Abstract
Background:
Little is known about the effects of antipsychotic medications on gray matter (GM) in schizophrenia. Although clozapine remains the most effective antipsychotic medication in treatment-refractory cases, it is unknown whether it has a differential effect on GM development.
Methods:
In an exploratory analysis, we used automated cortical thickness measurements and prospectively scanned childhood-onset schizophrenia (COS) patients who were maintained on one medication. Two atypical antipsychotic medications, clozapine (n=12, 37 scans) and olanzapine (n=12, 33 scans) were compared with respect to effects on cortical development, in contrast to GM trajectories of matched controls.
Results:
There were no significant differences in the trajectories of cortical thickness between the two treatment groups with the exception of a small circumscribed area in the right prefrontal cortex, where the olanzapine group showed thicker cortex. As expected, both groups showed thinner GM compared to matched controls.
Conclusions:
Although these analyses do not rule out effects of antipsychotic medications on GM development in schizophrenia, they show no differential effect between clozapine and olanzapine on GM trajectory.
Introduction
Clozapine was the first atypical antipsychotic agent to be discovered and was the landmark in schizophrenia treatment due to its efficacy in treatment-refractory cases (Kane et al., 1988a). Despite the availability of many newer atypical agents with less detrimental side effect profiles, clozapine remains the most effective medication for treatment resistant schizophrenia (Kane et al., 1988b, Kane, 1992, McEvoy et al., 2006). Childhood-onset schizophrenia (COS), the rare and severe pediatric form of the illness, is generally treatment-refractory. Thus, the majority of patients end up on clozapine (Gogtay and Rapoport, 2008). In the only head-to-head double-blind comparison trial of two atypical antipsychotic medications in COS, clozapine showed overall superiority to the atypical antipsychotic olanzapine (Shaw et al., 2006). The mechanism for this result remains unclear.
Progressive gray matter (GM) abnormalities are an established feature of schizophrenia. However, it remains unclear whether ongoing medication treatment influences cortical GM loss (DeLisi et al., 2006, Gogtay, 2008). First episode studies in adults and medication-naïve patients (Pantelis et al., 2003) with schizophrenia suggest that cortical abnormalities are likely to either precede or coincide with onset of psychosis and thus are less likely to be induced by medication (Hazlett et al., 2008). Studies in healthy first-degree relatives also suggest that GM loss could be a familial/trait marker and thus unlikely caused by drug treatment (Gogtay et al., 2007a, Goldman et al., 2009).
Some studies have suggested that exposure to typical antipsychotics is associated with increased basal ganglia and thalamic volumes (Gur et al., 1998), while exposure to atypical antipsychotics is associated with decreased basal ganglia volumes (Khorram et al., 2006, Corson et al., 1999, Scherk and Falkai, 2006). However, the differential effect of both typical and atypical antipsychotics on cortical GM volumes is unclear and findings are inconsistent. An earlier small study in patients with acute psychosis (n=7) showed no GM volume changes in relation to haloperidol treatment (Garver et al., 2005). In contrast, a study by Lieberman et al. (n=164) found a reduction in frontal and total GM volumes among first-episode patients taking haloperidol, which was not seen with olanzapine (Lieberman et al., 2005b). A follow-up study by the same group using cortical mapping methods and more frequent scan intervals (3 month; n = 36) showed that GM loss progressed in a parieto-frontal direction in haloperidol-treated patients, which was not seen in olanzapine-treated patients (Thompson et al., 2009). However, in both these studies the differential effects disappeared after one year.
A series of studies focusing on GM abnormalities in COS have shown widespread cortical loss that evolves in a parieto-frontal direction during adolescence (Giedd et al., 1999, Jacobsen et al., 1998, Rapoport et al., 1999, Thompson et al., 2001) merging into the adult pattern by early adulthood (Greenstein et al., 2006). Findings from longitudinal MRI studies of adolescent-onset psychosis also support the idea of progressive frontal GM changes (Reig et al., 2009, Moreno et al., 2005) bolstering support for COS as a progressive neurodevelopmental disorder with both early and late developmental aberrations (Arango et al., 2008). More recent studies with larger sample sizes have suggested that the GM thickness may be positively correlated to the overall functional outcome, thus raising the possibility that drug treatment could be influencing GM development (Greenstein et al., 2008, van Haren et al., 2008). We decided to explore whether the superiority of clozapine uniquely influenced GM trajectory in COS by comparing it to olanzapine. We identified two COS samples where patients were consistently treated with either clozapine or olanzapine for at least two prospective scans and matched them for baseline clinical severity to minimize disease effects.
Methods
Subjects
COS patients were recruited nationwide and most were diagnosed after a thorough inpatient observation that included a complete medication washout. Exclusionary criteria include history of significant medical or neurological illness, substance abuse, or IQ below 70 prior to onset of psychotic symptoms; details are described elsewhere (Kumra et al., 1996; McKenna et al., 1994). All patients were followed longitudinally at two-year intervals and anatomic MRI scans were obtained at each visit.
For this study, COS subjects with at least two or more successive scans while on the same medication (either clozapine (n= 12, 37 scans); or olanzapine (n= 12, 33 scans) were used. Both groups were matched for age, clinical severity (BPRS, CGI-SI scores at baseline), and IQ. Most subjects in the olanzapine group were originally part of a randomized double-blind clozapine-olanzapine comparison trial and assigned to treatment with olanzapine. At the conclusion of the trial, these patients showed improvement versus baseline and did not require clozapine initiation. Additionally, scans from a group of healthy controls (n=44) matched for age and sex were also used for comparative analysis. The Institutional Review Board of the National Institute of Mental Health approved this study.
MRI acquisition and image analysis
T1-weighted images with contiguous 1.5-mm slices in the axial plane were obtained using a 3-dimensional spoiled gradient recalled echo sequence in the steady state. Imaging parameters were echo time of 5 milliseconds, repetition time of 24 milliseconds, flip angle of 45°, acquisition matrix of 256 × 192, number of excitations equaled 1, and a 24-cm field of view. Head placement was standardized as previously described (Castellanos et al., 2001). Magnetic resonance images were registered into standardized space using a linear transformation and corrected for nonuniformity artifacts (Sled et al., 1998). Registered and corrected volumes were segmented with an advanced neural net classifier (Zijdenbos et al., 2002) and GM and white matter (WM) surfaces were fitted with a surface deformation algorithm, which first determines the WM surface, then expands outward to find the GM–cerebrospinal fluid intersection (Kim et al., 2005, MacDonald et al., 2000). Cortical thickness measurements, defined as the distance between linked vertices of the GM and WM boundaries using a 30-mm surface-based blurring kernel (which has been shown to maximize statistical power), have been previously validated and were calculated in native space at 40,962 cortical points (Lerch and Evans, 2005).
Statistical Analysis
Demographic differences between groups were tested using t-tests for continuous variables, and chi-square tests of independence for categorical variables. We performed a linear mixed effects regression model at each of the 40,962 GM points per hemisphere. At each point, the dependent variable was GM thickness. Fixed effects included group (clozapine and olanzapine), age (centered at sample average age), and group*age (difference in cortical thickness development between groups). We also included a random intercept per person to account for within-subject dependence. Type I error was controlled per hemisphere using the False Discovery Rate (FDR) procedure (Genovese et al., 2002) with q set at 0.05 (i.e. no more than 5% are false positives). The model was run with sex as a covariate.
Results
Demographics and clinical data
Sample demographics for the medication groups are shown in Table 1. There were no significant differences with respect to age, handedness, or duration of illness between patients treated with clozapine and those treated with olanzapine at the initial scan (Table 1). When all scans were included, the clozapine group had a significantly higher proportion of males versus the olanzapine group (x2 = .002). As a result, sex was included as a covariate in the analyses. There were no significant differences between the clozapine and olanzapine groups with respect to BPRS, CGI_SI, and IQ. Sample demographics for the control group are listed in Table 2.
Table 1.
Sample Demographics for Treatment Groups
| CLOZAPINE | OLANZAPINE | p values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Average Age | # of scans |
Sex (M,F) |
Hand (R,L,M) |
Average Age | # of scans |
Sex (M,F) |
Hand (R,L,M) |
Age | Sex | Handedness | |
| First Scan | 14.48 | 12 | 8,4 | 8,3,1 | 15.43 | 12 | 5,7 | 9,2,1 | 0.32 | 0.10 | 0.63 |
| Last Scan | 21.095 (STD 4.01) |

|
20.178 (STD 3.19) |

|
|||||||
| ALL SCANS* |
18.92 | 37 | 28,9 | 23,11,3 | 18.00 | 33 | 13,20 | 24,5,4 | 0.40 (t=0.85) |
0.002 (x2=9.4) |
0.33 (x2=2.2) |
| Clinical Severity |
Avg. CGI_SI | 5.50 (STD 1.19) | Avg. CGI_SI | 5.25 (STD 1.29) | p-0.52 (t=0.65) | ||||||
| Avg. BPRS | 67.42 (STD 16.9) | Avg. BPRS | 65.50 (STD 20.5) | p=0.68 (t=−0.41) | |||||||
| Avg. IQ | 73.91 (STD 22.2) | Avg. IQ | 74.94 (STD 21.1) | p=0.29 (t=1.07) | |||||||
“All Scans” includes first scans, last scans, and all scans in between
CGI_SI: Clinician's Global Impressions-Severity of Illness scale
BPRS: Brief Psychiatric Rating Scale
STD: Standard Deviation
IQ: Intelligence Quotient
Table 2.
Sample Demographics for Control Group
| CONTROLS | ||||
|---|---|---|---|---|
| Average Age | # of scans |
Sex (M,F) |
Hand (R,L,M) |
|
| Firal Scan | 14.65 | 44 | 28,16 | 39,2,3 |
| Last Scan | 20.53 (STD 4.32) |

|
||
| ALL SCANS* | 17.86 | 135 | 91,44 | 122,6,7 |
| Clinical Severity |
Avg. CGI_SI | NA | ||
| Avg. BPRS | NA | |||
| Avg. IQ | 129.96 (STD 107.44) | |||
“All Scans” includes first scans, last scans, and all scans in between
CGI_SI: Clinician's Global Impressions-Severity of Illness scale
BPRS: Brief Psychiatric Rating Scale
STD: Standard Deviation
IQ: Intelligence Quotient
Cortex analysis
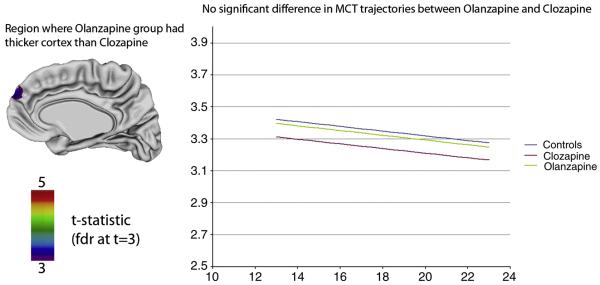
The trajectories for GM change in both groups were not significantly different over the entire cortex (Figure 1). The overall cortical thickness (GM amount) was also not significantly different between the clozapine and olanzapine treated groups in most brain regions. However, COS subjects treated with clozapine had thinner GM compared to those treated with olanzapine in one small region in the right prefrontal cortex (Figure 1). In this region, the deficit appeared to be fixed with no differences in the shapes of GM trajectories. There was no shape difference in trajectory between each treatment group and controls.
Figure 1.
Left: Cortical thickness (GM) differences between clozapine and olanzapine treated patients. The only significant difference in cortical thickness was seen in a small area in the right prefrontal cortex in which olanzapine treated patients had thicker cortices compared to the clozapine treated group. Right: Trajectories of mean cortical thickness between olanzapine and clozapine treated patients. The treatment groups did not differ significantly in the trajectories of cortical development (p=0.27).
Discussion
We examined whether clozapine differentially alters cortical GM loss in COS compared to olanzapine. We found no differences in GM trajectories between the clozapine and olanzapine treated patients suggesting that these medications do not differentially influence GM in COS. Similarly there were no significant differences in GM amount between the groups except for a small region in the right medial prefrontal cortex where the olanzapine treated group showed thicker cortex compared to the clozapine treated group. As expected, GM trajectories for both clozapine and olanzapine treated subjects showed thinner mean GM cortical thickness (and volume) compared to controls which is consistent with the GM loss seen in COS.
These results suggest that the superior outcome of clozapine is unlikely to be entirely mediated by influencing the GM trajectory. These observations indirectly support those made by Lieberman et al. (Lieberman et al., 2005a) where olanzapine-treated patients showed greater GM volume compared to the haloperidol-treated group. Similarly, in the present study the olanzapine group appeared to have qualitatively greater GM thickness in most areas, although they reach statistical significance only in a small region in the medial prefrontal cortex.
Human studies exploring the question of medication influence on GM are inconsistent, with some reporting no effect of antipsychotics on GM volumes (Ho et al., 2003, DeLisi et al., 2004), while others showed GM expansion after treatment with either atypical or typical antipsychotic drugs (Garver et al., 2005, Molina et al., 2005). There are fewer studies looking at the influence of antipsychotics on longitudinal GM trajectories and none that include clozapine. A study of 29 neuroleptic-naïve first-episode schizophrenia patients before and after eighteen months of a variety of typical antipsychotic treatment did not demonstrate significant changes in cortical volume (Chakos et al., 1994). Similarly, Keshavan et al. found no changes in regional or total brain volume after twelve months of typical antipsychotic treatment (Keshavan et al., 1994). Alternatively, two relatively small studies have shown GM reduction with typical antipsychotic treatment when observed within a shorter time period on the medication. Dazzan et al. reported that patients (n=32) receiving 8-9 weeks of treatment with typical antipsychotics had diffuse GM reduction when compared with patients not receiving medication (Dazzan et al., 2005). A recent double-comparison between haloperidol and olanzapine found a significant decrease in total and frontal GM in the haloperidol group after twelve weeks of treatment (Lieberman et al., 2005b). This was subsequently localized to the parietal and frontal cortices by cortical density mapping measures (Thompson et al., 2009). However, in the Lieberman study the differences disappeared after the first year, suggesting no longer-term influence. This study is consistent with many longitudinal studies that suggest prolonged exposure of atypical antipsychotics may not influence GM changes as well as some evidence for olanzapine showing increased GM in the medial prefrontal cortex.
More recent animal studies also support these findings. A study in macaques showed a significant reduction in postmortem brain weight and volume in olanzapine and haloperidol treated monkeys compared to controls, but the experimental groups did not differ from each other (Dorph-Petersen et al., 2005). Earlier studies from our group have also suggested a lack of differential effect of antipsychotic medication on GM changes in COS. Our initial study comparing progressive cortical GM loss in COS and in children with atypical psychoses (multidimensionally impaired, MDI) did not find a medication effect on GM change over a two-year period (Gogtay et al., 2004). A follow-up study which involved dynamic mapping of cortical development before and after onset of pediatric bipolar illness also showed no medication influence (including mood stabilizers) on GM trajectories (Gogtay et al., 2007b). However, in both studies the samples were medication-matched only at Time 1 and thus did not specifically address longer-term medication effects on GM trajectory, which is addressed in this study.
This study has several limitations. First, the sample size is small rendering limited power to the analyses. Thus, the results should be interpreted with caution. Although these results support the idea that there is no differential influence of clozapine and olanzapine on GM trajectory, it is possible that they both still influence GM development. As most longitudinal studies of COS have found decreases in GM over time, it is difficult to ascertain how much of this effect is due to progression of illness, differential brain effects of drug treatment, or interactions among these variables. Ideally, a comparative longitudinal sample of matched, unmedicated COS patients would answer this question, but clearly such a sample is not possible. COS subjects often had medication exposure prior to being started on either olanzapine or clozapine, which may have influenced GM development prior to the first scan, and particularly since the time period in which MRIs were obtained after initiation of clozapine or olanzapine varied among subjects. Finally, dosage effects of either medication could not be tested due to inadequate information during time between follow-up visits. Despite these limitations, these results suggest that clozapine does not appear to differentially influence longer-term GM trajectories in COS.
Acknowledgements
none
Role of funding
The present research was funded by the Intramural Research Program (IRP) at the National Institute of Mental Health (NIMH) in Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflicts of Interest
The authors do not have any disclosures.
References
- ARANGO C, MORENO C, MARTINEZ S, PARELLADA M, DESCO M, MORENO D, FRAGUAS D, GOGTAY N, JAMES A, RAPOPORT J. Longitudinal brain changes in early-onset psychosis. Schizophr Bull. 2008;34:341–53. doi: 10.1093/schbul/sbm157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTELLANOS FX, GIEDD JN, BERQUIN PC, WALTER JM, SHARP W, TRAN T, VAITUZIS AC, BLUMENTHAL JD, NELSON J, BASTAIN TM, ZIJDENBOS A, EVANS AC, RAPOPORT JL. Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58:289–95. doi: 10.1001/archpsyc.58.3.289. [DOI] [PubMed] [Google Scholar]
- CHAKOS MH, LIEBERMAN JA, BILDER RM, BORENSTEIN M, LERNER G, BOGERTS B, WU H, KINON B, ASHTARI M. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry. 1994;151:1430–6. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- CORSON PW, NOPOULOS P, MILLER DD, ARNDT S, ANDREASEN NC. Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. Am J Psychiatry. 1999;156:1200–4. doi: 10.1176/ajp.156.8.1200. [DOI] [PubMed] [Google Scholar]
- DAZZAN P, MORGAN KD, ORR K, HUTCHINSON G, CHITNIS X, SUCKLING J, FEARON P, MCGUIRE PK, MALLETT RM, JONES PB, LEFF J, MURRAY RM. Different effects of typical and atypical antipsychotics on grey matter in first episode psychosis: the AESOP study. Neuropsychopharmacology. 2005;30:765–74. doi: 10.1038/sj.npp.1300603. [DOI] [PubMed] [Google Scholar]
- DELISI LE, SAKUMA M, MAURIZIO AM, RELJA M, HOFF AL. Cerebral ventricular change over the first 10 years after the onset of schizophrenia. Psychiatry Res. 2004;130:57–70. doi: 10.1016/j.pscychresns.2003.08.004. [DOI] [PubMed] [Google Scholar]
- DELISI LE, SZULC KU, BERTISCH HC, MAJCHER M, BROWN K. Understanding structural brain changes in schizophrenia. Dialogues Clin Neurosci. 2006;8:71–8. doi: 10.31887/DCNS.2006.8.1/ldelisi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DORPH-PETERSEN KA, PIERRI JN, PEREL JM, SUN Z, SAMPSON AR, LEWIS DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30:1649–61. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- GARVER DL, HOLCOMB JA, CHRISTENSEN JD. Cerebral cortical gray expansion associated with two second-generation antipsychotics. Biol Psychiatry. 2005;58:62–6. doi: 10.1016/j.biopsych.2005.02.008. [DOI] [PubMed] [Google Scholar]
- GENOVESE CR, LAZAR NA, NICHOLS T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- GIEDD JN, JEFFRIES NO, BLUMENTHAL J, CASTELLANOS FX, VAITUZIS AC, FERNANDEZ T, HAMBURGER SD, LIU H, NELSON J, BEDWELL J, TRAN L, LENANE M, NICOLSON R, RAPOPORT JL. Childhood-onset schizophrenia: progressive brain changes during adolescence. Biol Psychiatry. 1999;46:892–8. doi: 10.1016/s0006-3223(99)00072-4. [DOI] [PubMed] [Google Scholar]
- GOGTAY N. Cortical brain development in schizophrenia: insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr Bull. 2008;34:30–6. doi: 10.1093/schbul/sbm103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOGTAY N, GREENSTEIN D, LENANE M, CLASEN L, SHARP W, GOCHMAN P, BUTLER P, EVANS A, RAPOPORT J. Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia. Arch Gen Psychiatry. 2007a;64:772–80. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- GOGTAY N, ORDONEZ A, HERMAN DH, HAYASHI KM, GREENSTEIN D, VAITUZIS C, LENANE M, CLASEN L, SHARP W, GIEDD JN, JUNG D, NUGENT TF, III, TOGA AW, LEIBENLUFT E, THOMPSON PM, RAPOPORT JL. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. J Child Psychol Psychiatry. 2007b;48:852–62. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- GOGTAY N, RAPOPORT J. Clozapine use in children and adolescents. Expert Opin Pharmacother. 2008;9:459–65. doi: 10.1517/14656566.9.3.459. [DOI] [PubMed] [Google Scholar]
- GOGTAY N, SPORN A, CLASEN LS, NUGENT TF, 3RD, GREENSTEIN D, NICOLSON R, GIEDD JN, LENANE M, GOCHMAN P, EVANS A, RAPOPORT JL. Comparison of progressive cortical gray matter loss in childhood-onset schizophrenia with that in childhood-onset atypical psychoses. Arch Gen Psychiatry. 2004;61:17–22. doi: 10.1001/archpsyc.61.1.17. [DOI] [PubMed] [Google Scholar]
- GOLDMAN AL, PEZAWAS L, MATTAY VS, FISCHL B, VERCHINSKI BA, CHEN Q, WEINBERGER DR, MEYER-LINDENBERG A. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch Gen Psychiatry. 2009;66:467–77. doi: 10.1001/archgenpsychiatry.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENSTEIN D, LERCH J, SHAW P, CLASEN L, GIEDD J, GOCHMAN P, RAPOPORT J, GOGTAY N. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J Child Psychol Psychiatry. 2006;47:1003–12. doi: 10.1111/j.1469-7610.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- GREENSTEIN DK, WOLFE S, GOCHMAN P, RAPOPORT JL, GOGTAY N. Remission Status and Cortical Thickness in Childhood-Onset Schizophrenia. J Am Acad Child Adolesc Psychiatry. 2008 doi: 10.1097/CHI.0b013e3181825b0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUR RE, MAANY V, MOZLEY PD, SWANSON C, BILKER W, GUR RC. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry. 1998;155:1711–7. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- HAZLETT EA, BUCHSBAUM MS, HAZNEDAR MM, NEWMARK R, GOLDSTEIN KE, ZELMANOVA Y, GLANTON CF, TOROSJAN Y, NEW AS, LO JN, MITROPOULOU V, SIEVER LJ. Cortical gray and white matter volume in unmedicated schizotypal and schizophrenia patients. Schizophr Res. 2008;101:111–23. doi: 10.1016/j.schres.2007.12.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HO BC, ANDREASEN NC, NOPOULOS P, ARNDT S, MAGNOTTA V, FLAUM M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60:585–94. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- JACOBSEN LK, GIEDD JN, CASTELLANOS FX, VAITUZIS AC, HAMBURGER SD, KUMRA S, LENANE MC, RAPOPORT JL. Progressive reduction of temporal lobe structures in childhood-onset schizophrenia. Am J Psychiatry. 1998;155:678–85. doi: 10.1176/ajp.155.5.678. [DOI] [PubMed] [Google Scholar]
- KANE J, HONIGFELD G, SINGER J, MELTZER H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Archives of general psychiatry. 1988a;45:789–96. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- KANE J, HONIGFELD G, SINGER J, MELTZER H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988b;45:789–96. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- KANE JM. Clinical efficacy of clozapine in treatment-refractory schizophrenia: an overview. Br J Psychiatry Suppl. 1992:41–5. [PubMed] [Google Scholar]
- KESHAVAN MS, BAGWELL WW, HAAS GL, SWEENEY JA, SCHOOLER NR, PETTEGREW JW. Changes in caudate volume with neuroleptic treatment. Lancet. 1994;344:1434. doi: 10.1016/s0140-6736(94)90599-1. [DOI] [PubMed] [Google Scholar]
- KHORRAM B, LANG DJ, KOPALA LC, VANDORPE RA, RUI Q, GOGHARI VM, SMITH GN, HONER WG. Reduced thalamic volume in patients with chronic schizophrenia after switching from typical antipsychotic medications to olanzapine. Am J Psychiatry. 2006;163:2005–7. doi: 10.1176/ajp.2006.163.11.2005. [DOI] [PubMed] [Google Scholar]
- KIM JS, SINGH V, LEE JK, LERCH J, AD-DAB'BAGH Y, MACDONALD D, LEE JM, KIM SI, EVANS AC. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27:210–21. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- LERCH JP, EVANS AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24:163–73. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN JA, STROUP TS, MCEVOY JP, SWARTZ MS, ROSENHECK RA, PERKINS DO, KEEFE RS, DAVIS SM, DAVIS CE, LEBOWITZ BD, SEVERE J, HSIAO JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005a;353:1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN JA, TOLLEFSON GD, CHARLES C, ZIPURSKY R, SHARMA T, KAHN RS, KEEFE RS, GREEN AI, GUR RE, MCEVOY J, PERKINS D, HAMER RM, GU H, TOHEN M. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005b;62:361–70. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- MACDONALD D, KABANI N, AVIS D, EVANS AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12:340–56. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- MCEVOY JP, LIEBERMAN JA, STROUP TS, DAVIS SM, MELTZER HY, ROSENHECK RA, SWARTZ MS, PERKINS DO, KEEFE RS, DAVIS CE, SEVERE J, HSIAO JK. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163:600–10. doi: 10.1176/ajp.2006.163.4.600. [DOI] [PubMed] [Google Scholar]
- MOLINA V, REIG S, SANZ J, PALOMO T, BENITO C, SANCHEZ J, SARRAMEA F, PASCAU J, DESCO M. Increase in gray matter and decrease in white matter volumes in the cortex during treatment with atypical neuroleptics in schizophrenia. Schizophr Res. 2005;80:61–71. doi: 10.1016/j.schres.2005.07.031. [DOI] [PubMed] [Google Scholar]
- MORENO D, BURDALO M, REIG S, PARELLADA M, ZABALA A, DESCO M, BACA-BALDOMERO E, ARANGO C. Structural neuroimaging in adolescents with a first psychotic episode. J Am Acad Child Adolesc Psychiatry. 2005;44:1151–7. doi: 10.1097/01.chi.0000179055.46795.3f. [DOI] [PubMed] [Google Scholar]
- PANTELIS C, VELAKOULIS D, MCGORRY PD, WOOD SJ, SUCKLING J, PHILLIPS LJ, YUNG AR, BULLMORE ET, BREWER W, SOULSBY B, DESMOND P, MCGUIRE PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–8. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- RAPOPORT JL, GIEDD JN, BLUMENTHAL J, HAMBURGER S, JEFFRIES N, FERNANDEZ T, NICOLSON R, BEDWELL J, LENANE M, ZIJDENBOS A, PAUS T, EVANS A. Progressive cortical change during adolescence in childhood-onset schizophrenia. A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:649–54. doi: 10.1001/archpsyc.56.7.649. [DOI] [PubMed] [Google Scholar]
- REIG S, MORENO C, MORENO D, BURDALO M, JANSSEN J, PARELLADA M, ZABALA A, DESCO M, ARANGO C. Progression of brain volume changes in adolescent-onset psychosis. Schizophr Bull. 2009;35:233–43. doi: 10.1093/schbul/sbm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERK H, FALKAI P. Effects of antipsychotics on brain structure. Curr Opin Psychiatry. 2006;19:145–50. doi: 10.1097/01.yco.0000214339.06507.d8. [DOI] [PubMed] [Google Scholar]
- SHAW P, SPORN A, GOGTAY N, OVERMAN GP, GREENSTEIN D, GOCHMAN P, TOSSELL JW, LENANE M, RAPOPORT JL. Childhood-onset schizophrenia: A double-blind, randomized clozapine-olanzapine comparison. Arch Gen Psychiatry. 2006;63:721–30. doi: 10.1001/archpsyc.63.7.721. [DOI] [PubMed] [Google Scholar]
- SLED JG, ZIJDENBOS AP, EVANS AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- THOMPSON PM, BARTZOKIS G, HAYASHI KM, KLUNDER AD, LU PH, EDWARDS N, HONG MS, YU M, GEAGA JA, TOGA AW, CHARLES C, PERKINS DO, MCEVOY J, HAMER RM, TOHEN M, TOLLEFSON GD, LIEBERMAN JA. Time-lapse mapping of cortical changes in schizophrenia with different treatments. Cereb Cortex. 2009;19:1107–23. doi: 10.1093/cercor/bhn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMPSON PM, VIDAL C, GIEDD JN, GOCHMAN P, BLUMENTHAL J, NICOLSON R, TOGA AW, RAPOPORT JL. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98:11650–5. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN HAREN NE, HULSHOFF POL HE, SCHNACK HG, CAHN W, BRANS R, CARATI I, RAIS M, KAHN RS. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63:106–13. doi: 10.1016/j.biopsych.2007.01.004. [DOI] [PubMed] [Google Scholar]
- ZIJDENBOS AP, FORGHANI R, EVANS AC. Automatic "pipeline" analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21:1280–91. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]