Abstract
Parathyroid hormone (PTH) and PTH-related protein (PTHrP) activate one single receptor (PTH1R) which mediates catabolic and anabolic actions in bone. Activation of PTH1R modulates multiple intracellular signaling responses. We previously reported that PTH and PTHrP downregulate pERK1/2 and cyclin D1 in differentiated osteoblasts. In this study we investigate the role of MAPK phosphatase-1 (MKP-1) in PTHrP regulation of ERK1/2 activity in relation to osteoblast proliferation, differentiation and bone formation. Here we show that PTHrP increases MKP-1 expression in differentiated osteoblastic MC3T3-E1 cells, primary cultures of differentiated bone marrow stromal cells (BMSCs) and calvarial osteoblasts. PTHrP had no effect on MKP-1 expression in proliferating osteoblastic cells. Overexpression of MKP-1 in MC-4 cells inhibited osteoblastic cell proliferation. Cell extracts from differentiated MC-4 cells treated with PTHrP inactivate/dephosphorylate pERK1/2 in vitro; immunodepletion of MKP-1 blocked the ability of the extract to dephosphorylate pERK1/2; these data indicate that MKP-1 is involved in PTHrP induced pERK1/2 dephosphorylation in the differentiated osteoblastic cells. PTHrP regulation of MKP-1 expression is partially dependent on PKA and PKC pathways. Treatment of nude mice, bearing ectopic ossicles, with intermittent PTH for 3-weeks, upregulated MKP-1 and osteocalcin, a bone formation marker, with an increase in bone formation. These data indicate that PTH and PTHrP increase MKP-1 expression in differentiated osteoblasts; and that MKP-1 induces growth arrest of osteoblasts, via inactivating pERK1/2 and downregulating cyclin D1; and identify MKP-1 as a possible mediator of the anabolic actions of PTH1R in mature osteoblasts.
Keywords: PTH, PTHrP, osteoblast, signaling
1.1 INTRODUCTION
Bone is a dynamic tissue being continuously destroyed by osteoclasts and renewed by osteoblasts. During skeletal development and remodeling, parathyroid hormone (PTH) and PTH-related protein (PTHrP) play crucial roles documented by PTH, PTHrP and PTH/PTHrP receptor (or PTH1R) knockout studies [1–3], and by targeted overexpresion of PTHrP [4], or constitutively active PTH1R [5, 6]. These data suggest that PTH and PTHrP are important physiologically for the maturation and differentiation of osteoblasts.
In vivo, PTH and PTHrP can be both catabolic and anabolic depending on their method of administration [7]. The anabolic effects of PTH and PTHrP have been well established yet their exact mechanisms of action are not clearly delineated [7]. Increased proliferation and differentiation of bone forming osteoblastic cells in vitro and in vivo, decreased osteoblast apoptosis, and activation of bone lining cells are among the several mechanisms that have been proposed for PTH anabolic actions (for review please see [8]).
Important crosstalks between the cAMP signaling cascade and mitogen-activated protein kinases (MAPKs) have been described in several hormonal systems in relation to proliferation and differentiation of various cell types [9, 10]. PTH shows both up- and down-regulation of MAPK during osteoblast proliferation and differentiation respectively [11–15]. PTH stimulates ERK1/2 by multiple mechanisms in cell lines transfected with recombinant PTH1R [16]. Our previous study demonstrated a bidirectional effect of PTHrP on MAPK in osteoblasts, increasing phosphorylated ERK1/2 (pERK1/2) in undifferentiated MC3T3-E1 cells but decreasing pERK1/2 in differentiated cells [17]. In proliferating osteoblastic cells PTHrP upregulates pERK1/2, increases cyclin D1 expression and promotes cell proliferation [18]. In contrast, in differentiated osteoblasts, PTHrP downregulates pERK1/2, decreases cyclin D1 expression and induces osteoblastic cell growth arrest, allowing more cellular differentiation and bone matrix formation [19].
MAPKs are activated by a cascade of phosphorylation and inactivated by dephosphorylation. A number of protein phosphatases deactivate MAPKs, including tyrosine, serine/threonine, and dual- specificity phosphatases [20–22]. In mammalian cells, the dual-specificity protein phosphatases, the MAP Kinase Phosphatases (MKPs), are the primary phosphatases responsible for dephosphorylation/deactivation of ERK1/2 [20]. Ten MKPs have been identified, one of which, MKP-1, is activated by PTHrP in pancreatic beta-cells [23], murine osteoblastic cells [24] and UMR cells [25, 26]. MKP-1 expression has been shown to be induced by agents that increase intracellular cAMP in PC12 cells [27].
In this paper we show that the activation of PTH1R induces MKP-1 expression in three different osteoblastic cell models, MC3T3-E1 clone 4 (MC-4) cells, primary cultures of bone marrow stromal cells (BMSCs), and mouse calvarial osteoblasts. Using a tissue engineered bone growth model, derived from BMSCs implanted in mice, we demonstrate that the anabolic response of PTH involves developmental stage specific up-regulation of MKP-1.
2.1 MATERIALS AND METHODS
2.1.1 Antibodies and Reagents
Antibodies to cyclin D1, MKP-1 and actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti phospho-ERK and total ERK antibodies were from Cell Signaling (Beverly, MA). Secondary antibody HRP conjugates to rabbit, mouse or goat immunoglobulins were obtained from Amersham (Piscataway, NJ) and Santa Cruz Biotechnology. Human PTH and PTHrP (1-34) were purchased from Bachem (Torrance, CA). Forskolin, PMA, GF109203X and H-89 were obtained from Calbiochem (San Diego, CA) and Lipofectamine reagent was from Invitrogen (Carlsbad, CA). A PTH analog, Gly1, Arg19-hPTH(1-28) NH2, which is more selective for cAMP stimulation [28] was kindly provided by Dr. Thomas Gardella of Mass General Hospital. 3-(4,5 Dimethylthiazol-2yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) was obtained from Sigma-Aldrich (St. Louis, MO).
2.1.2 Cell Culture, Differentiation and Treatments
MC3T3-E1 subclone 4 cells (MC-4) with high osteoblast differentiation potential were maintained and passaged every 4–5 days as previously described [18, 19]. Briefly, cells were cultured in minimum essential medium alpha (αMEM) (Invitrogen, Carlsbad, CA) containing 100 units/ml penicillin and streptomycin and 10% fetal bovine serum. For proliferation studies MC-4 cells were plated at a less than 5,000 cells/cm2 overnight followed by synchronization via serum starvation for 36– 48 h prior to 100 nM PTHrP (1-34) treatment as described previously [18]. Cells were plated at 40–50,000 cells/cm2 and differentiation was induced with the addition of ascorbic acid (50 μg/ml) for 7 days [19]. The culture medium was changed at days 1, 3, 5, and 7. Cells were subsequently treated with vehicle or PTHrP at 5– 1000 nM for 30 – 240 min as indicated. For some experiments cells were pretreated with H-89 (20 μM) or GF109203X (1μM) for 30 min before the addition of PTHrP (100 nM). In additional experiments the cAMP agonist Forskolin (10 μM) or PKC agonist PMA (0.1μM) was added to the cells for 1h without PTHrP treatment [18, 19]. The cell layers were harvested [19], and subsequent experiments were performed. Primary mouse calvarial cells were isolated as previously described [19]. Briefly, calvaria of mice were dissected, isolated and subjected to sequential digestions in collagenase A (2mg/ml) and 0.25% trypsin for 20, 40, and 90 min. Cells from the third digest were washed, counted and plated in αMEM with 10% FBS containing 100 U/ml of penicillin and streptomycin. Harvesting of BMSCs was performed as described [18, 29]. Primary cultures were used without passage.
2.1.3 MKP-1 Overexpression
A mammalian expression vector encoding MKP-1 (pSRα-Flag-Srf 1/MKP-1) construct was created by replacing the HA-JNK1 coding sequence with a Flag-MKP-1 sequence PCR- amplified from rat MKP-1 cDNA [30], kindly provided by Yusen Liu (Department of Pediatrics, The Ohio State University) and Keith L Kirkwood (Medical University of South Carolina). Proliferating MC-4 cells were seeded in 6 well plates at a density of 1×105cells/well in 10% FBS and α-MEM without penicillin and streptomycin. Cells were grown to 80% confluence before transfection. Cells were transfected with pSRα-Flag-Srf 1/MKP-1 expression vector or control empty vector in 1ml with Lipofectamine reagent (Invitrogen) and OPTI-MEM (Invitrogen) according to manufacturer’s instructions replacing the growth media. Stable transfections were performed along with a puro plasmid, pSELECT-puro-mcs (InvivoGen, San Diego, CA) to allow the selection of transfected clones. Transfections were performed at a concentration of 0.9–1.0 μg per 105 cells. Each transfection contained a combination of 0.7 μg of the MKP-1 or vector control plasmid and 0.2 μg of puro plasmid. Transfection was done by incubation for 6–8 hours at 37° C and 5% CO2 before adding additional 1ml of growth media with 20% FBS. Medium was changed with 10% FBS containing media after 24 h transfection. Selection was made with 4 μg/ml puromycin and the stable cells were maintained in the same selection media. Stable colonies were developed within 6 to 8 weeks and overexpression of MKP-1 was confirmed by quantitative real-time PCR with MKP-1 specific primers and by Western, using antibodies to MKP-1 protein. Selected highly expressed clones were then seeded in 12 well plates at equal densities (5000 cells/well) for MKP-1 and empty vector transfected cells. Cell growth was monitored over 8 days and viable cell growth rate was determined using MTT reduction activity as an index of the cellular proliferation.
2.1.4 MTT assay
Cell proliferation analysis was performed by the MTT reduction to formazan in living cells as a measure of mitochondrial activity. PBS solution of MTT (0.5 mg/ml) was incubated with cells in 12 well plates for 4 hours. The resulting formazan crystals were dissolved by the addition of 1 ml isopropyl alcohol to each well and optical density read at a wavelength of 595 nm.
2.1.5 Analysis of mRNA by Real-time PCR
mRNA isolation of cells were performed with TRIZOL reagent (Invitrogen), and cDNAs were prepared using the TaqMan® Reverse Transcription assay system (Applied Biosystems, Foster City, CA). Real- time PCR was performed using StepOne Plus real-time PCR system (Applied Biosystems) with FAM labeled primers assay systems (cyclin D1 #Mm00432359; MKP-1 # Mm01309842; ALKP # 01187117; OCN part# 4331348; GAPDH # 99999915) from Applied Biosystems. GAPDH was used as an internal control.
2.1.6 SDS-PAGE and Western Analysis
SDS-PAGE and Western analysis were performed as described previously [18, 19]. Cells were washed twice with cold PBS, scraped and lysed for 30 min at 4°C following sonication with RIPA buffer containing protease inhibitors (Sigma). Cell lysates were cleared by centrifugation at 14,000 × g for 45 min. An aliquot of each lysate was removed for protein concentration determination. SDS-PAGE was performed in 10–12% polyacrylamide. Each lane contained between 40–80 μg of cell (lysate) protein. For a given western analysis each lane received equal protein loads. Pre-stained molecular weight standards were run in parallel lanes. After electrophoresis, the proteins were transferred to PVDF membrane (Bio-Rad laboratories, Inc., Hercules, CA) in buffer containing 25 mM Tris-HCL, 192 mM glycine, 20% v/v methanol, 0.01% SDS (pH 8.5). Residual protein binding sites on the membrane were blocked by incubation for 3 hr to overnight in TBST buffer (20 mM Tris-HCL, pH7.6, 137 mM NaCl, 0.5% Tween-20) containing 5% nonfat dry milk. The membrane was then incubated with primary antibody for 2 to 12 hr. After washing with TBST, secondary antibody (anti IgG conjugated with horseradish peroxidase) was added and incubated for 20–60 min. Finally the proteins were visualized by autoradiography using an enhanced chemiluminescence (ECL) detection system (Pierce, Rockford, IL, USA). The protein band intensities on ECL Western autoradiograms (all with exposures within the linear range of the film) were quantitated using Scion software (Frederick, MD).
2.1.7 In Vitro De-phosphorylation Assay
Both proliferating and differentiated MC-4 cells were used for this study. Proliferating cells were serum starved and treated with 100 nM PTHrP (1-34) or PMA for 10 min [18] to induce pERK1/2 and generate the substrate lysate. Differentiated MC-4 cells were treated with 100 nM PTHrP (1-34) for 1h to induce MKP-1 expression and generate the phosphatase lysate. After treatment, cellular extracts were prepared as above and protein concentrations measured. For in vitro de-phosphorylation, equal quantities of protein (50 μg) from phosphatase lysate and substrate lysate were mixed and incubated at 30° C for 45 min. De-phosphorylation reaction was stopped by addition of sample buffer and heating at 95° C for 10 min. For some experiments MKP-1 was immunodepleted from the phosphatase lysate. Immunodepletion was carried out with agarose conjugated MKP-1 antibody (Santa Cruz) and immune complexes were removed by centrifugation. For complete depletion the procedure was repeated three times and loss of MKP-1 protein was verified by Western blot analysis of the depleted lysate. The MKP-1 depleted lysate was incubated with the substrate lysate and reaction terminated as above. As a negative control phosphatase lysate were incubated with an unrelated antibody (anti JunB) and immune complexes were removed before the dephosphorylation reaction as above. Samples were resolved by SDS-PAGE and Western analysis was performed with antibodies to p-ERK1/2 and total ERK. To determine the basal MKP-1 activity in differentiated lysate, MC-4 cell lysates were prepared after differentiation without PTHrP treatment, incubated with substrate lysate and processed as above.
2.1.8 Generation of Ectopic Ossicles (Tissue Engineered Bone)
Harvesting of BMSCs, cell implantation and generation of ectopic ossicles was performed as described [18, 29]. Four to 8 week old C57BL/6 mice were used to isolate BMSC. Bone marrow flushed with αMEM from the femoral, tibial, and humeral cavities, was placed into a 75-cm2 culture flask in 30 ml of growth media and maintained at 37°C. When an adherent confluent layer was formed, the cells were passaged and maintained for 5–7 days before implantation. BMSC pellets were incorporated into pre- soaked 3–5mm gelatin sponges and implanted subcutaneously in 4–6 week old male nude mice (NIH III Nude; Charles River Laboratories, Wilmington, MA) following anesthesia. Blunt dissection was used to form subcutaneous pouches and each animal received four implants. All animal protocols were performed in compliance with the Institutional Animal Care and Use Committee for the Use and Care of Animals.
2.1.9 In vivo Injection of PTH and Harvesting Ossicle
At one week post-implantation the animals were either injected subcutaneously with a single dose of recombinant human PTH (20 μg/kg) for 8h or 12h to evaluate the acute effects, or, administered daily subcutaneous injection of either PTH (40 μg/kg) or vehicle (0.9% sodium chloride) for one week or three weeks. Mice were then sacrificed at the end of each treatment period and ectopic ossicles were harvested. Ossicles were flash-frozen in liquid nitrogen and total RNA was isolated as previously described [18, 29]. Finally, cDNA was prepared using the TaqMan® Reverse Transcription assay system (Applied Biosystems). Real time PCR was performed using either the ABI PRISM 7700 or StepOne Plus real-time PCR system (Applied Biosystems) with a FAM labeled primer assay system (Applied Biosystems) as above. GAPDH was used as an internal control.
2.1.10 Micro-computed tomography (microCT)
Ossicles were scanned on a 3D microCT scanner (eXplore Locus, GE Healthcare Biosciences, London, ON) located at John D. Dingell VA Medical Center. Images were reconstructed with an isotropic resolution of 27 μm. Scanning procedure also included the use of a calibration phantom (array of materials at known densities). Analysis of bone parameters was performed using MicroView software (MicroView, GE Healthcare Biosciences). Bone regions of interest were manually segmented using the Advanced Region of Interest (ROI) tool in MicroView. Contours were drawn around ossicles in sequential 2D image sections. The contours were then interpolated and a 3D ROI was created. Histograms were then generated to select a global mineralized tissue threshold that delineated bone from all other tissues. Bone volume (BV) and total volume (TV) was analyzed within this ROI using the Bone analysis module in MicroView.
2.1.11 Densitometry and Statistical Analysis
Autoradiograms (all with exposures within the linear range of the film) were quantitated using Scion software (Frederick, MD). Results were analyzed using ANOVA followed by a Tukey-Kramer multiple comparison test or Student’s t test, with the Instat 2.1 biostatistics program (GraphPad Software, San Diego, CA) and data expressed as mean ± SEM.
3.1 RESULTS
3.1.1 PTHrP up-regulates MKP-1 protein in a dose and time dependent manner in differentiated MC- 4 osteoblasts
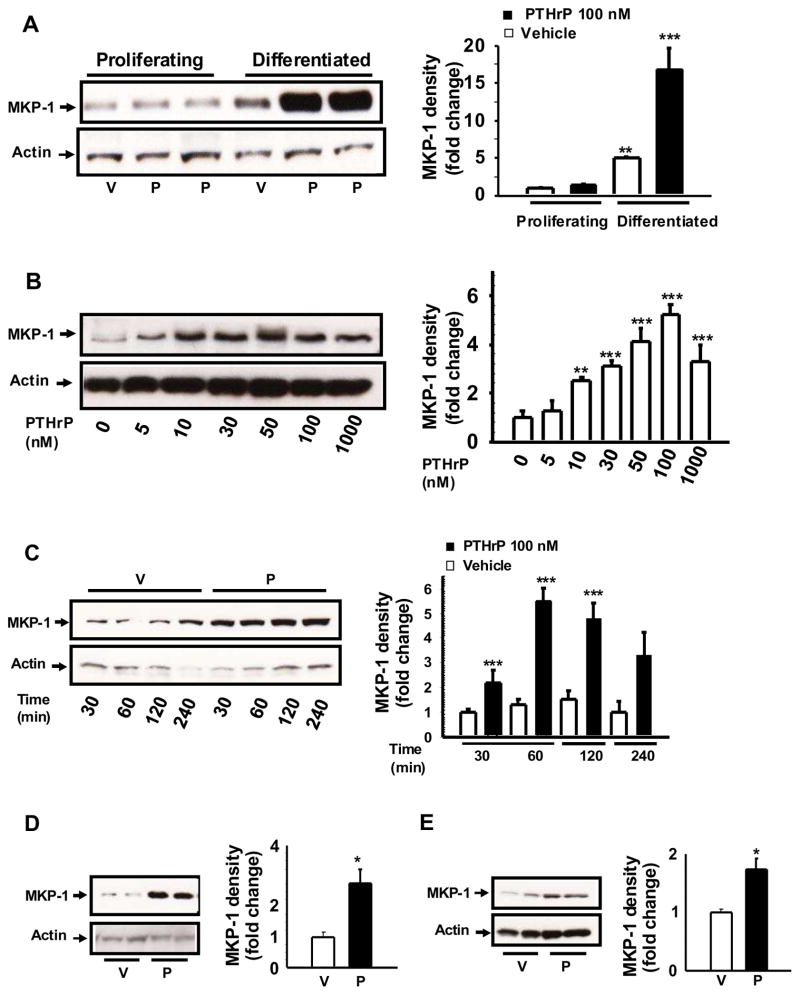
To assess the role of MKP-1 in PTHrP regulation of ERK1/2 activity we examined the effects of PTHrP on MKP-1 expression in differentiated MC-4 cells. The cells were differentiated in the presence of ascorbic acid for 7 days then challenged with increasing concentrations of PTHrP for different time periods. Western blot analyses of cellular extracts from differentiated and proliferating MC-4 cells showed that PTHrP increases MKP-1 protein in differentiated MC-4 (Fig. 1A). PTHrP had no effect on MKP-1 expression in the proliferating MC-4 cells (Fig 1A). While the basal expression of MKP-1 is increased 3–5 fold in differentiaed osteoblasts compared to proliferating cells, PTHrP induction of differentiated osteoblasts further upregulates the relative abundance of MKP-1 protein (Fig. 1A). Incubation of MC-4 cells with increasing concentrations of PTHrP (5–1000 nM) for 1 h dose-dependently increased MKP-1 protein level with a maximum effect at 100 nM (Fig 1B). To determine the optimal time point of MKP-1 up-regulation by PTHrP, differentiated MC-4 cells were treated with 100 nM PTHrP for different time periods (30 min – 4h, Fig 1C). MKP-1 levels increased at 30 min with maximum up-regulation at 1–2 h. Incubation with PTHrP for shorter time periods, 10 and 20 min, had no significant effect on MKP-1 (data not shown). Finally, Western blot analysis was performed with cellular extracts from 7 day differentiated primary BMSCs or calvarial osteoblasts following 1h PTHrP or vehicle treatment. Results demonstrate significant increase in MKP-1 protein in PTHrP induced BMSCs (Fig 1D) and in calvarial osteoblasts (Fig 1E) compared to vehicle reatment.
Figure 1. Relative protein abundance of MKP-1 protein after PTHrP treatment.
Representative Western blots from three to four independent experiments are shown. Cell plating, differentiation and PTHrP (1-34) (P) treatment were done as described [19]. A. MKP-1 protein levels in proliferating and differentiated MC-4 cells. Dose- dependence and time-course analysis of MKP-1 protein expression in differentiated MC-4 cells were performed in response to B. various concentrations (5 – 1000 nM) of P or vehicle (V) for 1 hour, and C. with 100 nM P or V for 30 min –4h. MKP-1 protein levels were also determined in primary cultures of D. BMSCs, and E. Calvarial osteoblasts following 1 hour of 100 nM P or V treatments. Total cellular protein was harvested and subjected to SDS-PAGE with equal protein loads. Western blot analysis was performed as described in materials and methods, using the anti MKP-1 antibody and visualized by an enhanced chemiluminescence (ECL) –based detection method. Whole cell lysates were also probed with anti Actin antibody as a protein loading control. Densitometric values were normalized with actin and plotted. Representative data from three to four independent experiments are shown. Values are mean ± SEM. ***p<0.001; **p< 0.05; *p<0.02 vs V.
3.1.2 Effects of PTHrP on MKP-1 mRNA expression
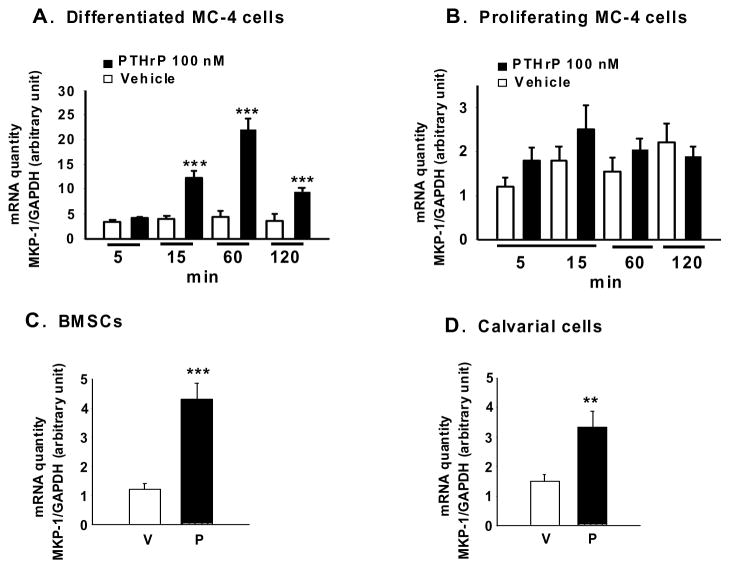
To further understand MKP-1 regulation by PTHrP, the levels of MKP-1 mRNA were evaluated by real- time PCR. MC-4 cells were differentiated for 7-day and treated with PTHrP (100 nM) for different time periods (5 min – 2 h). MKP-1 mRNA levels in differentiated MC-4 cells were up-regulated by 15 min PTHrP treatment with a maximal level at 60 min of incubation and remained elevated up to 2h (Fig. 2A). In contrast, PTHrP has no effects on MKP-1 mRNA levels in the proliferating MC-4 cells (Fig. 2B). We then examined PTHrP regulation of MKP-1 expression in primary mouse BMSCs and calvarial osteoblasts differentiated for 7 days. Incubation of the cells with PTHrP for 1 h increased MKP-1 mRNA levels in both BMSCs (Fig. 2C) and calvarial osteoblasts (Fig. 2D).
Figure 2. Effect of PTHrP on MKP-1 mRNA in differentiated MC-4 cells and primary osteoblasts.
Real-time PCR were used to analyze RNA extracted from A. 7-day differentiated MC-4 cells, B. Proliferating MC-4 cells, C. BMSCs and D. calvarial osteoblasts. Cells treated with 100 nM PTHrP or vehicle either for different time points (A, B) or for 1h (C, D). GAPDH was used as an internal control. Representative data from three independent experiments are shown. Results are expressed as mean ± SEM. ***p<0.001, **p<.01 vs vehicle. P, PTHrP (1-34); V, vehicle.
3.1.3 Intracellular Signals Involved in PTHrP up-regulation of MKP-1
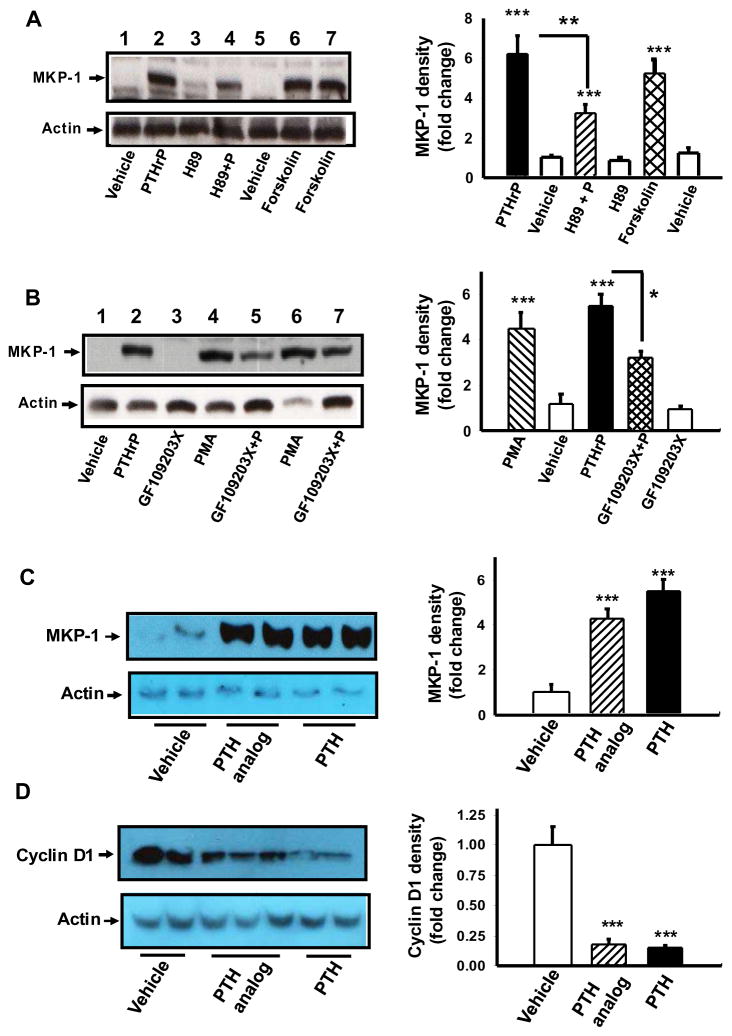
To gain insight into the signaling events involved in MKP-1 up-regulation by PTHrP in differentiated MC-4 cells, inhibitors and/or activators of PTHrP signaling pathways were used. The effect of PTHrP on MKP-1 was mimicked by Forskolin (Fig. 3A, lanes 6, 7) and PMA (Fig. 3B lanes 4, 6). Inhibitors of PKA (Fig. 3A, lane 4) and PKC (Fig. 3B, lanes 5, 7) partially blocked the effect of PTHrP on MKP-1. These data suggest involvement of both PKA and PKC signaling in PTHrP stimulation of MKP-1 expression in differentiated MC-4 osteoblastic cells.
Figure 3. Up-regulation of MKP-1 by PTHrP is cAMP, PKA and PKC dependent.
MC-4 cells were induced to differentiate with ascorbic acid for 7 days and differentiated cells were (A) pretreated with PKA inhibitor H-89 (20 μM) for 30 min, (B) pretreated with GF109203X (1 μM) for 30 min, followed by treatment with or without 100 nM PTHrP or vehicle for 1hr, To determine the effects of cAMP or PKC agonists on MKP-1 protein expression, differentiated cells were also exposed to (A) Forskolin (10 μM) or (B) PMA (0.1 μM) for 1h. Differentiated cells were also treated with either 100 nM PTH or PTH analog or vehicle for (C) 1h or (D) 4–5 h. Total cellular extracts prepared, SDS PAGE and Western blot analysis performed with anti MKP-1 or anti cyclin D1 or anti actin (as a loading control) as indicated. Representative data from three to four independent experiments are shown. Densitometric values were normalized to actin and plotted. Values are mean ± SEM. ***p<0.001; **p<0.002; *p<0.01 vs vehicle.
PTH analogs with selective signaling properties are useful for probing the signaling pathways involved in PTHrP action on MKP-1. [Gly1,Arg19]hPTH(1-29)NH2 (GR-PTH) selectively increases cAMP accumulation in PTH1R expressing cells with little effects, if any, on the phospholipase C and protein kinase C signaling cascade [28]. Both hPTH(1-34) and GR-PTH augmented (5–8 fold) MKP-1 protein expression in differentiated MC-4 cells (Fig. 3C). To gain insight into the signaling events that link PTHrP induced MKP-1 to the cell cycle regulatory machinery the effects of GR-PTH on cyclin D1 was determined in differentiated MC-4 cells. PTH and GR-PTH decreased cyclin D1 protein in these cells (Fig. 3D).
3.1.4 MKP-1 overexpression in MC-4 cells induces growth arrest
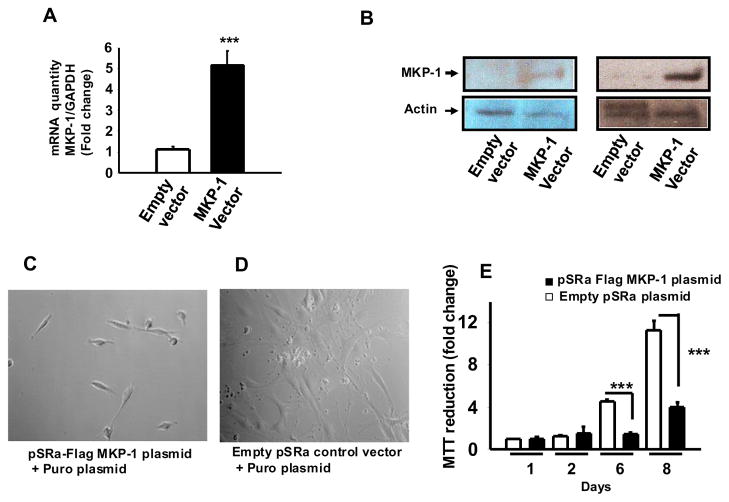
To determine the effect of MKP-1 on the growth of MC-4 cells, cells were stably transfected with an MKP-1 expression vector pSRα-Flag-MKP-1 and subclones over-expressing MKP-1 were generated and screened for MKP-1 mRNA and protein expression levels. The subclones showed 4–6 fold increase in MKP-1 mRNA (Fig. 4A) and 2–5 fold increase in MKP-1 protein levels (Fig. 4B).
Figure 4. Overexpression of MKP-1 induces MC-4 osteoblastic cell growth arrest.
MC-4 cells were transfected with an expression vector for MKP-1 (pSRα-Flag-MKP-1) or an empty vector control. (A) Real time PCR analysis of MKP-1 mRNA levels from MC-4 cell transfectants. Datta shown are mean ± SEM from six individual clones. Each clone was assayed in triplicates. (B) Representative Western Blot for MKP-1 protein levels in extracts from MC-4 cell transfectants. MKP-1 overexpressing cells showed higher mRNA and protein as compared with the respective controls. Growth of (C) pSRα-Flag-MKP-1 tranfected MC-4 cells or (D) empty pSRα-plasmid transfected cells cultured for 8 days. (E) MTT reduction activity. Representative data from three independent clones are shown. Values are mean ± SEM (n=4). ***p<0.001.
We used the highest expressing cell lines to evaluate the possible role of MKP-1 in osteoblast proliferation. Monitoring cultures for 8 days showed decreased cell density in MKP-1 overexpressing cells (Fig. 4C) compared to vector transfected cells (Fig. 4D). The MTT reduction activity was significantly decreased with increasing culture periods in MKP-1 overexpressing clones (Fig. 4E). This observation suggests that overexpression of MKP-1 decreases osteoblastic cell proliferation.
3.1.5 MKP-1 is responsible for PTHrP-induced dephosphorylation of p-ERK1/2
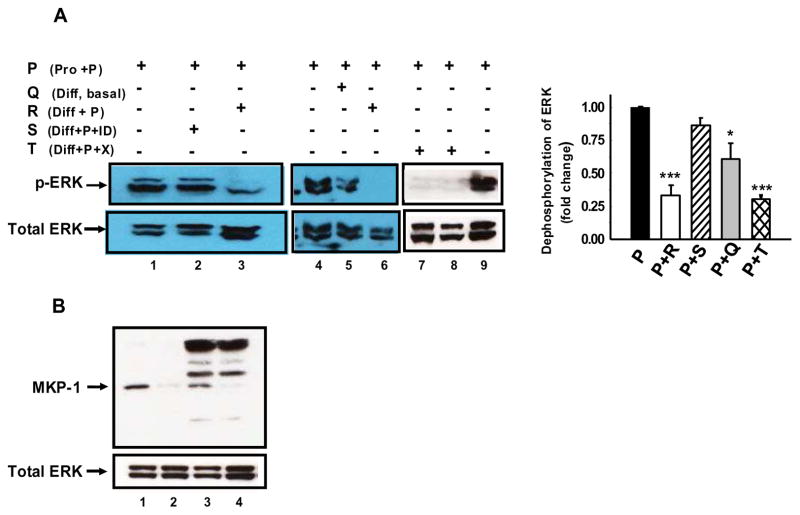
To examine if PTHrP induced up-regulation of MKP-1 accounts for the PTHrP induced dephosphorylation of pERK1/2, we performed an in vitro dephosphorylation assay by incubating cell lysate rich in pERK1/2 with cell lysate rich in MKP-1 from PTHrP treated cells. The pERK1/2 rich lysate was obtained from proliferating MC-4 cells challenged with PTHrP for 10 min whereas the MKP- 1- rich lysate was obtained from differentiated MC-4 cells challenged with PTHrP for 1 h. Equal quantities of MKP-1- rich and p-ERK-rich lysates were mixed and incubated at 30° C for 45 min (Fig. 5A). Compared to a control lysate, the MKP-1- rich lysate caused dephosphorylation of the pERK1/2- rich lysate (Fig 5A, lanes 3 and 6). Using a specific antiserum, we immunodepleted MKP-1 from the MKP-1- rich lysate (Fig. 5B, lane 4) and examined its effects on pERK1/2 dephosphorylation. The MKP- 1- depleted lysate did not cause any significant dephosphorylation of the pERK1/2- rich lysate (compare lanes 2 and 1, Fig 5A). In contrast, MKP-1-rich lysate incubated with an unrelated antibody, anti JunB, caused dephosphorylation of the pERK1/2-rich lysate (Fig. 5A, lanes 7 and 8); the presence of MKP-1 was confirmed in the JunB immune-depleted lysate (Fig. 5B, lane 3). These data suggest that MKP-1 upregulation by PTHrP is responsible for PTHrP-induced dephosphorylation of pERK1/2 in the differentiated osteoblasts.
Figure 5. MKP-1 is the phosphatase responsible for PTHrP induced ERK-MAPK dephosphorylation.
A. PTHrP induced ERK dephosphorylation. P (Pro+P): Lysate from proliferating cells treated with PTHrP, Q (Diff, basal): Lysate from differentiated cells without PTHrP treatment, R (Diff+P): Lysate from differentiated cells treated with PTHrP, S (Diff+P+ID): Lysate from differentiated cells treated with PTHrP followed by 3 round of MKP-1 immunodepletion. T (Diff+P+X): Lysate from differentiated cells treated with PTHrP followed by incubation with unrelated antibody. Mixing lysate (P) from proliferating cells with lysate (R) from differentiated cells treated with PTHrP dephosphorylate ERK (lanes 3 and 6). MKP-1 immune depletions prevent ERK dephosphorylation (lane 2). Mixing lysate (P) from proliferating cells with lysate (Q) from basal differentiated cells partially dephosphorylate ERK (lane 5). Incubation with unrelated antibody does not prevent ERK dephosphorylation. (lanes 7 and 8). Representative data from three independent experiments are shown. Densitometric values were normalized to Total ERK and plotted. Values are mean ± SEM. ***p<0.001; *p<0.05 vs P (Pro+P). B. Western blot analysis of lysate from differentiated cells treated with PTHrP (lane 1), lysate from proliferating cells treated with PTHrP (lane 2), lysate from differentiated cells treated with PTHrP followed by incubation with unrelated antibody beads as a negative control (lane 3), Lysate from differentiated cells treated with PTHrP followed by 3 rounds of MKP-1 immunodepletion (lane 4).
3.1.6 MKP-1 expression is induced in PTH treated mature ectopic ossicles implanted in nude mice
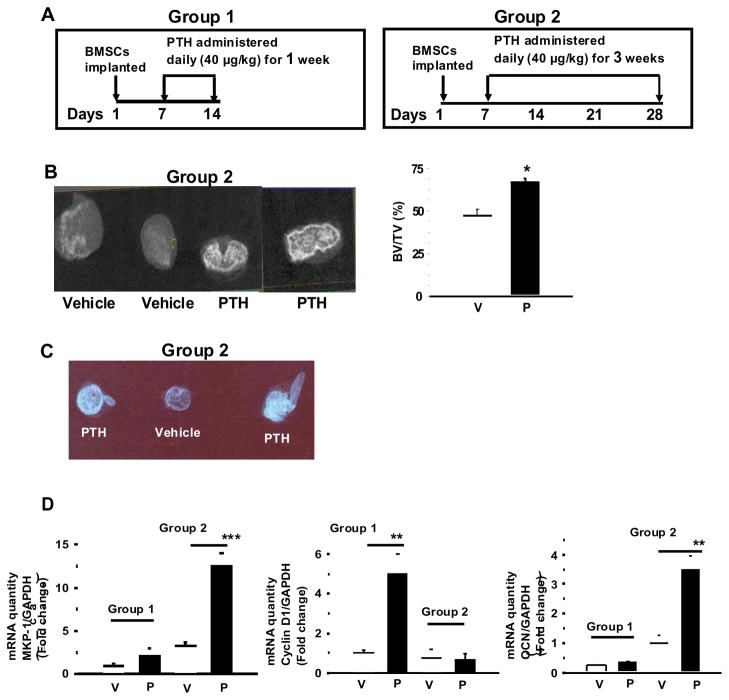
We have previously used ectopic ossicles as a model to study the anabolic effects of PTH in vivo and showed PTH regulation of cyclin D1 [18]. To further examine in vivo the biological effects of PTH during osteoblast proliferation and differentiation we studied the effects of PTH on ossicles generated in nude mice. Two groups of animals were subcutaneously injected with daily PTH (40 μg/kg) or vehicle, for one week or three weeks (Fig. 6A). Ectopic ossicles were collected and MicroCT and Microradiographic images of ossicles harvested from PTH or vehicle- treated mice were taken to evaluate the extent of mineralization and bone formation. Similar to previous observations [29] there was an increase in bone formation and a marked increase in radiopacity in the ossicles from 3 week PTH treated animals (Fig. 6B and 6C) compared to vehicle or one week PTH treatment (data not shown); these data confirm increased mineral content and bone formation.
Figure 6. Effect of anabolic PTH in vivo on ossicles of bone.
A. Experimental design used to characterize the response of BMSC-derived ectopic ossicles. Subcutaneous anabolic injections (1 or 3 week) of PTH (1-34) or vehicle (0.9% saline) were administered to immunocompromised mice one week after the implantation procedure. The animals were sacrificed at the end of the treatment period and RNA isolated. B. Micro-computed tomography (microCT) and C. Micro-radiographic images were obtained for representative group 2 ossicles harvested from PTH or vehicle treated mice. D. Real-time PCR were performed for mRNA quantity of MKP-1, cyclin D1 and OCN in Group 1 and Group 2 ossicles, normalized to GAPDH. Results are expressed as mean ± SEM (n = 10–15). BV, bone volume; TV, total volume; V, vehicle; P, PTH; ***p<0.001; **p<0.01; *p<0.02 vs. vehicle.
MKP-1 mRNA levels were measured by real-time PCR in total RNA prepared from the ectopic ossicles. Studies with 1 week old immature ossicles showed no significant difference in MKP-1 mRNA levels at 8 hour or 12 hour after single dose of PTH treatment (data not shown), or after daily PTH administration for 1 week (Fig. 6D, Group 1). In the more mature ossicles, MKP1 mRNA expression was significantly increased after 3 week daily PTH administrations (Fig. 6D, Group 2). On the other hand, the mRNA level of cyclin D1 was only increased in group 1 immature ossicles (Fig. 6D).
Real-time PCR was also performed for alkaline phosphatase and osteocalcin mRNA expression after 8 and 12 hour acute injection with PTH. There was no PTH effect on osteocalcin or alkaline phosphatase mRNA levels at 8 or 12h after one single injection (data not shown). In contrast, a significant rise in osteocalcin expression was noted only in group 2 after 3 weeks of daily PTH injections (Fig. 6D, Group 2).
4.1 DISCUSSION
Osteoblast differentiation is a critical step in the process of bone formation. PTH is known to increase net bone mass when administered intermittently, whereas when administered continuously PTH results in the opposite net effect on bone mass (reviewed in [31]). However, the molecular mechanism regulating this process remains unclear. Work from several laboratories established the involvement of several cell cycle regulatory proteins, but the definitive mechanism of PTH-induced bone formation remains controversial. Our previous studies in osteoblasts demonstrated that PTH and PTHrP regulate ERK-MAPK and several cell cycle regulatory proteins including cyclin D1, CDK1 and p27 [17–19]. PTH1R stimulation of ERK1/2 phosphorylation was observed in proliferating osteoblastic cells not in mature differentiated osteoblasts, even though the abundance of total ERK1/2 did not differ between the two states. Our data indicate that increased ERK1/2 signaling and cyclin D1 level are important for PTH1R regulation of early osteoblastic cells and that decreased ERK activation is required to induce osteoblastic cell growth arrest in mature cells and increased bone formation.
The role of ERK activation in osteoblast differentiation has been the subject of extensive investigation. ERK activation was reported to be required for osteoblast differentiation by several investigators [32, 33] whereas others have shown that ERK activation inhibits osteoblastic differentiation [13, 34]. Consistent with our data, studies showed that osteogenesis induced by the bone morphogenetic protein (BMP) and PTH is associated with down-regulation of pERK in BMSCs and MC3T3-E1 cells [35, 36].
MAPK activities are regulated by two opposing events (i.e. phosphorylation and dephosphorylation). Activation occurs through the reversible phosphorylation of both threonine and tyrosine residues of the TXY motif in the catalytic domain by upstream kinases (MEKs) [37, 38]. On the other hand, MAPKs are deactivated by members of the MAPK phosphatases such as MKP-1 [20]. The MKP-1 gene is an immediate early response gene whose expression is induced rapidly by a variety of extracellular stimuli. We hypothesized that MKP-1 may be involved in PTH1R regulation of pERK1/2 and cyclin D1 in osteoblastic cells. This study supports our hypothesis by demonstrating increased MKP- 1 protein and mRNA levels in differentiated MC-4, BMSCs, and calvarial osteoblasts following PTHrP treatment. We also found that MKP-1 up-regulation is sufficient to account for the decreased ERK1/2 activity in differentiated osteoblasts and that overexpression of MKP-1 in proliferating MC-4 cells induces growth arrest mimicking the effects of PTHrP, supporting the involvement of MKP-1 in PTH1R regulation of osteoblast functions.
PTH1R is known to signal primarily through PKA, which governs majority of its effects on osteoblasts. Here we show that pharamacological inhibition of PKA partially blocked MKP-1 up- regulation by PTHrP and that a signal-specific PTH analog, which selectively increases cAMP, increased MKP-1 maximally. In addition, using GF109203X, a pharmacological inhibitor of PKC, our studies also show that up-regulation of MKP-1 was partially dependent on PKC pathway. We previously reported p- ERK down-regulation by PTHrP in differentiated MC-4 cells [17]. In a preliminary study we also demonstrated a temporal regulation of p38 following PTHrP induction, being increased in earlier time points and down-regulated at later time points [39]. Taken together these data suggest a crosstalk between cAMP/PKA, PKC and MAPK pathways in PTH1R regulation of MKP-1 expression in differentiated osteoblasts.
The regulated dephosphorylation of MAPKs plays a key role in determining the magnitude and duration of kinase activation and hence the physiological outcome of signaling. By immune depletion experiments, we demonstrated that MKP-1 is responsible for the effects of PTHrP on pERK dephosphorylation in differentiated MC-4 cell lysates. We also found that the basal MKP-1 level in differentiated cells partly abrogated PTHrP induced ERK1/2 phosphorylation. One possible mechanism could be that, as osteoblasts begin to mature the level of basal MKP-1 increases, which dephosphorylates ERK, slows cell growth and promotes osteoblastic differentiation; such a mechanism is augmented by PTH1R. This is conistent with the hypothesis that ERK dephosphorylation is essential for osteoblast differentiation [13, 34].
Our previous study suggested cyclin D1 as a target for the proliferative effects of PTH and PTHrP in early osteoblastic cells both in vitro and in vivo [17–19]. BrdU incorporation also demonstrated osteoblast proliferation in early ectopic ossicles confirming PTH regulation of osteoblast proliferation in immature osteoblasts [40]. In this study intermittent PTH administration for 1 week in nude mice bearing oscicles did not cause significant increase in osteoblast differentiation markers, alkaline phosphatase or osteocalcin, and did not show any increase in MKP-1 expression, while cyclin D1 expression was increased. In contrast, intermittent PTH administration for 3 weeks increased bone formation and MKP1 expression. An increase in bone mass following PTH administration for 3-week in 1-week post implantation ossicles was also demonstrated previously determined by histological analysis (H & E staining), histomorphometry and osteocalcin gene expression [18, 29]. Taken together, our findings suggests that PTH1R regulation of MKP-1 and anabolic action in osteoblasts is developmental stage specific.
It has been shown that MKP-1 can inactivate all three major MAPKs, including ERK, JNK, and p38 [41–43]. Because ERK, JNK, and p38 are capable of inducing either apoptosis or cell proliferation, MKP-1 is believed to be involved in regulating the cell cycle or apoptosis [44–48]. We and others have shown that PTH1R regulates p38 in differentiating osteoblastic cells [39, 49]. Also, a constitutively active MKK6 transgene, that specifically activate p38, inhibits proliferation, cyclin D1 expression and delays endochondral bone formation [50]. Finally, MKP-1 knock out mice have confirmed the central function of MKP-1 in the feedback control of p38 and JNK activity as a negative regulator of the synthesis of pro-inflammatory cytokines in vivo [51]. Inflammation-induced bone loss [52], and PTH regulation of IL-6 [17], IL-18 [53] and high mobility group box 1 (HMGB1), an immunomodulatory cytokine in osteoblasts [54], raises the possibility that MKP-1 may play a role in inflammation-induced bone resorption.
In summary, we have shown that PTH induced MKP-1 is an important regulator of osteoblast cell cycle and may be involved in bone formation. Importantly, we showed that upregulation of MKP-1 by PTH and PTHrP is dependent on the maturation stage of osteoblasts thus promotes cell proliferation of immature pre-osteoblasts and further advances cell differentiation and bone formation in mature osteoblastic cells. Based on the data presented here and those in the literature we propose that modulation of MKP-1 may be an effective approach for the development of new treatments for osseous defects and bone regeneration.
Acknowledgments
The authors wish to thank Thomas Gardella (Mass General Hospital) for providing cAMP specific PTH analog, Yusen Liu (The Ohio State University) and Keith L Kirkwood (Medical University of South Carolina) for providing pSRα-Flag-Srf 1/MKP-1 expression vector, Renny Franceschi (University of Michigan) for MC3T3-E1 clone 4 cells. Acknowledgement is also due to animal care facilities, Wayne State University, for maintenance of the nude mice strain at Elliman building used during generation of ectopic ossicles in vivo. We thank Richard Miller, John D. Dingell VA Medical Center, Detroit, for our use of the microCT scanner at the VA Medical Center facility. We acknowledge Mohammad Habib for providing technical assistance. Funding was supported by National Institute of Health Grant DE016865 (to NSD), and DK062286 (to ABA).
Abbreviations
- PTH
parathyroid hormone
- PTHrP
PTH related protein
- PTH1R
PTH/PTHrP receptor
- MC-4
MC3T3-E1 clone-4
- BMSC
Bone marrow stromal cell
- MAPK
Mitogen Activated Protein Kinase
- MKP-1
MAPK Phosphatase-1, ERK, extracellular signal-regulated kinase
- MTT
3-(4,5 Dimethylthiazol-2yl)-2,5-diphenyl-2H-tetrazolium bromide
- OCN
osteocalcin
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miao D, He B, Karaplis AC, Goltzman D. J Clin Invest. 2002;109(9):1173–1182. doi: 10.1172/JCI14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karaplis AC, Luz A, Glowacki J, Bronson RT, Tybulewicz VL, Kronenberg HM, Mulligan RC. Genes Dev. 1994;8(3):277–289. doi: 10.1101/gad.8.3.277. [DOI] [PubMed] [Google Scholar]
- 3.Lanske B, Amling M, Neff L, Guiducci J, Baron R, Kronenberg HM. J Clin Invest. 1999;104(4):399–407. doi: 10.1172/JCI6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weir EC, Philbrick WM, Amling M, Neff LA, Baron R, Broadus AE. Proc Natl Acad Sci U S A. 1996;93(19):10240–10245. doi: 10.1073/pnas.93.19.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, Kronenberg HM, Baron R, Schipani E. J Clin Invest. 2001;107(3):277–286. doi: 10.1172/JCI11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schipani E, Lanske B, Hunzelman J, Luz A, Kovacs CS, Lee K, Pirro A, Kronenberg HM, Juppner H. Proc Natl Acad Sci U S A. 1997;94(25):13689–13694. doi: 10.1073/pnas.94.25.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goltzman D. Arch Biochem Biophys. 2008;473(2):218–224. doi: 10.1016/j.abb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Datta NS, Abou-Samra AB. Cell Signal. 2009;21(8):1245–1254. doi: 10.1016/j.cellsig.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stork PJ. Cell Cycle. 2002;1(5):315–317. doi: 10.4161/cc.1.5.145. [DOI] [PubMed] [Google Scholar]
- 10.Stork PJ, Schmitt JM. Trends Cell Biol. 2002;12(6):258–266. doi: 10.1016/s0962-8924(02)02294-8. [DOI] [PubMed] [Google Scholar]
- 11.Nakayama K, Tamura Y, Suzawa M, Harada S, Fukumoto S, Kato M, Miyazono K, Rodan GA, Takeuchi Y, Fujita T. J Bone Miner Res. 2003;18(5):827–835. doi: 10.1359/jbmr.2003.18.5.827. [DOI] [PubMed] [Google Scholar]
- 12.Franceschi RT, Xiao G. J Cell Biochem. 2003;88(3):446–454. doi: 10.1002/jcb.10369. [DOI] [PubMed] [Google Scholar]
- 13.Higuchi C, Myoui A, Hashimoto N, Kuriyama K, Yoshioka K, Yoshikawa H, Itoh K. J Bone Miner Res. 2002;17(10):1785–1794. doi: 10.1359/jbmr.2002.17.10.1785. [DOI] [PubMed] [Google Scholar]
- 14.Swarthout JT, Doggett TA, Lemker JL, Partridge NC. J Biol Chem. 2001;276(10):7586–7592. doi: 10.1074/jbc.M007400200. [DOI] [PubMed] [Google Scholar]
- 15.Verheijen MH, Defize LH. J Biol Chem. 1997;272(6):3423–3429. doi: 10.1074/jbc.272.6.3423. [DOI] [PubMed] [Google Scholar]
- 16.Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, Lefkowitz RJ. J Biol Chem. 2006;281(16):10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Koh AJ, Datta NS, Zhang J, Keller ET, Xiao G, Franceschi RT, D’Silva NJ, McCauley LK. J Biol Chem. 2004;279(28):29121–29129. doi: 10.1074/jbc.M313000200. [DOI] [PubMed] [Google Scholar]
- 18.Datta NS, Pettway GJ, Chen C, Koh AJ, McCauley LK. J Bone Miner Res. 2007;22(7):951–964. doi: 10.1359/jbmr.070328. [DOI] [PubMed] [Google Scholar]
- 19.Datta NS, Chen C, Berry JE, McCauley LK. J Bone Miner Res. 2005;20(6):1051–1064. doi: 10.1359/JBMR.050106. [DOI] [PubMed] [Google Scholar]
- 20.Keyse SM. Curr Opin Cell Biol. 2000;12(2):186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 21.Cobb MH. Prog Biophys Mol Biol. 1999;71(3–4):479–500. doi: 10.1016/s0079-6107(98)00056-x. [DOI] [PubMed] [Google Scholar]
- 22.English J, Pearson G, Wilsbacher J, Swantek J, Karandikar M, Xu S, Cobb MH. Exp Cell Res. 1999;253(1):255–270. doi: 10.1006/excr.1999.4687. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B, Hosaka M, Sawada Y, Torii S, Mizutani S, Ogata M, Izumi T, Takeuchi T. Diabetes. 2003;52(11):2720–2730. doi: 10.2337/diabetes.52.11.2720. [DOI] [PubMed] [Google Scholar]
- 24.Aghaloo TL, Pirih FQ, Shi A, Bezouglaia O, Tetradis S. J Periodontol. 2006;77(1):21–30. doi: 10.1902/jop.2006.77.1.21. [DOI] [PubMed] [Google Scholar]
- 25.Homme M, Schmitt CP, Mehls O, Schaefer F. J Am Soc Nephrol. 2004;15(11):2844–2850. doi: 10.1097/01.ASN.0000143472.13214.2C. [DOI] [PubMed] [Google Scholar]
- 26.Qin L, Li X, Ko JK, Partridge NC. J Biol Chem. 2005;280(4):3104–3111. doi: 10.1074/jbc.M409846200. [DOI] [PubMed] [Google Scholar]
- 27.Burgun C, Esteve L, Humblot N, Aunis D, Zwiller J. FEBS Lett. 2000;484(3):189–193. doi: 10.1016/s0014-5793(00)02153-0. [DOI] [PubMed] [Google Scholar]
- 28.Takasu H, Gardella TJ, Luck MD, Potts JT, Jr, Bringhurst FR. Biochemistry. 1999;38(41):13453–13460. doi: 10.1021/bi990437n. [DOI] [PubMed] [Google Scholar]
- 29.Pettway GJ, Schneider A, Koh AJ, Widjaja E, Morris MD, Meganck JA, Goldstein SA, McCauley LK. Bone. 2005;36(6):959–970. doi: 10.1016/j.bone.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Gorospe M, Yang C, Holbrook NJ. J Biol Chem. 1995;270(15):8377–8380. doi: 10.1074/jbc.270.15.8377. [DOI] [PubMed] [Google Scholar]
- 31.Hock JM, Fitzpatrick LA, Bilezikian JP. In: Principles of Bone Biology. 2. Bilezikian JP, Raisz LG, Rodan GA, editors. San Diego: Academic Press; 2002. pp. 463–482. [Google Scholar]
- 32.Chae HJ, Jeong BJ, Ha MS, Lee JK, Byun JO, Jung WY, Yun YG, Lee DG, Oh SH, Chae SW, Kwak YG, Kim HH, Lee ZH, Kim HR. Immunopharmacol Immunotoxicol. 2002;24(1):31–41. doi: 10.1081/iph-120003401. [DOI] [PubMed] [Google Scholar]
- 33.Xiao G, Jiang D, Thomas P, Benson MD, Guan K, Karsenty G, Franceschi RT. J Biol Chem. 2000;275(6):4453–4459. doi: 10.1074/jbc.275.6.4453. [DOI] [PubMed] [Google Scholar]
- 34.Kono SJ, Oshima Y, Hoshi K, Bonewald LF, Oda H, Nakamura K, Kawaguchi H, Tanaka S. Bone. 2007;40(1):68–74. doi: 10.1016/j.bone.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 35.Huang MS, Morony S, Lu J, Zhang Z, Bezouglaia O, Tseng W, Tetradis S, Demer LL, Tintut Y. J Biol Chem. 2007;282(29):21237–21243. doi: 10.1074/jbc.M701341200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osyczka AM, Leboy PS. Endocrinology. 2005;146(8):3428–3437. doi: 10.1210/en.2005-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang L, Karin M. Nature. 2001;410(6824):37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 38.Johnson GL, Lapadat R. Science. 2002;298(5600):1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 39.Datta NS, Kolailat R, Pettway GJ, Berry JE, McCauley LK. FASEB J. 2007;21:786, 784. [Google Scholar]
- 40.Pettway GJ, Meganck JA, Koh AJ, Keller ET, Goldstein SA, McCauley LK. Bone. 2008;42(4):806–818. doi: 10.1016/j.bone.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franklin CC, Kraft AS. J Biol Chem. 1997;272(27):16917–16923. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- 42.Noguchi T, Metz R, Chen L, Mattei MG, Carrasco D, Bravo R. Mol Cell Biol. 1993;13(9):5195–5205. doi: 10.1128/mcb.13.9.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun H, Charles CH, Lau LF, Tonks NK. Cell. 1993;75(3):487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 44.Li M, Zhou JY, Ge Y, Matherly LH, Wu GS. J Biol Chem. 2003;278(42):41059–41068. doi: 10.1074/jbc.M307149200. [DOI] [PubMed] [Google Scholar]
- 45.Brondello JM, McKenzie FR, Sun H, Tonks NK, Pouyssegur J. Oncogene. 1995;10(10):1895–1904. [PubMed] [Google Scholar]
- 46.Kenner L, Hoebertz A, Beil T, Keon N, Karreth F, Eferl R, Scheuch H, Szremska A, Amling M, Schorpp-Kistner M, Angel P, Wagner EF. J Cell Biol. 2004;164(4):613–623. doi: 10.1083/jcb.200308155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez-Perez I, Martinez-Gomariz M, Williams D, Keyse SM, Perona R. Oncogene. 2000;19(45):5142–5152. doi: 10.1038/sj.onc.1203887. [DOI] [PubMed] [Google Scholar]
- 48.Wu GS. Cancer Biol Ther. 2004;3(2):156–161. doi: 10.4161/cbt.3.2.614. [DOI] [PubMed] [Google Scholar]
- 49.Rey A, Manen D, Rizzoli R, Ferrari SL, Caverzasio J. Bone. 2007;41(1):59–67. doi: 10.1016/j.bone.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 50.Zhang R, Murakami S, Coustry F, Wang Y, de Crombrugghe B. Proc Natl Acad Sci U S A. 2006;103(2):365–370. doi: 10.1073/pnas.0507979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X, Liu Y. Cell Signal. 2007;19(7):1372–1382. doi: 10.1016/j.cellsig.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romas E, Gillespie MT. Rheum Dis Clin North Am. 2006;32(4):759–773. doi: 10.1016/j.rdc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Raggatt LJ, Qin L, Tamasi J, Jefcoat SC, Jr, Shimizu E, Selvamurugan N, Liew FY, Bevelock L, Feyen JH, Partridge NC. J Biol Chem. 2008;283(11):6790–6798. doi: 10.1074/jbc.M709909200. [DOI] [PubMed] [Google Scholar]
- 54.Yang J, Shah R, Robling AG, Templeton E, Yang H, Tracey KJ, Bidwell JP. J Cell Physiol. 2008;214(3):730–739. doi: 10.1002/jcp.21268. [DOI] [PubMed] [Google Scholar]