Abstract
To develop an investigative tool for the study of human osteosarcoma (OSA), we established a human OSA cell line, namely, SOSP-9607, which exhibits a potential for spontaneous pulmonary metastasis. Subsequently, we screened two related sublines (F5M2 and F4) that have different pulmonary metastatic potentials. An in vivo orthotopic transplantation assay confirmed spontaneous pulmonary metastasis in all mice (100%) transplanted with the more aggressive OSA cells (F5M2) and a lesser degree of metastases with smaller nodules in 33.3% mice transplanted with the less aggressive OSA cell subline (F4). In mice transplanted with F5M2 cells, death from metastasis occurred at a median of 71 days; however, in mice transplanted with F4, no death occurred even after 120 days. Therefore, the F5M2 and F4 sublines, which originated from the same parent cell line, differed with respect to metastasis-related properties such as proliferating ability and invasiveness. Hence, these well-characterized human OSA sublines can be used as valuable models for comparative studies of genetic determinants of OSA in the future.
Keywords: Osteosarcoma, Pulmonary metastasis, Cell line, Biology properties
Introduction
Osteosarcoma (OSA) is the most common type of malignant bone tumor. Even with amputation, this tumor had once been reported to have a poor prognosis. During the past 30 years, surgical intervention and neoadjuvant chemotherapy have been considered as successful treatment approaches for OSA and have improved limb salvage rate and considerably raised the survival of affected patients to 65–75%. However, approximately one-third of all the OSA patients develop lung metastasis with much lower survival rate (Mankin et al. 2004). A greater understanding of the course of metastasis of OSA and the identification of metastasis-associated molecules are essential. A comparative study of two cell sublines with different metastatic potentials has yielded important information regarding the metastasis of tumors other than OSAs (Minn et al. 2005; Steeg et al. 1988; Tavazoie et al. 2008; Toh et al. 1994). On these lines, to elucidate the mechanisms underlying the metastasis of OSA, it is necessary to establish OSA sublines with different metastatic potentials. Because of the limited tumorigenic and poor metastatic abilities of most of the available OSA cell lines, some researchers have established cell lines by transforming the cells with the relevant oncogenes or carcinogenic substances. However, such manipulation might change the biological behavior of the cells or alter the environmental conditions of the hosts (Luu et al. 2005). In 2000, Khanna described a syngeneic model of OSA and screened two different mice OSA cell lines—K7M2 and K12—that originated from the same parental cell line (Khanna et al. 2000). He compared the differences in the gene expression profiles of these 2 sublines, but the murine origin of these sublines limited their wider application in this study (Khanna et al. 2001). In our study, we used a human OSA cell line, namely, SOSP-9607 established at our laboratory, which has higher tumorigenic ability and potential for spontaneous pulmonary metastasis, to screen for two related sublines with high and low metastatic potentials; these sublines were named F5M2 and F4, respectively. We then compared the biological properties associated with the metastatic abilities of the cells of the F5M2 and F4 sublines, including proliferating ability, motility, cytoskeletal organization, invasiveness, adherence, and angiogenic potential. The results indicated that these two clonal variants of the OSA cell line exhibited different levels of cell proliferating and invasion abilities and could be used in the future for studying the mechanism of metastasis of OSA.
Materials and methods
Cell lines
Human osteosarcoma cell line SOSP-9607 was established in our laboratory from the specimen of a 17-year-old patient who was diagnosed with osteosarcoma of chondroblast subtype at the time of surgery. F4 and F5 are the cell clones isolated from the SOSP-9607 cell line using the limited dilution method (Grenman et al. 1989). All the cell lines were maintained in complete RPMI 1640 medium (HyClone) supplemented with 10% fetal calf serum (FCS) (Sijiqing Co.) at 37 °C under 5% CO2 atmosphere. Cells in log-phased growth were harvested and resuspended in serum-free medium (1×107 cells/mL). They were kept at 0 °C until they were used for mouse inoculation (0–2 h). Before cell injection, their viability was determined by trypan blue staining, and the experiments were continued if the cell viability was greater than 90%.
Animals and animal procedures
Four-week-old female nude mice (BALB/c, nu/nu; animal centre of the Fourth Military Medical University in China (FMMU)) were housed under pathogen-free conditions at 26–28 °C and 50–65% humidity. The mice were maintained under a 12-h light/12-h dark cycle and were fed autoclaved standard chow and water. All the animal operations were performed under the rules provided by Declaration of Helsinki and approved by FMMU Ethnics Committee. The anesthesia method and orthotopic transplantation procedures were based on the methods reported previously (Berlin et al. 1993; Khanna et al. 2000). Briefly, after mice were anesthetized by administering intraperitoneal injections of ketamine (0.45 mg/mouse) and xylazine (0.45 mg/mouse), tumor cells were aspirated into a 1-mL tuberculin syringe fitted with a 27-gauge needle, and the needle was inserted through the cortex of the anterior tuberosity of the tibia with a rotating movement to minimize cortical fracture. Once the cortex was traversed, the needle was pushed 3–5 mm into the axis of the tibia. A volume of 20 μL (2 × 105 cells) was injected into the bone. Before the cessation of the effect of anesthesia, radiograms (45 kV, 2.0 mA) of the legs injected with the tumor cells were examined. Radiograms were taken immediately after tumor injection and at a 2-week intervals until the time of killing. The tumor size was measured once a week, and the tumor volume was calculated by the formula of V = 1/6 × π × length × (width)2. Morbidity associated with metastases was characterized by ill thrift, anorexia, dehydration, decreased activity, grooming behavior and dyspnea. Mice with dyspnea were suspected to have lung metastasis and were killed. Pulmonary metastasis in mice was confirmed at the macro and micro levels by administering intratracheal injection of India ink and performing histological examination (hematoxylin-eosin (HE) staining). The percent of spontaneous pulmonary of the mice implanted with F5M2 and F4 cells and the number of nodules visible on the surface of lungs were compared. The difference in the survival of mice injected with these two cell sublines was analyzed for statistical significance by using the log-rank test.
Tumor cell-line proliferation
The tumor cells were seeded in 96-well plates at a density of 1 × 104 cells/well and allowed to adhere to the plates. Subsequently, plates were harvested at 0, 24, 48, and 72 h, and the cell number was quantified using the MTT assay (which utilizes the formazan salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) to detect living cells). The experiments were performed in quadruplicates and were repeated 3 times.
Angiogenesis assay
The tissues were prepared for staining by treatment with 10% formalin solution. The formalin-fixed frozen tissues were immunohistochemically stained for CD31 (Santa Cruz Biotech) and factor VIII (Dako), and the bound antibodies were detected using EnVison+ System kit (Dako) by hematoxylin counterstaining.
Actin cytoskeleton
Cells were grown on sterile cover slips, fixed with 4% p-formaldehyde in PBS for 30 min, and then extracted with 0.2% Triton X-100 for 10 min at room temperature. After fixing and extraction, 20 μL fluoresceinyl-amiomethyidithiolano-phalloidin (phalloidin (FITC)) (ALEXIS) was added to the cover slips, and the cells were incubated at room temperature. After 5 min, the cover slips were washed with PBS, air-dried, and mounted onto slides using mounting media. Cells were visualized on an Olympus microscope using a ×100 objective, and images were captured with a digital camera.
Motility and invasion assays
Millicell inserts (Millipore) with 8-μm filter membranes were precoated with 40 μL Matrigel (dilution; 1:3) (BD). The cells were introduced in the upper chamber, and the cell density was adjusted to 1 × 105 by adding 400 μL of serum-free complete medium, and 600 μL of completed medium with 5% FCS was introduced into the lower chamber. The entire chamber was incubated at 37 °C in 5% CO2 for 24 h. Fixation and staining was performed according to the manufacture’s manual. Briefly, at the end of the incubation period, the Millicell insert was removed from the plate and washed gently with PBS to remove growth medium and unattached cells. Cells on the unmigrated side were gently wiped off with a wet cotton tip applicator. The migrated cells were fixed with 3% glutaraldehyde in PBS for 15 min and washed twice by distilled water. Inserts were immerged in 0.5% Triton X™-100 for 5 min followed by water rinsing twice. After staining with Toluidine Blue (Sigma) to the apical cell side of membrane for 30–60 s, the stained cells were counted under the 20× objective of the microscope from five random fields.
Heterotypic adherence assay
A total of 1×105 tumor cells were introduced in each of the four 96-well flat-bottomed plates precoated with 1:10 Matrigel (BD). The plates were incubated at 37 °C in 5% CO2 for 0.5, 1.5, 12, 24, and 48 h. After incubation, medium was carefully suctioned out of each well, and every well was washed 3 times with 100 μL of PBS. Between each wash, the plates were manually rocked back and forth three times. Subsequently, 4 g/L of crystal violet was added to the wells for 15 min, and then the wells were rinsed for 3–5 times with PBS until no purple color was observed. Finally, the cells were extracted using 100 μL glacial acetic acid, and the absorbance was read at 490 nm to determine the number of adhered cells.
Results
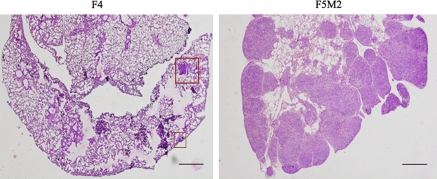
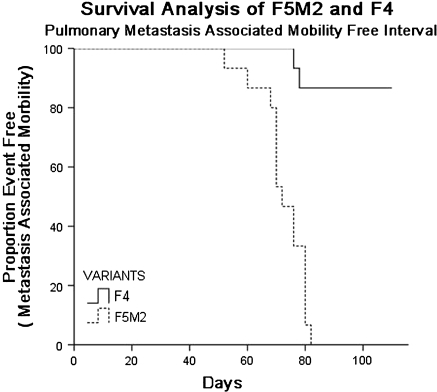
We isolated 12 cell clones by the limited dilution method and orthotopically inoculated nude mice with cells from these clones. The pulmonary metastatic abilities of these cell clones seemed different: for a given period, the mice bearing F4 and F5 tumor cells mostly developed primary tumors, while the former had rare macro- or micro-scopically pulmonary metastasis (Table 1). The metastatic nodules from F5-transplanted mice were isolated and expanded in vitro to develop a cell subline, named F5M0. After 2 months, F5M0 cells were inoculated into the tibia of the nude mice in the same manner. The abovementioned procedures were repeated 3 times to acquire the more aggressive subline named as F5M2. The histological appearances of spontaneous pulmonary metastasis of F4 and F5M2 cell lines are shown in Fig. 1. The percentage of mice with pulmonary metastasis was higher, and the number of metastatic modules throughout the surface of the lung was greater in the F5M2-implanted mice than in the F4-implanted ones. The pulmonary metastatic potential of the F5M2-transplanted model was significantly greater than that of the F4-transplanted model (Table 2). This difference is clearly demonstrated by the Kaplan–Meier survival analysis shown in Fig. 2.
Table 1.
Transplantable and spontaneous metastasis of SOSP-9607 variants
| SOSP 9607 variants | Time to autopsy after tumor injection (weeks) | Tumor take rate at inoculation site (mice with tumors/mice inoculated) | Mice with macroscopically visible tumors | Mice with microscopically visible tumors |
|---|---|---|---|---|
| D5 | 6 | 1/5 | 0/5 | 0/5 |
| D6 | 6 | 1/5 | 0/5 | 0/5 |
| E4 | 6 | 1/5 | 0/5 | 0/5 |
| E7 | 6 | 1/5 | 0/5 | 0/5 |
| F4 | 6 | 4/5 | 1/5 | 1/5 |
| F5 | 6 | 5/5 | 4/5 | 5/5 |
| F7 | 6 | 5/5 | 2/5 | 2/5 |
| F9 | 6 | 5/5 | 1/5 | 3/5 |
| G6 | 6 | 5/5 | 0/5 | 3/5 |
| G9 | 6 | 5/5 | 0/5 | 2/5 |
| H1 | 6 | 1/5 | 0/5 | 0/5 |
| H7 | 6 | 1/5 | 0/5 | 0/5 |
| 9607 | 6 | 2/5 | 2/5 | 2/5 |
Fig. 1.
Sections of lung metastasis (hematoxylin and eosin stain, ×40). F5M2 shows severe pulmonary metastasis and F4 reveals few metastasis, nodulus (square) in the same period. Bar 500 μm
Table 2.
F5M2 and F4: spontaneous pulmonary metastasis
| Cell sublines | Percent spontaneous pulmonary metastasis (%)a | Survival (spontaneous pulmonary metastasis) median (days ± SD)b | Number of spontaneous pulmonary metastasisc |
|---|---|---|---|
| F5M2 (n = 15) | 100 | 71 ± 2d | >200 |
| F4 (n = 15) | 33.3 | Not achieved at day 120 | 12 |
aPercentage of tumor bearing mice developing spontaneous pulmonary metastasis (χ2 test, p < 0.001)
bMetastasis-associated morbidity-free survival in mice with primary tumors
cEvaluation of pulmonary metastasis with injection of India ink 42 days post transplantation. Data represent evaluation of pulmonary metastases in ten mice
dMedian survival of mice transplanted with F5M2 cells is significantly shorter than those transplanted with F4 cells (Student’s t-test, p < 0.001)
Fig. 2.
Kaplan–Meir survival plot for orthotopic spontaneous metastasis in F5M2 models compared to F4 models. Compared with F4 models, the median event-free survivals was significantly shorter in F5M2 models
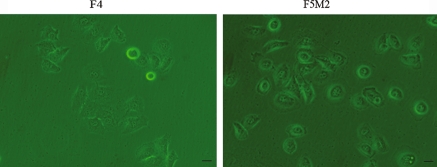
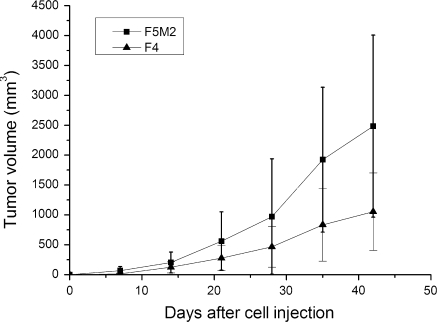
Microscopic analysis of these two cell lines revealed that both the F4 and F5M2 cells retained the epithelial cell-like shape and similar size, F4 cells is more polygonal while F5M2 shows near round shape (Fig. 3). The growth rate of F5M2 cells was greater than that of the less aggressive F4 cells: the doubling time of the F5M2 cells in the logarithmic phase was 20 ± 2 h, while that of the F4 cells was 31 ± 3 h (p < 0.05). The average days for transplanted tumors to reach 500 mm3 was compared, High metastatic potential subline F5M2 grew faster than the low metastatic potential cell subline in vivo (p < 0.05, Fig. 4) .
Fig. 3.
Morphological appearance of cultured low-metastatic subline F4 and high-metastatic subline F5M2 (×400). Bar 20 μm
Fig. 4.
Tumor growth after orthotopic injection of high- and low-metastatic cell sublines. Error bars indicate SD
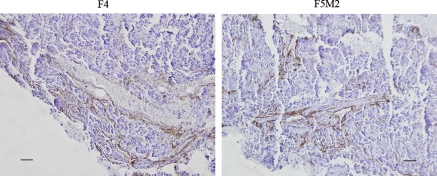
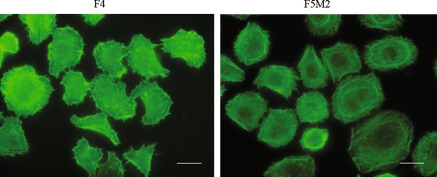
The tumor angiogenesis abilities of both the primary tumor and the pulmonary metastatic tumors did not differ remarkably as revealed by CD31 and factor VIII immunohistochemistry staining (Fig. 5). In cytoskeleton assay, F-actin staining by phalloidin (FITC) revealed that F4 cells had more larger cellular extensions while F5M2 had more actin stress fibers (Fig. 6).
Fig. 5.
CD31 staining with hematoxylin counterstaining of F5M2 and F4. Bar 50 μm
Fig. 6.
FITC-phalloidin staining of F-actin reveals that F4 cells had more larger cellular extensions, while F5M2 had more actin stress fibers (100× oil-immersion objective lens). Bar 20 μm
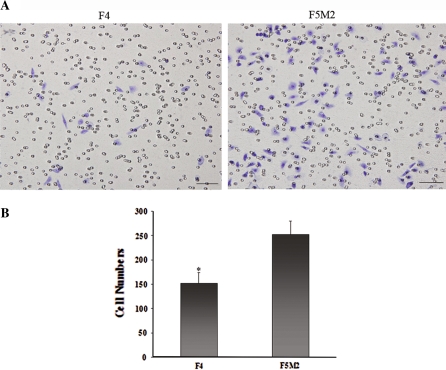
The invasiveness of these two cell sublines were evaluated by comparing their relative abilities to invade the tumor extracellular matrix as described in materials and methods. F5M2 cells were more aggressive than the F4 cells, with the number of cells migrating over the Matrigel being higher among the former than the latter (p < 0.01, Fig. 7). The difference in the heterotypic adherence (cell-to-substrate adherence) of F5M2 and F4 cells was evaluated using Matrigel as the substrate. Adherence was assessed at early (0.5 and 1.5 h) and late time points (12, 24, and 48 h). No significant difference was observed in the heterotypic adhesion ability of these two cell lines at all 5 time points (data not shown).
Fig. 7.
a Invasion of F4 and F5M2 cells with transwell chambers in vitro. Cells migrating to the lower surface of filters were stained with toluidine blue solution. Cells were counted from five fields per sample at ×200 and averaged. Bar 50 μm. b Values in the graph represent the average number of cells (mean ± SD). Compared with F5M2, the invasion capacity of F4 was significantly lower (p < 0.01)
Discussion
Despite the achievements in surgical limb salvage and chemotherapy management, the mechanisms underlying pulmonary metastasis on OSA remains to be elucidated partly because of the scarcity of a reliable and reproducible animal model. An ideal model should exhibit a high uptake rate of tumor cells during xenograft transplantation assay and present the clinical signs characteristic of that cancer. On the basis of the results of the previous study, we selected the orthotopic transplantation model of nude mice for this experiment. Since the rate of tumor uptake of the available OSA tumor cell lines, such as U2OS or HOS (Dass et al. 2006), by the transplantation model was low, we used the OSA cell line SOSP-9607 established in our laboratory as the parent cell line. This cell line was created by using cells from a tumor sample of a patient with pathologically diagnosed OSA. The SOSP-9607 line has been cultured to over 200 generations without any marked changes in cellular morphology. The in vitro assay of these cells revealed an epithelial appearance, multiploid karyotype, and other characteristics of human malignant tumors. Moreover, the histological examination and X-ray analysis of the orthotopic transplantation model revealed that the properties of the transplanted tumor were similar to those of the surgically excised primary tumor. Thus, SOSP-9607 can be considered as an ideal parent cell line for the screening of clonal variants with different metastatic abilities. Through limit dilution method, we obtained 12 cell clones from SOSP-9607. To select the more potential cell variants presenting differential metastasis ability in vitro assay later, we used all these 12 clones to perform the orthotopic transplantation. These cell subpopulations presented distinct tumor percent rate and spontaneous metastasis condition. Cell variants with poorer tumorigenesis were excluded, such as D5, D6, H1 and H7. Among the other variants, F4 showed only two micro-metastasis on the lung surface of only one mouse, while F5 exhibited micro-metastasis in all mice. Although the number of animals is limited, the result presumed the differential potential of these two cell clones.
The animal models of xenograft transplantation can be of two types: the first one is hetero-transplantation model, which includes the widely used subcutaneous transplantation model and the intravenous tail-vein injection or intracardiac injection model, and the other one is the orthotopic transplantation model. However, the subcutaneous transplantation method does not offer a paired microenvironment that might result in transplantation failure in some models. Further, injection of tumor cells through the tail vein or cardiac atrium failed to demonstrate the later events in the metastatic cascade (Poste and Fidler 1980). Because of these limitations of the heterotransplantation model, the orthotopic transplantation method has been inclined to use for establishing the metastatic models of human tumors. The sources of transplantation can also be consisted of two types. Transplantation with a small tissue mass that avoids the destruction of tumor cell–cell contacts might be considered as the best method that mimics the real clinical conditions (Crnalic et al. 1997). However, the transplantation of mice using this method in our study failed to result in the development of metastatic nodules, which were to be transplanted again into the primary site, partly because the primary tumor developed so fast that the host mouse became dyscrasic before the detection of pulmonary micrometastasis on gross examination. Amputation of the tumor-bearing limb was required to prevent morbidity associated with the primary tumor (Khanna et al. 2000). However, this operation was difficult to complete in our laboratory due to the complexed microsurgical techniques and necessary anesthetic conditions. As an alternative, we selected the orthotopic cell injection method to establish the transplantation models. The model established presented shorter primary tumor latency and higher metastasis occurrence. Further, we could not find the other remote organ’s metastasis except the lung at the time point of autopsy by the histological examination which coordinates with the organ specificity of osteosarcoma. Thus this model could be considered as an ideal method to screen the cell sublines with differential metastasis potentials.
Metastasis is a series of complex cascades or biological events, including proliferation of tumor cells at the primary site, reduction in the adherence ability of the cells to the baseline matrix, invasion of the tumor cells into the circulation system, evasion of immune surveillance, extravasation of the tumor to the secondary site, and development of metastases (Woodhouse et al. 1997). After reviewing relevant literature reports, we selected proliferating ability, adherence, angiogenic potential, motility, and invasiveness for the identification of metastasis-associated properties of the cell sublines. Through comparative studies, we found that these two cell sublines exhibiting remarkable metastatic abilities in the in vivo transplantation assays demonstrated increased proliferating and invasion abilities (F5M2 was more aggressive than F4 cell line), while other biological characteristics such as adherence, angiogenesis, and motility afforded similar results. Recent genomic analysis of a cell subline with a high metastatic potential confirmed that few genes that determine the cell growth also control the growth of the cells at the second site (Minn et al. 2005). Some researchers also hypothesize that few tumor cells from the metastatic site return to the primary site and increase the growth rate of the primary tumor. These findings suggest that a high growth rate of the primary tumor indicates a high possibility of tumor metastasis. Tumor cells secrete many cytokines, such as TGF β, through the paracrine pathway to improve other metastasis-associated abilities, such as angiogenesis, in order to facilitate metastasis (Padua et al. 2008). On the whole, the proliferation ability is probably the most important determinant of tumor metastasis. In our study, the F5M2 cell subline with high metastatic potential appears to possess a higher growth rate in vitro than the F4 subline with low metastasis potential. This is in accordance with the development of dyscrasia and early diagnosis of tumors at the primary site in mice transplanted with F5M2 cells than in mice transplanted with F4 cells. The invasive phenotype of F5M2 and F4 cells was assessed by comparing their relative abilities to invade tumor extracellular matrix (Matrigel) in the Millipore culture system. Tumor cells secrete many proteinases that dissolve the extracellular substrates to help invasion of the cells into the circulatory system. Matrix metalloproteinase (MMP) molecular family is one such important family of proteinases. In addition to facilitating tumor cell invasion, the polypeptide fragments generated by the proteinase function of MMP reversely combine with the receptors on the surface of the surrounding tumor cells to activate metastasis, initiating a pathway that eventually becomes a vicious circle (Gupta and Massague 2006). Of cause, the difference of proliferation and invasion ability between these two cell sublines cannot be completely contribute to metastatic behavior differences due to the limit validate possibility of these assays, but it can be presumed that there might be some unknown factors influencing the transplantation of these two cell sublines with different metastatic abilities. These factors might be related to other important factors influencing the tumor’s microenvironment, such as immune surveillance and the interaction between the cell and the microenvironment. A greater number of studies support the fact that not only does the tumor microenvironment provide essential factors for tumor growth but also help or educate the malignant cells to develop metastatic abilities (Gupta and Massague 2006).
Conclusion
In short, these two cell sublines with the different pulmonary metastatic potentials originate from the same parent cell line and possess similar biological properties, except proliferation and invasion abilities. Therefore, these cell lines are ideal comparative tools that can be used in future studies directed at discovering the genetic determinants of human OSA pulmonary metastasis by microarray analysis.
Acknowledgments
This work is supported by two grants from the National Natural Science Foundation of China (No. 30330610 and No. 30873027). We would like to thank Professor Qiu Xiuchun for his technical help and Zhao Hong for his efforts in taking care of animals. We also thank Dr. Ji Zhengang for his valuable advice.
Footnotes
Xiang Chen and Tong-Tao Yang contributed equally to this work.
References
- Berlin O, Samid D, Donthineni-Rao R, Akeson W, Amiel D, Woods VL Jr (1993) Development of a novel spontaneous metastasis model of human osteosarcoma transplanted orthotopically into bone of athymic mice. Cancer Res 53:4890–4895 [PubMed]
- Crnalic S, Hakansson I, Boquist L, Lofvenberg R, Brostrom LA (1997) A novel spontaneous metastasis model of human osteosarcoma developed using orthotopic transplantation of intact tumor tissue into tibia of nude mice. Clin Exp Metastasis 15:164–172 [DOI] [PubMed]
- Dass CR, Ek ET, Contreras KG, Choong PF (2006) A novel orthotopic murine model provides insights into cellular and molecular characteristics contributing to human osteosarcoma. Clin Exp Metastasis 23:367–380 [DOI] [PubMed]
- Grenman R, Burk D, Virolainen E, Buick RN, Church J, Schwartz DR et al (1989) Clonogenic cell assay for anchorage-dependent squamous carcinoma cell lines using limiting dilution. Int J Cancer 44:131–136 [DOI] [PubMed]
- Gupta GP, Massague J (2006) Cancer metastasis: building a framework. Cell 127:679–695 [DOI] [PubMed]
- Khanna C, Prehn J, Yeung C, Caylor J, Tsokos M, Helman L (2000) An orthotopic model of murine osteosarcoma with clonally related variants differing in pulmonary metastatic potential. Clin Exp Metastasis 18:261–271 [DOI] [PubMed]
- Khanna C, Khan J, Nguyen P, Prehn J, Caylor J, Yeung C et al (2001) Metastasis-associated differences in gene expression in a murine model of osteosarcoma. Cancer Res 61:3750–3759 [PubMed]
- Luu HH, Kang Q, Park JK, Si W, Luo Q, Jiang W et al (2005) An orthotopic model of human osteosarcoma growth and spontaneous pulmonary metastasis. Clin Exp Metastasis 22:319–329 [DOI] [PubMed]
- Mankin HJ, Hornicek FJ, Rosenberg AE, Harmon DC, Gebhardt MC (2004) Survival data for 648 patients with osteosarcoma treated at one institution. Clin Orthop Relat Res 429:286–291 [DOI] [PubMed]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD et al (2005) Genes that mediate breast cancer metastasis to lung. Nature 436:518–524 [DOI] [PMC free article] [PubMed]
- Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR et al (2008) TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133:66–77 [DOI] [PMC free article] [PubMed]
- Poste G, Fidler IJ (1980) The pathogenesis of cancer metastasis. Nature 283:139–146 [DOI] [PubMed]
- Steeg PS, Bevilacqua G, Kopper L, Thorgeirsson UP, Talmadge JE, Liotta LA et al (1988) Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst 80:200–204 [DOI] [PubMed]
- Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD et al (2008) Endogenous human microRNAs that suppress breast cancer metastasis. Nature 451:147–152 [DOI] [PMC free article] [PubMed]
- Toh Y, Pencil SD, Nicolson GL (1994) A novel candidate metastasis-associated gene, mta1, differentially expressed in highly metastatic mammary adenocarcinoma cell lines. cDNA cloning, expression, and protein analyses. J Biol Chem 269:22958–22963 [PubMed]
- Woodhouse EC, Chuaqui RF, Liotta LA (1997) General mechanisms of metastasis. Cancer 80:1529–1537 [DOI] [PubMed]