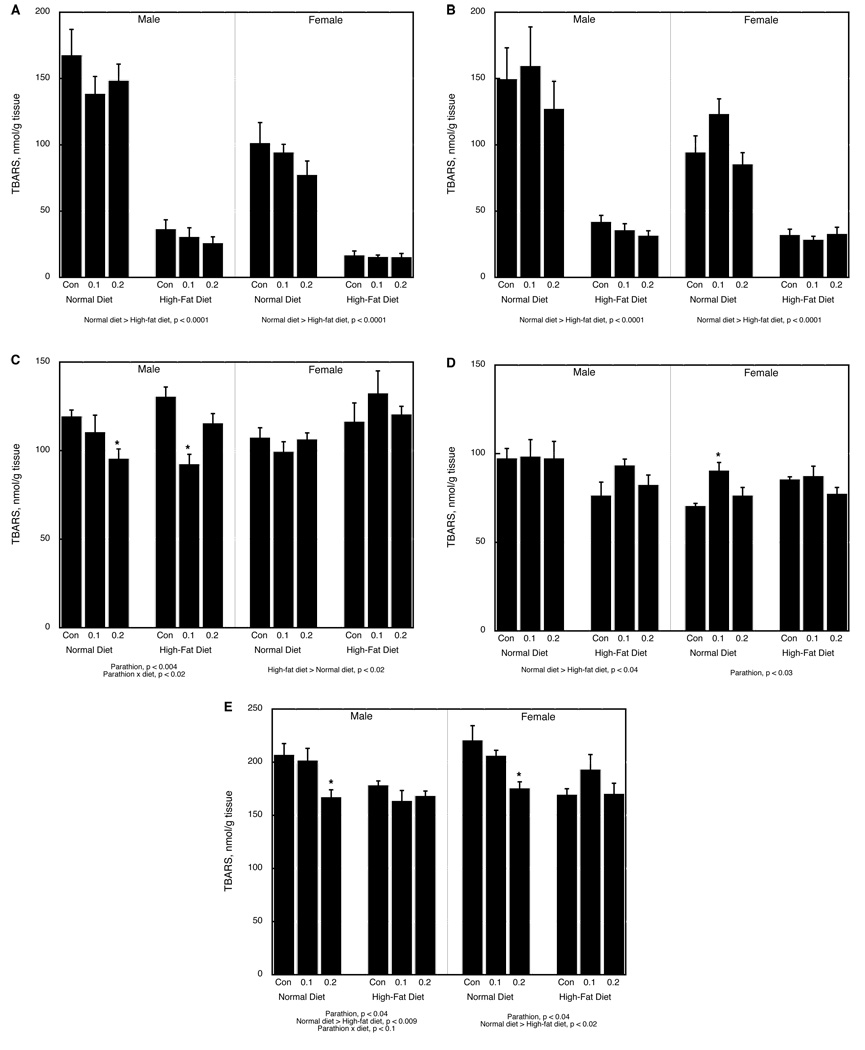
TBARS
We evaluated TBARS in three different tissue groupings: fat pads, brain regions, and peripheral tissues. In the fat pads, there was a main effect of 0.2 mg/kg parathion treatment (p < 0.05), diet (p < 0.0001), sex (p < 0.0001) and tissue (mesenteric vs. inguinal pad, p < 0.0002), along with interactions of diet × tissue (p < 0.0001) and sex × tissue (p < 0.02). In the mesenteric fat pad, both sexes showed a significant reduction caused by the high-fat diet but no effect of parathion or interaction of parathion with diet (Figure 3A); the inguinal fat pad showed similar diet-related differences (Figure 3B).
Figure 3.
TBARS in (A) mesenteric white fat pad, (B) inguinal white fat pad, (C) temporal/occipital cortex, (D) cerebellum, (E) gastrocnemius muscle. Data represent means and standard errors. ANOVA for each sex appears below the panels. Asterisks denote individual groups that differ from the corresponding control, evaluated only where permitted by the interaction terms; otherwise, only main effects are shown.
In contrast, the brain regions showed parathion effects that were interactive with diet, sex and region (p < 0.02 for parathion × region, p < 0.04 for parathion × diet × sex × region), along with main effects and interactions of the other variables (p < 0.0001 for region, p < 0.08 for diet × region, p < 0.01 for diet × sex, p < 0.002 for sex × region). The frontal/parietal cortex showed only a main effect of sex (p < 0.03), representing an average of 15% higher values in females, without any main effects or interactions with parathion or diet (data not shown). In the temporal/occipital cortex, however, there were significant effects of both parathion and diet that differed between males and females (p < 0.02 for main effect of diet, p < 0.05 for parathion × sex, p < 0.02 for parathion × diet × sex). Males maintained on a normal diet showed significant parathion-related reductions in TBARS; on the high-fat diet, values were restored in the 0.2 mg/kg parathion group, but not in the 0.1 mg/kg group. Females showed only an overall increase attributable to the high-fat diet (Figure 3C). In the cerebellum, parathion had little or no effect on TBARS in males and the only significant change was an overall reduction caused by the high-fat diet (Figure 3D). Females on a normal diet showed a significant increase in cerebellar TBARS attributable to neonatal parathion treatment, an effect that was reduced by the high-fat diet.
The peripheral tissues showed significant main effects of diet (p < 0.0001), sex (p < 0.0001) and tissue (p < 0.0001), along with interactions of parathion × tissue (p < 0.1), diet × tissue (p < 0.002), sex × tissue (p < 0.0001) and parathion × diet × tissue (p < 0.09). The liver showed no significant effects or interactions for any of the variables and the kidney displayed only a main effect of sex (p < 0.0001), representing an approximately 40% higher overall value in females (data not shown). In the heart, the high-fat diet caused small but significant overall reductions in TBARS regardless of sex (main effect, p < 0.0001) but without any interactions with parathion treatment (data not shown). Gastrocnemius muscle showed main effects of parathion treatment (p < 0.003) and diet (p < 0.0002), with a significant interaction between the two variables (p < 0.05). The main effect of parathion exposure represented the fact that, in either sex on a normal diet, there was a significant decrement in TBARS (Figure 3E). The main effect of diet represented a reduction caused by elevated fat intake, an effect that was also significant for both sexes. The effect of the high-fat diet obtunded the effect of parathion.
DISCUSSION
In our earlier work with neonatal exposure to either chlorpyrifos [38] or parathion [21], we found sex-selective changes in serum lipids and their response to a high-fat diet that were suggestive of defects in lipid metabolism. The present study provides some of the first verification that early-life organophosphate exposure disrupts major control points for lipid metabolism as well as evoking an inflammatory response in adipose tissue; further, we found evidence that effects on lipid metabolism interact with deficits in synaptic function that in turn may contribute to impaired behavioral performance. By itself, parathion exposure elicited a decrease in serum adiponectin levels in males as well as increases in TNFα in adipose tissues of both sexes. Adiponectin is depressed in prediabetes, an effect that is mechanistically linked to decreased insulin sensitivity [17] and to dyslipidemia [18]. Accordingly, our finding of a reduction in adiponectin is consistent with our earlier demonstration of impaired glucose utilization, hyperglycemia and altered serum lipid profiles in these animals, despite normal circulating insulin levels [21]. For leptin, the case is more complex. Ordinarily, leptin should increase in parallel with weight but in previous work with developmental chlorpyrifos exposure, we found dissociation of leptin from body weight [20]. Here, the animals given parathion and consuming a normal diet showed only slight changes in leptin but that could reflect the fact that parathion elicits smaller weight changes than those seen with the earlier chlorpyrifos study [20]. The introduction of a high-fat diet, however, uncovered the dysregulation of leptin in the parathion-exposed animals. As expected, the increase in fat intake, and associated weight gain, led to significant increases in serum leptin in all groups, and fasting reduced leptin levels. However, the effect of the high-fat diet was exaggerated in males exposed neonatally to 0.1 mg/kg parathion and attenuated in females exposed to 0.2 mg/kg, indicating a substantial shift in the relationship between leptin levels and body weight. Similarly, the dietary manipulation produced a rise in serum adiponectin that was enhanced by prior parathion exposure, restricted to males. Accordingly, neonatal organophosphate exposure not only alters basal adipokine concentrations but also alters their reactivity to dietary factors.
Adiponectin and leptin are released by lipid-storing tissues and our finding of increased TNFα levels in the white fat pads after neonatal parathion exposure is consistent with a chronic adipose inflammatory state akin to that associated with diabetes and obesity [3,16,45]. Thus, early-life parathion by itself induces a state of chronic adipose inflammation even in the absence of dietary manipulations, effects that could well provide a driving force for many of the other metabolic abnormalities seen here and in our previous work [21]. Further inferences can be drawn from the result that introduction of a high-fat diet actually reduced the effect of neonatal parathion exposure on TNFα. Earlier, we showed that some of the metabolic and synaptic defects evoked by parathion could actually be ameliorated by increasing the proportion of dietary calories derived from fat, likely reflecting a correction of defective fat utilization [21,39]. The reduction in adipose inflammation on the high-fat diet thus supports the view that early parathion exposure evokes defects in fat metabolism in association with an inflammatory response, but that the effects are surmountable by providing excess fat in the diet; of course, this strategy has its own, associated adverse health effects, but it does provide a proof-of-principle that specific dietary manipulations can modify the outcome of developmental toxicant exposure.
Adverse cellular effects of defective lipid metabolism also involve alterations in membrane function associated with lipid peroxidation, which increases when oxygen consumption and the generation of reactive oxygen species outstrip the antioxidant defense capacity of the cell. Consumption of a high-fat diet can, by itself, increase lipid peroxidation [4], so it may seem surprising that here, the introduction of a high-fat diet actually reduced TBARS in adipose tissues. This is likely a reflection of our use of a diet in which coconut oil provides the majority of the dietary fat. The high concentration of saturated fat limits the number of oxidizable double bonds in the fatty acid side-chains; this explains why, for example, a diet rich in unsaturated fats raises the concentration of lipid peroxides [9]. Additionally, coconut oil is a rich source of fat-soluble antioxidants such as Vitamin E, which, because they concentrate in adipose tissue, further reduce the endogenous level of lipid peroxides in the fat pads [27]. However, if this were the only factor contributing to the TBARS measurements, then all the tissues should show a similar pattern, although perhaps to a lesser extent than in adipose tissue, and there should be little or no relationship of the diet effect to prior parathion exposure. Instead, we found highly selective effects of the high-fat diet on peripheral tissues and brain regions, as well as effects of parathion alone and in combination with fat intake. In the periphery, there was a clear distinction between skeletal muscle, which utilizes fatty acids as a major source of energy, and visceral organs where fat provides a lesser source (heart, kidney, liver). Parathion by itself evoked a significant decrease in lipid peroxidation in the gastrocnemius muscle, consistent with an impairment of fat utilization [21] and a consequent reduction in oxidative capacity; again, this effect was eliminated by increasing the amount of fat in the diet, consistent with overcoming the underlying metabolic deficiency.
The regionally-selective effects of parathion on lipid peroxidation in the brain also are consistent with specific events related to cellular function. Parathion reduced TBARS in the temporal/occipital cortex, a region in which we earlier found evidence for a loss of synaptic function underlying impaired behavioral performance [37,39,41,46]. The brain is highly susceptible to lipid peroxidation because of its high oxygen consumption, low antioxidant capacity and unique membrane lipid composition [15]. Accordingly, the reduction in TBARS in the temporal/occipital cortex likely reflects reduced oxygen consumption consequent to the underlying loss of synaptic function; indeed, the effect on lipid peroxidation displayed the same sex-selectivity as seen for indices of cholinergic presynaptic activity in this region [37]. In turn, the fact that lipid peroxidation was increased by the high-fat diet in the temporal/occipital cortex, instead of showing the decrease that would be expected from the dietary composition, implies that synaptic activity is enhanced by the dietary change; for the group exposed to 0.2 mg/kg of parathion, this restored the TBARS values to those seen in control rats on a normal diet. Similarly, in the cerebellum, introduction of the high-fat diet eliminated the significant increase in TBARS caused by parathion alone. The alterations in synaptic activity caused by the dietary manipulation are thus likely to be directly related to the amelioration of some of the effects of parathion on synaptic function, as noted earlier [39]. At the same time, however, other synaptic effects may be worsened by the dietary intervention, seen here in the male temporal/occipital cortex at the lower parathion dose. This points out the complexities of the interaction between metabolic factors and the impact of toxicant exposures on the central nervous system, which will require a dissection of the specific cellular and synaptic mechanisms involved in each.
In conclusion, our results support the view that early-life organophosphate exposure disrupts lipid metabolism in adulthood, as evidenced by an uncoupling of the relationship between leptin and body weight and by alterations in adiponectin and TNFα consistent with adipose inflammation. Taken together with our earlier findings for effects of parathion as well as chlorpyrifos on glucose and lipid metabolism [20,21,38], it is evident that organophosphates, although generally classified as developmental neurotoxicants, target important homeostatic mechanisms that govern metabolism, growth, and the risk factors contributing to diabetes, obesity and cardiovascular disease. In light of the widespread human exposures to these agents, our findings reinforce the idea that environmental toxicants are contributing to the increasing incidence of obesity and diabetes. Finally, the interaction between the metabolic changes wrought by dietary fat intake and indices of synaptic function in the brain indicates that metabolic disruption and neurobehavioral consequences of early-life organophosphate exposure may be related, thus offering the opportunity for therapies in adulthood that can ameliorate the adverse effects of early-life toxicant exposures.
Acknowledgments/disclaimers
Research was supported by NIH ES10356 and by the Leon Golberg Postdoctoral Fellowship. TAS has provided expert witness testimony in the past three years at the behest of the following law firms: The Calwell Practice (Charleston WV), Frost Brown Todd (Charleston WV), Snyder Weltchek & Snyder (Baltimore MD), Finnegan Henderson Farabow Garrett & Dunner (Washington DC), Frommer Lawrence Haug (Washington DC), Carter Law (Peoria IL), Corneille Law (Madison WI), Angelos Law (Baltimore MD), Kopff, Nardelli & Dopf (New York NY), and Gutglass Erickson Bonville & Larson (Madison WI).
Abbreviations
- ANOVA
analysis of variance
- ROS
reactive oxygen species
- TBARS
thiobarbituric acid reactive species
- TNFα
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors do not have any conflicts of interest. However, TAS has provided expert witness testimony in the past three years at the behest of the following law firms: The Calwell Practice (Charleston WV), Frost Brown Todd (Charleston WV), Snyder Weltchek & Snyder (Baltimore MD), Finnegan Henderson Farabow Garrett & Dunner (Washington DC), Frommer Lawrence Haug (Washington DC), Carter Law (Peoria IL), Corneille Law (Madison WI), Angelos Law (Baltimore MD), Kopff, Nardelli & Dopf (New York NY), and Gutglass Erickson Bonville & Larson (Madison WI).
REFERENCES
- 1.Abdollahi M, Donyavi M, Pournourmohammadi S, Saadat M. Hyperglycemia associated with increased hepatic glycogen phosphorylase and phosphoenolpyruvate carboxykinase in rats following subchronic exposure to malathion. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2004;137:343–347. doi: 10.1016/j.cca.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Ahima RS. Adipose tissue as an endocrine organ. Obesity. 2006;14:242S–249S. doi: 10.1038/oby.2006.317. [DOI] [PubMed] [Google Scholar]
- 3.Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, Kalofoutis A. Inflammatory process in type 2 diabetes: the role of cytokines. Ann. N.Y. Acad. Sci. 2006;1084:89–117. doi: 10.1196/annals.1372.039. [DOI] [PubMed] [Google Scholar]
- 4.Beltowski J, Wojcicka G, Gorny D, Marciniak A. The effect of dietary-induced obesity on lipid peroxidation, antioxidant enzymes and total plasma antioxidant capacity. J. Physiol. Pharmacol. 2000;51:883–896. [PubMed] [Google Scholar]
- 5.Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem. Res. Toxicol. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- 6.Clandinin MT, Foot M, Robson L. Plasma membrane: can its structure and function be modulated by dietary fat? Comp. Biochem. Physiol. B. 1983;76:335–339. doi: 10.1016/0305-0491(83)90079-2. [DOI] [PubMed] [Google Scholar]
- 7.Clegg DJ, van Gemert M. Determination of the reference dose for chlorpyrifos: proceedings of an expert panel. J. Toxicol. Environ. Health. 1999;2:211–255. doi: 10.1080/109374099281179. [DOI] [PubMed] [Google Scholar]
- 8.Curl CL, Fenske RA, Kissel JC, Shirai JH, Moate TF, Griffith W, Coronado G, Thompson B. Evaluation of take-home organophosphorus pesticide exposure among agricultural workers and their children. Environ. Health Perspect. 2002;110:A787–A792. doi: 10.1289/ehp.021100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Aquino M, Benedetti PC, Felice MDi, Gentili V, Tomassi G, Maiorino M, Ursini F. Effect of fish oil and coconut oil on antioxidant defence system and lipid peroxidation in rat liver. Free Radic. Res. Commun. 1991;12:147–152. doi: 10.3109/10715769109145779. [DOI] [PubMed] [Google Scholar]
- 10.Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ. Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenske RA, Kissel JC, Lu C, Kalman DA, Simcox NJ, Allen EH, Keifer MC. Biological based pesticide dose estimates for children in an agricultural community. Environ. Health Perspect. 2000;108:515–520. doi: 10.1289/ehp.00108515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geiser F. Influence of polyunsaturated and saturated dietary lipids on adipose tissue, brain and mitochondrial membrane fatty acid composition of a mammalian hibernator. Biochim. Biophys. Acta. 1990;1046:159–166. doi: 10.1016/0005-2760(90)90183-x. [DOI] [PubMed] [Google Scholar]
- 13.Guan ZZ, Yu WF, Nordberg A. Dual effects of nicotine on oxidative stress and neuroprotection in PC12 cells. Neurochem. Intl. 2003;43:243–249. doi: 10.1016/s0197-0186(03)00009-3. [DOI] [PubMed] [Google Scholar]
- 14.Gudbjarnason S, Benediktsdottir VE. Regulation of β-adrenoceptor properties and the lipid milieu in heart muscle membranes during stress. Mol. Cell. Biochem. 1996;164:137–143. doi: 10.1007/978-1-4613-1289-5_16. [DOI] [PubMed] [Google Scholar]
- 15.Gupta RC. Brain regional heterogeneity and toxicological mechanisms of organophosphates and carbamates. Toxicol. Mech. Meth. 2004;14:103–143. doi: 10.1080/15376520490429175. [DOI] [PubMed] [Google Scholar]
- 16.Hotamisligil GS. Mechanisms of TNF-α-induced insulin resistance. Exp. Clin. Endocrinol. Diabetes. 1999;107:119–125. doi: 10.1055/s-0029-1212086. [DOI] [PubMed] [Google Scholar]
- 17.Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, Matsuzawa Y. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 18.Kazumi T, Kawaguchi A, Hirano T, Yoshino G. Serum adiponectin is associated with high-density lipoprotein cholesterol, triglycerides, and low-density lipoprotein particle size in young healthy men. Metabolism. 2004;53:589–593. doi: 10.1016/j.metabol.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Kelly JF, Joseph JA, Denisova NA, Erat S, Mason RP, Roth GS. Dissociation of striatal GTPase and dopamine release responses to muscarinic cholinergic agonists in F344 rats: influence of age and dietary manipulation. J. Neurochem. 1995;64:2755–2764. doi: 10.1046/j.1471-4159.1995.64062755.x. [DOI] [PubMed] [Google Scholar]
- 20.Lassiter TL, Brimijoin S. Rats gain excess weight after developmental exposure to the organophosphorothionate pesticide, chlorpyrifos. Neurotoxicol. Teratol. 2008;30:125–130. doi: 10.1016/j.ntt.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Lassiter TL, Ryde IT, MacKillop EA, Brown KK, Levin ED, Seidler FJ, Slotkin TA. Exposure of neonatal rats to parathion elicits sex-selective reprogramming of metabolism and alters the response to a high-fat diet in adulthood. Environ. Health Perspect. 2008;116:1456–1462. doi: 10.1289/ehp.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meggs WJ, Brewer KL. Weight gain associated with chronic exposure to chlorpyrifos in rats. J. Med. Toxicol. 2007;3:89–93. doi: 10.1007/BF03160916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer A, Seidler FJ, Slotkin TA. Developmental effects of chlorpyrifos extend beyond neurotoxicity: critical periods for immediate and delayed-onset effects on cardiac and hepatic cell signaling. Environ. Health Perspect. 2004;112:170–178. doi: 10.1289/ehp.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molina PE, Qian L, Schuhlein D, Naukam R, Wang H, Tracey KJ, Abumrad NN. CNI-1493 attenuates hemodynamic and pro-inflammatory responses to LPS. Shock. 1998;10:329–334. doi: 10.1097/00024382-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Morgan DP, Lin LI, Saikaly HH. Morbidity and mortality in workers occupationally exposed to pesticides. Arch. Environ. Contam. Toxicol. 1980;9:349–382. doi: 10.1007/BF01057414. [DOI] [PubMed] [Google Scholar]
- 26.Morgan MK, Sheldon LS, Croghan CW, Jones PA, Robertson GL, Chuang JC, Wilson NK, Lyu CW. Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environments. J. Exposure Anal. Environ. Epidemiol. 2005;15:297–309. doi: 10.1038/sj.jea.7500406. [DOI] [PubMed] [Google Scholar]
- 27.Nevin KG, Rajamohan T. Virgin coconut oil supplemented diet increases the antioxidant status in rats. Food Chem. 2006;99:260–266. [Google Scholar]
- 28.Newbold RR, Padilla-Banks E, Jefferson WN, Heindel JJ. Effects of endocrine disruptors on obesity. Intl. J. Androl. 2008;31:201–208. doi: 10.1111/j.1365-2605.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 29.Perera FP, Rauh V, Whyatt RM, Tang D, Tsai WY, Bernert JT, Tu YH, Andrews H, Barr DB, Camann DE, Diaz D, Dietrich J, Reyes A, Kinney PL. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology. 2005;26:573–587. doi: 10.1016/j.neuro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Petchuay C, Visuthismajarn P, Vitayavirasak B, Hore P, Robson MG. Biological monitoring of organophosphate pesticides in preschool children in an agricultural community in Thailand. Intl. J. Occup. Environ. Health. 2006;12:134–141. doi: 10.1179/oeh.2006.12.2.134. [DOI] [PubMed] [Google Scholar]
- 31.Ponsard B, Durot I, Delerive P, Oudot F, Cordelet C, Grynberg A, Athias P. Cross-influence of membrane polyunsaturated fatty acids and hypoxia-reoxygenation on α- and β- adrenergic function of rat cardiomyocytes. Lipids. 1999;34:457–466. doi: 10.1007/s11745-999-0385-5. [DOI] [PubMed] [Google Scholar]
- 32.Qiao D, Seidler FJ, Slotkin TA. Oxidative mechanisms contributing to the developmental neurotoxicity of nicotine and chlorpyrifos. Toxicol. Appl. Pharmacol. 2005;206:17–26. doi: 10.1016/j.taap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Roegge CS, Timofeeva OA, Seidler FJ, Slotkin TA, Levin ED. Developmental diazinon neurotoxicity in rats: later effects on emotional response. Brain Res. Bull. 2008;75:166–172. doi: 10.1016/j.brainresbull.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothlein J, Rohlman D, Lasarev M, Phillips J, Muniz J, McCauley L. Organophosphate pesticide exposure and neurobehavioral performance in agricultural and nonagricultural Hispanic workers. Environ. Health Perspect. 2006;114:691–696. doi: 10.1289/ehp.8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saldana TM, Basso O, Hoppin JA, Baird DD, Knott C, Blair A, Alavanja MCR, Sandler DP. Pesticide exposure and self-reported gestational diabetes mellitus in the Agricultural Health Study. Diabetes Care. 2007;30:529–534. doi: 10.2337/dc06-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 37.Slotkin TA, Bodwell BE, Ryde IT, Levin ED, Seidler FJ. Exposure of neonatal rats to parathion elicits sex-selective impairment of acetylcholine systems in brain regions during adolescence and adulthood. Environ. Health Perspect. 2008;116:1308–1314. doi: 10.1289/ehp.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slotkin TA, Brown KK, Seidler FJ. Developmental exposure of rats to chlorpyrifos elicits sex-selective hyperlipidemia and hyperinsulinemia in adulthood. Environ. Health Perspect. 2005;113:1291–1294. doi: 10.1289/ehp.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slotkin TA, Lassiter TL, Ryde IT, Wrench N, Levin ED, Seidler FJ. Consumption of a high-fat diet in adulthood ameliorates the effects of neonatal parathion exposure on acetylcholine systems in rat brain regions. Environ. Health Perspect. 2009;117:916–922. doi: 10.1289/ehp.0800459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slotkin TA, Levin ED, Seidler FJ. Comparative developmental neurotoxicity of organophosphate insecticides: effects on brain development are separable from systemic toxicity. Environ. Health Perspect. 2006;114:746–751. doi: 10.1289/ehp.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slotkin TA, Levin ED, Seidler FJ. Developmental neurotoxicity of parathion: progressive effects on serotonergic systems in adolescence and adulthood. Neurotoxicol. Teratol. 2009;31:11–17. doi: 10.1016/j.ntt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slotkin TA, Tate CA, Ryde IT, Levin ED, Seidler FJ. Organophosphate insecticides target the serotonergic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environ. Health Perspect. 2006;114:1542–1546. doi: 10.1289/ehp.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slotkin TA, Wrench N, Ryde IT, Lassiter TL, Levin ED, Seidler FJ. Neonatal parathion exposure disrupts serotonin and dopamine synaptic function in rat brain regions: modulation by a high-fat diet in adulthood. Neurotoxicol. Teratol. 2009 doi: 10.1016/j.ntt.2009.07.003. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snedecor GW, Cochran WG. Statistical Methods. Ames, Iowa: Iowa State University Press; 1967. [Google Scholar]
- 45.Tataranni PA, Ortega E. A burning question: does an adipokine-induced activation of the immune system mediate the effect of overnutrition on type 2 diabetes? Diabetes. 2005;54:917–927. doi: 10.2337/diabetes.54.4.917. [DOI] [PubMed] [Google Scholar]
- 46.Timofeeva OA, Sanders D, Seemann K, Yang L, Hermanson D, Regenbogen S, Agoos S, Kallepalli A, Rastogi A, Braddy D, Wells C, Perraut C, Seidler FJ, Slotkin TA, Levin ED. Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Res. Bull. 2008;77:404–411. doi: 10.1016/j.brainresbull.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trasande L, Cronk C, Durkin M, Weiss M, Schoeller DA, Gall EA, Hewitt JB, Carrel AL, Landrigan PJ, Gillman MW. Environment and obesity in the National Children's Study. Environ. Health Perspect. 2009;117:159–166. doi: 10.1289/ehp.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol. Appl. Pharmacol. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]
- 49.Whyatt RM, Camann D, Perera FP, Rauh VA, Tang D, Kinney PL, Garfinkel R, Andrews H, Hoepner L, Barr DB. Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth. Toxicol. Appl. Pharmacol. 2005;206:246–254. doi: 10.1016/j.taap.2004.11.027. [DOI] [PubMed] [Google Scholar]