Summary
Because the brain undergoes dramatic changes during fetal development it is vulnerable to environmental insults. There is evidence that maternal stress and anxiety during pregnancy influences birth outcome but there are no studies that have evaluated the influence of stress during human pregnancy on brain morphology. In the current prospective longitudinal study we included 35 women for whom serial data on pregnancy anxiety was available at 19 (±0.83), 25 (±0.9) and 31 (±0.9) weeks gestation. When the offspring from the target pregnancy were between six to nine years of age, their neurodevelopmental stage was assessed by a structural MRI scan. With the application of voxel based morphometry, we found regional reductions in gray matter density in association with pregnancy anxiety after controlling for total gray matter volume, age, gestational age at birth, handedness and postpartum perceived stress. Specifically, independent of postnatal stress, pregnancy anxiety at 19 weeks gestation was associated with gray matter volume reductions in the prefrontal cortex, the premotor cortex, the medial temporal lobe, the lateral temporal cortex, the postcentral gyrus as well as the cerebellum extending to the middle occipital gyrus and the fusiform gyrus. High pregnancy anxiety at 25 and 31 weeks gestation was not significantly associated with local reductions in gray matter volume.
This is the first prospective study to show that a specific temporal pattern of pregnancy anxiety is related to specific changes in brain morphology. Altered gray matter volume in brain regions affected by prenatal maternal anxiety may render the developing individual more vulnerable to neurodevelopmental and psychiatric disorders as well as cognitive and intellectual impairment.
Keywords: pregnancy anxiety, pregnancy, prenatal stress, longitudinal, MRI, gray matter volume
Introduction
Programming refers to the action of a factor during a sensitive developmental period affecting the organization and maturity of specific organs. One key assumption of the programming hypothesis is that biological systems undergoing rapid developmental changes are especially vulnerable to organizing and disorganizing influences (Nathanielsz et al., 2003; Seckl & Meaney, 2004). Considering the extended pre- and postnatal developmental trajectory of the brain, it is apparent that early life experiences have the potential to sculpt brain morphology. Such sculpting of the immature brain is an interactive process between genetic programming, cell function and the environment (Andersen, 2003). Growing evidence suggests that abnormal development of the brain during gestation contributes to many neurological disorders that are manifested throughout the entire lifespan (Rees et al., 2008).
The immature brain can be considered “under construction” (Connors et al., 2008) with the development of the human central nervous system following a protracted, neatly orchestrated chain of specific ontogenetic events. Although brain change and adaptation are part of a lifelong process, the earliest phases of maturation during fetal development and childhood are perhaps the most dramatic and important (Toga et al., 2006). Understanding the timing of neurodevelopmental events is essential for determining how particular environmental disturbances can selectively affect certain functions. During fetal life, neurons proliferate, migrate, and aggregate, providing the “hardware” for the developing brain. Neural proliferation before birth has been estimated at an average rate of 250,000 cells per minute (Cowan, 1979). Between gestational ages 8 and 16 weeks, migrating neurons form the subplate zone and await connections from afferent neurons originating in the thalamus, basal forebrain, and brainstem (Kostovic et al., 2002). Once neurons reach their final destination at about the 16th fetal week, they arborize and branch in an attempt to establish appropriate connections (Sidman & Rakic, 1973). Axon collaterals connect to numerous regions in the brain before the neuron finishes migrating to its target location (Jones et al., 1985). Neurotrophins influence the migration or retraction of neurons (Jones et al., 1993) and ephrins guide neurons further by establishing a chemical gradient to follow (Knoll & Drescher, 2002). During development of the human brain, little synapse formation occurs before the beginning of the third trimester, when it accelerates to approximately 40,000 synapses per minute (Bourgeois, 1997).
The presence of periods with specific neurodevelopmental events results in windows of specific vulnerability for adverse influences. In animal models, changes in brain morphology have been observed in offspring of mothers exposed to prenatal stress. In non-human primates, daily acute prenatal stress is associated with 10-12% reductions in hippocampal volume and inhibition of neurogenesis in the dentate gyrus (Coe et al., 2003) as well as altered size of the corpus callosum (Coe et al., 2002). Several lines of evidence from studies in rodents indicate that the cytoarchitecture of the rat hippocampus is altered as a consequence of prenatal stress (Hayashi et al., 1998; Gould & Tanapat, 1999; Lemaire et al., 2000). Impaired neurogenesis and associated cognitive impairment have been repeatedly reported in prenatally stressed animals (Lemaire et al., 2000; Fujioka et al., 2006; Lemaire et al., 2006). Furthermore, prenatal stress has the potential to alter synaptic plasticity by impairing long-term potentiation but facilitating long-term depression (Yaka et al., 2007; Yang et al., 2007). Although prenatal stress associated changes in the hippocampal formation have received major attention, morphological changes in other brain regions have been shown as well. For example significantly expanded dimensions of the lateral nucleus of the amygdala were observed in prenatally stressed offspring (Salm et al., 2004). Also, reduced spine densities and significant reduction of dendritic length of pyramidal neurons in the dorsal anterior cingulate and orbitofrontal cortex have been reported in offspring of mothers exposed to stress during pregnancy (Murmu et al., 2006). Since the rodent brain is less mature at birth than the human brain, it has been suggested that brain maturation occurring in the early postnatal period in the rat is analogous to maturational changes that occur in humans in late gestation (Clancy et al., 2001). Therefore, it is interesting to note that there is an impressive body of evidence from rodent studies showing changes in brain morphology in association with manipulation of the early postnatal environment (e.g. Meaney et al., 1991; Pham et al., 1999; Helmeke et al., 2001; Huot et al., 2002; Roceri et al., 2002; Bredy et al., 2003; Ovtscharoff et al., 2006; Fabricius et al., 2008).
In humans, changes in brain morphology have been reported in individuals born prematurely. Thus, low birth weight as well as preterm birth have been related to changes in regional brain volumes (e.g. Peterson et al., 2000; Abernethy et al., 2002; Nosarti et al., 2002; Huizink et al., 2004; Buss et al., 2007; Beauchamp et al., 2008). Adverse birth outcomes may be markers of in utero stress exposure (e.g. Wadhwa, 2005) but the changes in brain morphology may also be due to perinatal complications that are often associated with premature delivery. To the best of our knowledge no study to date in humans has investigated the association between prenatal stress exposure and brain morphology in the offspring.
Pregnancy anxiety has been suggested to be a more sensitive predictor of birth outcomes than general anxiety (Wadhwa et al., 1993; DiPietro et al., 2004; Roesch et al., 2004; Kramer et al., 2009) and has been suggested as a distinctive syndrome (Huizink et al., 2004). Further evidence suggests that measures of pregnancy specific stress are better than measures of generalized psychological distress for predicting developmental outcomes including, fetal behavior (DiPietro et al., 2002), infant cognitive and motor development (Huizink et al., 2003; Dipietro et al., 2006; Davis & Sandman, in press) and infant emotional regulation (Dipietro et al., 2006). The objective of the current study was therefore to test in a prospective longitudinal study the associations between pregnancy anxiety, measured repeatedly over the course of gestation, and reductions in gray matter volume in their 6-9 year-old offspring.
Methods
Participants
Pregnant women were recruited for study participation between 1998 and 2002. Five hundred and fifty seven pregnant women, who received prenatal care from the faculty obstetric practice at the University of California, Irvine Medical Center or Cedars-Sinai Hospital in Los Angeles, were recruited by the 15th week of gestation and provided written, informed consent. All methods and procedures were approved by the Institutional Review Board of the participating institutions. Study participants were English-speaking adult women (>18 years age) with singleton, intrauterine pregnancies. Exclusion criteria included tobacco, alcohol, or other drug use in pregnancy; uterine or cervical abnormalities; or presence of any condition potentially associated with dysregulated neuroendocrine function such as endocrine, hepatic or renal disorders or corticosteroid medication use. While not an exclusion criteria, none of the women in this sample were treated for any psychiatric disorders. In the context of an on-going study on the effects of prenatal stress exposure on child brain development that started in 2008, women were re-contacted and invited to participate in a follow-up study of their children. Three hundred and forty women of the initial sample were located. Fifty-two children have undergone and MRI scan to date; of those one MRI scan had to be excluded due to morphological abnormalities and two MRI scans due to severe motion artifacts. Among the 49 mother-child dyads with usable MRI data, 35 women had provided complete maternal stress data at three time points between 19 and 31 weeks gestation as well as postpartum and are included in the current report. Among these 35 children were two siblings; thus one mother was enrolled in the study with two subsequent pregnancies and consequently two children for the follow-up study. Women whose children participated in the follow-up study of brain development did not differ in sociodemographic characteristics (maternal age and education, annual household income) from women in the initial sample (all p’s >0.4).
Assessments in pregnant women
For all pregnant women gestational age was determined by best obstetric estimate with a combination of last menstrual period and early uterine size, and was confirmed by obstetric ultrasonographic biometry before 20 weeks using standard clinical criteria (O’Brien et al., 1981). Medical risk was defined as the presence of certain medical conditions in the index pregnancy or previous pregnancies (e.g. vaginal bleeding, pregnancy-induced hypertension, preeclampsia, infection, Hobel, 1982). Risk conditions were determined through interview and extensive medical chart review. The sum of medical risk factors was calculated as an indicator of presence of any current or historical risk conditions. Information on birth outcomes were retrieved from medical charts after delivery. Sociodemographic characteristics and birth outcomes are summarized in Table1.
Table 1.
Sociodemographic characteristics and birth outcomes
| Mother-child dyads (N=35) | |
|---|---|
| Maternal Age | 32.7±6.5 |
| Race/ethnicity | |
| Non-Hispanic White | 37.1% |
| Hispanic White | 20.0% |
| African American | 8.6% |
| Asian | 31.4% |
| Other | 2.9% |
| Annual household income | |
| $0 to $30,000 | 32.4% |
| $30,001 to $60,000 | 11.8% |
| $60,001 to $100,000 | 26.4% |
| Over $100,000 | 29.4% |
| Pregnancy anxiety | |
| 19 weeks gestation | 19.7±6.5 |
| 25 weeks gestation | 17.7±4.3 |
| 31 weeks gestation | 17.3±4.4 |
| Perceived Stress postpartum | 2.1±0.7 |
| Primiparous | 44.1% |
| Child sex | |
| Male | 51.4% |
| Female | 48.6% |
| Length of gestation (weeks) | 38.8±1.83 (11% 34-36.9 weeks) |
| Birth weight (grams) | 3527.5±574.2 (0% <2500g) |
Pregnancy anxiety
Pregnancy anxiety was assessed over the course of gestation at 19 (±0.83, SD), 25 (±0.9) and 31 (±0.9) weeks gestation, and for all 35 children included in the current analyses complete data was available. A 10-item pregnancy anxiety scale, which assesses a woman’s feelings about her health during pregnancy, the health of her baby, and her feelings about labor and delivery, was administered at all three study visits. Answers were given on a 4-point scale and included items such as: “I am fearful regarding the health of my baby,” “I am concerned or worried about losing my baby,” and “I am concerned or worried about developing medical problems during my pregnancy.” The final score on this measure could range from 10 to 40. This reliable measure (α=0.75-0.85) was specifically developed for use in pregnancy research (Rini et al., 1999; Glynn et al., 2008).
Pregnancy anxiety and medical risk
To test whether the effects of pregnancy anxiety on the developing brain could be mediated by the presence of medical risk, correlation analyses were performed between pregnancy anxiety and the number of medical risk conditions. At none of the assessments, significant correlations could be observed (19 weeks: r= -0.14, p= 0.42; 26 weeks: r= -0.14, p= 0.44, 31 weeks: r= 0.02, p= 0.92).
Pregnancy anxiety, sociodemographic characteristics and postpartum stress
To test whether the effects of pregnancy anxiety on the developing brain could be mediated by the quality of the postnatal environment, correlation analyses were performed between pregnancy anxiety and sociodemographic characteristics as well as postpartum stress. Generalized stress was assessed at 8.2 (±2.9) weeks postpartum (range: 5-19 weeks) using a modification of the 10-item version of the Perceived Stress Scale (Cohen & Williamson, 1988). As shown in Table 2, pregnancy anxiety was not significantly associated with maternal sociodemographic characteristics, while highly significant correlations between pregnancy anxiety at all time points during gestation and postpartum perceived stress were observed.
Table 2.
Correlations between pregnancy anxiety during pregnancy and sociodemographic characteristics and perceived stress postpartum
| Pregnancy anxiety at 19 weeks GA | Pregnancy anxiety at 25 weeks GA | Pregnancy anxiety at 31 weeks GA | |
|---|---|---|---|
| Maternal age | -0.11 (p=0.54) | -0.17 (p=0.92) | -0.08 (p=0.66) |
| Maternal education (years of school completed) | 0.14 (p=0.43) | 0.19 (p=0.27) | 0.18 (p=0.31) |
| Annual household gross income | 0.17 (p=0.34) | 0.27 (p=0.12) | 0.16 (p=0.35) |
| Perceived Stress postpartum | 0.44 (p=0.01) | 0.36 (p=0.04) | 0.56 (p=0.00) |
Assessments in children
All children included in the study had a stable neonatal course (all Apgar scores >8) and no emotional or physical conditions were reported in a structured interview using the MacArthur Health and Behavior Questionnaire (Armstrong & Goldstein, 2003) at the ages of 6 and 9 years (mean: 7.2 ± 0.86), when they participated in an MRI scan. Child’s handedness was assessed with a modified version of the Edinburgh Handedness Inventory (Oldfield, 1971). For the majority (86%) of children the dominant hand was the right.
MRI Acquisition
Each child underwent an MRI scan conducted on a 3-T Philips Achieva system. To minimize head motion, padding was placed around the head. Ear protection was given to all children. To further increase compliance and reduce motion, children were fitted with head phones and allowed to watch a movie of their choice while in the scanner. Following the scanner calibration and pilot scans, a high resolution T1 anatomical scan was acquired in the sagittal plane with 1mm3 isotropic voxel dimensions. An Inversion-Recovery Spoiled Gradient Recalled Acquisition (IR-SPGR) sequence with the following parameters was applied: Repetition rate (TR)= 11ms, Echo Time (TE)= 3.3ms, Inversion Time (TI)= 1100ms, Turbo Field Echo factor (TFE)= 192, Number of slices: 150, no SENSE acceleration, Flip angle=180, Shot interval (time from inversion pulse to the center of acquisition) = 2200ms. Acquisition time for this protocol was seven minutes. Variations of these parameters were tested on volunteers to obtain an optimal set that gave us the best gray-white matter contrast, sharpness and high resolution while ensuring that there were no discernible artifacts. The purpose was to keep the total acquisition time at a tolerable length for children. Automatic brain segmentation software was tested on these pilot scans to ensure gray-white matter segmentation with minimal errors.
Processing of MRI data
Images were visually assessed by a neurologist for normal anatomic appearance. The structural images were bias field corrected, and segmented using an integrated generative model (unified segmentation, Ashburner & Friston, 2005). Unified segmentation involves alternating between segmentation, bias field correction, and normalization to obtain local optimal solutions for each process. The pediatric CCHMC a priori templates (Wilke et al., 2002) were used to segment and normalize (affine and 16 iteration non-linear transformations) the children’s images. The resulting images were modulated to correct voxel signal intensities for the amount of volume displacement during normalization. The normalized and segmented images were averaged across the children’s datasets to produce gray matter, white matter, and CSF sample specific a priori templates. The process was then repeated using the sample specific a priori templates resulting in VBM probability maps of 1mm isotropic voxels. The normalized, segmented, and modulated images were then smoothed using a 12 mm kernel to ensure that the data were normally distributed and to limit the number of false positive findings (Salmond et al., 2002). Coordinates of clusters (centroids) were converted from original Montreal Neurological Institute (MNI) coordinates to those of the Talairach brain atlas (Talairach & Tournoux, 1988) using the mni2tal utility (Matthew Brett 1999 GPL). Anatomical locations of the significant areas are based on the best estimate from the Talairach atlas using the Tailairach Daemon Client (http://www.talairach.org/client.html).
Total gray matter analysis
Total gray matter volume for each child was estimated based on the volumes of the segmented and modulated images. The sum of the nonzero voxel values in the image was calculated and multiplied by the voxel size to obtain an estimate of total gray matter volume. Partial correlation analyses were performed to test the association between pregnancy anxiety at each of the three pregnancy visits and total gray matter volume controlling for age, sex, gestational age at birth and postpartum stress.
Voxel Based Morphometry (VBM) analysis
To examine reductions in regional gray matter volume in association with pregnancy anxiety, a multiple regression model was employed with pregnancy anxiety at 19, 25 and 31 weeks gestation as the predictors of interest. Normalization for global differences in gray matter concentration across subjects was performed by controlling for total gray matter volume. Consequently, the analysis detected regional difference rather than overall, large-scale variations in gray matter concentrations. By controlling for total gray matter volume, differences in overall brain size were accounted for that varies by sex. We furthermore controlled for child’s age at assessment. Also, gestational age at birth was controlled for in these analyses because preterm delivery has been shown to be associated with reductions in gray matter volume (e.g. Gimenez et al., 2004). In addition, handedness of the child was controlled for because structural asymmetries of the brain may be associated with handedness (Toga & Thompson, 2003). Because pregnancy anxiety and postpartum stress were highly correlated, we included postpartum stress as an additional covariate in order to address the association between prenatal stress and gray matter volume independent of postnatal stress. Relative threshold masking (threshold >0.3) was used to minimize gray-white matter boundary effects, and implicit masking was used to disregard voxels with zero values. Analyses for detection of brain regions that showed significantly reduced gray matter density in association with high pregnancy anxiety were performed at p<0.001 uncorrected, but only those voxels within a cluster of at least 100 voxels that reached a False Discovery Rate (FDR) threshold of p<0.05 are reported.
Results
Pregnancy anxiety over the course of gestation
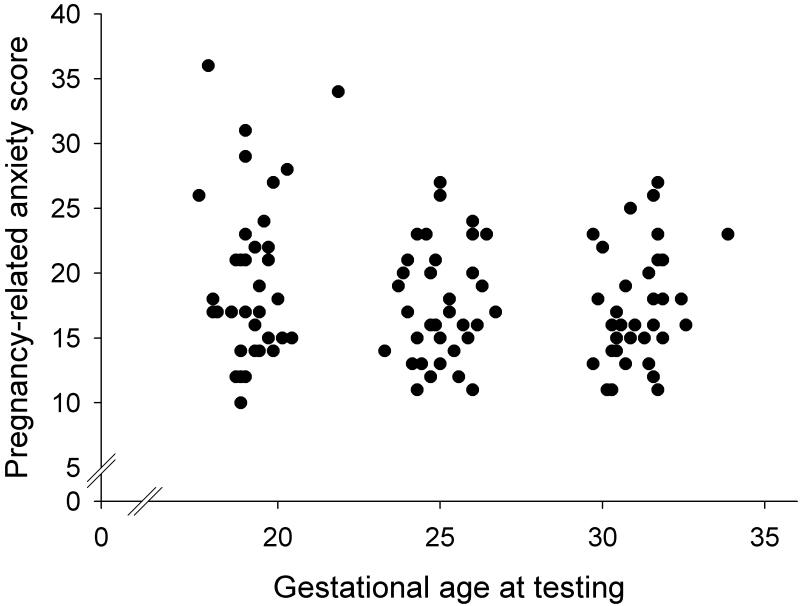
Table 1 shows mean pregnancy anxiety scores at each pregnancy visit and Figure 1 presents the distribution of pregnancy anxiety scores over the course of the three pregnancy visits. With advancing gestational age pregnancy anxiety scores significantly decreased (F(1.8, 56.8)=5.4, p=0.01) resulting in lower scores at the second and third assessments as compared to the first. As depicted in Table 3, Spearman’s rho correlation coefficients suggested significant rank stability in pregnancy anxiety scores over the course of gestation.
Figure 1.
Pregnancy anxiety scores over the course of gestation. With advancing gestational age, pregnancy anxiety decreases.
Table 3.
Rank correlations between pregnancy anxiety scores across pregnancy visits
| Pregnancy anxiety at 19 weeks GA | Pregnancy anxiety at 25 weeks GA | Pregnancy anxiety at 31 weeks GA | |
|---|---|---|---|
| Pregnancy anxiety at 19 weeks GA | - | 0.64 (p<0.001) | 0.65 (p<0.001) |
| Pregnancy anxiety at 25 weeks GA | - | - | 0.74 (p<0.001) |
| Pregnancy anxiety at 31 weeks GA | - | - | - |
Pregnancy anxiety and global reductions in gray matter volume
Pregnancy anxiety at any of the three time points during pregnancy was not correlated with total gray matter volume (T1: r=0.05, p=0.81; T2: r=0.06, p=0.75; T3: r=-0.27, p=0.15).
Pregnancy anxiety and regional reductions in gray matter volume
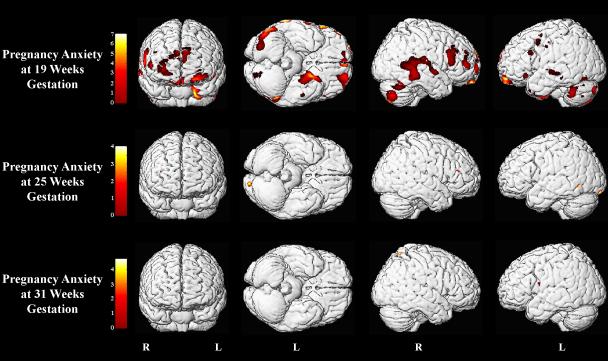
The VBM analysis revealed lower gray matter density in several brain areas in association with high pregnancy anxiety. The significance of the relation between pregnancy anxiety and gray matter volume reductions varied across gestation (see Table 4 and Figure 2). High pregnancy anxiety at 19 weeks gestation was associated with significant, mostly bilateral, gray matter volume reductions in the anterior (Brodmann Area (BA) 10), orbitofrontal (BA 11 and BA 47), dorsolateral (BA 46 and BA 9) and ventrolateral prefrontal cortex (BA 45). Also, lower gray matter density was observed with high pregnancy anxiety at 19 weeks gestation in the left precentral gyrus extending to the middle frontal gyrus (BA 6). Reduced gray matter volume in association with high pregnancy anxiety at 19 weeks gestation was furthermore observed in the left medial temporal lobe, uncus, extending to the entorhinal cortex (BA 28) and the parahippocampal gyrus (BA 36) as well as in the left temporal pole (BA 38) and the left inferior temporal gyrus (BA 20). Bilateral reductions in gray matter volume were found in children whose mothers reported higher pregnancy anxiety in the lateral temporal cortex extending from the superior temporal gyrus (BA 22) to the middle temporal gyrus (BA 21) and on the right side to the postcentral gyrus. Reduction in gray matter volume in association with high pregnancy anxiety at 19 weeks gestation was also observed in the left postcentral gyrus as well as in the left supramarginal gyrus (BA 39) and the right angular gyrus (BA 39). Furthermore, in children of mothers with high pregnancy anxiety at 19 weeks gestation, pronounced bilateral gray matter volume reduction was found in the cerebellum extending to the middle occipital gyrus (BA 19) and to the fusiform gyrus (BA 37). Clusters of voxels with reduced gray matter density were found in association with high pregnancy anxiety at 25 and 31 weeks (see Table 4) but these did not survive FDR correction and are therefore not reported here. Excluding either child of the sibling pair did not significantly change the reported results.
Table 4.
Local reductions in gray matter density in association with high pregnancy anxiety: Talairach coordinates and z-scores for the most significant voxel in each of the clusters, and volumes for all clusters in the gray matter SPMs are displayed
| Cluster No | Cerebral region | Talairach coordinates |
Cluster size | t-value | Voxel p (uncor) | Voxel p (FDR-cor) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
|
Pregnancy Anxiety at 19 weeks gestation | ||||||||
| 1 | Left Superior Frontal Gyrus (BA 10) | 0 | 67 | 25 | 5523 | 7.0 | <0.001 | 0.02 |
| Right Superior Frontal Gyrus (BA 10) | 34 | 67 | -4 | 4.8 | <0.001 | 0.02 | ||
| 2 | Left Superior Frontal Gyrus (BA 10) | -28 | 69 | -9 | 3582 | 5.5 | <0.001 | 0.02 |
| Left Superior Frontal Gyrus (BA 11) | -23 | 53 | -19 | 5.4 | <0.001 | 0.02 | ||
| Left Middle Frontal Gyrus (BA 10) | -39 | 62 | -11 | 4.8 | <0.001 | 0.02 | ||
| 3 | Left Superior Frontal Gyrus (BA 10) | -37 | 64 | 19 | 734 | 4.4 | <0.001 | 0.02 |
| Left Middle Frontal Gyrus (BA 10) | -50 | 57 | 4 | 3.9 | <0.001 | 0.03 | ||
| Left Inferior Frontal Gyrus (BA 46) | -54 | 51 | 5 | 3.7 | <0.001 | 0.03 | ||
| 4 | Left Inferior Frontal Gyrus (BA 9) | -62 | 23 | 30 | 442 | 3.9 | <0.001 | 0.03 |
| Left Middle Frontal Gyrus (BA 46) | -61 | 31 | 26 | 3.5 | 0.001 | 0.04 | ||
| 5 | Left Inferior Frontal Gyrus (BA 47) | -55 | 25 | -9 | 236 | 4.2 | <0.001 | 0.03 |
| 6 | Left Precentral Gyrus (BA 6) | -66 | -10 | 40 | 2854 | 4.3 | <0.001 | 0.02 |
| Left Middle Frontal Gyrus (BA 6) | -51 | 6 | 52 | 4.3 | <0.001 | 0.03 | ||
| 7 | Right Inferior Frontal Gyrus (BA 46) | 53 | 50 | 5 | 3794 | 4.9 | <0.001 | 0.02 |
| Right Middle Frontal Gyrus (BA 46) | 57 | 32 | 28 | 4.3 | <0.001 | 0.03 | ||
| Right Inferior Frontal Gyrus (BA 45) | 61 | 25 | 9 | 4.2 | <0.001 | 0.03 | ||
| 8 | Right Superior Frontal Gyrus (BA11) | 2 | 55 | -21 | 1543 | 4.7 | <0.001 | 0.03 |
| 9 | Left Uncus (BA 28) | -13 | 3 | -25 | 3398 | 5.3 | <0.001 | 0.02 |
| Left Uncus (BA 36) | -25 | -10 | -37 | 5.1 | <0.001 | 0.02 | ||
| Left Superior Temporal Gyrus (BA 38) | -20 | 13 | -28 | 4.7 | <0.001 | 0.02 | ||
| 10 | Left Superior Temporal Gyrus (BA 22) | -73 | -13 | 0 | 1375 | 4.3 | <0.001 | 0.03 |
| Left Middle Temporal Gyrus (BA 21) | -76 | -26 | -5 | 4.2 | <0.001 | 0.03 | ||
| 11 | Left Inferior Temporal Gyrus (BA 20) | -49 | -12 | -37 | 109 | 3.8 | <0.001 | 0.03 |
| 12 | Right Middle Temporal Gyrus (BA 21) | 75 | -33 | -8 | 6241 | 5.3 | <0.001 | 0.02 |
| Right Superior Temporal Gyrus (BA 22) | 74 | -35 | 14 | 4.9 | <0.001 | 0.02 | ||
| 13 | Left Middle Occipital Gyrus (BA 19) | -58 | -71 | -12 | 3969 | 5.9 | <0.001 | 0.02 |
| Left Cerebellum (Tuber) | -57 | -52 | -23 | 4.0 | <0.001 | 0.03 | ||
| 14 | Left Cerebellum (Tuber) | -32 | -93 | -28 | 4644 | 5.1 | <0.001 | 0.03 |
| 15 | Left Cerebellum (Pyramis) | -54 | -69 | -32 | 181 | 3.6 | 0.001 | 0.04 |
| 16 | Right Cerebellum (Tuber) | 61 | -64 | -19 | 2742 | 5.7 | <0.001 | 0.02 |
| Right Middle Occipital Gyrus (BA19) | 62 | -72 | -6 | 3.9 | <0.001 | 0.03 | ||
| Fusiform Gyrus (BA37) | 62 | -75 | 1 | 3.8 | <0.001 | 0.03 | ||
| 17 | Right Cerebellum (Pyramis) | 53 | -72 | -32 | 2747 | 5.3 | <0.001 | 0.02 |
| 18 | Right Cerebellum (Tuber) | 43 | -88 | -24 | 827 | 4.2 | <0.001 | 0.03 |
| 19 | Left Postcentral Gyrus (BA 2) | -56 | -28 | 59 | 348 | 4.4 | <0.001 | 0.02 |
| 20 | Right Angular Gyrus (BA 39) | 60 | -68 | 40 | 399 | 4.2 | <0.001 | 0.02 |
| 21 | Left Supramarginal Gyrus (BA 39) | -70 | -56 | 24 | 331 | 4.1 | <0.001 | 0.02 |
|
Pregnancy Anxiety at 25 weeks gestation | ||||||||
| 22 | Right Middle Frontal Gyrus (BA 10) | 33 | 37 | 12 | 115 | 3.8 | <0.001 | 0.94 |
| 23 | Left Middle Temporal Gyrus (BA 37) | -42 | -58 | -2 | 106 | 4.0 | <0.001 | 0.94 |
| 24 | Left Lingual Gyrus (BA 17) | -11 | -93 | -9 | 148 | 3.9 | <0.001 | 0.94 |
|
Pregnancy Anxiety at 31 weeks gestation | ||||||||
| 25 | Right Inferior Frontal Gyrus (BA 47) | 32 | 25 | 2 | 617 | 4.7 | <0.001 | 0.95 |
| 26 | Left Insula (BA 13) | -38 | 6 | 17 | 200 | 3.9 | <0.001 | 0.95 |
| 27 | Right Postcentral Gyrus (BA 7) | 18 | -53 | 65 | 121 | 3.9 | <0.001 | 0.95 |
Figure 2.
Areas of reduced gray matter volume in association with pregnancy anxiety at 19, 25 and 31 weeks gestation. Voxels with p < 0.001 (uncorrected) are displayed.
Discussion
We present the first evidence in humans that prenatal maternal anxiety is associated with brain morphology in the developing individual within specific sensitive time periods. Specifically, pregnancy anxiety at 19 weeks gestation was associated with gray matter volume reductions in the prefrontal cortex, the premotor cortex, the medial temporal lobe, the lateral temporal cortex, the postcentral gyrus as well as the cerebellum extending to the middle occipital gyrus and the fusiform gyrus. These associations with gray matter density were confined to pregnancy anxiety reported at 19 weeks gestation, as reports of pregnancy anxiety at 25 and 31 weeks gestation were not significantly associated with gray matter volume. Our findings are consistent with accumulating evidence from animal studies that medial temporal and prefrontal cortical regions are shaped by early experience (e.g. Coe et al., 2003; Salm et al., 2004; Fujioka et al., 2006; Murmu et al., 2006).
Scales measuring pregnancy anxiety have been suggested to better assess anxieties and worries related specifically to pregnancy than general scales of stress, depression and anxiety (Huizink et al., 2004; Dipietro et al., 2006). This is emphasized by observations of pregnancy anxiety having a higher predictive quality for birth outcomes and fetal/child development than general stress scales (Wadhwa et al., 1993; Huizink et al., 2003; DiPietro et al., 2004; Roesch et al., 2004; Dipietro et al., 2006; Kramer et al., 2009; Davis & Sandman, in press) and is furthermore supported by the highly significant association between self-reported maternal pregnancy anxiety and brain morphology in this study.
The brain regions that we have found to be affected by pregnancy anxiety are areas specifically associated with cognitive performance. The prefrontal cortex is sometimes described as the “highest” structure of the brain because it is involved in executive cognitive functions such as reasoning, planning, attention, working memory, and some aspects of language (e.g. Connolly et al., 2002). Structures in the medial temporal lobe, including areas connected to the hippocampus (entorhinal, perirhinal, parahippocampal cortex), have been proposed to constitute a “medial temporal lobe memory system” with the primary functions of these areas related to the storage and recall of facts and events (Squire et al., 2004). The temporal polar cortex appears to be involved in social and emotional processing including recognition and semantic memory (Nakamura & Kubota, 1996; Hoistad & Barbas, 2008). A network in the temporal-parietal cortex consisting of the middle temporal gyrus (BA 21), the superior temporal gyrus (BA 22) and the angular gyrus (BA 39) has been shown to be important in processes related to auditory language processing in children (Ahmad et al., 2003). Also involved in language learning seems to be another network of brain regions affected by pregnancy anxiety (the inferior frontal gyrus (BA 45), the middle temporal gyrus (BA 21) and the parahippocampal gyrus, Mestres-Misse et al., 2008).
Importantly and consistent with the primary functions of the affected brain regions, a small but growing literature indicates that prenatal stress influences both cognitive development as well as temperament. Thus, elevated prenatal maternal stress/anxiety is associated with infant inability to attend and with delayed cognitive development (Brouwers et al., 2001; Huizink et al., 2002; O’Connor et al., 2002; Buitelaar et al., 2003; Huizink et al., 2003; Davis & Sandman, in press), lower academic achievement in school (Niederhofer & Reiter, 2004), higher infant behavioral reactivity (Davis et al., 2004; Davis et al., 2005; Davis et al., 2007) and emotional/ behavioral problems that persisted until adolescence (Van den Bergh et al., 2005; Van den Bergh et al., 2008). Furthermore, offspring of women who were exposed to a natural disaster during their pregnancies had poorer general intellectual functioning and language development (Laplante et al., 2004; Laplante et al., 2008) and maternal exposure to natural disasters, war or stressful life events have been associated with increased prevalence of psychopathology in the offspring (van Os & Selten, 1998; Selten et al., 1999; Watson et al., 1999; Beversdorf et al., 2005; Khashan et al., 2008). Our observations of reduced gray matter density in the premotor cortex and the cerebellum may provide the anatomical basis for previous observations of delayed motor development in association with prenatal stress/ anxiety (Buitelaar et al., 2003; Huizink et al., 2003).
Interestingly, a recent functional MRI study in humans found that prefrontal regions, that we found are affected by high pregnancy anxiety, are involved in the regulation of stress hormone secretion (Pruessner et al., 2008). These same brain regions appear to be particularly vulnerable under conditions of chronic stress due to their high density of glucocorticoid receptors (Sapolsky et al., 1990). Thus, by its effect on these brain regions, high maternal prenatal anxiety may increase the risk for higher stress susceptibility and reactivity in the developing individuals. This may result in higher concentrations of stress hormones which could further delay brain development. These assumptions are consistent with reports of higher baseline and stress-reactive cortisol concentrations in children born to mothers with high anxiety levels during pregnancy (Gutteling et al., 2005; O’Connor et al., 2005; Van den Bergh et al., 2008).
Reduced gray matter density in the precentral and postcentral gyrus in association with pregnancy anxiety is consistent with evidence for disturbed development of the nociceptive system and associated behavioral changes in association with prenatal stress (Smythe et al., 1994; Rokyta et al., 2008). Occipital-temporal areas (middle occipital gyrus (BA19) and fusiform gyrus), involved in visual processing, are furthermore affected by pregnancy anxiety (Brandt et al., 2000).
Limbic structures, especially the hippocampus, have been shown to be prominent targets for early life stress (e.g. Coe et al., 2003; Buss et al., 2007). Still, we did not observe a significant reduction in gray matter density in this region. Before concluding that this area is not affected by maternal pregnancy anxiety, alternative, potentially more sensitive, methods of analyses (e.g. manual segmentation, shape analyses) are required.
The fetus participates in a dynamic exchange of environmental (intrauterine) information with the maternal host over the course of gestation. All communication between the maternal and fetal compartments is mediated via the placenta, an organ of fetal origin. One of the major placental signals in pregnant primates is the peptide corticotrophin-releasing hormone (CRH) which has been shown to be stress-sensitive in in vitro studies (Petraglia et al., 1987; Petraglia et al., 1989; Petraglia et al., 1990). Other in vivo studies have found significant correlations among maternal pituitary-adrenal stress hormones (ACTH, cortisol) and placental corticotrophin-releasing hormone (pCRH) concentrations (Goland et al., 1992; Chan et al., 1993; Wadhwa et al., 1997; Hobel et al., 1999). Some (Hobel et al., 1999; Erickson et al., 2001), but not all studies (Petraglia et al., 2001), also have reported direct associations between maternal psychosocial stress and pCRH function. With the production and release of CRH from the placenta, regulation of the HPA axis changes dramatically during pregnancy. Maternal cortisol increases two- to four-fold over the course of normal gestation (Mastorakos & Ilias, 2003; Sandman et al., 2006) resulting from pCRH stimulating production of maternal cortisol (Sasaki et al., 1989). Maternal cortisol passes the placenta with 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) presenting a partial barrier (Brown et al., 1996). pCRH furthermore stimulates cortisol secretion from the fetal adrenals directly, as the CRH 1 receptor is present in human fetal adrenal tissue from mid-gestation (Smith et al., 1998).
At high concentrations pCRH as well as cortisol may inhibit growth and differentiation of the developing nervous system. Thus considerable evidence indicates that glucocorticoids are neurotoxic to hippocampal CA3 pyramidal cells (Sapolsky et al., 1985; Sapolsky et al., 1990; Magarinos et al., 1996), and fetal exposure to high levels of glucocorticoids produces irreversible damage to the hippocampus (Uno et al., 1990; Uno et al., 1994). Larger amounts of exogenously administered CRH increase limbic neuronal excitation leading to seizures (Ehlers et al., 1983; Baram et al., 1992; Baram et al., 1997) and may participate in mechanisms of neuronal injury (Baram & Hatalski, 1998). The potential mechanisms by which maternal stress and associated increases in stress-sensitive hormones (pCRH, cortisol) may produce long-lasting changes in brain function have been suggested from animal models and may include changes in neurotransmitter levels (Roceri et al., 2002; Kinnunen et al., 2003; Pickering et al., 2006), adult neurogenesis (Lemaire et al., 2000; Coe et al., 2003; Fujioka et al., 2006; Lemaire et al., 2006; Odagiri et al., 2008) as well as cell growth and survival (Roceri et al., 2002; Fumagalli et al., 2004; Van den Hove et al., 2006; Aisa et al., in press).
Interestingly, pCRH as well as cortisol concentrations during pregnancy predict fetal and infant development. Low concentrations of pCRH at the beginning of the second trimester are associated with precocious maturation of the human fetus (Class et al., 2008), while elevated concentrations of pCRH during the third trimester of gestation are associated with impaired fetal learning (Sandman et al., 1999). The developmental consequences of elevated concentrations of pCRH during pregnancy extend into postnatal life, as higher pCRH concentrations during pregnancy are associated with delayed neonatal physical and neuromuscular maturation (Ellman et al., 2008), more fearful temperaments in infants (Davis et al. 2005), and an increase in central adiposity in 3 year old children (Gillman et al., 2006). Endogenous maternal cortisol also plays a role in shaping human development. Prenatal exposure to elevated maternal cortisol has been shown to predict increased fussiness, negative behavior and fearfulness in infancy (de Weerth et al., 2003; Davis et al., 2007) and greater cortisol reactivity in childhood (Gutteling et al., 2005) as well as delays in mental (Huizink et al., 2003; Davis & Sandman, in press) and motor development (Huizink et al., 2003).
The results of the current study suggest that earlier in pregnancy, the effects of pregnancy anxiety on offspring’s gray matter volume are most pronounced. This effect of timing may be due to the fact that pregnancy anxiety is highest at 19 weeks gestation and decreases over the course of gestation, which is in line with previous observations of reduced physiological and psychological stress reactivity as pregnancy advances (Schulte et al., 1990; Glynn et al., 2001; Glynn et al., 2004; de Weerth & Buitelaar, 2005; Glynn et al., 2008) as well as with a recent observation that pregnancy anxiety early (around 15 weeks gestation) but not later in gestation predicts mental development at 12 months age (Davis & Sandman, in press). The effect of timing may also be related to the fact that different brain regions have a unique timetable for development and therefore specific periods of neural vulnerability. This possibility has been supported by observations in rhesus monkeys, where prenatal exposure to the same stressor had greater effects on postnatal motor development if it occurred earlier in gestation, when neuronal migration was at its peak, than if it occurred in mid- to late gestation, when synaptogenesis was at its peak (Schneider et al., 1999). The implication of these findings is that the impact of stress during pregnancy is not uniform but that stress earlier in pregnancy may have more pronounced consequences for brain development than at a later gestational stage.
It important to acknowledge that the observed consequences of prenatal programming not only depend on the timing of the insult and the brain region of interest but also on the stage of assessment. Studies on postnatal brain development have clearly shown regional and temporal patterns of dynamic maturational change continuing through childhood and adolescence. This implies that what we observed and reported here in children of this age range may not be final. It is possible that at a later maturational stage, prenatal stress exposure will confer a different morphological pattern. Therefore, following-up these children into adolescence and adulthood will provide valuable information on the persistence of prenatal stress effects on brain morphology.
It cannot be ruled out that the prenatal stress effects on brain morphology are moderated by postnatal exposures (Buss et al., 2007). By controlling for several relevant variables including postnatal maternal stress and socioeconomic status, it can be concluded though that the observed effects of pregnancy anxiety on brain structure were not mediated by these postnatal factors. Thus, the results suggest that, independent of postnatal maternal stress, prenatal stress has an impact on the offspring’s brain morphology.
It has to be noted that while there was no indication of psychiatric disorders and there was no report of treatment for any disorders in the structured interviews that probed such issues, the possibility of an undiagnosed disorder cannot be ruled out because clinical diagnostic assessments were not conducted. Pregnancy anxiety may be higher in women with undiagnosed psychiatric disorders and accompanying endocrine alterations could impact on neurodevelopment of the offspring.
This is the first study in healthy children to show that prenatal maternal anxiety is related to distinctive patterns of structural brain development. These morphological patterns may increase vulnerability for certain neurodevelopmental disorders and impair cognitive function. Therefore the results suggest that addressing mothers’ pregnancy-related concerns and anxiety should be a major focus for public health initiatives.
Acknowledgments
This research was supported by National Institute of Health grants NS-41298, HD-51852 and HD28413 to CAS. We gratefully acknowledge contributions made by staff of our Research Laboratory for data collection, especially Christina Canino and Cheryl Crippen. We also thank the mothers and children who participated.
Role of funding source
This research was supported by National Institute of Health grants NS-41298, HD-51852 AND HD28413 to CAS. The NIH had no further role in study design, in the collection, analysis, and interpretation of data, in the writing of this report, or in the decision to submit this report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors acknowledge no conflict of interest.
References
- Abernethy LJ, Palaniappan M, Cooke RW. Quantitative magnetic resonance imaging of the brain in survivors of very low birth weight. Arch Dis Child. 2002;87:279–283. doi: 10.1136/adc.87.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Z, Balsamo LM, Sachs BC, Xu B, Gaillard WD. Auditory comprehension of language in young children: neural networks identified with fMRI. Neurology. 2003;60:1598–1605. doi: 10.1212/01.wnl.0000059865.32155.86. [DOI] [PubMed] [Google Scholar]
- Aisa B, Elizalde N, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Hippocampus. Effects of neonatal stress on markers of synaptic plasticity in the hippocampus: Implications for spatial memory. in press. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Armstrong JM, Goldstein LH. Manual for the MacArthur Health and Behavior Questionnaire (HBQ 1.0) MacArthur Foundation Research Network on Psychopathology and Development University of Pittsburgh; 2003. [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baram TZ, Chalmers DT, Chen C, Koutsoukos Y, De Souza EB. The CRF1 receptor mediates the excitatory actions of corticotropin releasing factor (CRF) in the developing rat brain: in vivo evidence using a novel, selective, non-peptide CRF receptor antagonist. Brain Res. 1997;770:89–95. doi: 10.1016/s0006-8993(97)00759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21:471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Hirsch E, Snead OC, 3rd, Schultz L. Corticotropin-releasing hormone-induced seizures in infant rats originate in the amygdala. Ann Neurol. 1992;31:488–494. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MH, Thompson DK, Howard K, Doyle LW, Egan GF, Inder TE, Anderson PJ. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain. 2008;131:2986–2994. doi: 10.1093/brain/awn227. [DOI] [PubMed] [Google Scholar]
- Beversdorf DQ, Manning SE, Hillier A, Anderson SL, Nordgren RE, Walters SE, Nagaraja HN, Cooley WC, Gaelic SE, Bauman ML. Timing of prenatal stressors and autism. J Autism Dev Disord. 2005;35:471–478. doi: 10.1007/s10803-005-5037-8. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP. Synaptogenesis, heterochrony and epigenesis in the mammalian neocortex. Acta Paediatr Suppl. 1997;422:27–33. doi: 10.1111/j.1651-2227.1997.tb18340.x. [DOI] [PubMed] [Google Scholar]
- Brandt T, Stephan T, Bense S, Yousry TA, Dieterich M. Hemifield visual motion stimulation: an example of interhemispheric crosstalk. Neuroreport. 2000;11:2803–2809. doi: 10.1097/00001756-200008210-00039. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Grant RJ, Champagne DL, Meaney MJ. Maternal care influences neuronal survival in the hippocampus of the rat. Eur J Neurosci. 2003;18:2903–2909. doi: 10.1111/j.1460-9568.2003.02965.x. [DOI] [PubMed] [Google Scholar]
- Brouwers EP, Van Baar AL, Pop VJ. Maternal anxiety during pregnancy and subsequent infant development. Infant Behavior and Development. 2001;24:95–106. [Google Scholar]
- Brown RW, Diaz R, Robson AC, Kotelevtsev YV, Mullins JJ, Kaufman MH, Seckl JR. The ontogeny of 11 beta-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology. 1996;137:794–797. doi: 10.1210/endo.137.2.8593833. [DOI] [PubMed] [Google Scholar]
- Buitelaar JK, Huizink AC, Mulder EJ, de Medina PG, Visser GH. Prenatal stress and cognitive development and temperament in infants. Neurobiol Aging. 2003;24(Suppl 1):S53–60. doi: 10.1016/s0197-4580(03)00050-2. discussion S67-58. [DOI] [PubMed] [Google Scholar]
- Buss C, Lord C, Wadiwalla M, Hellhammer DH, Lupien SJ, Meaney MJ, Pruessner JC. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J Neurosci. 2007;27:2592–2595. doi: 10.1523/JNEUROSCI.3252-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EC, Smith R, Lewin T, Brinsmead MW, Zhang HP, Cubis J, Thornton K, Hurt D. Plasma corticotropin-releasing hormone, beta-endorphin and cortisol inter-relationships during human pregnancy. Acta Endocrinol (Copenh) 1993;128:339–344. doi: 10.1530/acta.0.1280339. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Class QA, Buss C, Davis EP, Gierczak M, Pattillo C, Chicz-DeMet A, Sandman CA. Low levels of corticotropin-releasing hormone during early pregnancy are associated with precocious maturation of the human fetus. Dev Neurosci. 2008;30:419–426. doi: 10.1159/000191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lulbach GR, Schneider ML. Prenatal disturbance alters the size of the corpus callosum in young monkeys. Dev Psychobiol. 2002;41:178–185. doi: 10.1002/dev.10063. [DOI] [PubMed] [Google Scholar]
- Cohen S, Williamson GM. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health. Sage; Newbury Park, CA: 1988. pp. 31–67. [Google Scholar]
- Connolly JD, Goodale MA, Menon RS, Munoz DP. Human fMRI evidence for the neural correlates of preparatory set. Nature neuroscience. 2002;5:1345–1352. doi: 10.1038/nn969. [DOI] [PubMed] [Google Scholar]
- Connors SL, Levitt P, Matthews SG, Slotkin TA, Johnston MV, Kinney HC, Johnson WG, Dailey RM, Zimmerman AW. Fetal mechanisms in neurodevelopmental disorders. Pediatr Neurol. 2008;38:163–176. doi: 10.1016/j.pediatrneurol.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Cowan WM. The development of the brain. Sci Am. 1979;241:113–133. [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Dunkel Schetter C, Hobel C, Chicz-Demet A, Sandman CA. Corticotropin-releasing hormone during pregnancy is associated with infant temperament. Dev Neurosci. 2005;27:299–305. doi: 10.1159/000086709. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal Exposure to Maternal Depression and Cortisol Influences Infant Temperament. J Am Acad Child Adolesc Psychiatry. 2007;46:737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. The Timing of Prenatal Exposure to Maternal Cortisol and Psychosocial Stress is Associated with Human Infant Cognitive Development. Child Dev. doi: 10.1111/j.1467-8624.2009.01385.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Snidman N, Wadhwa P, Dunkel-Schetter C, Glynn LM, Sandman CA. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy. 2004;6:319–331. [Google Scholar]
- de Weerth C, Buitelaar JK. Physiological stress reactivity in human pregnancy--a review. Neurosci Biobehav Rev. 2005;29:295–312. doi: 10.1016/j.neubiorev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- de Weerth C, van Hees Y, Buitelaar JK. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Hum Dev. 2003;74:139–151. doi: 10.1016/s0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Ghera MM, Costigan K, Hawkins M. Measuring the ups and downs of pregnancy stress. J Psychosom Obstet Gynaecol. 2004;25:189–201. doi: 10.1080/01674820400017830. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Hilton SC, Hawkins M, Costigan KA, Pressman EK. Maternal stress and affect influence fetal neurobehavioral development. Developmental Psychology. 2002;38:659–668. [PubMed] [Google Scholar]
- Dipietro JA, Novak MF, Costigan KA, Atella LD, Reusing SP. Maternal psychological distress during pregnancy in relation to child development at age two. Child Dev. 2006;77:573–587. doi: 10.1111/j.1467-8624.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Henriksen SJ, Wang M, Rivier J, Vale W, Bloom FE. Corticotropin releasing factor produces increases in brain excitability and convulsive seizures in rats. Brain Res. 1983;278:332–336. doi: 10.1016/0006-8993(83)90266-4. [DOI] [PubMed] [Google Scholar]
- Ellman LM, Schetter CD, Hobel CJ, Chicz-Demet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: effects on newborn physical and neuromuscular maturation. Dev Psychobiol. 2008;50:232–241. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K, Thorsen P, Chrousos G, Grigoriadis DE, Khongsaly O, McGregor J, Schulkin J. Preterm birth: associated neuroendocrine, medical, and behavioral risk factors. J Clin Endocrinol Metab. 2001;86:2544–2552. doi: 10.1210/jcem.86.6.7607. [DOI] [PubMed] [Google Scholar]
- Fabricius K, Wortwein G, Pakkenberg B. The impact of maternal separation on adult mouse behaviour and on the total neuron number in the mouse hippocampus. Brain Struct Funct. 2008;215:403–416. doi: 10.1007/s00429-007-0169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka A, Fujioka T, Ishida Y, Maekawa T, Nakamura S. Differential effects of prenatal stress on the morphological maturation of hippocampal neurons. Neuroscience. 2006;141:907–915. doi: 10.1016/j.neuroscience.2006.04.046. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Bedogni F, Perez J, Racagni G, Riva MA. Corticostriatal brain-derived neurotrophic factor dysregulation in adult rats following prenatal stress. Eur J Neurosci. 2004;20:1348–1354. doi: 10.1111/j.1460-9568.2004.03592.x. [DOI] [PubMed] [Google Scholar]
- Gillman MW, Rich-Edwards JW, Huh S, Majzoub JA, Oken E, Taveras EM, Rifas-Shiman SL. Maternal corticotropin-releasing hormone levels during pregnancy and offspring adiposity. Obesity. 2006;14:1647–1653. doi: 10.1038/oby.2006.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez M, Junque C, Narberhaus A, Caldu X, Salgado-Pineda P, Bargallo N, Segarra D, Botet F. Hippocampal gray matter reduction associates with memory deficits in adolescents with history of prematurity. Neuroimage. 2004;23:869–877. doi: 10.1016/j.neuroimage.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Schetter CD, Hobel CJ, Sandman CA. Pattern of perceived stress and anxiety in pregnancy predicts preterm birth. Health Psychol. 2008;27:43–51. doi: 10.1037/0278-6133.27.1.43. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Schetter CD, Wadhwa PD, Sandman CA. Pregnancy affects appraisal of negative life events. J Psychosom Res. 2004;56:47–52. doi: 10.1016/S0022-3999(03)00133-8. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Sandman CA. When stress happens matters: effects of earthquake timing on stress responsivity in pregnancy. Am J Obstet Gynecol. 2001;184:637–642. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- Goland RS, Conwell IM, Warren WB, Wardlaw SL. Placental corticotropin-releasing hormone and pituitary-adrenal function during pregnancy. Neuroendocrinology. 1992;56:742–749. doi: 10.1159/000126302. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Buitelaar JK. Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology. 2005;30:541–549. doi: 10.1016/j.psyneuen.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Nagaoka M, Yamada K, Ichitani Y, Miake Y, Okado N. Maternal stress induces synaptic loss and developmental disabilities of offspring. Int J Dev Neurosci. 1998;16:209–216. doi: 10.1016/s0736-5748(98)00028-8. [DOI] [PubMed] [Google Scholar]
- Helmeke C, Poeggel G, Braun K. Differential emotional experience induces elevated spine densities on basal dendrites of pyramidal neurons in the anterior cingulate cortex of Octodon degus. Neuroscience. 2001;104:927–931. doi: 10.1016/s0306-4522(01)00201-9. [DOI] [PubMed] [Google Scholar]
- Hobel CJ. Identification of the patient at risk. In: Schwartz RH, Schneider J, editors. Perinatal Medicine: Management of the high risk fetus and neonate. Williams & Wilkins; Baltimore: 1982. pp. 3–28. [Google Scholar]
- Hobel CJ, Dunkel Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;180:S257–263. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- Hoistad M, Barbas H. Sequence of information processing for emotions through pathways linking temporal and insular cortices with the amygdala. Neuroimage. 2008;40:1016–1033. doi: 10.1016/j.neuroimage.2007.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. Psychological measures of prenatal stress as predictors of infant temperament. J Am Acad Child Adolesc Psychiatry. 2002;41:1078–1085. doi: 10.1097/00004583-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJ, Robles de Medina PG, Visser GH, Buitelaar JK. Is pregnancy anxiety a distinctive syndrome? Early Hum Dev. 2004;79:81–91. doi: 10.1016/j.earlhumdev.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Robles de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry. 2003;44:810–818. doi: 10.1111/1469-7610.00166. [DOI] [PubMed] [Google Scholar]
- Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- Jones JI, Gockerman A, Busby WH, Jr., Wright G, Clemmons DR. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the alpha 5 beta 1 integrin by means of its Arg-Gly-Asp sequence. Proc Natl Acad Sci U S A. 1993;90:10553–10557. doi: 10.1073/pnas.90.22.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LS, Gauger LL, Davis JN, Slotkin TA, Bartolome JV. Postnatal development of brain alpha 1-adrenergic receptors: in vitro autoradiography with [125I]HEAT in normal rats and rats treated with alpha-difluoromethylornithine, a specific, irreversible inhibitor of ornithine decarboxylase. Neuroscience. 1985;15:1195–1202. doi: 10.1016/0306-4522(85)90262-3. [DOI] [PubMed] [Google Scholar]
- Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, Kenny LC, Mortensen PB. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008;65:146–152. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- Kinnunen AK, Koenig JI, Bilbe G. Repeated variable prenatal stress alters pre- and postsynaptic gene expression in the rat frontal pole. J Neurochem. 2003;86:736–748. doi: 10.1046/j.1471-4159.2003.01873.x. [DOI] [PubMed] [Google Scholar]
- Knoll B, Drescher U. Ephrin-As as receptors in topographic projections. Trends Neurosci. 2002;25:145–149. doi: 10.1016/s0166-2236(00)02093-2. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Judas M, Rados M, Hrabac P. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 2002;12:536–544. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Lydon J, Seguin L, Goulet L, Kahn SR, McNamara H, Genest J, Dassa C, Chen MF, Sharma S, Meaney MJ, Thomson S, Van Uum S, Koren G, Dahhou M, Lamoureux J, Platt RW. Stress pathways to spontaneous preterm birth: the role of stressors, psychological distress, and stress hormones. Am J Epidemiol. 2009;169:1319–1326. doi: 10.1093/aje/kwp061. [DOI] [PubMed] [Google Scholar]
- Laplante DP, Barr RG, Brunet A, Galbaud du Fort G, Meaney ML, Saucier JF, Zelazo PR, King S. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatr Res. 2004;56:400–410. doi: 10.1203/01.PDR.0000136281.34035.44. [DOI] [PubMed] [Google Scholar]
- Laplante DP, Brunet A, Schmitz N, Ciampi A, King S. Project Ice Storm: Prenatal Maternal Stress Affects Cognitive and Linguistic Functioning in 5(1/2)-Year-Old Children. J Am Acad Child Adolesc Psychiatry. 2008;47:1063–1072. doi: 10.1097/CHI.0b013e31817eec80. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Lamarque S, Moal ML, Piazza PV, Abrous DN. Postnatal Stimulation of the Pups Counteracts Prenatal Stress-Induced Deficits in Hippocampal Neurogenesis. Biol Psychiatry. 2006;59:786–792. doi: 10.1016/j.biopsych.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann NY Acad Sci. 2003;997:136–149. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Bhatnagar S, Sapolsky RM. Postnatal handling attenuates certain neuroendocrine, anatomical, and cognitive dysfunctions associated with aging in female rats. Neurobiol Aging. 1991;12:31–38. doi: 10.1016/0197-4580(91)90036-j. [DOI] [PubMed] [Google Scholar]
- Mestres-Misse A, Camara E, Rodriguez-Fornells A, Rotte M, Munte TF. Functional neuroanatomy of meaning acquisition from context. J Cogn Neurosci. 2008;20:2153–2166. doi: 10.1162/jocn.2008.20150. [DOI] [PubMed] [Google Scholar]
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kubota K. The primate temporal pole: its putative role in object recognition and memory. Behav Brain Res. 1996;77:53–77. doi: 10.1016/0166-4328(95)00227-8. [DOI] [PubMed] [Google Scholar]
- Nathanielsz PW, Berghorn KA, Derks JB, Giussani DA, Docherty C, Unno N, Davenport A, Kutzlers M, Koenen S, Visser GH, Nijland MJ. Life before birth: effects of cortisol on future cardiovascular and metabolic function. Acta Paediatr. 2003;92:766–772. [PubMed] [Google Scholar]
- Niederhofer H, Reiter A. Prenatal maternal stress, prenatal fetal movements and perinatal temperament factors influence behavior and school marks at the age of 6 years. Fetal Diagn Ther. 2004;19:160–162. doi: 10.1159/000075142. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Al-Asady MH, Frangou S, Stewart AL, Rifkin L, Murray RM. Adolescents who were born very preterm have decreased brain volumes. Brain. 2002;125:1616–1623. doi: 10.1093/brain/awf157. [DOI] [PubMed] [Google Scholar]
- O’Brien GD, Queenan JT, Campbell S. Assessment of gestational age in the second trimester by real-time ultrasound measurement of the femur length. Am J Obstet Gynecol. 1981;139:540–545. doi: 10.1016/0002-9378(81)90514-7. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005;58:211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180:502–508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- Odagiri K, Abe H, Kawagoe C, Takeda R, Ikeda T, Matsuo H, Nonaka H, Ebihara K, Nishimori T, Ishizuka Y, Hashiguchi H, Ishida Y. Psychological prenatal stress reduced the number of BrdU immunopositive cells in the dorsal hippocampus without affecting the open field behavior of male and female rats at one month of age. Neurosci Lett. 2008;446:25–29. doi: 10.1016/j.neulet.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ovtscharoff W, Jr., Helmeke C, Braun K. Lack of paternal care affects synaptic development in the anterior cingulate cortex. Brain Res. 2006;1116:58–63. doi: 10.1016/j.brainres.2006.07.106. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. Jama. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Garuti GC, De Ramundo B, Angioni S, Genazzani AR, Bilezikjian LM. Mechanism of action of interleukin-1 beta in increasing corticotropin-releasing factor and adrenocorticotropin hormone release from cultured human placental cells. Am J Obstet Gynecol. 1990;163:1307–1312. doi: 10.1016/0002-9378(90)90711-f. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Hatch MC, Lapinski R, Stomati M, Reis FM, Cobellis L, Berkowitz GS. Lack of effect of psychosocial stress on maternal corticotropin-releasing factor and catecholamine levels at 28 weeks’ gestation. J Soc Gynecol Investig. 2001;8:83–88. [PubMed] [Google Scholar]
- Petraglia F, Sawchenko PE, Rivier J, Vale W. Evidence for local stimulation of ACTH secretion by corticotropin-releasing factor in human placenta. Nature. 1987;328:717–719. doi: 10.1038/328717a0. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Sutton S, Vale W. Neurotransmitters and peptides modulate the release of immunoreactive corticotropin-releasing factor from cultured human placental cells. Am J Obstet Gynecol. 1989;160:247–251. doi: 10.1016/0002-9378(89)90130-0. [DOI] [PubMed] [Google Scholar]
- Pham TM, Soderstrom S, Winblad B, Mohammed AH. Effects of environmental enrichment on cognitive function and hippocampal NGF in the non-handled rats. Behav Brain Res. 1999;103:63–70. doi: 10.1016/s0166-4328(99)00019-4. [DOI] [PubMed] [Google Scholar]
- Pickering C, Gustafsson L, Cebere A, Nylander I, Liljequist S. Repeated maternal separation of male Wistar rats alters glutamate receptor expression in the hippocampus but not the prefrontal cortex. Brain Res. 2006;1099:101–108. doi: 10.1016/j.brainres.2006.04.136. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 2008;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Rees S, Harding R, Walker D. An adverse intrauterine environment: implications for injury and altered development of the brain. Int J Dev Neurosci. 2008;26:3–11. doi: 10.1016/j.ijdevneu.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Rini CK, Dunkel-Schetter C, Wadhwa PD, Sandman CA. Psychological adaptation and birth outcomes: the role of personal resources, stress, and sociocultural context in pregnancy. Health Psychol. 1999;18:333–345. doi: 10.1037//0278-6133.18.4.333. [DOI] [PubMed] [Google Scholar]
- Roceri M, Hendriks W, Racagni G, Ellenbroek BA, Riva MA. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Mol Psychiatry. 2002;7:609–616. doi: 10.1038/sj.mp.4001036. [DOI] [PubMed] [Google Scholar]
- Roesch SC, Dunkel-Schetter C, Woo G, Hobel C. Modeling the types and timing of stress in pregnancy. Anxiety, Stress & Coping. 2004;17:87–102. [Google Scholar]
- Rokyta R, Yamamotova A, Slamberova R, Franek M, Vaculin S, Hruba L, Schutova B, Pometlova M. Prenatal and perinatal factors influencing nociception, addiction and behavior during ontogenetic development. Physiol Res. 2008;57(Suppl 3):S79–88. doi: 10.33549/physiolres.931602. [DOI] [PubMed] [Google Scholar]
- Salm AK, Pavelko M, Krouse EM, Webster W, Kraszpulski M, Birkle DL. Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Brain Res. 2004;148:159–167. doi: 10.1016/j.devbrainres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Ashburner J, Vargha-Khadem F, Connelly A, Gadian DG, Friston KJ. Distributional assumptions in voxel-based morphometry. NeuroImage. 2002;17:1027–1030. [PubMed] [Google Scholar]
- Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-Demet A, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): Priming the placental clock. Peptides. 2006;27:1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa PD, Chicz-DeMet A, Porto M, Garite TJ. Maternal corticotropin-releasing hormone and habituation in the human fetus. Dev Psychobiol. 1999;34:163–173. doi: 10.1002/(sici)1098-2302(199904)34:3<163::aid-dev1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Shinkawa O, Yoshinaga K. Placental corticotropin-releasing hormone may be a stimulator of maternal pituitary adrenocorticotropic hormone secretion in humans. J Clin Invest. 1989;84:1997–2001. doi: 10.1172/JCI114390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Roughton EC, Koehler AJ, Lubach GR. Growth and development following prenatal stress exposure in primates: an examination of ontogenetic vulnerability. Child Dev. 1999;70:263–274. doi: 10.1111/1467-8624.00020. [DOI] [PubMed] [Google Scholar]
- Schulte HM, Weisner D, Allolio B. The corticotrophin releasing hormone test in late pregnancy: lack of adrenocorticotrophin and cortisol response. Clin Endocrinol. 1990;33:99–106. doi: 10.1111/j.1365-2265.1990.tb00470.x. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Meaney MJ. Glucocorticoid programming. Ann NY Acad Sci. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- Selten JP, van der Graaf Y, van Duursen R, Gispen-de Wied CC, Kahn RS. Psychotic illness after prenatal exposure to the 1953 Dutch Flood Disaster. Schizophr Res. 1999;35:243–245. doi: 10.1016/s0920-9964(98)00143-1. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Smith R, Mesiano S, Chan EC, Brown S, Jaffe RB. Corticotropin-releasing hormone directly and preferentially stimulates dehydroepiandrosterone sulfate secretion by human fetal adrenal cortical cells. J Clin Endocrinol Metab. 1998;83:2916–2920. doi: 10.1210/jcem.83.8.5020. [DOI] [PubMed] [Google Scholar]
- Smythe JW, McCormick CM, Rochford J, Meaney MJ. The interaction between prenatal stress and neonatal handling on nociceptive response latencies in male and female rats. Phys Behav. 1994;55:971–974. doi: 10.1016/0031-9384(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic at;as of the human brain; 3-dimensional proportional system: an approach to cerebral imaging. Thieme; New York: 1988. [Google Scholar]
- Toga AW, Thompson PM. Temporal dynamics of brain anatomy. Annu Rev Biomed Eng. 2003;5:119–145. doi: 10.1146/annurev.bioeng.5.040202.121611. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, Holden J. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994;28:336–348. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- Uno H, Lohmiller L, Thieme C, Kemnitz JW, Engle MJ, Roecker EB, Farrell PM. Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I. Hippocampus. Brain Res. 1990;53:157–167. doi: 10.1016/0165-3806(90)90002-g. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Mennes M, Oosterlaan J, Stevens V, Stiers P, Marcoen A, Lagae L. High antenatal maternal anxiety is related to impulsivity during performance on cognitive tasks in 14- and 15-year-olds. Neurosci Biobehav Rev. 2005;29:259–269. doi: 10.1016/j.neubiorev.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2008;33:536–545. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- Van den Hove DL, Steinbusch HW, Scheepens A, Van de Berg WD, Kooiman LA, Boosten BJ, Prickaerts J, Blanco CE. Prenatal stress and neonatal rat brain development. Neuroscience. 2006;137:145–155. doi: 10.1016/j.neuroscience.2005.08.060. [DOI] [PubMed] [Google Scholar]
- van Os J, Selten JP. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br J Psychiatry. 1998;172:324–326. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, Chicz-DeMet A, Porto M. Placental CRH modulates maternal pituitary adrenal function in human pregnancy. Ann NY Acad Sci. 1997;814:276–281. doi: 10.1111/j.1749-6632.1997.tb46163.x. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. Am J Obstet Gynecol. 1993;169:858–865. doi: 10.1016/0002-9378(93)90016-c. [DOI] [PubMed] [Google Scholar]
- Watson JB, Mednick SA, Huttunen M, Wang X. Prenatal teratogens and the development of adult mental illness. Dev Psychopathol. 1999;11:457–466. doi: 10.1017/s0954579499002151. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Hum Brain Mapp. 2002;17:48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, Salomon S, Matzner H, Weinstock M. Effect of varied gestational stress on acquisition of spatial memory, hippocampal LTP and synaptic proteins in juvenile male rats. Behav Brain Res. 2007 doi: 10.1016/j.bbr.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Yang J, Hou C, Ma N, Liu J, Zhang Y, Zhou J, Xu L, Li L. Enriched environment treatment restores impaired hippocampal synaptic plasticity and cognitive deficits induced by prenatal chronic stress. Neurobiol Learn Mem. 2007;87:257–263. doi: 10.1016/j.nlm.2006.09.001. [DOI] [PubMed] [Google Scholar]