Abstract
Recent evidences have suggested that humoral factors released from the appropriate co-cultured cells influenced the expansion and differentiation of mesenchymal stem cells (MSCs). However, little is known about the proliferation and differentiation of MSCs subjected to co-culture condition with tenocytes. In this study, we aimed to establish a co-culture system of MSCs and tenocytes and investigate the proliferation and tendon/ligament related gene expression of MSCs. MTT assay was used to detect the expansion of MSCs. Semi-quantitative RT-PCR was performed to investigate the expression of proliferation associated c-fos gene and tendon/ligament related genes, including type I collagen (Col I), type III collagen (Col III), tenascin C and scleraxis. Significant increase in MSCs expansion was observed after 3 days of co-culture with tenocytes. The c-fos gene expression was found distinctly higher than for control group on day 4 and day 7 of co-culture. The mRNA expression of four tendon/ligament related genes was significantly up-regulated after 14 days of co-culture with tenocytes. Thus, our research indicates that indirect co-culture with tenocytes promotes the proliferation and mRNA expression of tendon/ligament related genes in MSCs, which suggests a directed differentiation of MSCs into tendon/ligament.
Keywords: MSCs, Proliferation, Differentiation, Tenocyte, Co-culture
Introduction
Tendon is a tough band of fibrous connective tissue that usually connects muscle to bone and is capable of withstanding tension. When overused or subjected to sudden high strain, injuries such as inflammation, degeneration of the tendon might occur, which may eventually lead to rupture. However, probably because tenocytes are highly differentiated cells and have limited potential to replicate, the instinct healing ability of tendon is poor and replacement tissues are usually required for tendon regeneration (Woo et al. 1999; Violini et al. 2009). Recently, using adult bone marrow derived MSCs as seed cells to regenerate functional tendon attracted great interests with the promising use of MSCs in engineering tissue construction and transplantation without allograft rejection.
MSCs can be easily obtained from adult bone marrow, fat, umbilical cord blood and fetal tissues and expanded in vitro while maintaining their multipotency (Caplan 2007; Chamberlain et al. 2007). A number of protocols have been developed to achieve MSCs differentiation by proper stimulations of physical factors, hormones, chemical molecules or growth factors (Aaron and Ciombor 1996; Chamberlain et al. 2007). Under the appropriate condition, MSCs are capable to differentiate into multiple mesenchymal lineages including osteoblasts, chondrocytes, adipocytes, myocytes and tenocytes as well as hepatocytes in endoderm and neural cells in ectoderm (Caplan 2007; Chamberlain et al. 2007; Brazelton et al. 2002; Krampera et al. 2007; Lange et al. 1999).
The potential application of MSCs to accelerate tendon repair in vivo have been described in previous literatures. Ouyang et al. directly delivered the allogeneic MSCs into the wounded rabbit patella tendon and found the seeded MSCs remained viable within the wounded site and had the potential to influence the formation and remodeling of injured tendon (Ouyang et al. 2004). Seeded on the scaffold, MSCs have been illustrated to repair and regenerate injured tendons after implanted into animal models together with the scaffold (Ouyang et al. 2003; Awad et al. 2003; Juncosa-Melvin et al. 2005). Juncosa-Melvin et al. further showed that mechanical stimulation of MSCs-collagen sponges constructs significantly improved tendon repair in rabbit model (Juncosa-Melvin et al. 2006). In vitro, MSCs have been successfully induced into tenocytes by exposure to growth factors BMP-12 (Violini et al. 2009) or low-dose FGF-2 (Hankemeier et al. 2005). Meanwhile, GDF-5 has also been reported to increase mRNA expression of Col I and SCX in MSCs which are believed to be the markers of tendon/ligament differentiation (Farng et al. 2008). Ouyang et al. showed when assembled with frozen tendon graft in vitro, previously made MSCs sheets were well-incorporated within the tissue sheath around the tendon and adopted characteristic spindle-shaped morphology of tenocyte-like cells (Ouyang et al. 2006). Distinctively, mechanical measures were more recommended for tenogenic differentiation of MSCs than other phenotypes of differentiation. It has been demonstrated that cyclic mechanical stretch not only up-regulated mRNA level expression of tendon/ligament-related genes such as Col I, Col III, TNC (Farng et al. 2008; Chen et al. 2008; Altman et al. 2002; Lee et al. 2007) but also was required for maintaining the expression of those tendon makers (Kuo and Tuan 2008). The MSCs-collagen sponges construct engineered tendon tissue subjected to cyclic mechanical stretch before transplantation has been demonstrated to significantly improve tendon repair and show better mechanical properties than control group (Juncosa-Melvin et al. 2006).
Several groups have illustrated that MSCs underwent site-specific differentiation into typical lineages when site-directed delivered into healthy or injured tissues (Chamberlain et al. 2007; Ouyang et al. 2004), which suggested that microenvironment and intercellular crosstalk play a critical role in cell fate determination of MSCs. Recently, co-culture systems have been developed to simulate the in vivo microenvironment and managed to induce MSCs differentiation, which was probably due to the humoral factors released by the appropriate cells. Moreover, this in vitro culture system made MSCs fate more controllable than in vivo and easier for understanding the molecular events within the crosstalk of MSCs and the local environment. Co-culturing with appropriate cell types, MSCs have been elucidated to commit to corresponding lineages, such as osteoblasts (Csaki et al. 2009), chondrocytes (Chen et al. 2009), ligament cells (Lee et al. 2007), cardiomyocytes (Wang et al. 2006), hepatocytes (Lange et al. 1999) and smooth muscle cells (Wang et al. 2006; Ball et al. 2004). Interestingly, direct cell-to-cell contact has been proven to be indispensable for the commitment of a sort of cell types, such as smooth muscle cells and cardiomyocytes (Wang et al. 2006; Ball et al. 2004).
Though MSCs graft was proven to support wounded tendon healing in vivo as described previously (Ouyang et al. 2003, 2004; Juncosa-Melvin et al. 2005, 2006; Awad et al. 2003), little is known about the exact MSCs commitment process and the mechanism of differentiation still remains unclear. Besides in vivo researches, in vitro co-culture system might offer another view to enucleate these questions. So far, the methods established for tenogenic differentiation of MSCs mostly based on mechanical loading and the changes of MSCs subjected to the microenvironment with full differentiated tenocytes are seldom investigated. In this study, we aimed to establish an indirect cell-to-cell contact co-culture system of rat MSCs and primary tenocytes and elucidate the proliferation and differentiation of MSCs affected by the microenvironment with tenocytes.
Materials and methods
MSCs and tenocyte isolation and culture
Adult Sprague–Dawley (SD) rats were obtained from Animal Center of the Third Military Medical University (Chongqing, China) and all procedures have been approved by the care of Experimental Animals Committee of Chongqing University, Chongqing, China. Bone marrow of 2-month-old SD rat was aspirated from rat thighbones and shinbones with 10 mL syringe containing 5 mL of low glucose DMEM (L-DMEM) medium (Gibco, Grand Island, NY, USA). Rat bone marrow MSCs were obtained by density gradient centrifugation with 1.073 g/mL Percoll (Sigma, Saint Louis, MO, USA) at 2,500 rpm/min for 30 min. The isolated MSCs were cultured in L-DMEM medium supplemented with 10% FBS (Hyclone, Logan, UT, USA), 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C with 5% CO2 in the incubator. Fresh medium was changed every 3 days and 1–3 passaging was performed when MSCs achieved 90% confluence. Cells at the 3rd passage (P3) were used for experiments.
To isolate rat primary tenocytes, the Achille tendons were separated from rat forefeet and cut into pieces with the size around 1 mm3. The tissue pieces were placed onto culture surface of a 50 mL flask evenly, which was leaved upside down in the incubator with 3 mL culture medium and turned around after 24 h. Some colonies formed within 10 days. The culture medium used for tenocytes was the same as for MSCs. After about 15 days, the cells were ready for passaging. Cells at P3 passage were used for co-culture experiments with MSCs.
MSCs characterization
The determination of MSCs characteristics were performed by both flow cytometry and cell fate induction. Briefly, MSCs were trypsinized, washed with PBS, resuspended and stained with FITC-conjugated mouse anti-rat CD44 (Serotec, Oxford, UK), CD90 (Serotec) and PE-conjugated mouse anti-rat CD34 antibodies (Santa Cruz, CA, USA) according to the manufactures’ instructions, while FITC or PE conjugated mouse IgG1 (Santa Cruz) staining cells were used as isotype controls. The expression of cell-surface antigens was detected by FACScalibur (BD Bioscience, San Jose, CA, USA) and analyzed by flowjo software (TreeStar, Ashland, OR, USA). To determine the multipotent differentiation ability, MSCs were induced into osteoblasts, adipocytes with the published protocols (Pittenger et al. 1999).
Co-culture condition
The co-culture system was constructed with a transwell chamber (Millipore, Bedford, MA, USA) which can be inserted into the well of 6-well plates. MSCs were seeded on 6-well tissue culture polystyrene plates (Costar, NY, USA) at 2 × 104/well while at the ratio of 1:1, tenocytes were seeded on the membrane (with pore size of 1 μm) of the transwell chamber. The chamber were inserted into the well of the plate and 3 mL of L-DMEM medium was added to cover both upper layer tenocytes and lower layer MSCs, allowing the crosstalk of the two cell types. Half of the culture medium was changed every 3 days.
MSCs proliferation assay
When investigating proliferation in the co-culture system, MSCs were firstly synchronized by serum starvation for 24 h in incubator before seeded into 6-well plate. Flow cytometry was performed to verify synchronization by cell cycle analysis using PI (propidium iodide) staining as previously described (Song et al. 2004). The number of 2 × 104 synchronized MSCs or tenocytes were seeded into the well or inserted chamber, respectively, at day 0. The expansion of MSCs was detected by MTT assay everyday from day 1 to day 7. For MTT assay, the inserted chamber was removed and 0.6 mL of 5 mg/mL MTT was added into the well. After incubation for 4 h at 37 °C, the supernatant was discarded and 4 mL dimethyl sulfoxide (DMSO) was added to completely dissolve the MTT formazan. The formazan solution of 200 μL was removed into a well of a 96 well plate and the optical density (OD) value was measured at a wavelength of 490 nm with a microtiter plate reader (Model 550, Bio-Rad, Hercules, CA, USA).
Semi-quantitative RT-PCR
Total RNA was isolated using RNA extraction kit (Qiagen, Valencia, CA, USA) and the potential genomic DNA contamination was digested by RNase free DNase I (Invitrogen, Carlsbad, CA, USA). Reverse transcription was performed with Oligo dT primer (Invitrogen) and SuperScript II reverse transcriptase (Invitrogen) following the manufacture’s instructions. Primers for semi-quantitative PCR were designed at similar Tm value around 60 °C by Primer Premier 5.0 software based on the reference mRNA sequences from NCBI Genebank and synthesized by Invitrogen (Shanghai, China). The primer sequence, product length and Genebank No. of reference mRNA sequence were shown in Table 1. 2 × PCR Master Mix (Fermentas, Shenzhen, China) were used to make PCR reaction mixtures with primers, cDNA and water. The reaction mixtures were incubated for 3 min at 94 °C, followed by 94 °C for 30 s, 55 °C for 30 s and 72 °C for 60 s up to 35 cycles, and then 72 °C for 7 min. For each gene, a reaction mixture with water instead of cDNA template was run at the same time as a negative control. The PCR products were analyzed with 1.5% agarose (Takara, Dalian, China) gel electrophoresis (Gel Doc XR + system, Bio-Rad) and Quantity One 1-D Analysis software (Bio-Rad).
Table 1.
Primer sequences for semi-quantitative PCR
| Target gene | Primer sequence | Product length (bp) | Gene bank no. | |
|---|---|---|---|---|
| Collagen I | Sense | 5′ GAGCGATTACTACTGGATTGACCC 3′ | 506 | NM 053356.1 |
| Anti-sense | 5′ CAAGGAATGGCAGGCGAGAT 3′ | |||
| Collagen III | Sense | 5′ AAGAGCGGAGAATACTGGG 3′ | 532 | AJ 005395.1 |
| Anti-sense | 5′ CAATGTCATAGGGTGCGATA 3′ | |||
| Scleraxis | Sense | 5′ GACCGCACCAACAGCGTGAA 3′ | 382 | NM 01130508.1 |
| Anti-sense | 5′ GTGGACCCTCCTCCT TCTAACTTC 3′ | |||
| Tenascin C | Sense | 5′ AGATGCTACTCCAGACGGTTTC 3′ | 200 | NM 053861.1 |
| Anti-sense | 5′ CACGGCTTATTCCATAGAGTTCA 3′ | |||
| c-fos | Sense | 5′ ATCCGAAGGGAAAGGAATAAGA 3′ | 246 | NM 022197.2 |
| Anti-sense | 5′ CAAGTCCAGGGAGGTCACAGA 3′ | |||
| β-actin | Sense | 5′ CTGCCGCATCCTCTTCCTC 3′ | 398 | NM_031144.2 |
| Anti-sense | 5′ CTCCTGCTTGCTGATCCACAT 3′ | |||
Statistics
All experiments were repeated 6 or 7 times. Data were analyzed with two-tail t-test using Excel software (Microsoft, USA). Differences were considered significant when p < 0.05.
Results
Characterization of MSCs and morphology of tenocytes
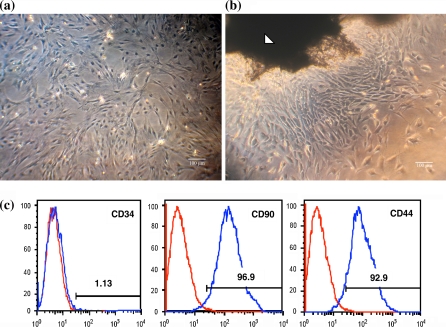
The isolated MSCs were homogeneously adhered histoleucocytes and expanded rapidly in L-DMEM medium with 10% FBS. The majority morphology of cultured MSCs was narrow spindle-shaped (Fig. 1a). MSCs showed fingerprint-like orientation when achieving confluence. MSCs express a number of surface markers, however none of which is specific for MSCs. It is generally agreed that MSCs do not express the hematopoietic makers such as CD34, CD45, CD14 or CD11, and do express CD44, CD90, CD29, CD73 and CD105 (Chamberlain et al. 2007). Thus, to identify the characteristics of rat bone marrow derived MSCs, the expression of three surface antigens, CD34, CD44 and CD90 was detected by flow cytometry. 92.9% of MSCs expressed CD44 and 96.9% expressed CD90 while only 1.13% expressed CD34 (Fig. 1c). Meanwhile, the expression of these antigens did not vary much between the different passages before passage five, neither between different donors (data not shown). The multipotent differentiation ability of MSCs was also confirmed with cells at the third passage by successful differentiation into osteoblasts, adipocytes using the published protocols (data not shown).
Fig. 1.
Morphology of MSCs at P3 passage (a) and tenocytes at P0 passage (b) observed by inverted phase contrast microscope (Scale bar represents 100 μm). The white arrow indicates a piece of tendon tissue for primary tenocyte culture. c Flow cytometry determination of MSCs surface markers of CD34, CD44 and CD90. The percentage of positive population for each antibody is shown in the corresponding histogram
The primary tenocyte colonies formed around the tissue pieces within 10 days and the cells presented a typical long fusiform and homogeneous morphology (Fig. 1b).
Proliferation of MSCs co-cultured with tenocytes
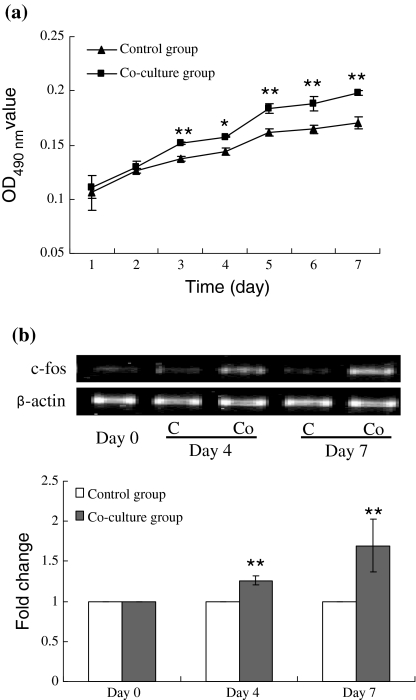
To clarify whether co-culture of MSCs and full differentiated tenocytes can stimulate the expansion of MSCs, the proliferation of MSCs was detected by both hemocytometer and MTT assay every day from day 1 to day 7. A parallel group without tenocytes seeded on the inserted chamber was set as control. The MTT results showed that there was no significant growth difference between control and co-culture group at the first 2 days of co-culture. After day 3, MSCs in co-culture group grew faster than control and a significant difference was found (Fig. 2a).
Fig. 2.
Effect of co-culture on the proliferation and c-fos gene expression of MSCs. a Expansion of MSCs with or without co-culture with tenocytes measured by MTT assay (n = 6); b Fold change of c-fos expression of MSCs in co-culture group compared to control group (n = 7). Band intensity was analyzed by Quantity-one software and the relative expression of c-fos gene was normalized to β-actin. * p < 0.05, ** p < 0.01. C control group; Co co-culture group
c-fos, a member of a family of intermediate early genes (IEGs), is identified as a proto-oncogene in virology research and the expression of which plays an important role in cell proliferation (Ovitt and Ruther 1989; Angle and Karin 1991). c-fos was also believed to be associated with the activation of protein mediating cell growth (Karin 1995). The expression of c-fos gene was assayed by semi-quantitative RT-PCR at day 0, day 4 and day 7, respectively, and the relative expression of c-fos was normalized to β-actin. Fold changes of expression were counted by normalizing to the relative expression amount of corresponding control groups. The result showed that the expression of c-fos gene was up-regulated after co-culture with tenocytes for 4 and 7 days, which were 1.2 and 1.6 folds higher than that of control (Fig. 2b).
mRNA expression of tendon/ligament related genes in MSCs co-cultured with tenocytes
TNC and SCX, especially SCX, have been recently reported to be the tendon specific markers which were higher expressed in tenocytes or ligament cells than in other fibroblasts (Schweitzer et al. 2001; Altman et al. 2002; Oldfield and Evans 2003). SCX has been illustrated as the specific marker for tendon development and expressed in earlier tendon progenitor cells and continuously expressed through tendon development (Schweitzer et al. 2001). Col I and Col III, are the main collagens found in connective tissues matrix (Liu et al. 1995), hence their up-regulation of expression are considered as primary reference for tenogenic differentiation.
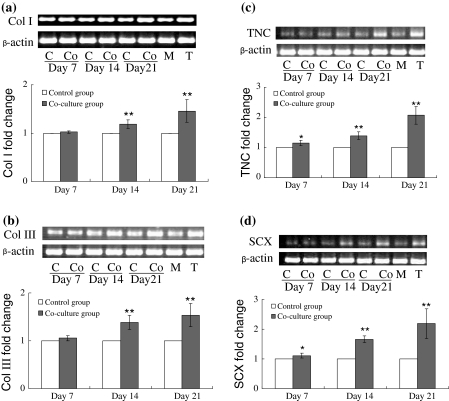
The mRNA expression of Col I, Col III, SCX and TNC was detected by semi-quantitative RT-PCR. In primary tenocytes, these genes, especially TNC and SCX, were expressed at a higher level than in MSCs (Fig. 3). To clarify the expression change of Col I, Col III, SCX and TNC in MSCs with or without co-culture with tenocytes, semi-quantitative RT-PCR was performed to investigate the mRNA expression on day 7, day 14 and day 21. After co-culture of 7 days, Col I and Col III expression in MSCs was not significantly changed, while the two tendon specific genes, TNC and SCX, were found significantly up-regulated. When detected on day 14 and day 21 of co-culture, the relative expression of the four genes was significant higher than control groups. Especially, the expression of TNC and SCX was shown more than twofold increased (Fig. 3).
Fig. 3.
Fold change of Col I (a), Col III (b), TNC (c) and SCX (d) expression after 7, 14 and 21 days of co-culture with tenocytes. The gene expression at mRNA level was detected by semi-quantitative RT-PCR. Band intensity was analyzed by Quantity-one software and the relative expression of each gene was normalized to β-actin. n = 7, * p < 0.05, ** p < 0.01. C control group; Co co-culture group; M MSCs; T tenocytes
Discussion
To take MSCs into tendon tissue engineering use, how to vast expand and differentiate MSCs into tenocytes are the basic issues which need to be considered. It has been illustrated that growth factors such as FGF-2, FGF-4, BMP-2, EGF, PDGF, promoted the proliferation of in vitro cultured MSCs (Martin et al. 1997; Liu et al. 2009; Choi et al. 2008; Tamama et al. 2006). Meanwhile, when co-cultured with ligament cells (Mizuno et al. 2008) or vascular endothelial cells (Sun et al. 2007), the proliferation of MSCs was elevated which was believed due to the humoral factors released from the co-culture cells. Our previous researches also elucidated cyclic mechanical stretch enhanced the proliferation of rat and human bone marrow derived MSCs (Song et al. 2007a, b). However, the effect on the proliferation of MSCs when they were subjected to the co-culture condition with tenocytes, was poorly described. In this study, we showed that after co-culture for 3 days, the expansion of MSCs was significantly faster than those without co-culture. Moreover, c-fos gene, a proto-oncogene and its up-regulation was believed to be the symbol of the promotion of cell proliferation, was found up-regulated on day 4 and day 7. These results indicated that with the dose-cumulation of factors released from the co-cultured tenocytes, MSCs were stimulated to proliferate faster comparing to those without co-culture. The expression of c-fos was also enhanced from day 1 to day 4 due to the dose-cumulation of factors released from tenocytes, and the factors kept c-fos continuously expressed at a higher level than that for the control group from day 4 to day 7.
Yet, little is known about the critical factors promoting MSCs expansion in the co-culture system. Tendon/ligament cells were reported to secrete a number of growth factors when healing or stimulated, such as IGF, TGF-β, FGF-2 and PDGF (Lyngstadaas et al. 2001; Dahlgren et al. 2005), among which FGF-2, PDGF and BMP-2, a member of TGF-β superfamily, have been illustrated to promote MSCs division in vitro (Martin et al. 1997; Liu et al. 2009; Choi et al. 2008; Tamama et al. 2006). In our co-culture system, the exact factors playing the critical role in MSCs proliferation need to be further clarified.
MSCs transplantation was found to be an effective strategy to accelerate tendon regeneration in many literature references (Ouyang et al. 2003, 2004; Juncosa-Melvin et al. 2005, 2006; Awad et al. 2003), which suggested that contact or crosstalk of MSCs and neighboring cells influenced the proliferation and differentiation of MSCs. The humoral factors released form the neighboring cells were probably responsible for these changes. Co-culture system allowed the contact of different cell types in vitro which ideally simulated the coexisting microenvironment of cells in vivo, thereby made it possible to investigate the molecular events during the crosstalk and made cell fate determination more controllable than directly injecting MSCs into tissues. The differentiation of MSCs affected by full differentiated cells in direct or indirect co-culture systems were investigated by a number of groups and differentiation of MSCs were achieved or promoted (Lange et al. 1999; Lee et al. 2007; Csaki et al. 2009; Chen et al. 2009; Wang et al. 2006; Ball et al. 2004). However, little is known about the proliferation and commitment of MSCs subjected to co-culture conditions with primary tenocytes.
Formerly, the lack of attention given to tendons/ligaments genesis can be largely attributed to the absence of specific early molecular markers for these tissues. Recently, scleraxis, a basic helix-loop-helix transcription factor, was discovered specifically associated with embryonic tendon and ligament development (Schweitzer et al. 2001). In addition, TNC, an extracellular matrix glycoprotein, was reported to be expressed in adult tendon-associated tissues. Thus, the expression of SCX and TNC is considered as the marker of tenogenic differentiation in vitro (Schweitzer et al. 2001; Hsia and Schwarzbauer 2005; Chiquet-Ehrismann and Tucker 2004). Though, it was illuminated later that in some fibroblasts these two genes were slightly expressed, the expression level in tenocytes was much higher (Pak et al. 2003; Seo et al. 2004). On the other hand, Collagen types I, III, V, IX, X, XI, XII and XIV are known or thought to be involved in bone, tendon or ligament healing, especially Col I and Col III are the main collagens found in connective tissues, such as tendon and ligament. Thus, the up-regulation of these two genes is also believed to be the symbol of tenogenic differentiation. Our study elucidated that after 14 days of indirect co-culture with tenocytes, the tendon-related genes, including Col I, Col III, TNC and SCX, were significantly up-regulated, though no significant changes of Col I and Col III were detected on day 7 of co-culture. The expression of these genes was even more increased after 21 days of co-culture, with 1.3-fold change for Col I, 1.5-fold change for Col III and more than twofold change for TNC and SCX than seen in control MSCs.
It should be noted that due to the lack of commercial antibody reacting with rat SCX, we did not explore the expression of this tendon/ligament related gene at a protein level. This is a limitation of this paper since combination of expression of those tendon/ligament related genes at mRNA level by RT-PCR and protein level by immunocytochemistry or western blot are important to obtain a clearer insight.
In our experiments, indirect co-culture was performed with an inserted chamber, hereby MSCs and tenocytes were separated by the membrane of the inserted chamber, and only soluble factors were allowed to diffuse. Though direct contact was reported to be inevitable for differentiation in some of the published studies (Wang et al. 2006; Ball et al. 2004), in our study, the mRNA expression of tenocyte related genes was up-regulated in the absence of direct contact of MSCs and tenocytes, which suggested that direct contact of MSCs and tenocytes was not indispensable for MSCs differentiation. However, it is interesting to study whether direct contact of MSCs and tenocytes promote tenogenic differentiation with higher efficiency. In our experiments, we showed the tenogenic differentiation of MSCs was triggered by the soluble factors in the co-culture system but the critical factors resulting in the differentiation remained unclarified, which is a worthwhile work for our future study.
In summary, we investigated the effect of rat MSCs and tenocyte co-culture microenvironment on the proliferation and tenogenic differentiation of MSCs. We showed that MSCs expansion was promoted after 3 days of co-culture with tenocytes and proliferation-related c-fos gene expression was enhanced after 4 days of co-culture. Meanwhile, semi-quantitative RT-PCR showed that the tenogenic differentiation related genes, Col I, Col III, SCX and TNC, continuously up-regulated after 7 days of co-culture with tenocytes, which suggested the tenogenic differentiation of MSCs.
Acknowledgments
This work was supported by grants from Natural National Science Foundation of China (No. 30770530), Chongqing Natural Science Foundation (CSTC-2009BB5040) and the 111 Project (No. 06023).
References
- Aaron RK, Ciombor DM (1996) Acceleration of experimental endochondral ossification by biophysical stimulation of the progenitor cell pool. J Orthop Res 14:582–589. doi:10.1002/jor.1100140412 [DOI] [PubMed]
- Altman GH, Horan RL, Martin I, Farhadi J, Stark PR, Volloch V, Richmond JC, Vunjak-Novakovic G, Kaplan DL (2002) Cell differentiation by mechanical stress. FASEB J 16:270–272. doi:10.1096/fj.01-0656fje [DOI] [PubMed]
- Angle P, Karin M (1991) The role of Jun, Fos and the AP-1 complex in cell proliferation and transformation. Biochim Biophys Acta 1072:129–157. doi:10.1016/0304-419X(91)90011-9 [DOI] [PubMed]
- Awad HA, Boivin GP, Dressler MR, Smith FN, Young RG, Butler DL (2003) Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res 21:420–431. doi:10.1016/S0736-0266(02)00163-8 [DOI] [PubMed]
- Ball SG, Shuttleworth AC, Kielty CM (2004) Direct cell contact influences bone marrow mesenchymal stem cell fate. Int J Biochem Cell Biol 36:714–727. doi:10.1016/j.biocel.2003.10.015 [DOI] [PubMed]
- Brazelton TR, Rossi FM, Keshet GI, Blau HM (2002) From marrow to brain: expression of neuronal phenotypes in adult mice. Science 290:1775–1779. doi:10.1126/science.290.5497.1775 [DOI] [PubMed]
- Caplan AI (2007) Adult mesenchymal stem cell for tissue engineering versus regenerative medicine. J Cell Physiol 213:341–347. doi:10.1002/jcp.21200 [DOI] [PubMed]
- Chamberlain G, Fox J, Ashton B, Middleton J (2007) Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem cells 25:2739–2749. doi:10.1634/stemcells.2007-0197 [DOI] [PubMed]
- Chen YJ, Huang CH, Lee IC, Lee YT, Chen MH, Young TH (2008) Effects of cyclic mechanical stretching on the mRNA expression of tendon/ligament-related and osteoblast-specific genes in human mesenchymal stem cells. Connect Tissue Res 49:7–14. doi:10.1080/03008200701818561 [DOI] [PubMed]
- Chen WH, Lai MT, Wu AT, Wu CC, Gelovani JG, Lin CT, Hung SC, Chiu WT, Deng WP (2009) In vitro stage-specific chondrogenesis of mesenchymal stem cells committed to chondrocytes. Arthritis Rheum 60:450–459. doi:10.1002/art.24265 [DOI] [PubMed]
- Chiquet-Ehrismann R, Tucker RP (2004) Connective tissues: signalling by tenascins. Int J Biochem Cell Biol 36:1085–1089. doi:10.1016/j.biocel.2004.01.007 [DOI] [PubMed]
- Choi SC, Kim SJ, Choi JH, Park CY, Shim WJ, Lim DS (2008) Fibroblast growth factor-2 and -4 promote the proliferation of bone marrow mesenchymal stem cells by the activation of the PI3K-Akt and ERK1/2 signaling pathways. Stem Cells Dev 17:725–736. doi:10.1089/scd.2007.0230 [DOI] [PubMed]
- Csaki C, Matis U, Mobasheri A, Shakibaei M (2009) Co-culture of canine mesenchymal stem cells with primary bone-derived osteoblasts promotes osteogenic differentiation. Histochem Cell Biol 131:251–266. doi:10.1007/s00418-008-0524-6 [DOI] [PubMed]
- Dahlgren LA, Mohammed HO, Nixon AJ (2005) Temporal expression of growth factors and matrix molecules in healing tendon lesions. J Orthop Res 23:84–92. doi:10.1016/j.orthres.2004.05.007 [DOI] [PubMed]
- Farng E, Urdaneta AR, Barba D, Esmende S, McAllister DR (2008) The effects of GDF-5 and uniaxial strain on mesenchymal stem cells in 3-D culture. Clin Orthop Relat Res 466:1930–1937. doi:10.1007/s11000-00800300-X [DOI] [PMC free article] [PubMed]
- Hankemeier S, Keus M, Zeichen J, Jagodzinski M, Barkhausen T, Bosch U, Krettek C, Van Griensven M (2005) Modulation of proliferation and differentiation of human bone marrow stromal cells by fibroblast growth factor 2: potential implications for tissue engineering of tendons and ligaments. Tissue Eng 11:41–49. doi:10.1089/ten.2005.11.41 [DOI] [PubMed]
- Hsia HC, Schwarzbauer JE (2005) Meet the tenascins: multifunctional and mysterious. J Biol Chem 280:26641–26644. doi:10.1074/jbc.R500005200 [DOI] [PubMed]
- Juncosa-Melvin N, Boivin GP, Galloway MT, Gooch C, West JR, Sklenka AM, Butler DL (2005) Effects of cell-to-collagen ratio in mesenchymal stem cell-seeded implants on tendon repair biomechanics and histology. Tissue Eng 11:448–457. doi:10.1089/ten.2005.11.448 [DOI] [PubMed]
- Juncosa-Melvin N, Shearn JT, Boivin GP, Gooch C, Galloway MT, West JR, Nirmalanandhan VS, Bradica G, Butler DL (2006) Effects of mechanical stimulation on the biomechanics and histology of stem sell—collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng 12:2291–2300. doi:10.1089/ten.2006.12.2291 [DOI] [PubMed]
- Karin M (1995) The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 270:16483–16486 [DOI] [PubMed]
- Krampera M, Marconi S, Pasini A, Galiè M, Rigotti G, Mosna F, Tinelli M, Lovato L, Anghileri E, Andreini A, Pizzolo G, Sbarbati A, Bonetti B (2007) Induction of neural-like differentiation in human mesenchymal stem cells derived from bone marrow, fat, spleen and thymus. Bone 40:382–390. doi:10.1016/j.bone.2006.09.006 [DOI] [PubMed]
- Kuo CK, Tuan RS (2008) Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A 14:1615–1627. doi:10.1089/ten.tea.2006.0415 [DOI] [PubMed]
- Lange C, Bassler P, Lioznov MV, Bruns H, Kluth D, Zander AR, Fiegel HC, Woo SL, Hidebrand K, Watanabe N, Fenwick JA, Papageorgiou CD, Wang JH (1999) Tissue engineering of ligament and tendon healing. Clin Orthop Relat Res Suppl 367:312–323 [DOI] [PubMed]
- Lee IC, Wang JH, Lee YT, Young TH (2007) The differentiation of mesenchymal stem cells by mechanical stress or/and co-culture system. Biochem Biophys Res Commun 352:147–152. doi:10.1016/j.bbrc.2006.10.170 [DOI] [PubMed]
- Liu SH, Yang RS, al-Shaikh R, Lane JM (1995) Collagen in tendon, ligament and bone healing. Clin Orthop Relat Res 318:265–278 [PubMed]
- Liu SB, Hu PZ, Hou Y, Li P, Cao W, Tian Q (2009) Recombinant human bone morphogenetic protein-2 promotes the proliferation of mesenchymal stem cells in vivo and in vitro. Chin Med J (Engl) 122:839–843 [PubMed]
- Lyngstadaas SP, Lundberg E, Ekdahl H, Andersson C, Gestrelius S (2001) Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative. J Clin Periodontol 28:181–188. doi:10.1034/j.1600-051x.2001.028002181.x [DOI] [PubMed]
- Martin I, Muraglia A, Campanile G, Cancedda R, Quarto R (1997) Fibroblast growth factor-2 supports ex vivo expansion and maintenance of osteogenic precursors from human bone marrow. Endocrinology 138:4456–4462 [DOI] [PubMed]
- Mizuno N, Ozeki Y, Shiba H, Kajiya M, Nagahara T, Takeda K, Kawaguchi H, Abiko Y, Kurihara H (2008) Humoral factors released from human periodontal ligament cells influence calcification and proliferation in human bone marrow mesenchymal stem cells. J Periodontol 79:2361–2370. doi:10.1902/jop/2008.070577 [DOI] [PubMed]
- Oldfield SF, Evans DJR (2003) Tendon morphogenesis in the developing avian limb: plasticity of fetal tendon fibroblasts. J Anat 202:153–164. doi:10.1046/j.1469-7580.2003.00145.x [DOI] [PMC free article] [PubMed]
- Ouyang HW, Goh JC, Thambyah A, Teoh SH, Lee EH (2003) Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit achilles tendon. Tissue Eng 9:431–439. doi:10.1089/107632703322066615 [DOI] [PubMed]
- Ouyang HW, Goh JC, Lee EH (2004) Viability of allogeneic bone marrow stromal cells following local delivery into patella tendon in rabbit model. Cell Transplant 13:649–657 [DOI] [PubMed]
- Ouyang HW, Cao T, Zou XH, Heng BC, Wang LL, Song XH, Huang HF (2006) Mesenchymal stem cell sheets revitalize nonviable dense grafts: implications for repair of large-bone and tendon defects. Transplantation 82:170–174. doi:10.1097/01.tp.0000226232.79106.72 [DOI] [PubMed]
- Ovitt CE, Ruther U (1989) The proto-oncogene c-fos: structure, expression, and functional aspects. Oxf Surv Eukaryot Genes 6:33–51 [PubMed]
- Pak HN, Qayyum M, Kim DT, Hamabe A, Miyauchi Y, Lill MC, Frantzen M, Takizawa K, Chen LS, Fishbein MC, Sharifi BG, Chen PS, Makkar R (2003) Mesenchymal stem cell injection induces cardiac nerve sprouting and increased tenascin expression in a Swine model of myocardial infarction. J Cardiovasc Electrophysiol 14:841–848. doi:10.1046/j.1540-8167.2003.03124.x [DOI] [PubMed]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147. doi:10.1126/science.284.5411.143 [DOI] [PubMed]
- Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ (2001) Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128:3855–3866 [DOI] [PubMed]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149–155. doi:10.1016/S0140-6736(04)16627-0 [DOI] [PubMed]
- Song G, Luo Q, Qin J, Wang B, Cai S (2004) Expression of integrin beta1 and its roles on adhesion between different cell cycle hepatocellular carcinoma cells (SMMC-7721) and human umbilical vein endothelial cells. Colloids Surf B Biointerfaces 34:247–252. doi:10.1016/j.colsurfb.2004.01.009 [DOI] [PubMed]
- Song G, Ju Y, Soyama H, Ohashi T, Sato M (2007a) Regulation of cyclic longitudinal mechanical stretch on proliferation of human bone marrow mesenchymal stem cells. Mol Cell Biomech 4:201–210 [PubMed]
- Song G, Ju Y, Shen X, Luo Q, Shi Y, Qin J (2007b) Mechanical stretch promotes proliferation of rat bone marrow mesenchymal stem cells. Colloids Surf B Biointerfaces 58:271–277. doi:10.1016/j.colsurfb.2007.04.001 [DOI] [PubMed]
- Sun H, Qu Z, Guo Y, Zang G, Yang B (2007) In vitro and in vivo effects of rat kidney vascular endothelial cells on osteogenesis of rat bone marrow mesenchymal stem cells growing on polylactide-glycoli acid (PLGA) scaffolds. Biomed Eng Online 6:41. doi:10.1186/1475-925X-6-41 [DOI] [PMC free article] [PubMed]
- Tamama K, Fan VH, Griffith LG, Blair HC, Wells A (2006) Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells 24:686–695. doi:10.1634/stemcells.2005-0176 [DOI] [PubMed]
- Violini S, Ramelli P, Pisani LF, Gorni C, Mariani P (2009) Horse bone marrow mesenchymal stem cells express embryo stem cell marker and show the ability for tenogenic differentiation by in vitro exposure to BMP-12. BMC Cell Biol 10:29. doi:10.1186/1471-2121-10-29 [DOI] [PMC free article] [PubMed]
- Wang T, Xu Z, Jiang W, Ma A (2006) Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. Int J Cardiol 109:74–81. doi:10.1016/j.ijcard.2005.05.072 [DOI] [PubMed]
- Woo SL, Hidebrand K, Watanabe N, Fenwick JA, Papageorgiou CD, Wang JH (1999) Tissue engineering of ligament and tendon healing. Clin Orthop Relat Res 367(Suppl):S312–S323 [DOI] [PubMed]