Abstract
Historically, it was assumed that the light-evoked neural signals driving the human pupillary light reflex (PLR) originated exclusively from rod and cone photoreceptors. However, a novel melanopsin-containing photoreceptive cell class has recently been discovered in the mammalian retina. These intrinsically photosensitive retinal ganglion cells (ipRGCs) project to the pretectum, the retinorecipient area of the brain responsible for the PLR. This study was therefore designed to examine the relative contribution of rod, cone and the melanopsin photoresponses of ipRGCs to the human PLR. We establish that the melanopsin photoresponse of ipRGCs contributes significantly to the maintenance of half maximal pupilloconstriction in response to light stimuli of 30 seconds or longer, even at low photopic irradiances. Furthermore, we show that the melanopsin photoresponse contributes significantly to three- quarter maximal pupilloconstriction in response to light stimuli as short as 2 seconds. We also demonstrate that cone photoresponses driving pupilloconstriction adapt considerably and contribute little after 30 seconds, but rod photoresponses adapt less and contribute significantly to the maintenance of pupilloconstriction in response to steady-state light stimuli at irradiance levels which are below the threshold of the melanopsin photoresponse.
Keywords: Human, pupil, rods, cones, ipRGC, melanopsin
1. Introduction
The pupillary light reflex (PLR) is a well studied neurological reflex characterized by a reduction in pupil diameter in response to an increase in retinal illumination. The PLR is an important clinical metric of retinal, midbrain and autonomic function (Girkin, 2003, Kawasaki, 2005) as well as being a major determinate of retinal image quality (Campbell & Gregory, 1960, Hirata, Yamaji, Sakai & Usui, 2003, McDougal & Gamlin, 2008). Although it is well accepted that the major afferent influence on pupil diameter is environmental light levels, the nature of the light signal and the receptors responsible for its origin have historically been the subject of much disagreement (e.g Alpern & Campbell, 1962, Loewenfeld & Lowenstein, 1993, ten Doesschate & Alpern, 1965). Such disagreements are now more understandable given the recent discovery of the retinal photopigment, melanopsin (Provencio, Rodriguez, Jiang, Hayes, Moreira & Rollag, 2000), expressed by a novel class of retinal ganglion cells, which have been shown to contribute to the human PLR (Gamlin, McDougal, Pokorny, Smith, Yau & Dacey, 2007, Mure, Cornut, Rieux, Drouyer, Denis, Gronfier & Cooper, 2009, Young & Kimura, 2008).
1.1 Intrinsically Photosensitive Retinal Ganglion Cells
Recently, in mice and non-human primates, a class of retinal ganglion cells has been reported that express melanopsin (Dacey, Liao, Peterson, Robinson, Smith, Pokorny, Yau & Gamlin, 2005, Gooley, Lu, Chou, Scammell & Saper, 2001, Hattar, Liao, Takao, Berson & Yau, 2002), and are intrinsically photosensitive (Berson, Dunn & Takao, 2002, Dacey et al., 2005). In addition to their intrinsic photosignal, these cells receive rod and cone inputs (Dacey et al., 2005, Jusuf, Lee, Hannibal & Grunert, 2007). These cells have been termed intrinsically-photosensitive retinal ganglion cells (ipRGCs). The three primary projections of ipRGCs are the pretectum, the midbrain region associated with the PLR, the suprachiasmatic nucleus (SCN), the area of the brain responsible for circadian rhythms, and the the intergeniculate leaflet (Dacey et al., 2005, Hattar, Kumar, Park, Tong, Tung, Yau & Berson, 2006, Hattar et al., 2002). Although ipRGCs receive rod and cone inputs, their unique intrinsic photosensitivity ensures that they encode photic information differently from all other retinal ganglion cell types. In response to a pulse of light, these cells show a characteristic transient burst of firing at stimulus onset, which rapidly decays to a plateau of sustained firing that often extends well past stimulus offset (Berson et al., 2002, Dacey et al., 2005, Tu, Zhang, Demas, Slutsky, Provencio, Holy & Van Gelder, 2005, Wong, Dunn, Graham & Berson, 2007). It has been suggested that the initial burst of firing at stimulus onset is mediated by photoreceptors of the outer retina, while the sustained firing is driven by the melanopsin mediated intrinsic response (Dacey et al., 2005). In addition, more recent studies have provided evidence that outer retinal photoreceptors also contribute to sustained firing during long duration light stimuli (Drouyer, Rieux, Hut & Cooper, 2007, Wong et al., 2007).
1.2 The Role of ipRGCs in the Mammalian Pupillary Light Reflex
Initial studies investigating the influence of ipRGCs on the PLR utilized the mouse model, which allowed for the genetic manipulation of the different photoresponses involved in the reflex. It was shown that the PLR was present in rodless/coneless (rd/rd cl) mice, although the latency to maximal constriction was increased, and the irradiance needed to produce an equivalent constriction was higher than in wild type mice (Lucas, Douglas & Foster, 2001). A subsequent study investigating the PLR in melanopsin knockout mice (opn4 -/-) found the PLR to be aberrant at high irradiances in these animals (Lucas, Hattar, Takao, Berson, Foster & Yau, 2003).
Studies involving human and non-human primates have demonstrated a role for ipRGCs in the primate PLR. Gamlin and colleagues (2007) found that when outer retinal photoreceptive signals were blocked pharmacologically, the PLR persisted in macaques, and that the spectral sensitivity of the residual response was closely matched by the spectral sensitivity of melanopsin, which is maximally sensitive to 483 nm light. In addition, this study found that in both humans and macaques, the melanopsin photoresponse of ipRGCs is responsible for the post-illumination pupillary constriction which is seen following a period of high intensity light stimuli (Alpern & Ohba, 1972, Newsome, 1971).
Studies conducted prior to the discovery of melanopsin, also suggest that ipRGCs contribute to the human PLR. It has been shown that the pupils of rod achromats continues to respond to light increments well over levels commonly accepted to saturate rod photoreceptors (Alpern, Falls & Lee, 1960), thus implying that an additional photopigment is involved in the pupillary responses of these individuals. Additionally, spectral sensitivity measurements of pupillary constriction to steady state illumination have shown short wavelength sensitivity that is not well matched by either rod or S-cone contributions (Bouma, 1962, Laurens, 1923). Historical investigations of the response dynamics of the PLR are also suggestive of a role for ipRGCs in the behavior of the pupil in response to light increments. Several investigators have proposed models of pupillary dynamics which utilize both a transient and sustained component to the PLR (Kohn & Clynes, 1969, Privitera & Stark, 2006, Young, Han & Wu, 1993). These transient/sustained dynamics are very similar to the cellular response of ipRGCs.
Two recent studies have investigated the influence of melanopsin on the human PLR. A brief report by Young and Kimura (2008), which reanalyzed previous data, reported the relative contribution of short and long wavelength light to the sustained component of the PLR, and suggested that melanopsin plays a role in the response. However, since this study did not examine the complete spectral sensitivity of the PLR, a more rigorous investigation is required to confirm the influence of melanopsin on the human PLR. In addition, Young and Kimura (2008) examined light-driven pupillary responses of 10 seconds or less, while the full contribution of the melanopsin photoresponse to steady-state pupil constriction is expected to develop with longer stimulus durations. A study by Mure et al. (2009) utilized the spectral sensitivity of the human PLR to investigate the possibility that melanopsin acts as a bistable photopigment similar to that of invertebrate opsins. Although spectral sensitivity data was generated for multiple stimulus durations, the bulk of their analysis was directed at ascertaining the existence of melanopsin bistability. Therefore, the present study was undertaken to more fully describe the relative contributions of rod, cone, and melanopsin light responses to the spectral sensitivity and response dynamics of the pupil during long light stimuli, and to compare these responses to those obtained with briefer stimuli.
2. Methods
2.1 Subjects
Six subjects participated in at least one of the three different experimental conditions of this study. All subjects had normal corrected visual acuity and normal color vision as measured by the Nagel anomaloscope, Farnsworth D-15, and HRR plate test. Subjects A, B, D, and F were males ages 33, 29, 51, and 27 years respectively. Subjects C and E were females ages 33 and 40 years respectively. Subjects D and E required approximately +2 diopters of visual correction. Three subjects (A,B, & F) participated in experiment 1. Five subjects (A-E) participated in experiment 2. Three subjects (A-C) participated in experiment 3. All experimental procedures were approved by the UAB Institutional Review Board, and were undertaken with the understanding and written consent of each subject.
2.2 Recording procedures
During experimental sessions, both of the subject's eyes were visualized under infrared illumination via video camera. Pupil diameters were measured in both eyes using ISCAN RK406 pupillometer systems, which were calibrated with apertures of known diameters placed at the plane of the subject's eyes. The positions of the right eye, left eye, and pupil diameters were sampled at 500 Hz. All samples were stored on computer disk for later analysis.
2.3 Behavioral task
Measurement of the subject's consensual PLR in response to monochromatic light stimuli was determined during the following behavioral task. Prior to each experimental session, the subject's right eye was dilated with topical 1% tropicamide in order to keep the pupillary response in an open- loop condition. Throughout an experimental session, the subjects' right eye was precisely aligned with the optical system and a 2° black cross was visible to the subject's right eye at all times. At the onset of a behaviour trial, a target generated on a computer monitor (2° white cross, 1 cd/m2) was presented to the subject's left eyes at optical infinity via a badal lens system (see Bennett, Rabbetts & Bennett, 1998). The subject was instructed to fuse both targets and during this time a baseline measurement of pupil diameter was recorded. Throughout the behavioural task, the subject could visualize both targets, and was instructed to minimize and report any blurring or disassociation of the two targets which would indicate an undesirable change in accommodation or vergence angle. In this way, changes in accommodation and vergence angle, which could act as confounding influences in these experiments, were minimized.
Approximately twelve seconds after the onset of the white cross, a monochromatic light stimulus subtending 36° was presented in Maxwellian view to the right eye for approximately 4, 12, 34, or 110 seconds depending on the duration condition being assessed. The monochromatic light stimulus (9.5 – 15 log quanta/ cm2/sec) was generated with ten narrow band pass interference filters (8-10 nm full width at half maximum, Andover Corp.) between 450 nm and 650 nm. The spectral transmission through each of the interference filters used was shown to be reduced by at least 3 log units within a 15 nm deviation from the wavelength of peak transmission, as measured by a PR-680 spectroradiometer (Photo Research, Inc). The exact timing of the stimulus onset and offset relative to the start of the behavioral task was varied randomly in order to prevent anticipation of the former by the subjects. In all cases, stimulus generation was under computer control, including a stepper-controlled counter rotating variable neutral-density filters, a 10-position filter wheel, and a mechanical shutter. An IL1700 Radiometer/ Photometer System was used to calibrate retinal irradiance at each wavelength.
2.4 Experimental Procedure
The spectral sensitivity data collected in the present study follows the general procedures set out by Webster and colleagues (1968), which allows for the more rapid assessment of a predetermined pupillary criterion response. In short, the real time measurement of the changes in pupil diameter allows for the use of a modified method of adjustment in which the irradiance necessary to produce a given criterion response at any wavelength of stimuli can quickly be estimated. In the present experiment this entailed modifying the intensity of the stimuli presented to the subject at each wavelength based on real time measurements of the change in pupil diameter induced by prior stimuli, i.e., increasing stimulus intensity if previous stimuli induced a response which was below the criterion response or vice versa. In this way, a rough estimate of the irradiance necessary to produce a criterion response could be estimated within 2-4 stimulus presentations. Data were then collected only at a narrow range of irradiances (+/- .75 log quanta cm2/sec) above and below this estimate. A more precise measure of the irradiance necessary to produce the criterion response at each wavelength was determined through post hoc analysis (see section 2.5 for details). An additional benefit of this approach is that the calculation of the irradiance required to produce the criterion response is generated from multiple repeats within a narrow range of irradiances, thus more precisely defining the irradiance response relationship at or near the criterion response. In addition, this method prevents potentially confounding influences hampering earlier studies of spectral sensitivity of the consensual PLR (i.e Alpern & Campbell, 1962, Wagman & Gullberg, 1942) (see section 4.6 for more detail).
The same general procedures were utilized in each experimental session, and were as follows. Given the suggestion that the intrinsic response of ipRGCs is mediated by a bistable photopigment (Melyan, Tarttelin, Bellingham, Lucas & Hankins, 2005, Mure et al., 2009, Mure, Rieux, Hattar & Cooper, 2007), we were concerned that a pseudorandom presentation of the different wavelength stimuli could introduce a confounding potentiation of subsequent wavelength responses. Therefore, to control for any such confounds, we systematically moved through the different wavelength stimuli in an alternating sequence from short to long wavelengths and from long to short wavelengths. Each session started by measuring a subject's responses to three stimuli of either the longest or shortest wavelength used in a particular condition, followed by three repeats at the next wavelength, etc., until the subject had been tested at all wavelengths used in a particular condition. The subject was then offered a break, and subsequently this procedure was repeated, but the wavelengths were presented in reverse order, i.e., either from short to long wavelength or long to short wavelength based on the previous sequence. The same procedure was followed a third and final time following a brief break. In this way the subjects' pupillary responses at each wavelength were assessed a total of nine times during an experimental session. There was no evidence of a sequence effect in the measured pupillary responses. Inter-trial intervals were approximately two minutes in duration during which the subject was either kept in darkness in experiment 1 or was continually exposed to an adapting background in experiments 2 and 3 (see section 3.2). A slight deviation from this procedure was necessary for the 100 second duration condition, in which time permitted the measurement of only one trial per wavelength per sequence for a total of three trials per wavelength.
2.5 Data analysis
The pupil diameter of the subject's left eye was continuously recorded during each behavioural trial and these data were collected to computer via an A/D convertor board (National Instruments Corp.) for analysis offline. The procedures for these analyses were as follows. Baseline pupillary diameter was measured during the 5 seconds immediately preceding the onset of the test stimulus. Initially, the average baseline pupil diameter for all trials in a given experimental session was calculated and used to determine the precise value of the criterion response, i.e., 1/2 or 3/4 maximal pupillary constriction, for that particular experimental condition. These values varied as much as 0.8 mm between subjects based on their typical resting pupil diameter, yet they were very consistent (+/- 0.1 mm) for the same subject over different experimental sessions. Any trials with baselines that deviated +/- 0.5 mm from the average baseline for the session were excluded from further analysis due to the possibility that this deviation indicated a possible confounding influence on pupil diameter during that trial, e.g. a reduction in pupil diameter due to subject fatigue or sleepiness.
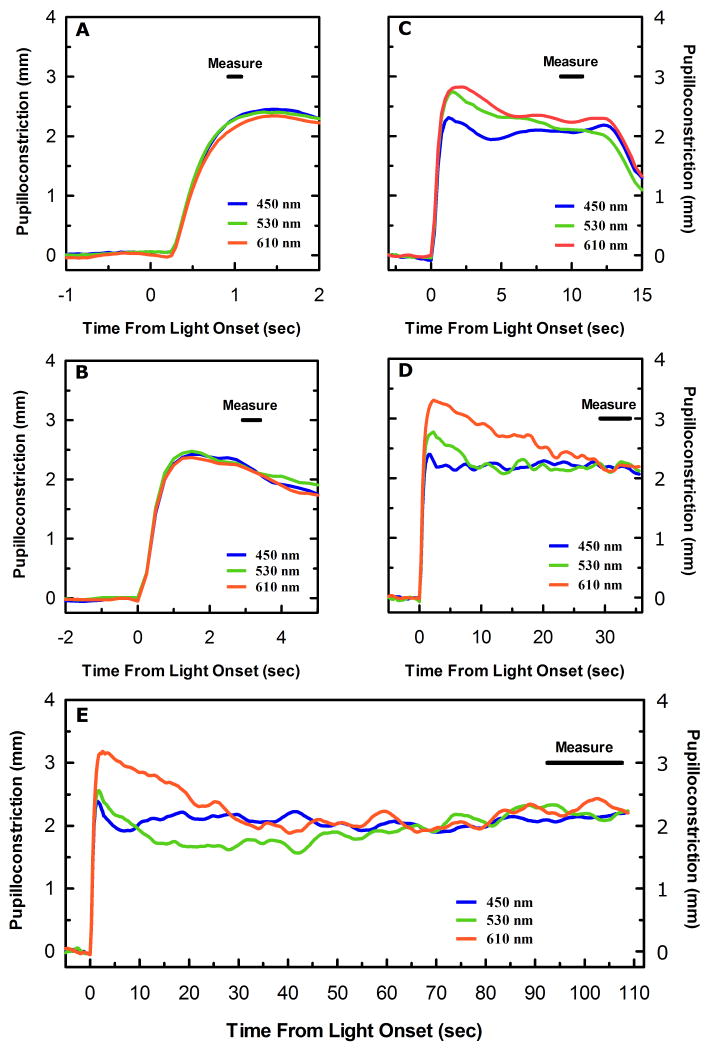
The change in pupil diameter produced by a given stimulus was determined by subtracting the baseline pupil diameter from the pupil diameter measured during a specific interval immediately prior to the cessation of the test stimulus. The light induced pupillary diameter was always measured for an interval centered at 1, 3.16, 10, 17.8, 31.6 or 100 seconds (0 - 2 log seconds) with the interval defined by 15% of the stimulus duration (Figure 1). For example, the light induced change in pupillary diameter produced by a 10 second trial would be generated by measuring the average diameter of a subjects pupil during an interval from 9.25 seconds to 10.75 seconds after stimulus onset (1.5 second interval centered at 10 seconds), which is then subtracted from the average pupil diameter measured during an interval from 0 to 5 seconds before stimulus onset for that individual trial.
Figure 1.
Average pupillary light responses to three different monochromatic light stimuli, 450 nm (blue trace), 530 nm (green trace), and 610 nm (red trace) necessary to produce approximately a half-maximal pupillary constriction at (A) 1 second, (B) 3.16 seconds, (C) 10 seconds, (D) 31.6 seconds, and (E) 100 seconds (n=6). The black bar in the upper right hand corner of each panel indicates the measurement interval utilized in each of the five duration conditions. Note the increase in the disparity of the initial response to each of the three wavelengths as stimulus duration is increased.
The differences in pupil diameter produced by each presentation of a single monochromatic stimulus were plotted as a function of irradiance. Previous findings indicate a linear relationship between irradiance and pupillary constriction at half maximal constriction in primates (Gamlin et al., 2007), therefore a linear regression analysis was performed on the scatter plot and a regression function was generated for each individual wavelength used during the session. This function was then used to predict the irradiance necessary to produce the criterion response at that wavelength. An individual spectral sensitivity plot was then generated using the data from all wavelength utilized, and corrected for prerecepotoral filtering based on the subject's age using the method of Pokorny, Smith and Lutze (1987). Average spectral sensitivities across subjects were produced by aligning each of the subject's spectral sensitivity plots relative to each other in order to produce the least scatter between all the curves via the Excel solver routine. Once the scatter had been minimized, an average of the values at each wavelength was generated.
2.6 Curve Fitting
To produce a smooth function through the average spectral sensitivities, we sought to combine the known sensitivities of the photoreceptive processes influencing ipRGCs. That is to say, that the sensitivity to any particular wavelength would be produced by the combination of the sensitivity of all the photoreceptive processes, both intrinsic and extrinsic, that are sensitive to that particular wavelength. Given that ipRGCs receive outer retinal inputs from rod and cone photoreceptors (Dacey et al., 2005, Tu et al., 2005, Viney, Balint, Hillier, Siegert, Boldogkoi, Enquist, Meister, Cepko & Roska, 2007, Wong et al., 2007), the simplest estimation of the spectral sensitivity of ipRGCs would have the form
| (1) |
where Sinner represents the spectral sensitivity of the inner retinal photoreceptive mechanisms of ipRGCs, i.e., the melanopsin mediated intrinsic response, and Souter represents the outer retinal inputs received by ipRGCs. If we first concern ourselves with the Souter term, we can further define this as a combination of rod and cone inputs.
| (2) |
To move from the theoretical model of equation (1) to a more specific mathematical prediction of the spectral sensitivity of the outer retinal photoreceptive signal impinging on ipRGCs, one must address how the spectral sensitivity of each individual cell type is combined to produce the composite sensitivity. It has been proposed by Quick (1974) that the total sensitivity of an array of elements each having different individual sensitivities can be modeled with a function of the form
| (3) |
where S is sensitivity of the entire array and the parameter k defines how the individual sensitivities are combined. This model, often termed the Quick pooling model, has been successfully used to model the sensitivity of a variety of visual functions, such as contrast sensitivity (Robson & Graham, 1981), mesopic spectral sensitivity (Kurtenbach, Meierkord & Kremers, 1999) and increment threshold spectral sensitivity (Miyahara, Pokorny & Smith, 1996). A more complete description of the Quick pooling model can be found in Graham (2001).
To produce a more precise estimation of the spectral sensitivity of the entire array of outer retinal photoreceptive elements, we can convert equation (2) into the form of equation (3), yielding
| (4) |
where the parameters r and c allow for the relative contribution of rods and cones to the composite spectral sensitivity of function. For the purposes of the current study, we further defined the sensitivity of the rod and cone signals as
| (5) |
and
| (6) |
where SV'lambda is the CIE scotopic luminosity function, and where Slws and Smws are the LWS and MWS Stockman and Sharpe 10° cone fundamentals respectively, (Stockman & Sharpe, 2000), and p defines the LWS/MWS cone ratio. In order to limit the free parameters involved in fitting the function to the data, the LWS/MWS ratio was fixed at 1.625 (p = 0.62), which is the LWS/MWS ratio of the standard observer (Pokorny, Jin & Smith, 1993). The addition or subtraction of a SWS cone signal did not improve the fit of the function to that of any spectral sensitivity data collected in the present study, and therefore was omitted from the current model. This was expected, as it has been previously suggested that primate ipRGCs receive only MWS and LWS ON inputs (Dacey et al., 2005). All spectral sensitivity templates were corrected for prereceptoral filtering in order to convert the corneal sensitivity functions to retinal sensitivity functions. The prereceptoral filtering estimates were produced by using the average age of the subjects involved in studies on which the templates were generated (e.g. Crawford, 1949, Wald, 1945) and predicting the average prereceptoral filtering based on this average age using the method of Pokorny et al. (1987).
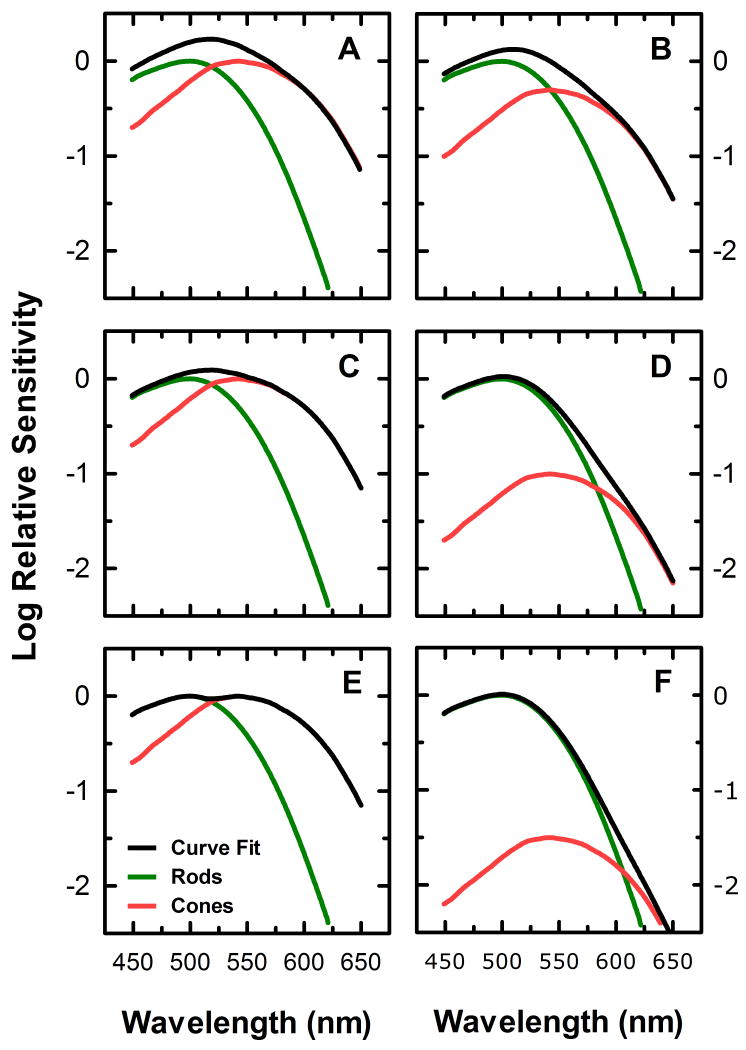
Figure 2 illustrates the effect of changing the parameters k1 in equation (4) on the composite spectral sensitivity of outer retinal photoreceptive responses. In the simplest case, k=1, the total sensitivity is defined by the linear sum of the rod and cone sensitivities (Fig. 2A). As k increases to values greater than 1, a nonlinear addition of the individual sensitivities occurs, which can be used to approximate a situation of probability summation, where the sensitivity of the array is augmented at the points at which the sensitivities of individual elements in the array overlap over the parameter of interest (Fig. 2C). Furthermore, as k is increased towards infinity, the sensitivity of the array approaches a situation of “winner take all’, where the most sensitive element in an array at a particular wavelength defines the total sensitivity of the array at that wavelength (Fig. 2E).
Figure 2.
Illustration of the effect of changing the curve fitting parameters of equation (4) on the composite spectral sensitivity derived from the combination of rod and cone spectral sensitivities. Panels A, C, and E demonstrate the effect of changing the value of the parameter k in equation (4) to 1 (A), 2 (C), and 100 (E). Panels B, D, and F demonstrate the effect of changing the relative contribution of the rod and cone signals on the spectral sensitivity of the overlying function, by setting c = 0.5r (B), c = 0.1r (D), and c = 0.03r (F).
Figure 2 also illustrates the effect of changing the relative sensitivities of the rod and cone signal, i.e., varying the relative values of r and c in equation (4), on the composite function while keeping k constant at a value of 1. For example, if a large mesopic stimulus is used to assess spectral sensitivity, the relative contribution of rod photoreceptor sensitivity to the composite sensitivity would be larger than that of cones. This could be modeled by reducing the value of the c parameter relative to r. If c is one half the value of r, this would yield a function produced by the combination of rod sensitivity with a cone sensitivity 0.316 log units less sensitive than the rod signal (Fig. 2B). Similarly, if c = 0.1r (Fig. 2D) or c=0.03r (Fig. 2F), the composite function would be comprised of the cone sensitivity reduced by 1 and 1.5 log units respectively relative to the rod sensitivity.
Following similar reasoning to our combination of outer photoreceptive mechanisms just discussed, equation (1) can be converted into the form of equation (3),
| (7) |
Given the differing origins and physiology of the photoreceptive mechanisms responsible for the Sinner and Souter terms, we allowed for the possibility these two mechanisms might combine differently than the rod and the cone signals in the Souter term. This is reflected in the differentiation of the two k parameters between equation (4) and equation (7), where k1 reflects the combination rule for the outer retinal sensitivities and k2 reflects the combination of the outer retinal signals with the melanopsin photoresponse. Expanding equation (7) to reflect the specific spectral sensitivity estimates used in this study and combining it with equation (4) yields
| (8) |
where the parameters m, along with the previously discussed parameters r, and c, allow the relative weights of the melanopsin, cone, and rod photoreceptive influences on the total spectral sensitivity to be adjusted, and where Sopn4 is a Baylor nomogram (Baylor, Nunn & Schnapf, 1987) with a lambda max at 483 nm. The Microsoft Excel solver routine was used to fit equation (8) to each of the spectral sensitivity plots generated in the study. Specifically, the parameters m, c, and r were varied to minimize the sum of squares of the residuals of the function from the collected data. That is to say, that the relative gain of the melanopsin, cone, and rod photoresponses were systematically changed until the deviation of the function from the actual data points at each wavelength measured was minimized. The values of the combination parameters k1 and k2 were also optimized by comparing their influence on the goodness of fit of the function to the data. Once the optimal values were determined, they were kept constant for all functional fits to the data (see section 2.7 for more detail).
2.7 Combination of photoresponses
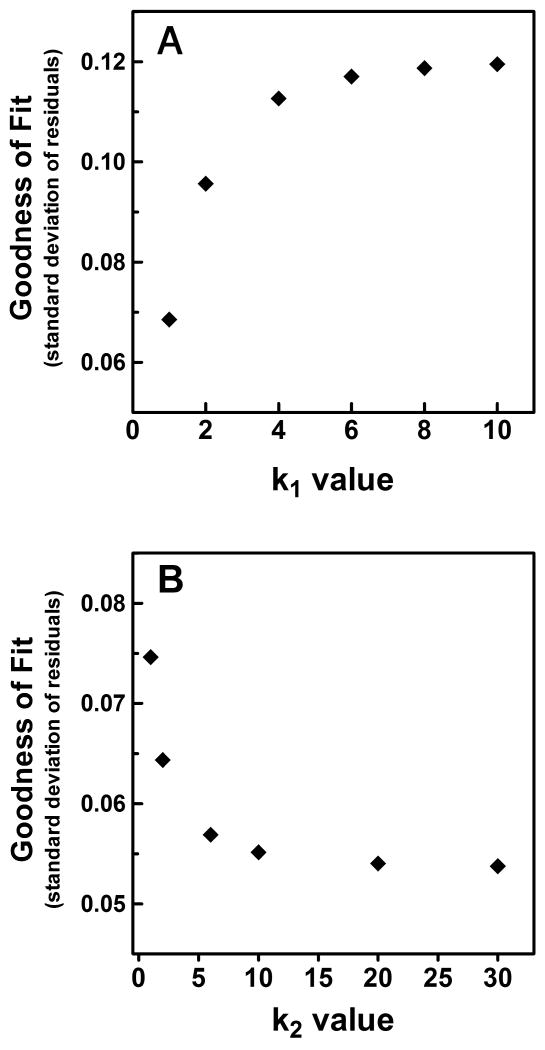
The parameters k1 and k2 of equation (8) represent the rules of combination for the individual outer retinal photoresponses (k1), and the combination of this composite outer retinal signal with that of the melanopsin photoresponse of ipRGCs (k2). To limit the free parameters involved in the fitting of equation (8) to the individual spectral sensitivity plots, we optimized these two parameters, thus allowing a fixed value to be used for each of our curve fitting procedures. To accomplish this optimization, we first sought to determine what k1 value best represented the combination of rod and cone signals in the outer retinal component of the response. Given that the spectral sensitivities from the 1 and 3.16 second duration conditions of experiment 1 and 2 were driven exclusively by outer retinal photoresponses (see Results), we systematically changed the value of k1 used in our curve fitting of these data and determined its effect on the average goodness of fit (Figure 3A). We found that as the value of k1 approached 1, the goodness of fit improved. This suggests that the outer retinal signal driving the PLR is the result of the linear summation of rod and cone photoresponses. To determine the optimum value of k2, we fixed the value of k1 at 1, and calculated the effect of changing the value of k2 on the average goodness of fit to the data for long duration conditions which clearly were influenced by the melanopsin photoresponse of ipRGCs (Figure 3B). We found that as k2 became larger, the goodness of fit improved. Given the results of this optimization, the values of k1 and k2 were fixed at 1 and 10, respectively, for all subsequent analyses.
Figure 3.
Optimization of the combination parameters of equation (8) for inner and outer retinal signals. The average effect on the deviation of the composite spectral sensitivity function from the measured average spectral sensitivity data as (A) the value of k1 is increased from 1 to 10 (n=4); (B) The value of k2 is increased from 1 to 30 (n=6). Only spectral sensitivity data at duration and experimental conditions which clearly were not influenced by the melanopsin photoresponse, i.e., short duration, half maximal responses, were used to optimize k1. For the optimization of k2, the value of k1 was set at 1, and spectral sensitivity data were used only at duration and experimental conditions at which the melanopsin photoresponse was clearly influencing composite spectral sensitivity.
3. Results
The present study was conducted to determine the influence of the melanopsin photoresponse of ipRGCs on the human pupillary light reflex. As with any photoreceptive process, the melanopsin response of ipRGCs has a unique photic sensitivity that can be utilized to indicate its influence on visually driven behaviors. By determining the spectral sensitivity of a behavior and comparing it to known spectral sensitivities of the photoreceptive processes of the human eye, one can ascertain the degree to which each of these process drive the behavior in question.
3.1 Experiment 1
Given the known differences in the speed and magnitude of the light adaptation of the inner and outer retinal photoresponses (Dacey et al., 2005, Wong et al., 2007) we first sought to determine if the melanopsin photoresponse of ipRGCs would act to compensate for the light adaptation of outer retinal photoresponses and drive the PLR in response to steady-state light increments. To test this hypothesis, we measured the spectral sensitivity of half-maximal pupillary constriction for light durations increasing from 1 second to 100 seconds at one-half log second intervals. If our hypothesis was correct, we would expect the spectral sensitivities of the short duration light stimuli to be indicative of outer retinal photoreceptive processes, while the spectral sensitivities of the long duration light responses would indicate a major contribution from the melanopsin photoresponse.
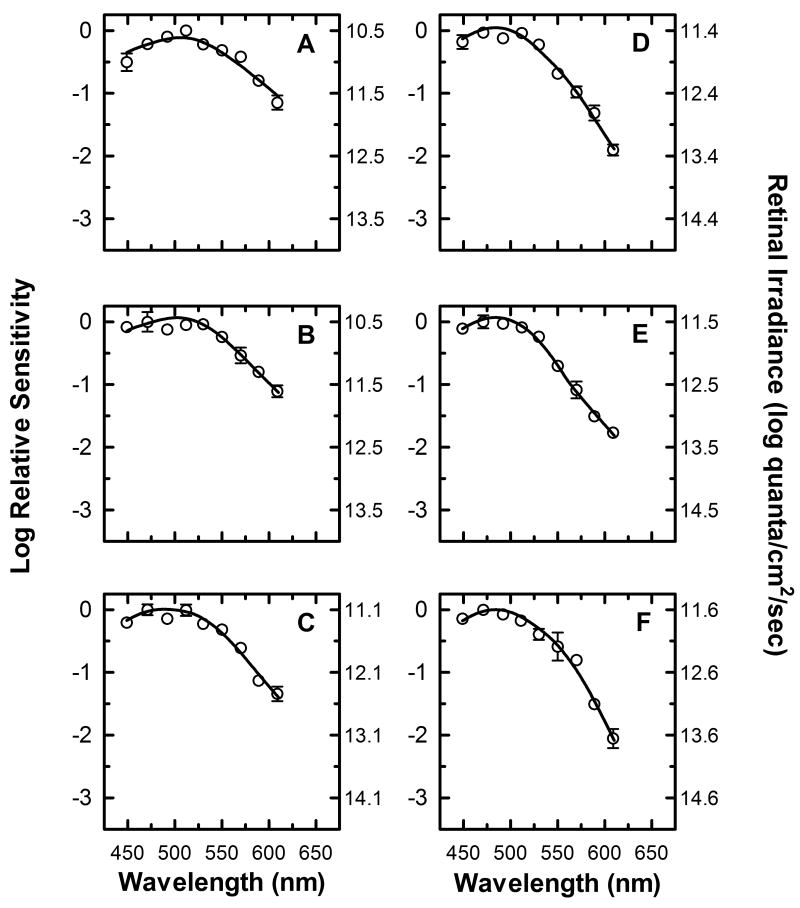
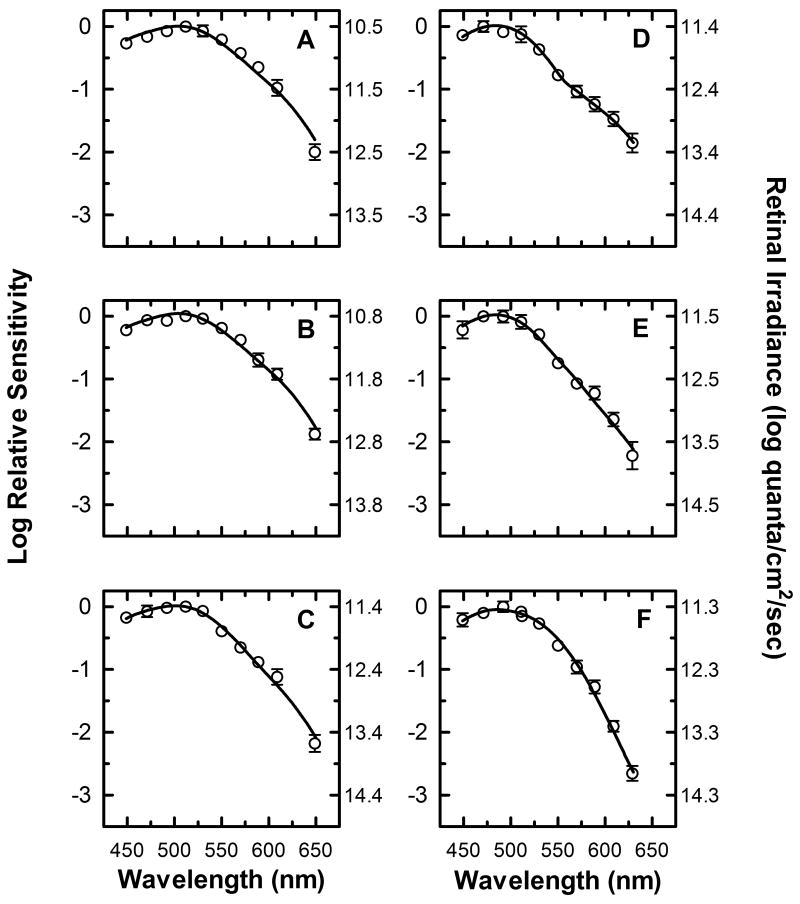
The average spectral sensitivity plots of three subjects are shown in figure 4. As the duration of the light stimulus increases, the wavelength of peak sensitivity changes from 510 nm (Fig. 4 A, B) to 470 nm (Fig. 4 E, F). The spectral sensitivities at all durations show a reduced sensitivity to long wavelength light, with the sensitivity to long wavelengths relative to short wavelength decreasing from -1 log units for the shortest duration stimulus (Fig. 4A, 610 nm vs. 510 nm) to -2 log units for the longest duration stimulus (Fig. 4E, 610 nm vs. 470nm). Furthermore, the absolute irradiance necessary to produce half-maximal pupillary constriction at the wavelength of peak sensitivity increases from 10.5 log quanta/cm2/sec for short duration stimuli (Fig. 4 A, B) to ∼11.5 log quanta/cm2/sec for long duration stimuli (Fig. 4 E, F). Taken together, these results demonstrate that the peak relative spectral sensitivity of the pupillary response not only shifts towards shorter wavelengths as the duration of the light stimuli increase, but there is also a decrease in the absolute sensitivity of the response.
Figure 4.
Spectral sensitivity of half-maximal pupillary constriction with no adapting field present. Mean spectral sensitivity measurements (n=3) at nine different wavelengths are represented by (○) for six different stimulus duration conditions, (A) 1 second, (B) 3.16 seconds, (C) 10 seconds, (D) 17.8 seconds, (E) 31.6 seconds, and (F) 100 seconds (In this and subsequent figures, error bars are SEM, and are smaller than symbol size when not shown). The left y-axis represents the log spectral sensitivity relative to the most sensitive wavelength at each duration condition. The right y-axis indicates the retinal irradiance necessary to produce the criterion response. The smooth curve through the data points represents the optimal fit to the data using equation (8), a mathematical combination of rod, cone, and melanopsin spectral sensitivities based on the Quick pooling model of visual sensitivity (see Methods and table 1 for details).
None of the spectral sensitivity plots in figure 4 are well fit by a single photopigment nomogram, and therefore must be the result of a combination of two or more different photopigments. In order to ascertain the underlying mechanisms and their relative contributions to the response at each of the duration conditions, a smooth function combining the spectral sensitivities of rods, cones and melanopsin [equation (8)] was fit to the data (continuous line in panels A-F). Table 1 list the values of the parameters r, c, and m of equation (8) that produce the best fit to the data at each duration condition. These values can be thought of as the gain of each of the photoreceptive signals needed to fit the data, and they provide an insight into the relative contribution of the melanopsin, rod, and cone photoresponses to the spectral sensitivity at each duration condition. It is clear from the data of table 1, that the outer retinal photoreceptive mechanisms drive the response at 1 second, 3.16 seconds, and 10 seconds. Additionally, by comparing the gain values of the rod and cone photoresponses at these duration conditions, it can be concluded that the rod response dominates the spectral sensitivities at these durations, and that the relative contribution of the cone photoresponse decreases systematically from 1 to 10 seconds. Furthermore, these data indicate that the melanopsin photoresponse contributes significantly to the spectral sensitivity of the response at 17.8, 31.6, seconds and 100 seconds. It should be noted that the values in table 1 only reflect the relative contribution of the melanopsin, rod, and cone photoresponses at each time interval without regard to the decrease in overall absolute sensitivity seen as stimulus duration is increased.
Table 1.
Relative contribution of the three photoreceptive mechanisms of equation (8) to the spectral sensitivity curve fits in experiment 1
| Photoreceptive Mechanism | 1 sec | 3.16 sec | 10 sec | 17.8 sec | 31.6 sec | 100 sec |
|---|---|---|---|---|---|---|
| Rods | 0.63 | 1.07 | 0.98 | 0.59 | 0.32 | 0.70 |
| Cones | 0.22 | 0.17 | 0.08 | .020 | 0.03 | 0.003 |
| Melanopsin Photoresponse | – | – | – | 1.11 | 1.17 | 1.00 |
3.2 Experiment 2
The most unexpected result of experiment 1 was the significant contribution of the rod photoresponse to the outer retinal photoreceptor component of the spectral sensitivity plots at all duration conditions. Previous investigations of the spectral sensitivity of the human PLR to transient stimulation have reported a greater contribution by L and M-cone photoresponses (Alpern & Campbell, 1962, Kimura & Young, 1995) than that found in our experiment. These previous experiments were conducted under conditions that may have produced a saturation of the rod photoresponses, and therefore we sought to repeat our initial experiments using a steady-state 510 nm adapting field to selectively adapt the rod photoresponse without stimulating the intristic photoresponse, and therefore we utilized a 50 scotopic troland (10.4 log quanta/cm2/sec) adapting background. This intensity was chosen because it was subthreshold for activation of the melanopsin photoresponse and had previously been shown to produce a significant adaptation of the rod mediated visual processes (Adelson, 1982).
A further modification of experiment 2 from experiment 1 was the use of an additional long wavelength monochromatic light stimulus. This long wavelength stimulus was added in order to better characterize the cone contribution of the response and to verify that the decrease in long wavelength sensitivity seen in experiment 1 was not due to an opponent mechanism similar to that responsible for the Sloan notch observed in increment threshold spectral sensitivities (e.g. Sperling & Harwerth, 1971).
The results of experiment 2 are remarkably similar to those of experiment 1 (Figure 5). The spectral sensitivity plots at each duration condition show greatest sensitivity to short wavelength light and a marked insensitivity to long wavelength light. This long wavelength insensitivity is evident at both 630 nm and 650 nm, thus showing no evidence of chromatic opponency in the spectral sensitivity of the cone contribution to the PLR. As in experiment 1, there is again a shift in peak sensitivity from 510 nm for the shorter duration conditions (Fig. 5A-C) to 470 nm and 490 nm in the longer duration conditions (Fig. 5D -F), and again, as stimulus duration increased, an increase in the absolute irradiance was needed to produce a half maximal response.
Figure 5.
Spectral sensitivity of half-maximal pupillary constriction with a 50 troland adapting field present. Mean spectral sensitivity measurements (n=5) at ten different wavelengths are represented by (○) for six different stimulus duration conditions, (A) 1 second, (B) 3.16 seconds, (C) 10 seconds, (D) 17.8 seconds, (E) 31.6 seconds, and (F) 100 seconds (SEM error bars). The left y-axis represents the log spectral sensitivity relative to the most sensitive wavelength at each duration condition. The right y-axis indicates the retinal irradiance necessary to produce the criterion response. The smooth curve through the data points represents the optimal fit to the data using equation (8), a mathematical combination of rod, cone, and melanopsin spectral sensitivities based on the Quick pooling model of visual sensitivity (see Methods and table 2 for details).
A function of the form of equation (8) was again used to fit a smooth curve to the spectral sensitivity plots (smooth line in each panel of figure 5). The values of the parameters of r, c, and m necessary to produce the best fit to the data in experiment 2 are displayed in table 2. These parameters show the same general pattern as that found in experiment 1, i.e., the rod photoresponse predominates in the outer retinal signal, and the emergence of the melanopsin photoresponse at the 17.8, 31.6, and 100 second duration conditions.
Table 2.
Relative contribution of the three photoreceptive mechanisms of equation (8) to the spectral sensitivity curve fits in experiment 2
| Photoreceptive Mechanism | 1 sec | 3.16 sec | 10 sec | 17.8 sec | 31.6 sec | 100 sec |
|---|---|---|---|---|---|---|
| Rods | 0.89 | 0.96 | 0.98 | 0.64 | 0.44 | 0.79 |
| Cones | 0.22 | 0.24 | 0.12 | 0.03 | 0.04 | 0.004 |
| Melanopsin Photoresponse | – | – | – | 1.20 | 1.07 | 0.88 |
3.3 Experiment 3
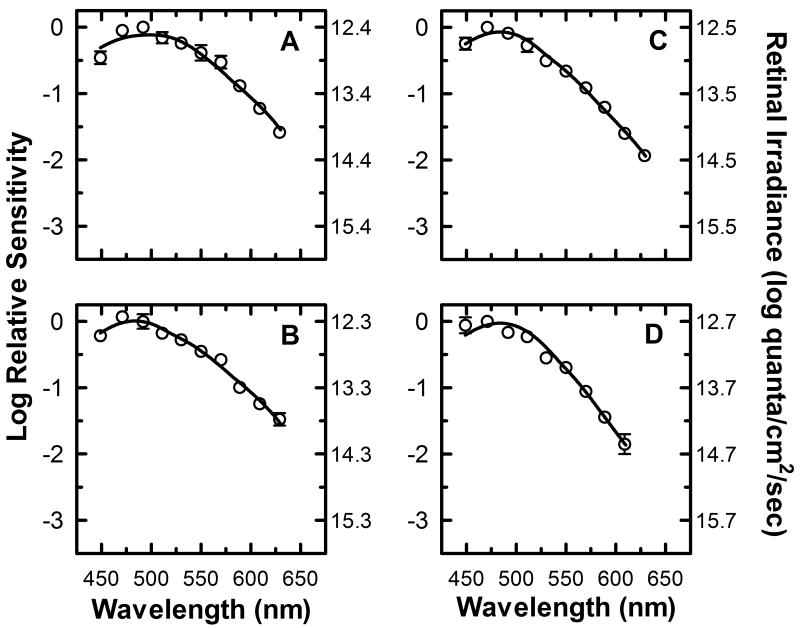
It has been demonstrated in numerous studies that a decrease in pupil diameter has a significant impact on retinal image quality by increasing depth of focus (Campbell, 1957, Tucker & Charman, 1975) and reducing the effects of optical aberrations (Campbell & Gubisch, 1966, Williams & Chalupa, 1983, Woodhouse, 1975). This improvement in retinal image quality leads to a subsequent improvement in visual acuity, and it has been found that a pupil diameter of ∼ 3 mm is optimum for visual acuity (Campbell & Gregory, 1960, Campbell & Gubisch, 1966, Tucker & Charman, 1975, Woodhouse, 1975). Additionally, it has been shown in the murine PLR that the melanopsin photoresponse of ipRGCs is necessary for complete pupillary constriction (Lucas et al., 2003). Taken together, these finding suggest that the melanopsin photoresponse should dominate the spectral sensitivity of pupillary constrictions to diameters optimal for visual acuity, even for short duration stimuli. To investigate this possibility, we sought to use a paradigm similar to that of experiment 2, except the criterion response was a 3/4 maximal pupil response rather than a 1/2 maximal response. This criterion results in an average pupil diameter near that reported for maximal visual acuity, but is sufficiently below maximal constriction to allow for accurate irradiance estimates using the methods of experiment 1 and 2.
The results of experiment 3 using three subjects from experiment 1 and 2 are shown in figure 6. The 1 second duration condition used in experiments 1 and 2 was replaced by a 1.78 second duration condition (Figure 6A) in experiment 3, since the sluggish nature of the iris musculature prevented 3/4 maximal constriction within 1 second of light onset. The spectral sensitivity plots for all four stimulus duration conditions again showed an increased sensitivity to short wavelength light. As seen in experiments 1 and 2, the insensitivity to long wavelength light increases as the duration of the light stimulus increases. Two major differences between the results of experiment 3 and experiments 1 and 2 were observed. First, as expected the average absolute irradiance needed to produce the three-quarter maximal response increased to ∼12.5 log quanta/cm2/sec from an average of ∼11.0 log quanta/cm2/sec necessary to produce a half maximal constriction in experiments 1 and 2. Second, for all duration conditions, the wavelength of peak sensitivity is either 490 nm or 470 nm. This suggests that the melanopsin photoresponse is responsible for the peak sensitivity of the response at all duration conditions. This is borne out by the curve fitting parameters used to produce the smooth function through the plots (table 3), which indicate the dominance of the melanopsin photoresponse at all duration conditions.
Figure 6.
Spectral sensitivity of three quarter-maximal pupillary constriction with a 50 td adapting field present. Mean spectral sensitivity measurements (n=3) at ten different wavelengths are represented by (○) for four different stimulus duration conditions, (A) 1.78 seconds, (B) 3.16 seconds, (C) 10 seconds, and (D) 31.6 seconds (SEM error bars). The left y-axis represents the log spectral sensitivity relative to the most sensitive wavelength at each duration condition. The right y-axis indicates the retinal irradiance necessary to produce the criterion response. The smooth curve through the data points represents the optimal fit to the data using equation (8), a mathematical combination of rod, cone, and melanopsin spectral sensitivities based on the Quick pooling model of visual sensitivity (see Methods and table 3 for details).
Table 3.
Relative contribution of the three photoreceptive mechanisms of equation (8) to the spectral sensitivity curve fits in experiment 3
| Photoreceptive Mechanism | 1.78 sec | 3.16 sec | 10 sec | 31.6 sec |
|---|---|---|---|---|
| Rods | 0.67 | 0.57 | 0.43 | 0.44 |
| Cones | 0.15 | 0.15 | 0.06 | 0.02 |
| Melanopsin Photoresponse | 0.70 | 1.02 | 0.85 | 0.94 |
3.4 Adaptation of photoresponses
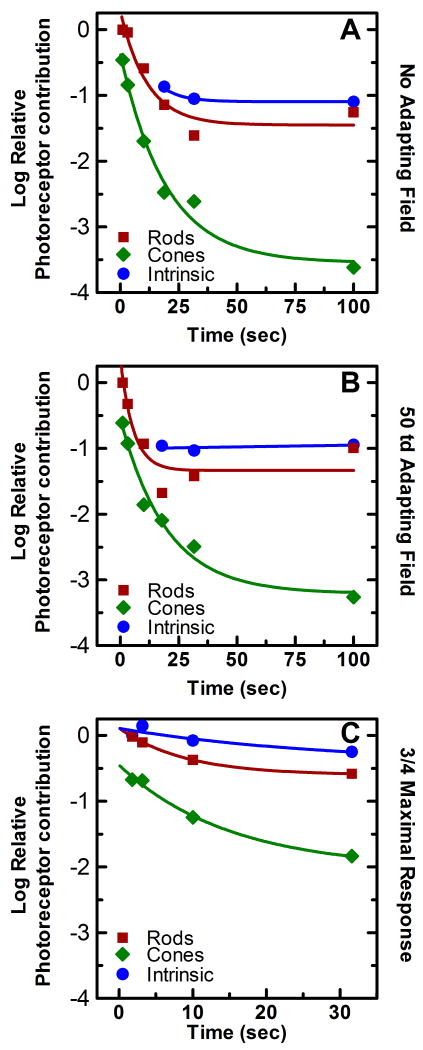
The change in the relative gains of the outer retinal photoresponses over time was an important result of experiments 1 and 2, and much can also be elucidated about the adaptation of the melanopsin photoresponse in experiment 3. By incorporating the decrease in the absolute sensitivity of the response over time with the values of relative gains of each of the three photoresponses involved in the response (tables 1-3), it is possible to plot the absolute change in the gain of the photoresponses due to time of light exposure, or more simply their rate of light adaptation. To incorporate the two values in question into a single plot, we first took the log of the curve fitting parameters r, c, and m, thus allowing the comparison of the relative sensitivities of the responses on a log scale. This allowed for the addition of the decrease in log absolute sensitivity as stimulus duration was increased to the log of the gain components. This combination produced a plot which incorporated both changes in the relative gains of the photoresponses and the global changes in gain in all photoresponses over time (Figure 7). In this plot, the change in the absolute gain of each photoresponse relative to the most sensitive response at the shortest time duration is plotted as a function of time for each of the three experiments.
Figure 7.
Relative contribution of the rod, cone, and melanopsin photoresponse to the spectral sensitivity of the PLR over time. The time course of light adaptation of the rod (■), cone (◆), and melanopsin (●) photoresponses while maintaining a half maximal PLR with (A) no background present, (B) a 50 td adapting background, and (C) a three quarter maximal PLR with a 50 td adapting background. Light adaptation was calculated by the combining the difference in absolute irradiance necessary to maintain these responses with the change in relative contribution of each of the photoresponses to the composite spectral sensitivity function generated for each duration condition of each of the three experiments (see section 2.4 for details). Each point is relative to the most sensitive photoresponse at the shortest duration condition. The smooth line through each data set is the best fit of a three parameter single exponential decay function to the data. The decay parameters and R2 values for each curve are reported in table 4.
A three parameter single exponential decay function of the form,
| (9) |
was fit to the photoresponse data (smooth lines in figure 7 A-C) using the curve fitting function in Sigma Plot (SPSS Inc.). The parameters of equation (9) give an indication of the rate and magnitude of the adaptation, where A estimates the magnitude of the total loss in log sensitivity from time 0, and the reciprocal of α is the time constant of the decay. Table 4 lists the values of the total loss in log sensitivity, the time constant of the decay, and the R2 values of the curve fitting for each photoreceptive mechanism under each of the three experimental conditions. Two alterations were made to the above curve fitting process for the melanopsin photoresponse data in experiment 2 and 3. The adaptation data produced for the melanopsin photoresponse in experiment 2 was not well fit by any decay function (see Fig. 7B), therefore the decay parameters for this condition were omitted from table 4. Additionally, the adaptation data produced for the melanopsin photoresponse in experiment 3 indicates an increase in sensitivity from 1.78 to 3.16 seconds, therefore equation (9) was only fit to the data points produced from duration conditions greater than 3.16 seconds (see Fig. 7C). This result is not unexpected, as it is consistant with previous studies which showed that the melanopsin photoresponse of ipRGCs often peaked well after light onset (Berson et al., 2002, Dacey et al., 2005, Wong, Dunn & Berson, 2005).
Table 4.
Representation of curve fitting parameters used to fit equation (9) to the photoreceptor adaptation data for each experimental condition.
| Rods | Cones | Melanopsin Photoresponse | ||
|---|---|---|---|---|
| Experiment 1 | ||||
| Total loss of sensitivity | 1.71 log units | 3.18 log units | 0.23 log Units* | |
| Time constant | 11.6 sec | 20.3 sec | 8.46 sec* | |
| R2 | 0.93 | 0.98 | 1.00 | |
| Experiment 2 | ||||
| Total loss of sensitivity | 1.45 log units | 2.67 log units | – | |
| Time constant | 5.9 sec | 15.3 sec | – | |
| R2 | 0.94 | 0.98 | – | |
| Experiment 3 | ||||
| Total loss of sensitivity | 0.71 log units | 1.58 log units | 0.41 log units | |
| Time constant | 8.9 sec | 14.6 sec | 8.6 sec | |
| R2 | 1.00 | 0.99 | 1.00 |
represents magnitude and time constant of adaptation from 17.8 seconds to 100 seconds only. See section 4.2.3 for details.
The photoresponsive mechanisms all showed light adaptation over time in each of the three experimental paradigms except for the melanopsin photoresponse in experiment 2. In addition, the adaptation parameters associated with each photoresponse showed the same general trend across all three experimental conditions. Although the cone photoresponse adapted at the slowest rate (larger time constants), this photoresponse showed a far greater magnitude of adaption (measured as total loss of sensitivity) than either the melanopsin or the rod photoresponse. Both the rod and melanopsin photoresponses adapted at similar rates, although the rod photoresponse had a greater magnitude of adaptation than the melanopsin photoresponse.
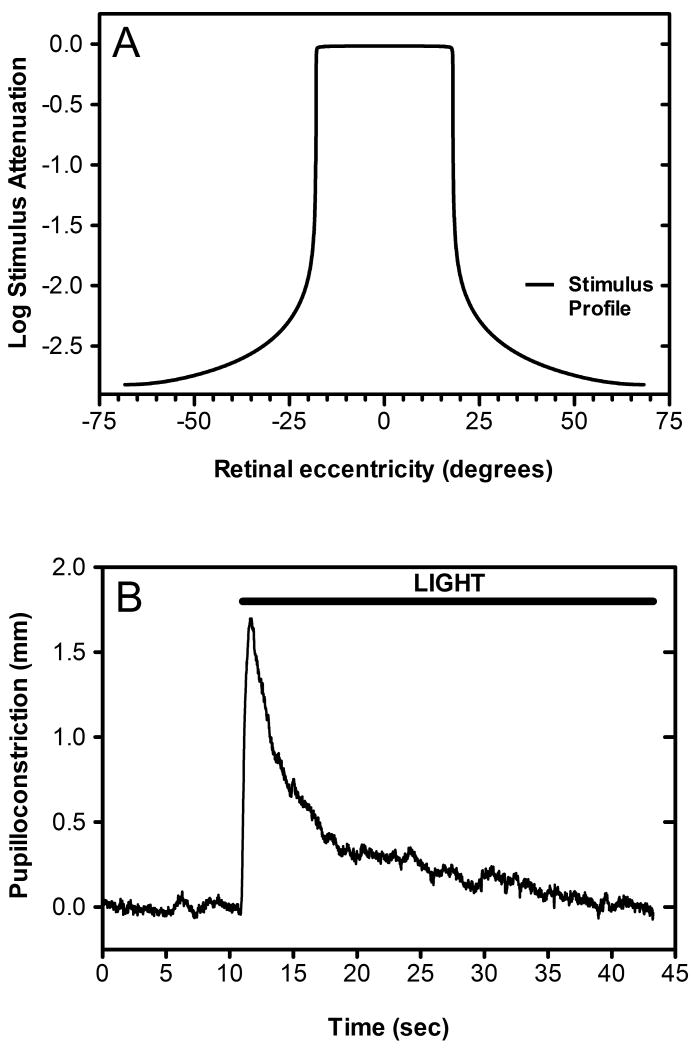
3.5 Does light scatter account for observed rod response?
One hypothesis for the apparent tonic contribution of rod photoreceptors to the human PLR is that dark adapted rods in the peripheral retina were unintentionally tonically stimulated due to intraocular light scatter. In order to investigate this hypothesis, we obtained a calculation of the theoretical intensity of intraocular light scatter as a function of retinal eccentricity, which was based on CIE collection 135-1999 (Figure 8A). Then, using a back-projection screen and Electrohome Marquee 8500 projector, we generated a light annulus which precisely matched the light scatter produced by the light stimulus used in experiment 2. This stimulus was presented to a dark-adapted subject for 35 seconds in order to ascertain its impact, if any, on steady-state pupilloconstriction. We determined that following a robust, transient pupillary constriction, the subject's pupil rapidly redilated, returning to baseline levels within thirty seconds of stimulus onset (Figure 8B).
Figure 8.
The effect of intraocular light scatter on the rod contribution to our spectral sensitivity measurements. In order to ascertain whether intraocular light scatter may have affected the calculated contribution of the rod photoresponse to our composite spectral sensitivity function, we calculated the actual stimulus profile striking the retina given our 36 degree stimulus and the effect of intraocular light scatter as specified in CIE collection 135-1999. Panel A is a two-dimensional representation of the actual stimulus profile impinging on the retina and demonstrates the attenuation of our light stimulus for retinal eccentricities greater that +/- 18 degrees from the central fixation. Panel B shows the average pupillary light response over time (n=6) of subject A to an annulus (36°-140 °) which matched the irradiance profile of the light scatter produced by the stimulus used to produce a half maximal PLR in subject A during experiment 1. Note that within 30 seconds of light onset the subject shows no increase in pupilloconstriction over that measured during the baseline measurement interval ten seconds prior to stimulus onset.
4. Discussion
Given that the existence of ipRGCs was completely unknown during the bulk of the research investigating the human PLR, there is a large gap in the understanding of the light evoked signals driving the response. The goal of the present study was to address this situation by determining the relative contribution of rods, cones, and the melanopsin photoresponse of ipRGCs to the human PLR at a variety of stimulus parameters. The major findings of this study are that the melanopsin photoresponse of ipRGCs contributes not only to pupillary constriction at high irradiances, but also acts to maintain pupillary diameter in steady-state photopic lighting conditions. We have also characterized how the photic signals of the inner and outer retina dynamically combine to produce pupillary constriction. We found that in response to steady-state light steps, within 10 seconds of light onset, cones contribute minimally to the maintenance of steady-state pupillary diameter at both low and high photopic irradiances. Furthermore, we have shown that rod contributions to the PLR also adapt over time, but reach a steady state at which they contribute to steady-state pupillary constriction at irradiances which are below threshold for the melanopsin photoresponse. In addition to their relevance to human PLR, our findings also add to the body of knowledge pertaining to the physiology of ipRGCs themselves. Given the recent evidence that ipRGCs provide all photic signals which drive the murine PLR (Guler, Ecker, Lall, Haq, Altimus, Liao, Barnard, Cahill, Badea, Zhao, Hankins, Berson, Lucas, Yau & Hattar, 2008), coupled with our previous study demonstrating a close correspondence between ipRGC physiology and the behavior of the human and non-human primate PLR (Gamlin et al., 2007), we believe that human pupillary responses to light provide a powerful model of the light sensitivity of human ipRGCs.
4.1 Comparison to previous studies of the human PLR
Although most previous investigations into the spectral sensitivity of the human PLR were conducted prior to the discovery of ipRGCs, their results are largely in agreement with the results of the present study and therefore add validity to our findings. Re-evaluation of the results of these studies is also useful as they provide additional insights into the role of ipRGCs in the human PLR beyond the finding of the present study. Previous studies of the spectral sensitivity of the human PLR can be grouped into two categories based on stimulus duration and methodology. One type of experiment investigated the spectral sensitivity of the PLR to transient light stimulation and the other investigated the spectral sensitivity of the PLR to steady-state light stimuli. The former set of studies generated transient light responses either by the rapid exchange of monochromatic lights (Alpern & Campbell, 1962, Young & Alpern, 1980), the presentation of brief light stimuli (Krastel, Alexandridis & Gertz, 1985), or the measurement of the transient portion of the response to extended light stimuli (Kimura & Young, 1995, Kimura & Young, 1999). With the exception of Alpern and Campbell (1962), these studies presented their stimuli on either a bright white or monochromatic adapting field in a paradigm similar to that utilized in psychophysical increment threshold spectral sensitivity experiments (e.g. Sperling & Harwerth, 1971). In contrast to the spectral sensitivities reported in the present study, the spectral sensitivities generated in these experiments were reminiscent of the three lobed increment threshold spectral sensitivities of the parvocellular cortical visual pathway. It is generally agreed that these spectral sensitivities are a result of descending cortical influences on midbrain pupillary centers and not reflective of the spectral sensitivity of the direct retino-pretectal fibers (Barbur, 1995, Weiskrantz, Cowey & Barbur, 1999, Wilhelm, Wilhelm, Moro & Barbur, 2002). Furthermore, in contrast to the half maximal and three- quarter maximal criterion response utilize in the present study, the criterion pupillary responses used in the previous mentioned studies were generally at or near threshold (Alpern & Campbell, 1962, Kimura & Young, 1995, Krastel et al., 1985, Young & Alpern, 1980). Taken together, these results show that the spectral sensitivity of the PLR to very brief light stimuli using small criterion responses is not a result of influences of photoresponses originating in ipRGCs, and thus explains the deviations of the spectral sensitivities of the present study from these previous results.
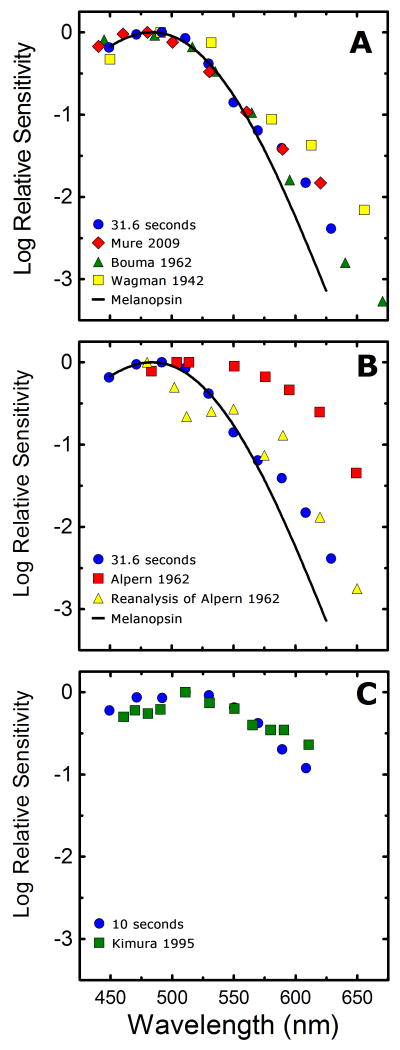
Historical studies investigating the spectral sensitivity of the PLR in response to steady-state light stimuli were primarily focused on the determination of the relative contribution of rod and cones to the PLR. Very similar to the present study, the spectral sensitivity of the response was compared to the known spectral sensitivities of rod and cone driven visual responses, thus producing an estimate of the relative contribution of the two known human photoreceptor classes to the PLR. In an effort to refute earlier claims that the human PLR was driven exclusively by cones (Brown & Page, 1939), Wagman and Gullberg (1942) examined the spectral sensitivity of the human steady-state PLR for a 0.5 mm criterion response and generated a spectral sensitivity well matched by the scotopic luminosity function and therefore rod dominated. This result is not unreasonable given the small light evoked change in pupil diameter chosen as a criterion response, and it is consistent with the findings of previous and subsequent studies using the same criterion (Alpern & Campbell, 1962, Laurens, 1923). It is interesting to note that, although a 0.5 mm criterion response was chosen by the authors, data throughout the complete range of pupillary diameters was collected and published. Using these published irradiance response plots, it is possible to generate a spectral sensitivity of half maximal pupillary constriction to the steady-state light stimulation. When the data from that study are analyzed in a manner similar to the current study and corrected for prereceptoral filtering (Figure 9A), the results are well matched by our data (Figure 9A), with a slight deviation at longer wavelengths. Two subsequent studies of the spectral sensitivity of the steady-state PLR produced results that either closely matched our present results (Bouma, 1962) (Figure 9A), or deviated significantly from our results (Campbell & Alpern, 1962) (Figure 9B).
Figure 9.
Comparison of the steady-state spectral sensitivities of the current study with previous studies. (A) Our data ( ) shows good concordance with previous reports by Mure 2009 (
) shows good concordance with previous reports by Mure 2009 ( ), Bouma 1962 (
), Bouma 1962 ( ), and Wagman 1942 (
), and Wagman 1942 ( ) of the spectral sensitivity of the human PLR in response to steady-state light stimuli. Conversely, (B) Our data (
) of the spectral sensitivity of the human PLR in response to steady-state light stimuli. Conversely, (B) Our data ( ) is not in agreement with the report by Alpern 1962 (
) is not in agreement with the report by Alpern 1962 ( ) of the spectral sensitivity of steady-state PLR. However, when the data of Alpern 1962 is reanalyzed to correct for possible errors produced by incorrect baseline measurements (
) of the spectral sensitivity of steady-state PLR. However, when the data of Alpern 1962 is reanalyzed to correct for possible errors produced by incorrect baseline measurements ( ) (see section 4.6 for details), the results are similar to the results of the current study. (C) Our measured spectral sensitivity of the PLR for shorter stimulus durations (
) (see section 4.6 for details), the results are similar to the results of the current study. (C) Our measured spectral sensitivity of the PLR for shorter stimulus durations ( ) is also in good concordance with the spectral sensitivity measured by Kimura 1995 (
) is also in good concordance with the spectral sensitivity measured by Kimura 1995 ( ) for similar stimulus durations. All previously reported data were converted from corneal illuminance to retinal irradiance when necessary in order to facilitate the comparisons between studies. In panels A and B, the absorbance spectrum of melanopsin is represented by a Baylor nomogram (Baylor et al., 1987) with a lambda max at 483 nm.
) for similar stimulus durations. All previously reported data were converted from corneal illuminance to retinal irradiance when necessary in order to facilitate the comparisons between studies. In panels A and B, the absorbance spectrum of melanopsin is represented by a Baylor nomogram (Baylor et al., 1987) with a lambda max at 483 nm.
The deviations of the data of these previous studies from our current work may be explained by the different experimental methodologies utilized in these studies. Both the Wagman and Gullberg (1942), and Alpern and Campbell (1962) studies utilized an experimental design which collected the data on each wavelength during separate experimental sessions, which were often conducted days or weeks apart from each other. It is well known that pupillary responses to light stimuli of similar intensities can fluctuate from day to day in the same individual (Loewenfeld & Lowenstein, 1993), and given this experimental design, these fluctuations would likely differentially affect the results of each wavelength, thus introducing confounding influences. Additionally, only a single measure of baseline pupillary diameter was established for each wavelength tested. This measurement was taken prior to the onset of the first light stimulus which made up a series of light increments taking between 10 and 20 minutes to complete. It has been demonstrated that pupillary diameter can be significantly influenced by non photic processes such as changes in accommodative state (Busettini, Davison & Gamlin, 2007, Ishikawa, Asakawa & Yoshitomi, 2004, Kasthurirangan & Glasser, 2005, Marg & Morgan, 1949), changes in state of arousal (Aston-Jones & Cohen, 2005, Lowenstein, Feinberg & Loewenfeld, 1963, McLaren, Hauri, Lin & Harris, 2002, Morad, Lemberg, Yofe & Dagan, 2000, Wilhelm, Giedke, Ludtke, Bittner, Hofmann & Wilhelm, 2001, Yoss, Moyer & Hollenhorst, 1970), or cognitive activity (Beatty, 1982, Beatty & Lucero-Wagoner, 2000, Beatty & Wagoner, 1978, Hess & Polt, 1960). Any non-photic induced change in baseline pupil diameter during the initial measurement period or during the subsequent light stimuli would produce a cofounding influence on the subject's pupil diameter that would be incorrectly attributed to the light stimulus. This is particularly apparent in the study by Alpern and Campbell (1962), in which the baseline pupillary diameter measured for the 502 nm and 480 nm stimuli were significantly elevated from that of the other seven wavelength utilized in the study, thus inducing a perceived reduction in the effectiveness of those wavelengths to produce an equivalent change in pupil diameter from baseline. It seems likely that this elevation in baseline pupillary diameter was only transient, thus skewing the measurement of the data collected later in the experiment. If one removes the effect of these likely erroneous baseline measurements by selecting a criterion response of an absolute pupillary diameter of 3.5 mm, thus assuming a similar average baseline for every experimental session, the spectral sensitivity plot produced (Figure 9B) more closely matched the data of the current study (Figure 9A).
A more contemporary study investigating the spectral sensitivity of the PLR to steady-state light stimuli was conducted as part of a study by Kimura and Young (1995). This study utilized a measurement paradigm similar to the present study in which baseline pupillary diameter was assessed prior to each trial and therefore any non-photic pupillary influences on baseline pupil diameter were appropriately controlled for. Their results for an ∼ 0.1 mm criterion response at 3.7 seconds after stimulus onset utilizing a 1000 td white background (Figure 9C) are in very good agreement with our results measured at a similar time interval (Figure 9C). In general, historical studies of the spectral sensitivity of the PLR are in close concordance with those generated in the present study, which serves to validate our analysis of the contribution of rod, cone, and melanopsin photoresponses to the PLR.
A recent study by Mure et al (2009) reports spectral sensitivities of the initial and steady-state human PLR which are largely in agreement with that of the current study (Figure 9A), although our analysis of the underlying mechanisms driving the response differ greatly. Mure and colleagues propose that the dynamic nature of the spectral sensitivity of the human PLR is due to the bistable nature of melanopsin, and that as stimulus duration is increased the absorbance spectrum is shifted by the light induced transformation of M-state melanopsin into the R-state, with each state having a different peak absorbance. Furthermore, the authors contend that in the steady-state condition, in which a complete transition from M to R states is achieved, the action spectrum of melanopsin would shift to ∼460 nm, as opposed to the more commonly reported peak of ∼480 nm (e.g. Berson et al., 2002, Dacey et al., 2005, Gamlin et al., 2007, Hankins & Lucas, 2002, Hattar, Lucas, Mrosovsky, Thompson, Douglas, Hankins, Lem, Biel, Hofmann, Foster & Yau, 2003). Our data do not support this model, as the short wavelength portion (450 nm-530 nm) of all the spectral sensitivity curves for durations greater than ten seconds in experiments 1 and 2, and greater than 1.78 seconds in experiment 3 are well fit by vitamin A1 nonmogram with peak absorbance at 483 nm (Fig 4-6). In addition there is no evidence for a shift in peak sensitivity towards 460 nm as stimulus duration is extended, which would be indicated by a shift in peak sensitivity from the 470 nm or 490 nm stimuli to the 450 nm or 470 nm stimuli. Although our data cannot speak to the absence or existence of melanopsin bistability, which was the focus of Mure et al. (2009), we believe that the shift in the peak sensitivity of our spectral sensitivity data as stimulus duration is increased is best explained by the light adaptation of outer retinal inputs to ipRGCs along with the emergence of a melanopsin mediated light response, which results from the increases in stimulus intensity that are necessary to achieve criterion pupil responses at longer stimulus durations.
4.2 Combination of outer and inner retinal photoresponses
An intriguing result of the current study is that the three photoresponses driving the PLR in humans do not appear to linearly combine at the level of ipRGCs, but rather the outer and inner retinal signals act in a “winner take all” fashion. The results of our optimization of the combination parameters k1 (1.0) and k2 (10) in equation (8) suggest that once the melanopsin photoresponse has reached a specific threshold at any particular wavelength (not necessarily the absolute threshold of the response), the spectral sensitivity at that wavelength is completely determined by the spectral sensitivity of melanopsin, while the spectral sensitivity of the response at irradiances below this threshold is determined by a combination of rod and cone spectral sensitivities. This is most convincingly represented by our spectral sensitivity plots generated under steady-state condition, i.e., stimulus durations of 30 seconds or greater. In all three experiments, the overall steady-state spectral sensitivities were best fit by a combination of the melanopsin and rod spectral sensitivities, with very little contribution by cones. Importantly, at any specific wavelength, the best fit was accomplished by fitting with either the melanopsin (at shorter wavelengths) or rod (at longer wavelengths) spectral sensitivity.
Previous studies investigating the murine PLR have produced data which suggest a mechanism by which outer and inner retinal photoresponses combine to drive the PLR. Pupillometric studies of knockout mice lacking either function rods and cones (Lucas et al., 2001), or a functional melanopsin photoresponse (Lucas et al., 2003, Panda, Provencio, Tu, Pires, Rollag, Castrucci, Pletcher, Sato, Wiltshire, Andahazy, Kay, Van Gelder & Hogenesch, 2003) have shown that the loss of either outer or inner retinal photoresponses causes a defect in the irradiance response dynamics of the PLR as compared to wild type animals. More specifically, mice lacking the melanopsin photoresponse showed a reduced sensitivity to high intensity stimuli (greater than ∼ 12 log quanta/cm2/sec; 470-480 nm) (Lucas et al., 2003, Panda et al., 2003) while mice lacking functional rods and cones showed a reduced sensitivity to all but the highest intensity stimuli (less ∼13 quanta/cm2/sec; 470-506 nm) (Lucas et al., 2001, Lucas et al., 2003, Panda et al., 2003).
As stated in Lucas et al. (2003), given the complementary nature of these defects, these results suggest that the PLR in wild type animals may be the result of the linear addition of outer and inner retinal photoresponses in response to stimuli above the threshold of the melanopsin photoresponse (∼11 log quanta/cm2/sec; 480 nm). This is clearly a reasonable hypothesis, yet the results of the present study suggest that at a specific threshold of activation, the melanopsin photoresponse acts to shunt the outer retinal signals impinging on ipRGCs, and that the melanopsin photoresponse exclusively drives the PLR above this threshold.
Further support for the shunting of outer retinal signals by activation of the melanopsin photoresponse can be found in a report by Sekaran and colleagues (2007), which utilized the rodent PLR as an in vivo function assay of the melanopsin photoresponse. This study reported that an intravitreal injection of a cocktail of glutamate receptor blockers designed to eliminate all outer retinal inputs to ipRGCs caused no significant difference in the PLR evoked by a 480 nm light stimulus with an irradiance of 12 log quanta/cm2/sec. The stimulus parameters used by Sekaran et al. (2007) clearly fall within the zone of overlap between the PLR irradiance response curves measured in mice lacking either rods and cones or melanopsin, yet it appears that rods and cones are not normally contributing to the response at this retinal irradiance. It is likely that there is an underlying biophysical basis for this “winner take all” effect. Activation of the melanopsin phototransduction cascade results in the opening of numerous membrane channels, possibly transient receptor potential (TRP) channels, and as a result the input impedance of an ipRGC would significantly decrease. This decrease in input impedance could act to shunt the outer retinal signals impinging on ipRGCs via synaptic inputs from bipolar and/or amacrine cells.
This “winner take all” phenomenon has a profound influence on the interpretation of the relative influences of rods, cones, and the melanopsin response of ipRGCs in driving the human PLR under broad spectrum lighting conditions, i.e. a light source emitting photons at a wide range of wavelengths. The logical result of this “winner take all” effect is that pupillary constriction driven by broad spectrum illumination, such as sunlight and typical indoor lighting, would be driven predominantly by either outer retinal photoreceptors or the melanopsin photoresponse. The determination of which photoreceptors would drive the response would be solely dependent on whether the illumination was sub- or suprathresold for the shunting of outer retinal photoresponses by sufficient activation of the melanopsin photoresponse.
4.3 Relative contribution of rods to the human PLR
Although our data suggest that outer retinal photoreceptive inputs are shunted by activation of the melanopsin photoresponse, rods act as the primary drive for the human PLR for light intensities below the threshold for this phenomenon. We determined that following a period of rapid adaptation with a time constant of ∼8 sec, rods continue to provide a tonic light signal capable of driving a relatively sustained level of pupillary constriction in response to steady-state light stimuli. This tonic rod signal compensates for the relative insensitivity of the melanopsin photoresponse to light, and acts to drive and maintain pupillary constriction at all light intensities below the threshold of melanopsin as well as augmenting the sensitivity of the human PLR to long wavelength light.
This rapid adaptation of the rod photoresponse may also explain the small difference observed between the results of experiment 1 and experiment 2 despite the use of an adapting background in experiment 2. It seems likely that the rods adapted rapidly, and therefore were able to respond to the monochromatic stimuli despite the presence of the background field. This is not unreasonable, as a background of between 3-4 log trolands would be necessary to completely saturate the rod photoresponse (Adelson, 1982). However, it would have been impossible to utilize a background intensity such as this in the present study, as the use of adapting backgrounds with an intensity greater than 50 trolands activated the melanopsin photoresponse and had a significant influence on baseline pupillary diameters (unpublished observation).
Recent studies using multi-electrode arrays to record ipRGCs have produced data which supports the existence of a tonic rod signal similar to that found in the present study (Tu et al., 2005, Wong et al., 2007). In particular, Wong and colleagues (2007) reported that the activity of ipRGCs in response to light stimuli well below the threshold of the melanopsin mediated intrinsic response was characterized by a tonic firing rate quite unlike the responses of conventional RGCs, as it was maintained throughout the entire duration of the light stimuli. The authors presumed this to be mediated by rods, and the findings of the present study support this assumption.
It has been recently shown in the rat retina that rod bipolar cells synapse directly onto ipRGCs (Østergaard, Hannibal & Fahrenkrug, 2007), thus circumventing the conventional rod pathway through cone bipolar cell via AII amacrine cell gap junctions. This pathway could provide a conduit for a sustained rod signal which avoids the traditional shunting by cone responses which occurs in the conventional retinal circuitry at high irradiances. In addition, recent preliminary reports suggest that rod signals may reach ipRGCs via cone bipolar cells (Dumitrescu, Pucci, Wong & Berson, 2009, Hoshi, Liu, Massey & Mills, 2009, Weng & Berson, 2009).
Given the tonic nature of the melanopsin photoresponse in the initial investigations of ipRGC physiology, it was assumed that the melanopsin photoresponse was required for the maintenance of non-image forming (NIF) behavior in steady-state lighting conditions. Our findings suggest that rod photoresponses are also capable of maintaining the PLR under steady-state lighting conditions as well, and therefore suggest that this assumption may not be valid. A recent study of the spectral sensitivity of negative masking of locomotor behavior in mice also suggests a role for outer photoreceptive inputs in augmentation of the melanopsin photoresponse under steady-state lighting conditions (Thompson, Foster, Stone, Sheffield & Mrosovsky, 2008). These results indicate that the melanopsin photoresponse is not exclusively responsible for the maintenance of NIF functions under extended lighting conditions. As noted in Thompson et al. (2008), this serves to explain how significant functionality of the NIF behavior was maintained in studies of melanopsin knockout mice (Hattar et al., 2003, Lucas et al., 2003, Mrosovsky & Hattar, 2003, Panda et al., 2003).
It should be noted that there are alternative hypotheses for the apparent tonic contribution of rod photoreceptors to the human PLR. First, it is possible that dark adapted rods in the peripheral retina were being unintentionally stimulated due to intraocular light scatter. However, our control experiments strongly suggest that this is not the case (see section 3.5). Second, it is possible that melanopsin acts as a bistable photopigment such that previous exposure to certain wavelengths of light could potentially potentiate subsequent responses, thus skewing our spectral sensitivity measurements. However, our spectral sensitivity experiments were conducted in such a way as to minimize the influence of a bistable photopigment (see section 2.4), and therefore it seems unlikely that this potential effect confounded our data. Furthermore, although studies investigating NIF behavior, including the PLR, provide evidence that melanopsin is a bistable photopigment (Mure et al., 2009, Mure et al., 2007), other studies in rodents do not support this suggestion (Do, Kang, Xue, Zhong, Liao, Bergles & Yau, 2009, Mawad & Van Gelder, 2008).
4.4 Relative contribution of cones to the human PLR
Although it appears that rodent retina is becoming the preferred model for in vitro recording of ipRGCs (Wong et al., 2007), the close overlap in spectral sensitivity between rodent rods and M-cones, 498 nm and 508 nm respectively (Aggelopoulos & Meissl, 2000, Lucas et al., 2001, Thompson et al., 2008) combined with the lower cone to rod ratio (100:1) of the rodent retina (Szel & Rohlich, 1992), make it difficult to assess the relative contribution of cone photoresponses to ipRGC physiology. Thus, previous rodent studies addressing the relative contribution of rod, cone, and melanopsin photoresponses to ipRGC physiology and NIF visual function generally make no distinction between rod and cone photoresponse, and group them together as outer retinal inputs (Berson et al., 2002, Guler et al., 2008, Hattar et al., 2003, Lucas et al., 2003, Mrosovsky & Hattar, 2003, Panda et al., 2003, Panda, Sato, Castrucci, Rollag, DeGrip, Hogenesch, Provencio & Kay, 2002). Given the larger spectral distinction between primate rods (498 nm) and M- and L-cones (533 nm and 564 nm respectively) (Dowling, 1987) as well as the higher cone to rod ratio compared to rodents, (20:1) in humans (Curcio, Sloan, Kalina & Hendrickson, 1990) and non-human primates (Finlay, Franco, Yamada, Crowley, Parsons, Muniz & Silveira, 2008), human and non-human primate studies may be better suited to address the roles of rods and cones in NIF visual functions.
In the present study we determined that L and M-cone driven influences on the human PLR rapidly adapt, losing ∼ 3 log units of sensitivity within 100 seconds of light onset, and therefore do not contribute significantly to maintenance of pupillary constriction at any intensity of steady-state light stimuli. In vitro recording of primate ipRGCs by Dacey and colleagues (2005) was also able to address the relative contribution of cone photoresponses to ipRGC physiology. This study provided evidence that the L and M-cone signals driving the ON response quickly adapted to steady-state light stimuli. The findings of the present study match well with these conclusions. These findings suggest that cone photoresponses have little impact on NIF visual behavior in primates, although there is evidence for involvement of UVS-cones in murine NIF behavior (Thompson et al., 2008).
4.5 Relative contribution of the melanopsin photoresponse to the human PLR
Very few studies have examined the relative contribution of the melanopsin photoresponse to ipRGC physiology and NIF behavior with outer photoreceptors signals still intact. It is becoming increasingly clear that the relative contribution of the melanopsin photoresponse to these behaviors is quite different when outer retinal influences remain viable (see discussion in Thompson et al., 2008). In the present study we determined that the melanopsin photoresponse indeed acts to compensate for the adaptation of outer retinal photoreceptors and maintain pupillary diameter in steady-state lighting conditions at a wide range of photopic irradiances. Given the “winner take all” nature of the contribution of the melanopsin photoresponse to the human PLR, as light intensity increases or the spectral nature of the light is increasingly influenced by short wavelengths, the melanopsin photoresponse becomes the dominant photoreceptive influence on the human PLR.
4.5.1 The influence of light adaptation of the melanopsin photoresponse on the human PLR
Few studies have addressed the light adaptation of the melanopsin photoresponse of ipRGCs; therefore relevant data generated in the current study are of more consequence. Prior to the discovery of ipRGCs, a series of papers by Nelson and Takahashi (1991, 1999) suggested that circadian entrainment was driven by a short wavelength photopigment, which resisted light adaptation and was capable of integrating light signals over tens of minutes. Subsequently, many of these findings have been confirmed; namely, the spectral sensitivity of the photopigment involved (Berson et al., 2002, Dacey et al., 2005, Hattar et al., 2003, Lucas et al., 2001) as well as its ability to integrate light signals over time (Dacey et al., 2005, Gamlin et al., 2007, Hut, Oklejewicz, Rieux & Cooper, 2008). Conversely, a study by Wong et al. (2005) found evidence for light adaptation in whole cell recordings of ipRGCs utilizing saturating light stimuli. Due to the inherent cellular disruption of this technique and the requisite blockade of outer retinal photoreceptive inputs to the cells, these results required further validation.
The results of the current study in regards to the light adaptation of the melanopsin photoresponse of ipRGCs are more consistent with the finding of Wong et al (2005) than that of Nelson and Takahashi (1991, 1999). We found evidence for light adaptation of the melanopsin photoresponse to steady-state light steps in both experiments 1 and 3 (Figure 7A and C). However, the results of experiment 3 provide the most reliable data on the kinetics of the adaptation. Our findings suggest that the rate of adaptation of the contribution of the melanopsin photoresponse to the human PLR is similar to that of rods, with a time constant of ∼8 sec. However, the data of experiment 3 indicate that the overall loss in sensitivity due to light adaptation of the melanopsin photoresponse (0.4 log units) is far less than the loss in sensitivity found in either the rod or cone photoresponses (0.7 and 1.6 log units respectively).
4.5.2 The influence of light integration of the melanopsin photoresponse on the human PLR
We found no evidence of the light integrating capacity of the melanopsin photoresponse during steady-state light exposure at the stimulus durations studied. This effect has been shown in a number of studies, but only following the cessation of light stimuli (Berson et al., 2002, Dacey et al., 2005, Gamlin et al., 2007, Wong et al., 2007). Our results suggest that the melanopsin photoresponse may act as a leaky integrator during steady-state light exposure. Alternatively, the integrative function of the melanopsin photoresponse may be intensity dependent as the irradiances of the stimuli in the current study are less than those that produced a significant integration in previous studies of both the PLR (Gamlin et al., 2007), and in vitro cellular recordings (Berson et al., 2002, Dacey, Peterson, Robinson & Gamlin, 2003, Wong et al., 2007).
4.6 Relative contribution of rod, cone, and melanopsin photoresponses to the dynamics of the PLR
Historically, many models of human PLR dynamics have been proposed by various research groups. A common characteristic of all these models are the parallel input of tonic and phasic signals driving pupillary constriction in response to light stimulation. Two of these models postulated independent visual processes driving the phasic and tonic portions of the response (Clynes, 1961, Kimura & Young, 1995, Kohn & Clynes, 1969, Young et al., 1993). Other more biometric theories of PLR dynamics did not explicitly specify the visual processes driving the phasic and tonic signals driving the response (Privitera & Stark, 2006, Sun, Krenz & Stark, 1983). These models of PLR dynamics were proposed to explain a fundamental feature of the behavioral dynamics of the PLR, which is characterized by an initial robust transient constriction at light onset followed by pupillary dilation to a larger pupil diameter which is then sustained for the remainder of the light stimulus. An example of this phenomenon can be seen in the red and green traces in panels C and D of figure 1. This behavior is often termed “pupillary escape” and until the discovery of the response properties of ipRGC, the visual processes driving this behavior were poorly understood.
The present study provides the first quantitative description of the relative influences of rod, cone, and melanopsin photoresponses on pupillary escape and other aspects of human pupillary dynamics. Given the previous models describing the transient and sustained nature of pupillary behavior and the phasic and tonic response properties of ipRGCs, it was initially assumed that the transient portion of the PLR was driven exclusively by rods or cones and the sustained portion was driven solely by the melanopsin photoresponse at photopic irradiances (Kawasaki & Kardon, 2007, Young & Kimura, 2008). The findings of the present study suggest that this generalization may be too simplistic. It is clear that at low photopic irradiances (experiments 1 and 2), maintenance of steady-state pupil diameters is seen in the absence of any contribution of the melanopsin photoresponse (see Fig. 1C). At these irradiance levels and stimulus durations, pupillary escape is seen and can be explained exclusively by the exponential decay of the outer retinal photoreceptor signals. For longer duration stimuli at these same irradiance levels, a more extensive pupillary escape is seen, especially in response to long wavelength light (e.g. Fig. 1D, 1E). Even for these long duration stimuli, the sustained portion of the response is not mediated solely by the melanopsin photoresponse, but is also augmented by outer receptor inputs in response to long wavelength lights. Interestingly,, a recent study by Kardon and colleagues (2009) demonstrates how the unique response dynamics of the human PLR can be successfully utilized as a diagnostic tool for the discrimination of retinal diseases of the inner and outer retina.
Acknowledgments
The authors thank Dr. Joel Pokorny for technical advice and comments on the manuscript, Sam Hayley for his technical assistance, and Drs. T.J.T.P van den Berg and Joris Coppens for their generous assistance in deriving an intraocular light scatter profile based on our light stimulus. This work was supported by NIH grants EY09380, P30 EY03039, and The EyeSight Foundation of Alabama.
References
- Adelson EH. Saturation and adaptation in the rod system. Vision Research. 1982;22(10):1299–1312. doi: 10.1016/0042-6989(82)90143-2. [DOI] [PubMed] [Google Scholar]
- Aggelopoulos N, Meissl H. Responses of neurones of the rat suprachiasmatic nucleus to retinal illumination under photopic and scotopic conditions. J Physiol. 2000;523(Pt 1):211–222. doi: 10.1111/j.1469-7793.2000.t01-1-00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern M, Campbell FW. The spectral sensitivity of the consensual light reflex. J Physiol. 1962;164:478–507. doi: 10.1113/jphysiol.1962.sp007033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern M, Falls HF, Lee GB. The enigma of typical total monochromacy. Am J Ophthalmol. 1960;50:996–1012. doi: 10.1016/0002-9394(60)90353-6. [DOI] [PubMed] [Google Scholar]
- Alpern M, Ohba N. The effect of bleaching and backgrounds on pupil size. Vision Res. 1972;12(5):943–951. doi: 10.1016/0042-6989(72)90016-8. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Barbur JL. A Study of Pupil Response Components in Human Vision. In: Robbins JG, Djamgoz MBA, Taylor A, editors. Basic and Clinical Perspectives in Vision Research : A Celebration of the Career of Hisako Ikeda. New York: Plenum Press; 1995. pp. 3–18. [Google Scholar]
- Baylor DA, Nunn BJ, Schnapf JL. Spectral sensitivity of cones of the monkey Macaca fascicularis. J Physiol. 1987;390:145–160. doi: 10.1113/jphysiol.1987.sp016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty J. Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychological bulletin. 1982;91(2):276–292. [PubMed] [Google Scholar]
- Beatty J, Lucero-Wagoner B. The Pupillary System. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 2nd. Cambridge University Press; 2000. pp. 142–162. [Google Scholar]
- Beatty J, Wagoner BL. Pupillometric signs of brain activation vary with level of cognitive processing. Science. 1978;199(4334):1216–1218. doi: 10.1126/science.628837. [DOI] [PubMed] [Google Scholar]
- Bennett AG, Rabbetts RB, Bennett AG. Bennett and Rabbetts' clinical visual optics. Oxford; Boston: Butterworth-Heinemann; 1998. pp. viii–451. [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Bouma H. Size of the static pupil as a function of wavelength and luminosity of the light incident on the human eye. Nature. 1962;193:690–691. doi: 10.1038/193690a0. [DOI] [PubMed] [Google Scholar]
- Brown RH, Page HE. Pupil dilatation and dark adaptation. Journal of Experimental Psychology. 1939;25(4):347–360. [Google Scholar]
- Busettini C, Davison RC, Gamlin PDR. The Near Triad: Vergence, Accommodation, and Pupilloconstriction. In: Squire L, editor. New Encyclopedia of Neuroscience. Oxford: Elsevier; 2007. [Google Scholar]
- Campbell FW. The depth of field of the human eye. Optica Acta. 1957;4(4):157–164. [Google Scholar]
- Campbell FW, Alpern M. Pupillomotor spectral sensitivity curve and color of the fundus. Osaka City Med J. 1962;52:1084. doi: 10.1364/josa.52.001084. [DOI] [PubMed] [Google Scholar]
- Campbell FW, Gregory AH. Effect of size of pupil on visual acuity. Nature. 1960;187:1121–1123. doi: 10.1038/1871121c0. [DOI] [PubMed] [Google Scholar]
- Campbell FW, Gubisch RW. Optical quality of the human eye. J Physiol. 1966;186(3):558–578. doi: 10.1113/jphysiol.1966.sp008056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynes M. Unidirectional rate sensitivity: a biocybernetic law of reflex and humoral systems as physiologic channels of control and communication. Ann N Y Acad Sci. 1961;92:946–969. doi: 10.1111/j.1749-6632.1961.tb40968.x. [DOI] [PubMed] [Google Scholar]
- Crawford BH. The Scotopic Visibility Function. Proc Phys Soc B 62. 1949;62(5):321–334. [Google Scholar]
- Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292(4):497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433(7027):749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Peterson BB, Robinson FR, Gamlin PD. Fireworks in the primate retina: In vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron. 2003;37(1):15–27. doi: 10.1016/s0896-6273(02)01143-1. [DOI] [PubMed] [Google Scholar]
- Do MTH, Kang SH, Xue T, Zhong H, Liao HW, Bergles DE, Yau KW. Photon capture and signalling by melanopsin retinal ganglion cells. Nature. 2009;457(7227):281–287. doi: 10.1038/nature07682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE. of plates. Vol. 284. Cambridge, Mass.: Belknap Press of Harvard University Press; 1987. The retina : an approachable part of the brain; pp. xii–282. [Google Scholar]
- Drouyer E, Rieux C, Hut RA, Cooper HM. Responses of suprachiasmatic nucleus neurons to light and dark adaptation: relative contributions of melanopsin and rod-cone inputs. J Neurosci. 2007;27(36):9623–9631. doi: 10.1523/JNEUROSCI.1391-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu ON, Pucci FG, Wong K, Berson DM. ON Bipolar Cell Output to the OFF Sublamina of the Inner Plexiform Layer: Contacts With Melanopsin Ganglion Cells and Dopaminergic Amacrine Cells. IOVS. 2009;50 doi: 10.1002/cne.22158. ARVO E-Abstract 5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BL, Franco EC, Yamada ES, Crowley JC, Parsons M, Muniz JA, Silveira LC. Number and topography of cones, rods and optic nerve axons in New and Old World primates. Vis Neurosci. 2008;25(3):289–299. doi: 10.1017/S0952523808080371. [DOI] [PubMed] [Google Scholar]
- Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47(7):946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girkin CA. Evaluation of the pupillary light response as an objective measure of visual function. Ophthalmol Clin North Am. 2003;16(2):143–153. doi: 10.1016/s0896-1549(03)00002-6. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4(12):1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- Graham NVS. Oxford psychology series; no 16. New York: Oxford University Press; 2001. Visual pattern analyzers; pp. xvi–646. [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453(7191):102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins MW, Lucas RJ. The primary visual pathway in humans is regulated according to long-term light exposure through the action of a nonclassical photopigment. Curr Biol. 2002;12(3):191–198. doi: 10.1016/s0960-9822(02)00659-0. [DOI] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. Journal of Comparative Neurology. 2006;497(3):326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295(5557):1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424(6944):76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess EH, Polt JM. Pupil size as related to interest value of visual stimuli. Science. 1960;132:349–350. doi: 10.1126/science.132.3423.349. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Yamaji K, Sakai H, Usui S. Function of the pupil in vision and information capacity of retinal image. Syst Comput Jpn. 2003;34(9):48–57. [Google Scholar]
- Hoshi H, Liu WL, Massey SC, Mills SL. ON Cone Bipolar Inputs Which Break the Stratification Rules of the Inner Plexiform Layer. IOVS. 2009;50 doi: 10.1523/JNEUROSCI.0912-09.2009. ARVO E-Abstract 5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hut RA, Oklejewicz M, Rieux C, Cooper HM. Photic sensitivity ranges of hamster pupillary and circadian phase responses do not overlap. J Biol Rhythms. 2008;23(1):37–48. doi: 10.1177/0748730407311851. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Asakawa K, Yoshitomi T. Pupillary near reflex. Neuro-Ophthalmology Japan. 2004;21(3):280–285. [Google Scholar]
- Jusuf PR, Lee SC, Hannibal J, Grunert U. Characterization and synaptic connectivity of melanopsin-containing ganglion cells in the primate retina. Eur J Neurosci. 2007;26(10):2906–2921. doi: 10.1111/j.1460-9568.2007.05924.x. [DOI] [PubMed] [Google Scholar]
- Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A. Chromatic Pupil Responses: Preferential Activation of the Melanopsin-mediated versus Outer Photoreceptor-mediated Pupil Light Reflex. Ophthalmology. 2009 doi: 10.1016/j.ophtha.2009.02.007. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- Kasthurirangan S, Glasser A. Characteristics of pupil responses during far-to-near and near-to-far accommodation. Ophthal Physl Opt. 2005;25(4):328–339. doi: 10.1111/j.1475-1313.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki A. Disorders of Pupillary Function, Accommodation, and Lacrimation. In: Miller NR, Walsh FB, Hoyt WF, editors. Walsh and Hoyt's Clinical Neuro-Ophthalmology. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 739–805. [Google Scholar]
- Kawasaki A, Kardon RH. Intrinsically photosensitive retinal ganglion cells. J Neuroophthalmol. 2007;27(3):195–204. doi: 10.1097/WNO.0b013e31814b1df9. [DOI] [PubMed] [Google Scholar]
- Kimura B, Young RSL. Nature of the pupillary responses evoked by chromatic flashes on a white background. Vision Research. 1995;35(7):897–906. doi: 10.1016/0042-6989(94)00188-r. [DOI] [PubMed] [Google Scholar]
- Kimura E, Young RS. S-cone contribution to pupillary responses evoked by chromatic flash offset. Vision Res. 1999;39(6):1189–1197. doi: 10.1016/s0042-6989(98)00154-0. [DOI] [PubMed] [Google Scholar]
- Kohn M, Clynes M. Color dynamics of the pupil. Ann N Y Acad Sci. 1969;156(2):931–950. doi: 10.1111/j.1749-6632.1969.tb14024.x. [DOI] [PubMed] [Google Scholar]
- Krastel H, Alexandridis E, Gertz J. Pupil increment thresholds are influenced by color opponent mechanisms. Ophthalmologica. 1985;191(1):35–38. doi: 10.1159/000309536. [DOI] [PubMed] [Google Scholar]
- Kurtenbach A, Meierkord S, Kremers J. Spectral sensitivities in dichromats and trichromats at mesopic retinal illuminances. Journal of the Optical Society of America A: Optics and Image Science, and Vision. 1999;16(7):1541–1548. [Google Scholar]
- Laurens H. Studies on the Relative Physiological Value of Spectral Lights: III. The Pupillomotor Effects of Wave-Lengths of Equal Energy Content. Am J Physiol. 1923;64(1):97–119. [Google Scholar]
- Loewenfeld IE, Lowenstein O. The Pupil : Anatomy, Physiology, and Clinical Applications. Iowa State University Press; 1993. [Google Scholar]
- Lowenstein O, Feinberg R, Loewenfeld IE. Pupillary movements during acute and chronic fatigue. Invest Ophthalmol. 1963;2:138–157. [Google Scholar]
- Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4(6):621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299(5604):245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Marg E, Morgan MW. The pupillary near reflex. Am J Optom. 1949;26:183–198. [PubMed] [Google Scholar]
- Mawad K, Van Gelder RN. Absence of Long-Wavelength Photic Potentiation of Murine Intrinsically Photosensitive Retinal Ganglion Cell Firing In Vitro. J Biol Rhythms. 2008;23(5):387–391. doi: 10.1177/0748730408323063. [DOI] [PubMed] [Google Scholar]
- McDougal DH, Gamlin PDR. Pupillary Control Pathways. In: Allan IB, Akimichi K, Gordon MS, Gerald W, editors. The Senses: A Comprehensive Reference. New York: Academic Press; 2008. pp. 521–536. [Google Scholar]
- McLaren JW, Hauri PJ, Lin SC, Harris CD. Pupillometry in clinically sleepy patients. Sleep Medicine. 2002;3(4):347–352. doi: 10.1016/s1389-9457(02)00017-5. [DOI] [PubMed] [Google Scholar]
- Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433(7027):741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- Miyahara E, Pokorny J, Smith VC. Increment threshold and purity discrimination spectral sensitivities of X-chromosome-linked color-defective observers. Vision Res. 1996;36(11):1597–1613. doi: 10.1016/0042-6989(95)00215-4. [DOI] [PubMed] [Google Scholar]
- Morad Y, Lemberg H, Yofe N, Dagan Y. Pupillography as an objective indicator of fatigue. Current Eye Research. 2000;21(1):535–542. [PubMed] [Google Scholar]
- Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int. 2003;20(6):989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- Mure LS, Cornut PL, Rieux C, Drouyer E, Denis P, Gronfier C, Cooper HM. Melanopsin Bistability: A Fly's Eye Technology in the Human Retina. PLoS ONE. 2009;4(6):e5991. doi: 10.1371/journal.pone.0005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mure LS, Rieux C, Hattar S, Cooper HM. Melanopsin-dependent nonvisual responses: Evidence for photopigment bistability in vivo. Journal of Biological Rhythms. 2007;22(5):411–424. doi: 10.1177/0748730407306043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus) Journal of Physiology. 1991;439:115–145. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Takahashi JS. Integration and saturation within the circadian photic entrainment pathway of hamsters. Am J Physiol. 1999;277(5 Pt 2):R1351–1361. doi: 10.1152/ajpregu.1999.277.5.R1351. [DOI] [PubMed] [Google Scholar]
- Newsome DA. Afterimage and pupillary activity following strong light exposure. Vision Res. 1971;11(3):275–288. doi: 10.1016/0042-6989(71)90191-x. [DOI] [PubMed] [Google Scholar]
- Østergaard J, Hannibal J, Fahrenkrug J. Synaptic contact between melanopsin-containing retinal ganglion cells and rod bipolar cells. Invest Ophthalmol Vis Sci. 2007;48(8):3812–3820. doi: 10.1167/iovs.06-1322. [DOI] [PubMed] [Google Scholar]
- Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301(5632):525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298(5601):2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- Pokorny J, Jin Q, Smith VC. Spectral-luminosity functions, scalar linearity, and chromatic adaptation. J Opt Soc Am A. 1993;10(6):1304–1313. doi: 10.1364/josaa.10.001304. [DOI] [PubMed] [Google Scholar]
- Pokorny J, Smith VC, Lutze M. Aging of the human lens. Appl Opt. 1987;26(8):1437. doi: 10.1364/AO.26.001437. [DOI] [PubMed] [Google Scholar]
- Privitera CM, Stark LW. A binocular pupil model for simulation of relative afferent pupil defects and the swinging flashlight test. Biol Cybern. 2006;94(3):215–224. doi: 10.1007/s00422-005-0042-8. [DOI] [PubMed] [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20(2):600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick RF., Jr A vector-magnitude model of contrast detection. Kybernetik. 1974;16(2):65–67. doi: 10.1007/BF00271628. [DOI] [PubMed] [Google Scholar]
- Robson JG, Graham N. Probability summation and regional variation in contrast sensitivity across the visual field. Vision Res. 1981;21(3):409–418. doi: 10.1016/0042-6989(81)90169-3. [DOI] [PubMed] [Google Scholar]
- Sekaran S, Lall GS, Ralphs KL, Wolstenholme AJ, Lucas RJ, Foster RG, Hankins MW. 2-Aminoethoxydiphenylborane is an acute inhibitor of directly photosensitive retinal ganglion cell activity in vitro and in vivo. J Neurosci. 2007;27(15):3981–3986. doi: 10.1523/JNEUROSCI.4716-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling HG, Harwerth RS. Red-green cone interactions in the increment-threshold spectral sensitivity of primates. Science. 1971;172(979):180–184. doi: 10.1126/science.172.3979.180. [DOI] [PubMed] [Google Scholar]
- Stockman A, Sharpe LT. The spectral sensitivities of the middle- and long-wavelength-sensitive cones derived from measurements in observers of known genotype. Vision Res. 2000;40(13):1711–1737. doi: 10.1016/s0042-6989(00)00021-3. [DOI] [PubMed] [Google Scholar]
- Sun F, Krenz WC, Stark LW. A systems model for the pupil size effect. I. Transient data. Biol Cybern. 1983;48(2):101–108. doi: 10.1007/BF00344393. [DOI] [PubMed] [Google Scholar]
- Szel A, Rohlich P. Two cone types of rat retina detected by anti-visual pigment antibodies. Exp Eye Res. 1992;55(1):47–52. doi: 10.1016/0014-4835(92)90090-f. [DOI] [PubMed] [Google Scholar]
- ten Doesschate J, Alpern M. Response of the pupil to steady-state retinal illumination: contribution by cones. Science. 1965;149(687):989–991. doi: 10.1126/science.149.3687.989. [DOI] [PubMed] [Google Scholar]
- Thompson S, Foster RG, Stone EM, Sheffield VC, Mrosovsky N. Classical and melanopsin photoreception in irradiance detection: negative masking of locomotor activity by light. European Journal of Neuroscience. 2008;27(8):1973–1979. doi: 10.1111/j.1460-9568.2008.06168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu DC, Zhang DY, Demas J, Slutsky EB, Provencio I, Holy TE, Van Gelder RN. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48(6):987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Tucker J, Charman WN. The depth-of-focus of the human eye for Snellen letters. American Journal of Optometry and Physiological Optics. 1975;52(1):3–21. doi: 10.1097/00006324-197501000-00002. [DOI] [PubMed] [Google Scholar]
- Viney T, Balint K, Hillier D, Siegert S, Boldogkoi Z, Enquist L, Meister M, Cepko C, Roska B. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr Biol. 2007;17(11):981–988. doi: 10.1016/j.cub.2007.04.058. [DOI] [PubMed] [Google Scholar]
- Wagman IH, Gullberg JE. The relationship between monochromatic light and pupil diameter the low internsity visibility curve as measured by pupillary measurements. Am J Physiol. 1942;137(4):769–778. [Google Scholar]
- Wald G. The spectal sensitivity of the human eye. J Opt Soc Am. 1945;35(3):187–196. [Google Scholar]
- Webster JG, Cohen GH, Boynton RM. Optimizing the use of the criterion response for the pupil light reflex. J Opt Soc Am. 1968;58(3):419–424. doi: 10.1364/josa.58.000419. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L, Cowey A, Barbur JL. Differential pupillary constriction and awareness in the absence of striate cortex. Brain. 1999;122(8):1533–1538. doi: 10.1093/brain/122.8.1533. [DOI] [PubMed] [Google Scholar]
- Weng S, Berson DM. Ganglion-Cell Photoreceptors Are Driven by the Most Sensitive Rod Pathway and by Cones. IOVS. 2009;50 doi: 10.1371/journal.pone.0066480. ARVO E-Abstract 2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm B, Giedke H, Ludtke H, Bittner E, Hofmann A, Wilhelm H. Daytime variations in central nervous system activation measured by a pupillographic sleepiness test. Journal of Sleep Research. 2001;10(1):1–7. doi: 10.1046/j.1365-2869.2001.00239.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm BJ, Wilhelm H, Moro S, Barbur JL. Pupil response components: Studies in patients with Parinaud's syndrome. Brain. 2002;125(10):2296–2307. doi: 10.1093/brain/awf232. [DOI] [PubMed] [Google Scholar]
- Williams RW, Chalupa LM. Development of the retinal pathway to the pretectum of the cat. Neuroscience. 1983;10(4):1249–1267. doi: 10.1016/0306-4522(83)90111-2. [DOI] [PubMed] [Google Scholar]
- Wong K, Dunn F, Graham D, Berson D. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol. 2007;582(Pt 1):279–296. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KY, Dunn FA, Berson DM. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48(6):1001–1010. doi: 10.1016/j.neuron.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Woodhouse JM. The effect of pupil size on grating detection at various contrast levels. Vision Res. 1975;15(6):645–648. doi: 10.1016/0042-6989(75)90278-3. [DOI] [PubMed] [Google Scholar]
- Yoss RE, Moyer NJ, Hollenhorst RW. Pupil size and spontaneous pupillary waves associated with alertness, drowsiness, and sleep. Neurology. 1970;20(6):545–554. doi: 10.1212/wnl.20.6.545. [DOI] [PubMed] [Google Scholar]
- Young RS, Alpern M. Pupil responses to foveal exchange of monochromatic lights. J Opt Soc Am. 1980;70(6):697–706. doi: 10.1364/josa.70.000697. [DOI] [PubMed] [Google Scholar]
- Young RS, Kimura E. Pupillary correlates of light-evoked melanopsin activity in humans. Vision Res. 2008;48(7):862–871. doi: 10.1016/j.visres.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Young RSL, Han BC, Wu PY. Transient and sustained components of the pupillary responses evoked by luminance and color. Vision Research. 1993;33(4):437–446. doi: 10.1016/0042-6989(93)90251-q. [DOI] [PubMed] [Google Scholar]