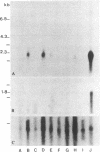
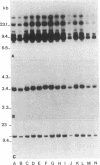
Abstract
Cells from various human nonlymphoreticular neoplasms show reduced HLA class I antigen expression. In this report, a system of human fibroblasts transformed by an avian retrovirus has been employed to investigate the mechanism of this phenomenon. Rous sarcoma virus has been used to transform in vitro human dermal fibroblasts, and clonal cell lines have been established from these cultures. In all the clones studied the integration of the provirus induced a reduction of cell-surface HLA-A, -B, -C framework antigen and beta 2-microglobulin expression when compared to levels for the respective parental fibroblasts. The reduction was correlated with a diminished intracellular synthesis of these molecules. Uninfected cells derived from an osteogenic sarcoma exhibited a reduced expression comparable to that of dermal diploid fibroblasts obtained from the same donor and transformed by Rous sarcoma virus. RNA gel blot analysis of total cellular RNA and of poly(A)+ cytoplasmic RNA showed a markedly decreased amount of HLA class I transcripts in the transformed cells. Southern blot study of genomic DNAs digested with several restriction endonucleases showed that the banding patterns of the HLA genes were not altered in the cells harboring the Rous sarcoma provirus. Our data are consistent with the hypothesis that the Rous sarcoma provirus that does not seem to be linked to the major histocompatibility complex class I gene superfamily may negatively control HLA gene expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Schwab M., Lin C. C., Varmus H. E., Bishop J. M. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1707–1711. doi: 10.1073/pnas.80.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Bartlett P. F., Edidin M. Effect of the H-2 gene complex rates of fibroblast intercellular adhesion. J Cell Biol. 1978 May;77(2):377–388. doi: 10.1083/jcb.77.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan A. K., DesMarais C. L. Immunohistologic characterization of major histocompatibility antigens and inflammatory cellular infiltrate in human breast cancer. J Natl Cancer Inst. 1983 Sep;71(3):507–516. [PubMed] [Google Scholar]
- Bodmer W. F. Evolutionary significance of the HL-A system. Nature. 1972 May 19;237(5351):139–passim. doi: 10.1038/237139a0. [DOI] [PubMed] [Google Scholar]
- Bohn W. A fixation method for improved antibody penetration in electron microscopical immunoperoxidase studies. J Histochem Cytochem. 1978 Apr;26(4):293–297. doi: 10.1177/26.4.77869. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degos L., Pla M., Colombani M. H-2 restriction for lymphocyte homing into lymph nodes. Eur J Immunol. 1979 Oct;9(10):808–814. doi: 10.1002/eji.1830091012. [DOI] [PubMed] [Google Scholar]
- Dobberstein B., Garoff H., Warren G., Robinson P. J. Cell-free synthesis and membrane insertion of mouse H-2Dd histocompatibility antigen and beta 2-microglobulin. Cell. 1979 Aug;17(4):759–769. doi: 10.1016/0092-8674(79)90316-7. [DOI] [PubMed] [Google Scholar]
- Douillard J. Y., Hoffman T., Herberman R. B. Enzyme-linked immunosorbent assay for screening monoclonal antibody production: use of intact cells as antigen. J Immunol Methods. 1980;39(4):309–316. doi: 10.1016/0022-1759(80)90231-8. [DOI] [PubMed] [Google Scholar]
- Doyle A., Martin W. J., Funa K., Gazdar A., Carney D., Martin S. E., Linnoila I., Cuttitta F., Mulshine J., Bunn P. Markedly decreased expression of class I histocompatibility antigens, protein, and mRNA in human small-cell lung cancer. J Exp Med. 1985 May 1;161(5):1135–1151. doi: 10.1084/jem.161.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eager K. B., Williams J., Breiding D., Pan S., Knowles B., Appella E., Ricciardi R. P. Expression of histocompatibility antigens H-2K, -D, and -L is reduced in adenovirus-12-transformed mouse cells and is restored by interferon gamma. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5525–5529. doi: 10.1073/pnas.82.16.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming K. A., McMichael A., Morton J. A., Woods J., McGee J. O. Distribution of HLA class 1 antigens in normal human tissue and in mammary cancer. J Clin Pathol. 1981 Jul;34(7):779–784. doi: 10.1136/jcp.34.7.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogusev J., Suskind G., Haguenau F., Rabotti G. Modification de l'expression des antigènes d'histocompatibilité (HLA) dans des fibroblastes humains transformés par le virus de la leucosarcomatose aviaire (ASV). C R Acad Sci III. 1985;301(16):701–706. [PubMed] [Google Scholar]
- Goodenow R. S., Vogel J. M., Linsk R. L. Histocompatibility antigens on murine tumors. Science. 1985 Nov 15;230(4727):777–783. doi: 10.1126/science.2997918. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Guild B. C., Erikson R. L., Strominger J. L. HLA-A2 and HLA-B7 antigens are phosphorylated in vitro by rous sarcoma virus kinase (pp60v-src) at a tyrosine residue encoded in a highly conserved exon of the intracellular domain. Proc Natl Acad Sci U S A. 1983 May;80(10):2894–2898. doi: 10.1073/pnas.80.10.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson L. A., Fisher C. A., Whelan J. P. Striking paucity of HLA-A, B, C and beta 2-microglobulin on human neuroblastoma cell lines. J Immunol. 1983 May;130(5):2471–2478. [PubMed] [Google Scholar]
- Lautenberger J. A., Schulz R. A., Garon C. F., Tsichlis P. N., Papas T. S. Molecular cloning of avian myelocytomatosis virus (MC29) transforming sequences. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1518–1522. doi: 10.1073/pnas.78.3.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liabeuf A., le Borgne de Kaouel C., Kourilsky F. M., Malissen B., Manuel Y., Sanderson A. R. An antigenic determinant of human beta 2-microglobulin masked by the association with HLA heavy chains at the cell surface: analysis using monoclonal antibodies. J Immunol. 1981 Oct;127(4):1542–1548. [PubMed] [Google Scholar]
- Malissen B., Rebai N., Liabeuf A., Mawas C. Human cytotoxic T cell structures associated with expression of cytolysis. I. Analysis at the clonal cell level of the cytolysis-inhibiting effect of 7 monoclonal antibodies. Eur J Immunol. 1982 Sep;12(9):739–747. doi: 10.1002/eji.1830120908. [DOI] [PubMed] [Google Scholar]
- Mittal K. K., Terasaki P. I. Cross-reactivity in the HL-A system. Tissue Antigens. 1972;2(2):94–104. doi: 10.1111/j.1399-0039.1972.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Momburg F., Degener T., Bacchus E., Moldenhauer G., Hämmerling G. J., Möller P. Loss of HLA-A,B,C and de novo expression of HLA-D in colorectal cancer. Int J Cancer. 1986 Feb 15;37(2):179–184. doi: 10.1002/ijc.2910370203. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Giacomini P., Bigotti A., Imai K., Nicotra M. R., Ng A. K., Ferrone S. Heterogeneity in the expression of HLA and tumor-associated antigens by surgically removed and cultured breast carcinoma cells. Cancer Res. 1983 Feb;43(2):660–668. [PubMed] [Google Scholar]
- Neefjes J. J., Doxiadis I., Stam N. J., Beckers C. J., Ploegh H. L. An analysis of class I antigens of man and other species by one-dimensional IEF and immunoblotting. Immunogenetics. 1986;23(3):164–171. doi: 10.1007/BF00373817. [DOI] [PubMed] [Google Scholar]
- Rabotti G., Teutsch B., Mariller M., Mongiat F. Transformation de fibroblastes diploïdes humains avec production virale après infection par des rétrovirus aviaires. C R Seances Acad Sci III. 1983;297(1):17–24. [PubMed] [Google Scholar]
- Rabotti G., Teutsch B., Mariller M., Pavloff N., Mongiat F., Auger J., Semmel M. Integration and expression of provirus in human cells transformed by avian sarcoma virus. J Natl Cancer Inst. 1987 May;78(5):817–829. [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Ser I. Plasma-insulin in a young diabetic after 13 years' oral therapy. Lancet. 1971 Apr 3;1(7701):706–707. doi: 10.1016/s0140-6736(71)92726-7. [DOI] [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam N. J., Spits H., Ploegh H. L. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986 Oct 1;137(7):2299–2306. [PubMed] [Google Scholar]
- Trowsdale J., Travers P., Bodmer W. F., Patillo R. A. Expression of HLA-A, -B, and -C and beta 2-microglobulin antigens in human choriocarcinoma cell lines. J Exp Med. 1980 Aug 1;152(2 Pt 2):11s–17s. [PubMed] [Google Scholar]
- Umpleby H. C., Heinemann D., Symes M. O., Williamson R. C. Expression of histocompatibility antigens and characterization of mononuclear cell infiltrates in normal and neoplastic colorectal tissues of humans. J Natl Cancer Inst. 1985 Jun;74(6):1161–1168. [PubMed] [Google Scholar]
- Vaessen R. T., Houweling A., van der Eb A. J. Post-transcriptional control of class I MHC mRNA expression in adenovirus 12-transformed cells. Science. 1987 Mar 20;235(4795):1486–1488. doi: 10.1126/science.3823900. [DOI] [PubMed] [Google Scholar]
- Whitwell H. L., Hughes H. P., Moore M., Ahmed A. Expression of major histocompatibility antigens and leucocyte infiltration in benign and malignant human breast disease. Br J Cancer. 1984 Feb;49(2):161–172. doi: 10.1038/bjc.1984.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. A., Hart D. N., Fabre J. W., Morris P. J. Distribution and quantitation of HLA-ABC and DR (Ia) antigens on human kidney and other tissues. Transplantation. 1980 Apr;29(4):274–279. doi: 10.1097/00007890-198004000-00002. [DOI] [PubMed] [Google Scholar]
- Zelený V., Matousek V., Lengerová A. Intercellular adhesiveness of H-2 identical and H-2 disparate cells. J Immunogenet. 1978 Feb;5(1):41–47. doi: 10.1111/j.1744-313x.1978.tb00629.x. [DOI] [PubMed] [Google Scholar]