Abstract
Primary culture of smooth muscle cells has been widely used as a valuable tool to study the molecular mechanisms underlying atherosclerosis and restenosis. Currently, tissue explants and enzymatic digestion methods are frequently applied to produce smooth muscle cells. Explants method is time consuming, usually taking several weeks. The enzymatic digestion method requires large amounts of proteolytic enzymes to generate enough cells for cardiovascular research. The present study reports an optimized method by combining both techniques to obtain high purity smooth muscle cells. The cultured cells exhibited the characteristic “hills and valleys” growth pattern as observed by phase contrast microscopy and showed α-SM-actin positive staining by indirect immunocytochemistry and immunofluorescence. Purity of the cells is guaranteed by the lack of von Willebrand Factor immunoreactivity. Finally, the cultured cells well proliferate on oxidized-LDL stimulation, suggesting the practical utility of this new method.
Keywords: Smooth muscle cell, Cell culture, Thoracic aorta, Protocol
Introduction
Vascular smooth muscle cells (VSMCs) are the only cell type present in the blood vessel wall of mammalian arteries. It must be therefore responsible for maintaining artery wall tension via contraction/relaxation and vascular remodeling (Chamley-Campbell et al. 1982). They are thus important for cardiovascular research such as atherosclerosis (Ray et al. 2001). A dominant feature of atherogenesis is the proliferation and migration of VSMCs into the intima of the arterial wall. In addition, another characteristic event at the atherosclerotic lesion is cholesterol accumulation and transformation of macrophages and VSMCs into foam cells (Strauss et al. 1996; Campbell and Campbell 1994; Zhou et al. 1996). Thus, primary culture of VSMCs provides a useful tool to study the molecular mechanisms of atherosclerotic diseases.
VSMCs are mainly derived from rat mesenteric arteries (Gunther et al. 1982), human umbilical cord arteries (Okker-Reitsma et al. 1985), human saphenous vein (Bond et al. 2001), coronary artery (Julia et al. 2007), rat and bovine thoracic aorta (Szöcs et al. 2007; Bellas et al. 1998) and so on. To the best of our knowledge, the techniques routinely used for high-yield isolation of vertebrate smooth muscle cells are explant method and enzymatic digestion method, which were originally described by Ross (1971) and Chamley et al. (1977), respectively. The biggest advantage of the explant method is that it is very simple and cost-effective. But the limitations are obvious; generally, several weeks are needed for the VSMCs to be sub-cultured (Koba et al. 1999). In addition, adventitial fibroblasts and intimal endothelial cells are often cross contaminants when this method is used. As for the enzymatic digestion method, it requires surgical dissection of media of artery and large amounts of proteolytic enzymes (elastase, collagenase) and soybean trypsin inhibitor (Campbell and Campbell 1994; Schwertschlag and Whorton 1988) to digest aorta segments, and thus is expensive. In addition, isolated VSMCs were reported to grow confluent after 3–6 days (Schwertschlag and Whorton 1988; Christen et al. 1999). Insufficient digestion resulted in low cell yield, whereas over-digestion subsequently causes non-attachment or weak contraction and cell viability of VSMCs (Gunther et al. 1982).
Therefore, in the present study, we describe an optimized protocol to isolate highly pure primary VSMCs which have the ability to proliferate upon stimulation. The whole process for primary culture is condensed to about 8 days and the purity of third passage cells can be as high as 95%.
Materials and methods
Materials
Tissue culture medium: Dulbeco’s Modified Eagle’s Medium (DMEM, Gibco), supplemented with 20% (v/v) heat-inactivated fetal bovine serum (Hang Zhou Sijiqing, 080219), 100U/mL Penicillin and 100 μg/mL Streptomycin (Gibco, 08022701), 2 mmol/L Glutamine (MBCHEM, M11033), pH 7.4
Digestion solution: freshly prepared 0.25% trypsin solution (Sigma, T8003), pH 7.4.
PBS solution (Wuhan Boster Biological Technology, AR0030), pH 7.4.
Oxidized-LDL (Ox-LDL) preparation: Ox-LDL was prepared as previously described (Chien et al. 2003), and the extent of LDL oxidation was determined by measuring thiobarbituric acid-reactive substances (TBARS) and relative electrophoretic mobility on agarose gels. The protein content of Ox-LDL was determined by BCA assay kit (Pierce). All preparations were filter sterilized (0.22 μm, Millipore, Billerica MA, USA), stored under an N2 atmosphere and used within 3 weeks. Endotoxin (LPS) levels were tested using the LAL (Limulus Amoebocyte Lysate) assay kit (Bio Whittaker, Walkersville, Md). Preparations containing less than 50 pg/mg of LPS were used for the experiments.
Sixty millimeter plastic petri dishes, 96-well microplates and T25 tissue culture flasks were from Costar (Costar Data Packaging, Cambridge, MA).
Antibodies: mouse anti-α-SM-actin primary antibody (BM0002, Wuhan Boster Biological Technology, China) was diluted to 1:200 or 1:400 with 0.5% BSA (made in PBS) for immunocytochemical and immunofluorescent studies, respectively. Anti- von Willebrand Factor antibody (BA0046, Boster) was diluted to 1:400 with 0.5% BSA (in PBS) then stored at 4 °C until use.
Streptavidin Biotin Complex (SABC) kit was purchased from Boster, Wuhan/China (SA1021).
FITC-conjugated goat anti-mouse secondary antibody was from Santa Cruz Biochem, CA/USA.
Procedures
Preparation of smooth muscle cell cultures
Anesthetization and sterilization: One 2-month-old Sprague–Dawley Rat (Experimental Animal Center of Sun Yat-sen University, Guangzhou, China) weighing 150 g was maintained on standard laboratory chow and tap water ad libitum, and was sacrificed by cervical dislocation, in accordance with Institutional Animal Care and Use Committee. The animal was decontaminated with 75% ethanol thoroughly, and transferred to a Laminar flow hood immediately.
Thoracic aorta obtaining: The chest was opened with sterilized forceps and scissors, and then the en bolc thoracic aorta was excised (about 1.5 cm long) quickly.
Dissection (performed with ice bath): The aorta segment was cleaned of fatty tissues immediately, and placed in a Petri dish containing ice-cold Ca2+ and Mg2+-free PBS buffer. After draining of blood by injecting fresh PBS into the lumen with a 1 mL sterilized syringe, the adventitial layer and outer portion of the media were carefully excised by straining the aorta oppositely with straight and angled forceps (the angled forceps to hold down the aorta and the straight forceps to peel off bits of tissue), guaranteeing to gain a cleaned “smooth” aorta. The whole aorta was rinsed with ice-cold PBS for 3 times, and then transferred to warmed DMEM supplemented with 20% FBS and washed 3 times. The aorta was cut open longitudally, and the endothelium was gently scrapped off with finely toothed forceps or a scalpel and minced with fine micro-dissecting scissors into approximately 1 mm2 squares.
Trypsinization: Following dissection procedures, the tissue pieces were digested in a conical shaker flask (Costar) with constantly stirring (150–200 rpm, IKA, Germany) at 37 °C for 8 min.
Repeating trypsinization: After trypsinization, endothelial cells and fibroblasts were dispersed from the tissue. The tissue pieces were then subjected to a second digestion.
Inoculation: Resulting suspensions were discarded from each digestion procedure. The digestion of the tunica was terminated by adding DMEM medium supplemented with 20% FBS. The undigested tissue pieces were evenly distributed into a T25 tissue culture flask (about 15 pieces per aorta, with a distance of 1 cm between individual pieces, no medium added into the flask), which was put upright at 37 °C into a CO2-incubator with a humidified atmosphere (5% CO2, 95% air, Thermo, USA) in order to facilitate the attachment of pieces on the surface of the flask.
Firm adherence: 4–6 h after inoculation (to guarantee the tissue pieces to have enough time to adhere firmly to the flask and not to be dislodged), the flask was put horizontally and then 2 mL of DMEM containing 20% FBS was carefully added to the flask.
Cultivation: Generally, about 15 tissue pieces isolated from one aorta segment can be seeded in one T25 tissue culture flask. It is important to avoid disturbing them during the initial 48 h. The culture medium was replenished after 72 h and thereafter every 48 h.
Cell viability evaluation
At passage 3, the number and viability of cultured cells were determined using a hemocytometer and the trypan blue (0.4%, Sigma) dye exclusion assay as previously described (Walford et al. 1964).
Immunocytochemical staining assay
Since α-SM-actin is regarded as a specific and well-recognized differentiation marker of VSMCs (Hu et al. 2003), a mouse monoclonal anti-α-SM-actin antibody (1:200, Boster) was applied as the primary antibody to identify VSMCs in the culture. A goat anti-mouse biotinylated immunoglobulin conjugated with avidin-biotinylated horseradish peroxidase was used as the secondary antibody, followed by SABC staining according to the manufacturer’s instructions with minor modifications. For the analysis of endothelial cell elimination, a primary antibody against von Willebrand Factor (vWF) was also applied as a negative control.
Immunofluorescent staining assay
To further identify the purity of VSMCs cultured with the optimized method, immunofluorescent staining against α-SM-actin was performed. Briefly, cells were incubated with mouse monoclonal antibody against α-SM-actin (1:200, Boster) at 4 °C overnight. After immuno-reaction with FITC-conjugated goat anti-mouse secondary antibody (1:50, Santa Cruz Biotechnology), cells were rinsed with PBS, and the nuclei were counter-stained with Hoechst 33342 (5 μg/mL, Sigma). The number of α-SM-actin positively stained cells was counted under an immunofluorescent microscope (Olympus, Japan).
Western blot analysis
Total protein extracted from different batches of cultured VSMCs were lysed on ice for 15 min with lysis buffer that contained protease inhibitor cocktail (Sigma) then centrifuged at 14,000× g at 4 °C for 20 min, and the supernatant was collected and aliquoted. The protein concentration was determined with a BCA assay kit according to the manufacturer’s instructions (Pierce). Equal amounts of total protein (30–50 μg) were subjected to SDS/PAGE under reducing conditions and then electrotransferred to polyvinylidene difluoride membrane (Millipore, Billerica, MA). The efficacy of protein loading and transfer to membranes was also assessed by Ponceau S staining. The membranes were blocked with 5% nonfat milk (Bio-rad) in Tris-Buffered Saline-0.05%Tween 20 (TBST) for 1 hour at room temperature and incubated with primary antibody against α-SM-actin overnight at 4 °C, then incubated with a horseradish peroxidase–conjugated secondary antibody (1:2000). Blots were visualized by a chemiluminescence detection system (SuperSignal West detection kit, Pierce). The maximal intensity of each band was quantified densitometrically using a high-resolution Gel Doc 2000 image analysis system and Quantity One software, Version 4.1.1 (Bio-Rad, Hercules, CA, USA).
MTT assay
Third passage VSMCs were counted and seeded into 96-well culture plates at a density of 20,000 cells/well. After 24 h, the medium was changed to serum-free DMEM to make them quiescent for 24 h. Cells were subsequently stimulated with different doses of Ox-LDL (0, 25, 50, 80, 100 μg/mL) for 24 h. Cell proliferation was assayed by MTT method as previously described with minor modifications (Yang et al. 2008; Fan et al. 2008). Briefly, a volume of 10 μL of 5 mg/mL MTT in PBS was added to each well and incubated for 4 h. Formazan crystals were dissolved in 150 μL of dimethyl sulfoxide (DMSO) and the absorbance was measured at a wavelength of 570 nm with an ELISA reader (BioRad 3550, Bio-Rad Laboratories).
Statistical analysis
All values were expressed as Mean ± SEM for three independent experiments. Data were analyzed by 2-tailed unpaired Student t test between two groups and by 1-way ANOVA followed by Bonferroni post hoc test when >2 groups were involved. p < 0.05 were considered statistically significant.
Results and discussion
The abnormal proliferation and migration of VSMCs play a pivotal role in the progression of atherosclerosis and development of restenosis as well as neo-intima formation after balloon surgery (Cary and Guan 1999; Schwartz et al. 1995; Newby and Zaltsman 2000; Nakagawa et al. 2007; Mnjoyan et al. 2008). In the current report, we excised the thoracic aorta first, and then removed adventitia and intima mechanically. The aorta was then cut into several pieces and subjected to enzymatic digestion to remove adventitia and intima as much as possible to avoid contamination with endothelial cells and fibroblasts. After that, the tissue pieces were re-plated and distributed evenly in the flask. Then the flask was put vertically, preventing the close contact of tissue segments with culture medium and thus allowing that the segments can firmly adhere to the flask. Finally, the flask was put horizontally after 4–6 h, and then culture medium was added. Compared with other available culture methods, our method not only increases cell yield up to 106 cells per aorta with high viability and insures a purity of more than 95%, but also reduces the primary culture period to 8 days. It does not require special equipment or large amounts of enzymes. In addition, our protocol is more detailed than any published protocol we are able to find in the literature. The comparison of pros and cons of the optimized method and both other methods is presented in Table 1.
Table 1.
The comparison of optimized method with explants and enzymatic digestion method
| Time required for primary culture | Contamination by other cells | Special equipment or reagent | |
|---|---|---|---|
| Optimized method | 8 days | Little | 0.25% Trypsin |
| Explant method | Several weeks | EC, Fb | No |
| Enzymatic method | Within 1 day but takes 3–6 Days for cells to reach confluence |
Little | Trypsin, collagenase, soybean trypsin inhibitor |
EC indicates endothelial cells; Fb indicates fibroblasts
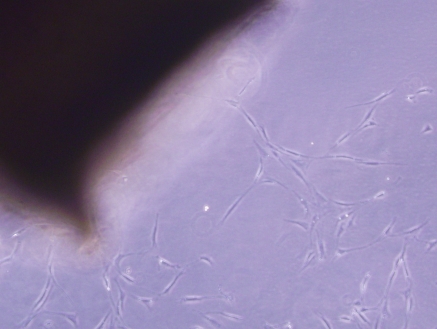
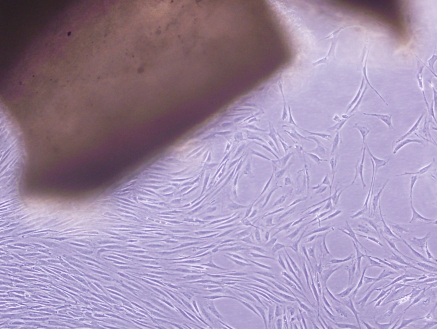
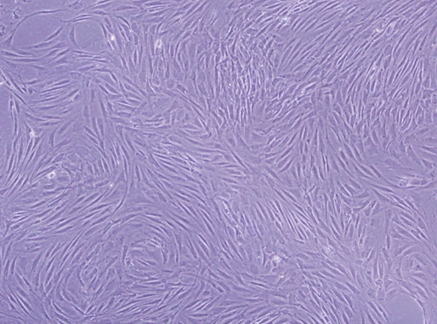
VSMCs were clearly identified after 3 days in culture (Fig. 1) through an inverted phase contrast microscope. VSMCs grew out slowly from explants of aortic tissues and spread to the surroundings, and had a doubling time of about 2 days (data not shown). Uninucleate or some binucleate cells could be observed. Most of the cells had a “ribbon-like” or “spindle” shape. After 5 days in culture, the clawed VSMCs began to contact each other and became sub-confluent (Fig. 2). Cells growing from the explants reached nearly 90% confluence within about 1 week (Fig. 3), appearing as a typical “hills and valleys” arrangement. The tissue blocks were detached and inoculated in another cell culture flask for further culture. The subculture of VSMCs was routinely conducted with trypsin digestion method.
Fig. 1.
VSMCs’ migration from explants of rat thoracic aorta tissue. VSMCs were shown to spread from the aorta after 3 days of cultivation, ×100
Fig. 2.
Phase contrast microscopy of VSMCs after 5 days of cultivation. VSMCs interlaced with each other to form a confluent monolayer, ×100. The figure shows the representative photographs from three independent experiments
Fig. 3.
Characteristic “hills and valleys” morphology of VSMCs from rat thoracic aorta in primary culture. The figure shows the “hills and valleys” growth pattern of VSMCs under light microscopy, ×100
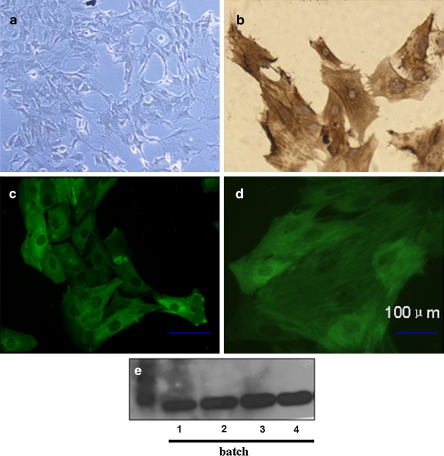
The viability of third passage cells was >96% (n = 6) as assessed by trypan blue exclusion test described in the Method section (data not shown). To demonstrate the purity of cultured VSMCs, we characterized the cells by immunocytochemistry and immunofluorescence using mouse monoclonal anti-α-SM-actin (1:200 and 1:400, respectively, Boster) and appropriate secondary antibodies. Immunostaining revealed that the vast majority of the cells were stained intensely with antibody against α-SM-actin in the cytosol as illustrated by Fig. 4b, c, and d. To confirm, western blot was used to analyze proteins derived from VSMCs whole cell lysates, and a clear band of α-SM-actin was observed (Fig. 4e). The rapid overgrowth and contamination with endothelial cells (EC) is a major problem in primary VSMCs culture systems. In order to exclude the contamination by EC, we conducted immunocytochemical staining of von Willebrand Factor (vWF) and the culture was devoid of vWF immunoreactivity as shown in Fig. 4a. The number of α-SM-actin positive staining cells per 100 cells was counted in three randomly selected fields per slide, and more than 95% positive cells were observed in our culture. We therefore regarded 95% as a conservative estimate of the purity of the smooth muscle cell population in culture.
Fig. 4.
Identification of cultured VSMCs by immunocytochemical, immunofluorescent staining and western blot analysis. a Negative immunocytochemical staining of VSMCs with anti-vWF primary antibody (1:200, Boster), ×100. b Immunocytochemical staining of third passage VSMCs with anti α-SM-actin antibody (1:200, Boster). Most (>95%) of the cells in the culture were positive for α-SM-actin, ×100. c Immunofluorescent staining of α-SM-actin by third passage VSMCs, ×100. Visualization on a fluorescent microscope shows the fibrillar pattern typical of α-smooth muscle actin. d Immunofluorescent staining of α-SM-actin with a magnification of 200. e Western blot of α-SM-actin (cell lysate) from different batches. The figure shows the representative photographs from three independent experiments. Bar = 100 μm
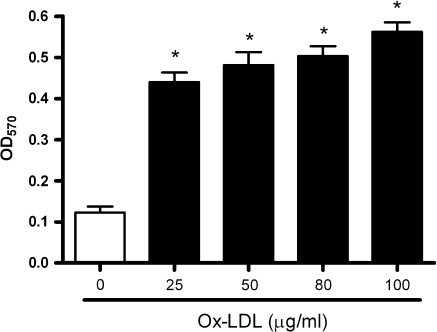
A wide variety of stimuli such as Ox-LDL, basic fibroblast growth factor (bFGF), angiotensin-II (Ang-II), endothelin-1 (ET-1), transforming growth factor-β(TGF-β) can enhance the migration and proliferation of VSMCs (Ross 1993). Among them, Ox-LDL is a potent and physiological pro-atherogenic molecule, and plays a pivotal role in the development of atherosclerotic plaque characterized by proliferation of VSMCs (Witztum 1993). Thus, in the present study, we also tested whether cultured VSMCs can respond to Ox-LDL. Figure 5 shows an example of the utility of this technique. Ox-LDL markedly stimulated the proliferation of VSMCs in a dose dependent manner. This result indicated that the present improved protocol produces high quality VSMCs that can be used in atherosclerosis-related research.
Fig. 5.
Dose dependent effect of oxidized LDL on VSMCs proliferation by MTT assay. VSMCs at passage 4 were cultured in 96-well plates. Growth medium was replaced by serum-free DMEM for 24 h before VMSC cultures were supplemented with increasing concentrations (0–100 μg/mL) of oxidized LDL for another 24 h. Results were from three experiments and each was performed in triplicate. * p < 0.001 versus the control group
Therefore, our optimized protocol presented herein would be useful for in vitro/ex vivo studies on the (patho-) physiology of atherosclerosis-related diseases as well as pharmacological and toxicological targets.
Acknowledgments
This study was supported by research grants from National Natural Science Foundation of China (No: 30472022), and Major Program in Key Field of People’s Government of Guangdong Province (P.R. of China) (No: 2003A30904), and Major Program in Scientific Research of Ministry of Education (P.R. of China) (No: 104146). We are grateful to Dr. Shaorui Chen and Dr. Minfeng Su for expert technical assistance. The authors extend their gratitude to the laboratory personnel for encouragement and support.
Footnotes
Suowen Xu and Jiajia Fu have equally contributed to this work.
References
- Bellas RE, Lee JS, Sonenshein GE (1998) Expression of a constitutive NF-KB-like activity is essential for proliferation of cultured bovine vascular smooth muscle cells. J Clin Invest 96:2521–2527. doi:10.1172/JCI118313 [DOI] [PMC free article] [PubMed]
- Bond M, Chase AJ, Baker AH, Newby AC (2001) Inhibition of transcription factor NF-kappaB reduces matrix metalloproteinase-1, -3 and -9 production by vascular smooth muscle cells. Cardiovasc Res 50:556–565. doi:10.1016/S0008-6363(01)00220-6 [DOI] [PubMed]
- Campbell JH, Campbell GR (1994) The role of smooth muscle cells in atherosclerosis. Curr Opin Lipidol 5:323–330 [DOI] [PubMed]
- Cary LA, Guan JL (1999) Focal adhesion kinase integrin-mediated signaling. Front Biosci 4:102–133 [DOI] [PubMed]
- Chamley JH, Campbell GR, McConnell JD, Gröschel-Stewart U (1977) Comparison of vascular smooth muscle cells from adult human, monkey and rabbit in primary culture and in subculture. Cell Tissue Res 177:503–522 [DOI] [PubMed]
- Chamley-Campbell JH, Campbell GR, Ross R (1982) Phenotype-dependent response of cultured aortic smooth muscle to serum mitogens. J Cell Biol 89:379–383 [DOI] [PMC free article] [PubMed]
- Chien MW, Chien CS, Hsiao LD, Lin CH, Yang CM (2003) OxLDL induces mitogen-activated protein kinase activation mediated via PI3-kinase/Akt in vascular smooth muscle cell. J Lipid Res 44:1667–1675. doi:10.1194/jlr.M300006-JLR200 [DOI] [PubMed]
- Christen T, Bochaton-Piallat ML, Neuville P, Rensen S, Redard M, van Eys G, Gabbiani G (1999) Cultured porcine coronary artery smooth muscle cells. A new model with advanced differentiation. Circ Res 85:99–107 [DOI] [PubMed]
- Fan YH, He CH, Liu GF, Zhang HB (2008) Optimization of the isolation and cultivation of Cyprinus carpio primary hepatocytes. Cytotechnology 58:85–92. doi:10.1007/s10616-008-9169-5 [DOI] [PMC free article] [PubMed]
- Gunther S, Alexander RW, Atkinson WJ, Gimbrone MA Jr (1982) Functional angiotensin II receptors in cultured vascular smooth muscle cells. J Cell Biol 92:289–298 [DOI] [PMC free article] [PubMed]
- Hu WY, Fukuda N, Ikeda Y, Suzuki R, Tahira Y, Takagi H, Matsumoto K, Kanmatsuse K, Mugishima H (2003) Human-derived vascular smooth muscle cells produce angiotensin II by changing to the synthetic phenotype. J Cell Physiol 196:284–292. doi:10.1002/jcp.10299 [DOI] [PubMed]
- Julia K, Angelika K, Sergey T, Krämer J, Haller H, Dietz R, Smith G, Dumler I (2007) Rosuvastatin regulates vascular smooth muscle cell phenotypic modulation in vascular remodeling: role for the urokinase receptor. Atherosclerosis 195:254–261. doi:10.1016/j.atherosclerosis.2006.12.030 [DOI] [PubMed]
- Koba S, Pakala R, Watanabe T, Katagiri T, Benedict CR (1999) Vascular smooth muscle proliferation: synergistic interaction between serotonin and low density lipoproteins. J Am Coll Cardiol 34:1644–1651. doi:10.1016/S0735-1097(99)00349-6 [DOI] [PubMed]
- Mnjoyan ZH, Doan D, Brandon JL, Felix K, Sitter CL, Rege AA, Brock TA, Fujise K (2008) The critical role of the intrinsic VSMC proliferation and death programs in injury-induced neointimal hyperplasia. Am J Physiol Heart Circ Physiol 294:H2276–H2284 [DOI] [PubMed]
- Nakagawa M, Ohno T, Maruyama R, Okubo M, Nagatsu A, Inoue M, Tanabe H, Takemura G, Minatoguchi S, Fujiwara H (2007) Sesquiterpene lactone suppresses vascular smooth muscle cell proliferation and migration via inhibition of cell cycle progression. Biol Pharm Bull 30:1754–1757. doi:10.1248/bpb.30.1754 [DOI] [PubMed]
- Newby AC, Zaltsman AB (2000) Molecular mechanisms in intimal hyperplasia. J Pathol 190:300–309. doi:10.1002/(SICI)1096-9896(200002)190:3<300 [DOI] [PubMed]
- Okker-Reitsma GH, Dziadkowiec IJ, Groot CG (1985) Isolation and culture of smooth muscle cells from human umbilical cord arteries. In Vitro Cell Dev Biol 21:22–25 [DOI] [PubMed]
- Ray JL, Leach R, Herbert JM, Benson M (2001) Isolation of vascular smooth muscle cells from a single murine aorta. Methods Cell Sci 23:185–188 [DOI] [PubMed]
- Ross R (1971) The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol 50:172–186 [DOI] [PMC free article] [PubMed]
- Ross R (1993) The pathogenesis of atherosclerosis: perspective for the 1990 s. Nature 362:801–809 [DOI] [PubMed]
- Schwartz SM, deBlois D, O’Brien ER (1995) The intima. Soil for atherosclerosis and restenosis. Circ Res 77:445–465 [DOI] [PubMed]
- Schwertschlag US, Whorton AR (1988) Platelet-activating factor-induced homologous and heterologous desensitization in cultured vascular smooth muscle cells. J Biol Chem 263:13791–13796 [PubMed]
- Strauss BH, Robinson R, Batchelor WB, Chisholm RJ, Ravi G, Natarajan MK, Logan RA, Mehta SR, Levy DE, Ezrin AM, Keeley FW (1996) In vivo collagen turnover following experimental balloon angioplasty injury and the role of matrix metalloproteinases. Circ Res 79:541–550 [DOI] [PubMed]
- Szöcs K, Lassègue B, Wenzel P, Wendt M, Daiber A, Oelze M, Meinertz T, Münzel T, Baldus S (2007) Increased superoxide production in nitrate tolerance is associated with NAD(P)H oxidase and aldehyde dehydrogenase 2 downregulation. J Mol Cell Cardiol 42:1111–1118. doi:10.1016/j.yjmcc.2007.03.904 [DOI] [PubMed]
- Walford RL, Gallagher R, Sjaarda JR (1964) Serologic typing of human lymphocytes with immune serum obtained after homografting. Science 144:868–870 [DOI] [PubMed]
- Witztum JL (1993) Role of oxidized low density lipoprotein in atherosclerosis. Br Heart J 69:S12–S18 [DOI] [PMC free article] [PubMed]
- Yang RF, Liu AJ, Ma XJ, Li L, Su D, Liu J (2008) Sodium tanshinone IIA sulfonate protects cardiomyocytes against oxidative stress-mediated apoptosis through inhibiting JNK activation. J Cardiovasc Pharmacol 51:396–401. doi:10.1097/FJC.0b013e3181671439 [DOI] [PubMed]
- Zhou YF, Guetta E, Yu ZX, Finkel T, Epstein SE (1996) Human cytomegalovirus increased modified low density lipoprotein uptake and scanvenger receptor mRNA expression in vascular smooth muscle cell. J Clin Invest 98:2129–2138 [DOI] [PMC free article] [PubMed]