Abstract
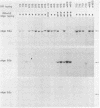
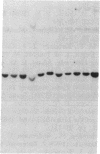
Recent progress in the molecular biology of human major histocompatibility complex class II genes (HLA-DP, -DQ, -DR) have shown that the genetic complexity and allelic polymorphism are greater than expected. In the case of HLA-DR, three DR beta-chain loci have been identified and linked, two of which (DR beta I and DR beta III, now assigned names HLA-DRIB and HLA-DR3B) are functional. We have shown that the HLA micropolymorphism detected at the DNA sequence level can easily be analyzed by hybridization with allele-specific oligonucleotides (HLA "oligotyping"). In the case of the HLA DRw52 supertypic specificity, which includes the DR3, DR5, DRw6, and DRw8 haplotypes, three alleles, referred to as DRw52a, DRw52b, and DRw52c, have recently been identified at the HLA-DR3B locus by DNA sequencing. Hybridization with locus- and allele-specific oligonucleotide probes (designated 52a, 52b, and 52c) has been performed on DNA from normal individuals forming a panel of 82 haplotypes to establish the distribution of these three alleles. Individuals of the DR3 haplotype had either the DRw52a or DRw52b allele, and individuals of extended haplotype HLA-A1,B8,DR3 had only the DRw52a allele. DR5 individuals all had the DRw52b allele, while individuals of DRw6 haplotype had the DRw52a, -52b, or -52c allele. None of these three alelles are found in DRw8 individuals. Analysis of this micropolymorphism, undetectable by common typing procedures, is therefore now operational for more accurate HLA matching for transplantation and for improving correlations between HLA and disease susceptibility.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelini G., de Preval C., Gorski J., Mach B. High-resolution analysis of the human HLA-DR polymorphism by hybridization with sequence-specific oligonucleotide probes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4489–4493. doi: 10.1073/pnas.83.12.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benacerraf B. Role of MHC gene products in immune regulation. Science. 1981 Jun 12;212(4500):1229–1238. doi: 10.1126/science.6165083. [DOI] [PubMed] [Google Scholar]
- Berdoz J., Gorski J., Termijtelen A. M., Dayer J. M., Irlé C., Schendel D., Mach B. Constitutive and induced expression of the individual HLA-DR beta and alpha chain loci in different cell types. J Immunol. 1987 Aug 15;139(4):1336–1341. [PubMed] [Google Scholar]
- Böhme J., Andersson M., Andersson G., Möller E., Peterson P. A., Rask L. HLA-DR beta genes vary in number between different DR specificities, whereas the number of DQ beta genes is constant. J Immunol. 1985 Sep;135(3):2149–2155. [PubMed] [Google Scholar]
- Chomczynski P., Qasba P. K. Alkaline transfer of DNA to plastic membrane. Biochem Biophys Res Commun. 1984 Jul 18;122(1):340–344. doi: 10.1016/0006-291x(84)90480-7. [DOI] [PubMed] [Google Scholar]
- Gorski J., Tilanus M., Giphart M., Mach B. Oligonucleotide genotyping shows that alleles at the HLA-DR beta III locus of the DRw52 supertypic group segregate independently of known DR or Dw specificities. Immunogenetics. 1987;25(2):79–83. doi: 10.1007/BF00364271. [DOI] [PubMed] [Google Scholar]
- Gorski J., Tosi R., Strubin M., Rabourdin-Combe C., Mach B. Serological and immunochemical analysis of the products of a single HLA DR-alpha and DR-beta chain gene expressed in a mouse cell line after DNA-mediated cotransformation reveals that the beta chain carries a known supertypic specificity. J Exp Med. 1985 Jul 1;162(1):105–116. doi: 10.1084/jem.162.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J. F., Auffray C., Korman A. J., Shackelford D. A., Strominger J. The class II molecules of the human and murine major histocompatibility complex. Cell. 1984 Jan;36(1):1–13. doi: 10.1016/0092-8674(84)90068-0. [DOI] [PubMed] [Google Scholar]
- Kaufman J. F., Strominger J. L. HLA-DR light chain has a polymorphic N-terminal region and a conserved immunoglobulin-like C-terminal region. Nature. 1982 Jun 24;297(5868):694–697. doi: 10.1038/297694a0. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Trowsdale J., Bodmer W. F. cDNA clones coding for the heavy chain of human HLA-DR antigen. Proc Natl Acad Sci U S A. 1982 Jan;79(2):545–549. doi: 10.1073/pnas.79.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. O., Wake C. T., Gorski J., Mach B. Complete sequence of an HLA-dR beta chain deduced from a cDNA clone and identification of multiple non-allelic DR beta chain genes. EMBO J. 1983;2(3):389–394. doi: 10.1002/j.1460-2075.1983.tb01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach B., Gorski J., Rollini P., Berte C., Amaldi I., Berdoz J., Ucla C. Polymorphism and regulation of HLA class II genes of the major histocompatibility complex. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):67–74. doi: 10.1101/sqb.1986.051.01.009. [DOI] [PubMed] [Google Scholar]
- Nagy Z. A., Baxevanis C. N., Ishii N., Klein J. Ia antigens as restriction molecules in Ir-gene controlled T-cell proliferation. Immunol Rev. 1981;60:59–83. doi: 10.1111/j.1600-065x.1981.tb00362.x. [DOI] [PubMed] [Google Scholar]
- Rollini P., Mach B., Gorski J. Linkage map of three HLA-DR beta-chain genes: evidence for a recent duplication event. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7197–7201. doi: 10.1073/pnas.82.21.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Spies T., Sorrentino R., Boss J. M., Okada K., Strominger J. L. Structural organization of the DR subregion of the human major histocompatibility complex. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5165–5169. doi: 10.1073/pnas.82.15.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termijtelen A., Schreuder G. M., Mickelson E. M., van Rood J. J. Ninth International Histocompatibility pre-workshop testing of Dw6 HTCs. Two subtypes of Dw6. Tissue Antigens. 1984 Jul;24(1):10–17. doi: 10.1111/j.1399-0039.1984.tb00391.x. [DOI] [PubMed] [Google Scholar]
- Tonnelle C., DeMars R., Long E. O. DO beta: a new beta chain gene in HLA-D with a distinct regulation of expression. EMBO J. 1985 Nov;4(11):2839–2847. doi: 10.1002/j.1460-2075.1985.tb04012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowsdale J., Young J. A., Kelly A. P., Austin P. J., Carson S., Meunier H., So A., Erlich H. A., Spielman R. S., Bodmer J. Structure, sequence and polymorphism in the HLA-D region. Immunol Rev. 1985 Jul;85:5–43. doi: 10.1111/j.1600-065x.1985.tb01129.x. [DOI] [PubMed] [Google Scholar]
- Ucla C., van Rood J. J., Gorski J., Mach B. Analysis of HLA-D micropolymorphism by a simple procedure: RNA oligonucleotide hybridization. J Clin Invest. 1987 Oct;80(4):1155–1159. doi: 10.1172/JCI113173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake C. T., Long E. O., Mach B. Allelic polymorphism and complexity of the genes for HLA-DR beta-chains--direct analysis by DNA-DNA hybridization. Nature. 1982 Nov 25;300(5890):372–374. doi: 10.1038/300372a0. [DOI] [PubMed] [Google Scholar]
- Wake C. T., Long E. O., Strubin M., Gross N., Accolla R., Carrel S., Mach B. Isolation of cDNA clones encoding HLA-DR alpha chains. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6979–6983. doi: 10.1073/pnas.79.22.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]